Simple Summary
Natural killer (NK)/T-cell lymphomas are aggressive extranodal Epstein–Barr virus (EBV)-positive malignancies. They can be divided into three subtypes: nasal (involving the nose and upper aerodigestive tract), non-nasal (involving skin, gastrointestinal tract, testis and other organs) and disseminated (involving multiple organs). Lymphoma cells are positive for CD3ε, CD56, cytotoxic molecules and EBV-encoded small RNA. There is a predilection for Asian and Central/South American populations. Genome-wide association studies have identified lymphoma susceptibility loci in Asians. Positron emission tomography computed tomography and plasma EBV DNA quantification are crucial at diagnosis and follow-up. Stage I/II patients receive non-athracycline asparaginse-containing regimens, together with sequential/concurrent radiotherapy. Anthracycline-containing regimens are ineffective. Stage III/IV patients receive asparaginase-containing regimens, followed by allogeneic haematopoietic stem cell transplantation (HSCT). Autologous HSCT does not improve outcome. In relapsed/refractory patients, novel approaches include PD1/PD-L1 targeting, EBV-specific cytotoxic T-cells, and monoclonal antibodies. Small molecules including histone deacetylase inhibitors may be beneficial.
Abstract
Natural killer (NK)/T-cell lymphomas are aggressive malignancies. Epstein–Barr virus (EBV) infection in lymphoma cells is invariable. NK/T-cell lymphomas are divided into nasal, non-nasal, and disseminated subtypes. Nasal NK/T-cell lymphomas involve the nasal cavity and the upper aerodigestive tract. Non-nasal NK/T-cell lymphomas involve the skin, gastrointestinal tract, testis and other extranodal sites. Disseminated NK/T-cell lymphoma involves multiple organs, rarely presenting with a leukaemic phase. Lymphoma cells are positive for CD3ε (not surface CD3), CD56, cytotoxic molecules and EBV-encoded small RNA. There is a predilection for Asian and Central/South American populations. Genome-wide association studies have identified lymphoma susceptibility loci in Asian patients. Positron emission tomography computed tomography and plasma EBV DNA quantification are crucial evaluations at diagnosis and follow-up. Stage I/II patients typically receive non-athracycline regimens containing asparaginse, together with sequential/concurrent radiotherapy. Anthracycline-containing regimens are ineffective. Stage III/IV patients are treated with asparaginase-containing regimens, followed by allogeneic haematopoietic stem cell transplantation (HSCT) in suitable cases. Autologous HSCT does not improve outcome. In relapsed/refractory patients, novel approaches are needed, involving PD1/PD-L1 targeting, EBV-specific cytotoxic T-cells, and monoclonal antibodies. Small molecules including histone deacetylase inhibitors may be beneficial in selected patients. Future strategies may include targeting of signalling pathways and driver mutations.
1. Introduction
Natural killer (NK) cells develop from a common lymphoid progenitor capable of differentiation to all lymphoid lineages [1]. Expression of the transcription regulators STAT5, NFIL3, NOTCH, PU.1, TCF1, RUNX3 and CBFB leads to NK lineage commitment [2]. NK-cells are part of the innate immune system and are responsible for detection and killing of tumour or virally infected cells [3]. They are strategically located at mucosal surfaces, the skin and gut, and they circulate between the blood and bone marrow [3]. The normal residency of NK-cells in these sites accounts for the predominant extranodal localization of malignancies arising from neoplastic NK-cells [4]. NK-cells express CD2, cytoplasmic CD3 epsilon (ε) (not surface CD3), CD16, CD56, CD94, and cytotoxic molecules (perforin, granzyme, T-cell intracellular antigen 1, TIA-1). Immunophenotypically, this pattern of antigen expression is preserved in NK-cell malignancies [4].
First reported more than seven decades ago, lethal midline granuloma was described as destructive midline facial malignancies that resulted inexorably in death [5]. Histologically, these lesions showed neoplastic lymphoid cells admixed with inflammatory cells and were morphologically referred to as polymorphic reticulosis. The initial detection of cytoplasmic CD3ε, together with the angiocentric and angiodestructive features of the tumour, resulted in its classification as an angiocentric T-cell lymphoma [6].
Further refinement of immunophenotyping revealed that these angiocentric T-cell lymphomas were in most cases surface CD3 negative, with T-cell receptor (TCR) genes in a germline configuration. Therefore, lymphoma cells showed feature typical of NK-cells. However, cases with similar anatomical localization and pathologic features may be of bona fide T-cell origin, expressing surface CD3 and possessing clonal TCR gene rearrangement [7]. Whether of putative NK-cell or T-cell derivations, these cases have indistinguishable clinicopathologic features and comparable treatment outcome. The nomenclature extranodal NK/T-cell lymphoma is currently adopted by the World Health Organization (WHO) classification to recognize their possible NK-cell and T-cell origins [8].
2. Clinical Subtypes of NK/T-Cell Lymphomas
NK/T-cell lymphomas are almost exclusively extranodal in their distribution, with the nasal cavity, nasopharynx, oropharynx, the Waldeyer’s ring, and the upper aerodigestive tract most commonly involved (Figure 1A,B). Clinically, they are referred to as nasal NK/T-cell lymphomas [9]. In about 10–20% of cases, other sites, including skin, testicles, gastrointestinal tract and salivary glands, are involved. Clinically, they are referred to as the non-nasal subtype (Figure 1C). Notably, the sites involved in non-nasal NK/T-cell lymphomas are also those that nasal NK/T-cell lymphomas disseminate to in advanced stages. Hence, for non-nasal NK/T-cell lymphomas, radiologic and/or histologic investigations ought to be performed to rule out nasal involvement. For an apparent non-nasal NK/T-cell lymphoma that can be shown to have nasal involvement, the case should be reclassified as disseminated nasal NK/T-cell lymphoma [9]. Rarely, the lymphoma is disseminated on presentation and may be associated with a leukaemic phase. Clinically, they are referred to as the aggressive leukaemia/lymphoma subtype (Figure 1D) [9].
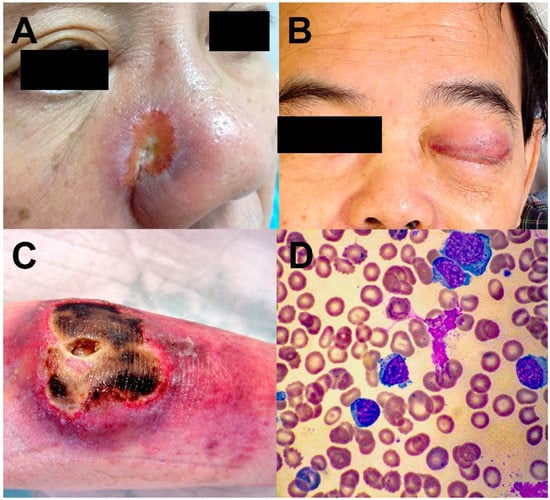
Figure 1.
Different clinical forms of NK/T-cell lymphomas. (A) Nasal NK/T-cell lymphoma, eroding from the nasal cavity into the skin. (B) Nasal NK/T-cell lymphoma eroding into the orbit and cavernous sinus, resulting in third nerve palsy and complete ptosis. (C) Non-nasal NK/T-cell lymphoma of the skin. (D) Aggressive NK/T-cell leukaemia/lymphoma. Circulating lymphoma cells were cytologically large granular lymphocytes.
These subtypes differ in clinical presentations. However, their underlying pathology and molecular alterations are comparable such that treatment strategies for these subtypes are similar.
3. Pathological Features of NK/T-Cell Lymphomas
The lymphomatous infiltrate is polymorphic, with median-sized malignant lymphoid cells intermixed with inflammatory cells. Cytologically, lymphoma cells show abundant cytoplasm with azurophilic granules, resembling large granular lymphocytes. Angiocentricity and angiodestruction may be found if blood vessels are included in the biopsy, explaining the frequent occurrence of coagulative necrosis. Lymphoma cells are typically CD2+, surface CD3−, cytoplasmic CD3ε+, CD56+, and cytotoxic molecules (perforin, granzyme, TIA1)+. Epstein Barr virus (EBV) is invariably present in an episomal form [4,8]. The current WHO classification requires presence of EBV for the diagnosis of NK/T-cell lymphoma [8]. Furthermore, there should also be expression of either CD56 or cytotoxic molecules. If CD56 and cytotoxic molecules are absent, the diagnosis becomes an EBV-positive peripheral T-cell lymphoma not otherwise specified [8].
Recently, exceptional cases of EBV-negative aggressive leukaemia/lymphoma of putative NK-cell derivation had been reported [10]. However, only few cases were described; thus, it remains unclear if they were biologically or clinically similar to NK/T-cell lymphoma.
4. Epidemiology and Genetic Susceptibilities
NK/T-cell lymphomas occur predominantly in the Asian and Central/South American populations [4,8,9]. Genome-wide association studies in Asian patients have identified three genetic loci, HLA-DPB1, IL18RAP and HLA-DRB1, in which amino-acid differences may increase the susceptibility to NK/T-cell lymphoma [11,12]. HLA-DPB1 is the β1 subunit of the HLA-DP heterodimer, which participates in extracellular antigen presentation to CD4+ T-cells [11]. IL18RAP (interleukin-18 receptor associated protein) is an accessory subunit of the heterodimeric IL-18 receptor. It increases the affinity of IL-18 receptor binding to IL-18, which results in enhanced cell-mediated immunity. IL18-RAP mutations are associated with Crohn’s disease [13], and susceptibility to coeliac disease and leprosy. HLA-DRB1 is the most prevalent β subunit of the HLA-DR heterodimer. Changes in HLA-DRB1 are associated with rheumatoid arthritis [14] and increased risks of nasopharyngeal carcinoma [12]. The changes in HLA-DRB1 in NK/T-cell lymphomas are predicted to alter the peptide-binding pocket 7 of HLA-DR [12]. The association of proteins involved in immune response with susceptibility to NK/T-cell lymphoma suggests that the racial predilection may be related to genetic differences in immune reaction against EBV infection. It remains to be defined if these genetic susceptibilities may be demonstrated in other ethnic populations at risk of NK/T-cell lymphomas.
5. Molecular Pathology of NK/T-Cell Lymphomas
In NK/T lymphoma cells, EBV infection shows a latency II pattern, with neoplastic cells expressing LMP1, LMP2 and EBNA1 [15]. EBV is present in a clonal form, suggesting that infection occurs before malignant transformation. Furthermore, LMP1, LMP2 and EBNA1 are oncoproteins, and may contribute to lymphomagenesis.
Early karyotypic studies showed that deletion of the long-arm of chromosome 6 (6q-) was the most consistent aberration observed [16], which was confirmed by comparative genomic hybridization [17] and loss of heterozygosity analyses [18]. Putative tumour suppressor genes in this segment of chromosome 6q that might be involved in NK/T-cell lymphomagenesis include HACE1 [19], PRMD1 [20], FOXO3 [21] and PTPRK [22]. In addition to inactivation/deletion of tumour suppressor genes, activation of putative oncogenes, including EZH2 [23] and RUNX3 [24], might also be involved. With gene expression profiling, other oncogenic mechanisms were also shown to be involved, including JAK/STAT and aurora kinase A activation [25] and overexpression of MYC and NF-κB [26].
Next generation sequencing (NGS) of NK/T-cell lymphoma samples from various patient populations had shown different results. Mutated genes defined by NGS included JAK3 [27], STAT3 and STAT5B (proliferation signalling) [28,29]; BCOR, KMT2D, ARID1A, EP300 and ASXL3 (epigenetic deregulation) [29,30]; TP53, MGA (tumour suppressor) [30], and DDX3X (RNA helicase, multiple functions) [30]. These gene mutations were predicted to have different oncogenic effects (Figure 2).
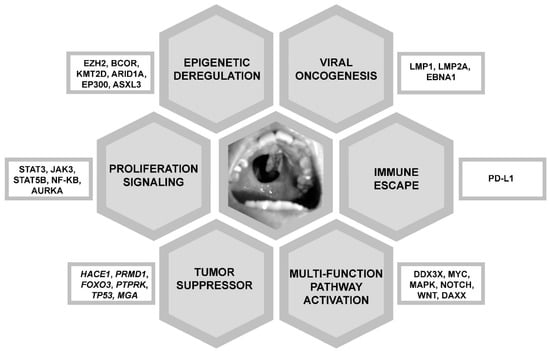
Figure 2.
Molecular pathogenesis of NK/T-cell lymphomas. These molecular pathways are targetable therapeutically. Shown in the centre is a typical hard palate perforation due to erosion from an NK/T-cell lymphoma in the nasal cavity, giving the classical appearance of a “lethal midline granuloma”.
Besides mutations, other mechanisms have been proposed to critically affect gene functions in NK/T-cell lymphomas. Promoter hypermethylation of BIM, DAPK1, SHP1, TET2 and SOCS6 resulted in down-regulation of these tumour suppressor genes [31]. Down-regulation of microRNAs miR-101, miR-26, miR-146a, miR-28-5 and miR-363 was predicted to result in deregulation of cell cycle-related, TP53 and MAPK signalling pathways [32]. These non-mutational mechanisms may contribute to NK/T-cell lymphomagenesis.
Overexpression of programmed cell death protein 1 ligand (PD-L1) had been shown to be a potential mechanism of immune evasion in EBV-infected lymphoid cells [33]. PD-L1 is the cognate ligand of the immune checkpoint protein PD1 expressed on T-cells. In NK/T-cell lymphomas, PD-L1 is overexpressed through a number of mechanisms [34]. The EBV oncoprotein LMP1 drives PD-L1 expression through the NF-κB pathway [35]. Furthermore, LMP1 positively regulates the expression of the transcription factor AP-1, which up-regulates PD-L1 [36]. Finally, the JAK/STAT pathway is activated in NK/T-cell lymphoma cells, which interacts with the interferon-stimulated response element located in the promoter region of PD-L1, driving its expression [37]. By binding to PD1 on T-cells and inhibiting their functions, PD-L1 enables NK/T lymphoma cells to escape immune detection, thereby enhancing their proliferation.
A combined genomic and transcriptomic approach has divided NK/T lymphoma cells into three molecular subtypes, TSIM, MB and HEA [38]. The TSIM subtype, accounting for about 55% of cases, was defined by mutations in the JAK-STAT pathway and TP53, amplifications of the 9p24.1/JAK2, 17q21.2/STAT3/5B/5A and 9p24.1/PD-L1/2 loci, and deletion of chromosome 6q21. Lymphoma cells in the TSIM subtype had a predominant NK-cell gene expression pattern, activation of the JAK/STAT pathway, overexpression of PD-L1/2 and genomic instability. The MB subtype, accounting for about 18% of cases, was defined by MGA mutation and loss of heterozygosity (LOH) of the 1p22.1/BRDT locus. Lymphoma cells in the MB subtype had a gene expression pattern intermediate between NK-cells and T-cells. Mutations in MGA led to increased MYC expression, and together with BRDT LOH resulted in MAPK, NOTCH and WNT pathway activations. The HEA subtype, accounting for about 27% of cases, was defined by mutations in HDAC9, EP300 and ARID1A. Lymphoma cells in the HES subtype had a predominant T-cell gene expression pattern, associated with overexpression of the histone chaperone DAXX, and activation of the NF-κB and T-cell receptor signalling pathways. From RNA-seq and immunohistochemical results, the TSIM, MB and HEA subtypes were characterized by overexpression of PD-L1, MYC and DAXX respectively. These findings might be of therapeutic implications.
6. Disease Presentations
Patients with nasal NK/T-cell lymphomas often have a long history of nasal blockage and discharge. On presentation, some destruction of nasal and facial structures is common. Nasal tumours can erode and destroy the hard palate, creating a communication between the nasal and oral cavities (the typical lethal midline granuloma), severely affecting phonation and ingestion. Invasion of the orbits may occur, affecting vision. In advanced nasal NK/T-cell lymphomas, systemic dissemination with a predilection for non-nodal organs occurs. In non-nasal NK/T-cell lymphomas, skin is the most common initial site of presentation [39]. However, virtually any anatomical sites can be involved. Interestingly, central nervous system (CNS) involvement on presentation is uncommon and usually occurs in advanced stages or relapsed disease [40].
Aggressive NK-cell leukaemia/lymphoma is currently referred to by WHO classification as aggressive NK-cell leukaemia [41]. Clinical manifestations include fever, lymphadenopathy, hepatosplenomegaly, pancytopenia, skin infiltration, deranged liver function, coagulopathy, hyperferritinaemia, and haemophagocytosis in the marrow and other reticuloendothelial organs [42]. Aggressive NK-cell leukaemia/lymphoma may arise de novo or may represent a terminal event in nasal or non-nasal NK/T-cell lymphomas.
7. Differential Diagnoses of NK/T-Cell Lymphomas
Plasmacytoid dendritic cell neoplasms, previously erroneously referred to as blastoid NK-cell lymphomas, are primarily a cutaneous neoplasm, although marrow infiltration occasionally occurs [43]. It can be differentiated from cutaneous NK/T-cell lymphomas by absence of CD3/CD3ε, cytotoxic molecules, and EBV infection. NK-cell lymphomatoid gastropathy/NK-cell enteropathy is a rare non-neoplastic NK-cell proliferation in the stomach, small and large bowels [44,45]. There is no EBV infection, and the disease is localized and often self-regressing. Chronic lymphoproliferative disorder of NK-cells is a rare condition, currently unclear if reactive or neoplastic. There is no EBV infection. The disease is indolent and may self-regress. No treatment is required.
8. Evaluation of Newly Diagnosed NK/T-Cell Lymphoma Patients
Histologic proof of organ involvement may not be straightforward, as the lymphoma infiltrate tends to be polymorphic, which is different from conventional lymphomas where a monomorphic infiltrate is found. Morphological evaluation can be supplemented by in situ hybridization for EBV encoded small RNA (EBER), which provides an accurate way of localizing lymphoma cells [8].
As lymphoma cells undergo apoptosis, fragments of EBV DNA are released into the circulation. Quantification of circulating EBV DNA therefore provides a molecular means of measuring tumour load [46]. Whole blood should not be used, because of the variable presence of circulating memory B-cells that may be infected by EBV, which introduces unpredictable errors [46]. Instead, plasma EBV DNA provides a more reliable assessment [46,47]. Plasma EBV DNA at diagnosis gives an accurate measurement of lymphoma load [48]. During treatment, plasma EBV DNA also provides a dynamic assessment of disease load, reflecting how well the lymphoma is responding to therapy [49]. Accordingly, on completion of treatment, quantifiable circulating EBV DNA indicates residual lymphoma and portends an unfavourable prognosis [50].
Different modalities of radiological examination have been adopted. NK/T-cell lymphoma cells are fluorine-18 fluorodeoxyglucose (FDG)-avid. Therefore, FDG positron emission tomography computed tomography (PET/CT) is the standard imaging modality for NK/T-cell lymphoma [51,52]. All patients should be evaluated with PET/CT on initial presentation for accurate staging. Furthermore, the five-point Deauville score should be adopted for the evaluation of PET/CT performed during and after completion of treatment [50,53].
9. Principles of Management of NK/T-Cell Lymphomas
Of all lymphoid cells, NK-cells express the highest level of P-glycoprotein, which confers a multidrug resistance (MDR) phenotype [54]. Anthracycline-containing (CHOP, cyclophosphamide, adriamycin, vincristine, prednisolone, or CHOP-like) regimens, designed for the treatment of conventional high-grade B-cell lymphomas, are MDR-dependent and hence ineffective [9]. Non-anthracycline-containing regimens have been developed for NK/T-cell lymphomas [9]. An important component of these regimens is asparaginase, which induces apoptosis of NK-cells in vitro [55]. Asparaginase has significant single-agent activity in relapsed/refractory (R/R) NK/T-cell lymphoma [9]. Currently, most recommended regimens for NK/T-cell lymphomas contain asparaginase or its pegylated form.
Prognostication and risk stratification are required for delivery of optimal therapy. Models developed in patients treated with CHOP or CHOP-like regimens, including the international prognostic index [56] and the Korean prognostic index [57], still retain prognostic value in patients treated with non-anthracycline regimens but have become somewhat obsolete in comparison with models that include biologic parameters. Two prognostic indices, prognostic index for NK/T-cell lymphoma (PINK) and PINK with EBV DNA (PINK-E), have been developed specifically for patients treated with non-anthracycline-containing regimens [58]. These prognostic models are based on presentation parameters. PINK includes only clinical parameters (age > 60 years, stage III/IV disease, distant lymph-node involvement, non-nasal type disease), whereas PINK-E considers additionally presentation EBV DNA (whether or not detectable), thereby incorporating one more important biologic prognosticator [48,49].
Prognostication based on dynamic parameters has also been proposed. Interim quantification of plasma EBV DNA and five-point Deauville scoring with PET/CT during treatment may predict outcome [49,53]. A combination of plasma EBV DNA quantification and PET/CT Deauville scoring at the end of treatment was also reported to be prognostic [50]. Such prognostic models will have to be validated in larger prospective studies.
9.1. Management of Stage I/II Disease
The treatment modalities for stage I/II NK/T-cell lymphomas include involved-field radiotherapy, chemotherapy or their combinations [59]. NK/T-cell lymphoma is radiosensitive, such that radiotherapy is conventionally considered a component of treatment. However, consensus lacks on how and when radiotherapy and chemotherapy should be used, viz., combined or sequenced [59]. This is because NK/T-cell lymphoma is uncommon even in countries where it is considered more prevalent such that large prospective phase III randomized trials to examine and compare different treatment approaches have not been conducted.
9.1.1. Concurrent Chemoradiotherapy
Concurrent chemoradiotherapy is predicated on augmented radiosensitivity when chemotherapy is given at the same time. Concurrent chemoradiotherapy with the DeVIC (dexamethasone, etoposide, ifosfamide and carboplatin) [60,61], VIPD (etoposide, ifosfamide, cisplatin and dexamethasone) [62], and VIDL (etoposide, ifosfamide, dexamethasone and L-asparaginase) [63] regimens and radiotherapy (40–50 Gy) in stage I/II patients gave overall response rates (ORR) of 78–90%, and complete remission rates (CR) of 75–87%. Five-year progression-free survivals (PFS) of 60–67% and overall survivals (OS) of 72–73% were achieved. Concurrent chemoradiotherapy is logistically difficult because radiotherapy is not always immediately available in most centres. Furthermore, the concomitant use of chemotherapy and radiotherapy has significant mucosal toxicity and may not be tolerated in older patients and those with poor performance status.
9.1.2. Sequential Chemotherapy and Radiotherapy
Sequential chemotherapy and radiotherapy involve delivering chemotherapy first, followed by interim or end-of-treatment radiotherapy. In a retrospective study of 303 patients with stage I/II NK/T-cell lymphoma, sequential chemotherapy and radiotherapy gave CR, PFS and OS that were comparable with those of concurrent chemoradiotherapy with or without subsequent consolidation chemotherapy [64]. The use of SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase and etoposide) [65], GELOX (gemcitabine, L-asparaginase, and oxaloplatin) [66,67], P-GEMOX (pegaspargase, gemcitabine, and oxaliplatin) [68], and MESA (methotrexate, etoposide, dexamethasone, and pegaspargase) [69], followed by or sandwiched with radiotherapy, also gave ORRs of 90–100%, and CRs of 74–91%, which resulted in 5-year PFS of 64–83% and OS of 64–90%. These excellent results depend critically on the use of asparaginase-containing regimens. In a large retrospective analysis of more than 2000 patients with early stage NK/T-cell lymphoma, the 5-year PFS and OS of patients receiving sequential radiotherapy and anthracycline-based regimens were poor at 54.4% and 65.3% [70]. In contrast, patients receiving non-anthracycline-based regimens had significantly superior 5-year PFS and OS of 67.7% and 77% [70]. In the same cohort (N = 376), the use of asparaginase-based chemotherapy regimens was associated with a significantly better 5-year OS (hazard ratio: 0.55), as compared with that of non-asparaginase based regimens when they were given sequentially with radiotherapy [71]. These results have underscored the importance of choosing the optimal chemotherapeutic regimens to be used with radiotherapy. Furthermore, these data also indicate that anthracycline-based regimens are inferior for NK/T-cell lymphoma and should be abandoned.
9.1.3. Radiotherapy
Radiotherapy as the sole modality in stage I/II NK/T-cell lymphoma is associated with unacceptably high failure rates. Therefore, radiotherapy is only considered an adjunct to chemotherapy. Incorporating radiotherapy in the treatment algorithm of stage I/II NK/T-cell lymphoma has been reported to improve outcome, particularly when the chemotherapy regimens used were not intensive [72,73]. The dose of radiotherapy should be at least 50Gy, in order to decrease in-field relapse [74,75]. Intensity modulated radiation therapy (IMRT) provides adequate tumour target coverage with sparing of normal surrounding tissues and is widely used [76]. In a retrospective study comparing the use of IMRT and three-dimensional conformal radiotherapy (3D-CRT) for early stage NK/T-cell lymphoma, patients receiving IMRT with or without chemotherapy had a superior 5-year PFS (69%) and OS (76%) as compared with those receiving 3D-CRT (5-year PFS and OS were 58% and 68%, respectively) [77]. Hence, radiotherapy is merely part of the treatment in NK/T-cell lymphoma and should be used alone only in patients too frail to tolerate any chemotherapy [59].
9.2. Management of Stage III/IV NK/T-Cell Lymphoma
The mainstay of treatment for stage III/IV NK/T-cell lymphomas is combination chemotherapy that incorporates asparaginase in the regimens [9]. Similar to stage I/II disease, anthracycline-based regimens have been shown to be ineffective and should not be used [70]. Regimens incorporating L-asparaginase or pegylated asparaginase including SMILE [65,78], AspaMetDex (L-asparaginase, methotrexate, and dexamethasone) [79], MEDA (methotrexate, etoposide, dexamethasone and pegylated asparaginase) [80] and P-GEMOX [81] have been evaluated in patients with newly diagnosed advanced-stage NK/T-cell lymphomas. With the SMILE regimen in treatment-naïve stage III/IV patients, 40–54% of cases achieved CR, with a 5-year OS of 47% [65]. In a phase III randomized study comparing DDGP (dexamethasone, gemcitabine, cisplatin and pegaspargase) with SMILE in newly diagnosed stage III/IV patients, the results of DDGP were apparently superior, with a higher CR (71%), and better 1-year PFS (86%) and 1-year OS (90%) [82]. However, the results of the SMILE group in this study was exceptionally poor, with CR of only 29% and a 1 year-OS of 57%; which were inferior to those of the phase II study [78] and the real-world setting [65]. Hence, the significance of this study remained doubtful.
The outcome of stage III/IV NK/T-cell lymphomas remains unfavourable, despite the reasonable CR rates with asparaginase-containing regimens. Therefore, the appropriate use of post-remission strategies in stage III/IV cases is important in order to improve long-term results.
10. Haematopoietic Stem Cell Transplantation (HSCT)
Early results of autologous HSCT in 18 patients showed that outcome was poor for patients transplanted not in remission, suggesting that at least some prior degree of disease control must be achieved [83]. These early observations were confirmed in a later retrospective analysis of frontline autologous HSCT in 62 patients. The 3-year PFS and OS were 64.5% and 67.6% for early stage patients, and 40.1% and 52.3% for advanced-stage patients, respectively. Multivariate analysis showed that patients with disease not under control before autologous HSCT had significantly inferior outcome [84]. In a recent phase II study of upfront autologous HSCT after VIDL induction chemotherapy in 27 patients with advanced-stage NK/T-cell lymphoma, 17 patients achieved CR or partial remission after 4 cycles of chemotherapy and underwent HSCT [85]. With a median follow up of 31.2 months, only 8 patients remained in CR after HSCT. It can be seen from these results that frontline HSCT did not appear to be superior to concurrent or sequential chemoradiotherapy for early stage disease and asparaginase-based regimens for advanced-stage disease. Therefore, frontline autologous HSCT is generally not recommended, because of its uncertain additional benefit.
Allogeneic HSCT offers a potential cure for high-risk patients, owing to its putative graft-versus-lymphoma effect. However, there is no randomized prospective study to evaluate its role. In highly selected patients with advanced-stage or relapsed/refractory (R/R) disease, a 5-year OS of more than 50% could be achieved with allogeneic HSCT [86,87]. However, allogeneic HSCT is associated with a high treatment-related mortality and there is a lack of prognostic and predictive markers for identification of high-risk patients who would benefit most from it.
11. Practical Recommendations for Management of NK/T-Cell Lymphomas
For stage I/II patients, asparaginase-containing regimens combined with radiotherapy can be considered the standard of care. Sequential chemotherapy and radiotherapy are used in most centres, owing to logistic simplicity. Furthermore, patients often have much better performance at the time of radiotherapy, which has fewer adverse effects when used alone. Concurrent chemoradiotherapy is hardly used, owing to logistic complexity and higher toxicity. Monitoring of plasma EBV DNA should be routinely performed. The aim should be to achieve non-quantifiable plasma EBV DNA after the initial two or three cycles of chemotherapy [49]. At end of-treatment, both EBV DNA and PET/CT should be normal, such that durable remission may be achieved [49,50,53]. Autologous HSCT has no role for stage I/II patients in clinical, molecular and radiologic remission. Regular monitoring of plasma EBV DNA should be undertaken during follow-up.
For stage III/IV patients, asparaginase-containing regimens are the standard. Patients who present with widespread disseminated disease should be carefully monitored for tumour lysis and liver function derangement, which may be related to cytokine released from the neoplastic lesions. Once disease control is achieved, allogeneic HSCT should be considered for suitable patients. CNS prophylaxis is not recommended for stage III/IV patients, because the incidence of CNS involvement or relapse is low. However, for regimens that do not contain intermediate to high dose methotrexate, which is included in SMILE or similar regimens, there appears to be an increased risk of CNS relapse [40]. Whether the use of CNS prophylaxis is justified in patients receiving such treatment should be further validated. Similar to stage I/II patients, the aim must be to achieve clinical, molecular and radiologic remission at end-of-treatment assessment.
12. R/R NK/T-Cell Lymphomas
The management of R/R NK/T-cell lymphomas remains challenging. For patients treated with first-line anthracycline-containing regimens, the use of asparaginase-containing regimens will achieve a high remission rate. However, for patients who have failed asparaginase-containing or other non-anthracycline-containing regimens, the outlook is bleak, with a median PFS of 4.1 months and OS of 6.4 months [88]. Therefore, novel treatment modalities are needed for these patients.
12.1. Immune Checkpoint Blockade Therapy for R/R NK/T-Cell Lymphomas
Histologic studies have shown overexpression of PD-L1 in NK/T-cell lymphomas, providing a theoretical basis of targeting the PD1/PD-L1 axis in R/R diseases [89]. In the first published series of seven patients treated with the anti-PD1 antibody pembrolizumab after failing asparaginase-based regimens and allogeneic HSCT, all patients responded after a median of seven cycles, with five patients achieving CR [90]. No treatment-related adverse events were observed. In another report of seven patients with R/R disease treated with pembrolizumab, four patients responded to treatment, and two of them achieved CR [91]. A durable response was observed in one patient who remained in CR after 18 cycles. In another 19 patients treated with pembrolizumab, there was no correlation between PD-L1 expression and response to treatment [92]. However, whole genome sequencing identified somatic mutations in the 3′-untranslated region of the PD-L1 gene as a predictive marker for response [92]. The effectiveness of the anti-PD1 antibody nivolumab in R/R NK/T-cell lymphoma has also been described in three patients, all of whom responded, with one case achieving continuous CR after nine cycles [93]. Another anti-PD1 antibody sintilimab was studied in 28 patients with R/R NK/T-cell lymphoma [94]. Although only two patients achieved CR, the ORR was 67.9%, and the 2-year OS was 78.6% [94].
Targeting anti-PD-L1 has also been studied. The use of avelumab, an anti-PD-L1 antibody, was reported in a phase II study of 21 patients [95]. The ORR was 38% and CR was 24%. Five patients have durable response after a median of 18 cycles. PD-L1 expression in lymphoma cells correlated with response [95]. No grade 4 adverse events were observed.
The disparity in responses to PD-1/PD-L1 blockade in these studies may be related to the relatively small sample size, heterogeneity of patient characteristics, and the use of anti-PD1 vs. anti-PD-L1 antibodies. Overall, the efficacy of PD-1/PD-L1 immune blockade therapy is promising for R/R disease, although further studies on the identification of predictive biomarkers for response are needed. The effectiveness of combining a PD1/PD-L1 blockade with chemotherapies or other novel treatment is warranted.
12.2. Other Immunotherapies including Cellular Therapy
The antigen CD38 is strongly expressed in about 50% of NK/T-cell lymphomas and has been shown to be a prognostic marker [96]. Anecdotal report of the efficacy of the anti-CD38 antibody daratumumab had been described in R/R NK/T-cell lymphoma [97]. However, in a phase II prospective study of daratumumab monotherapy in 32 patients with R/R NK/T-cell lymphomas, the ORR was merely 25%, with no CR attained [98]. Responses were independent of CD38 expression. With a median follow-up of 10 months, the median PFS and OS were 53 and 141 days only, suggesting that daratumumab monotherapy was not effective [98]. CD30 is expressed in 30–50% of NK/T-cell lymphomas. The anti-CD30 antibody conjugate brentuximab vedotin had been reported to be efficacious in two patients with R/R NK/T-cell lymphomas expressing CD30 [99,100], an observation requiring prospective validation.
Another strategy to harness anti-tumour immunity is adoptive cellular therapy. There has been strong evidence supporting the use of EBV-specific cytotoxic T-lymphocytes (CTL) to target EBV-associated lymphomas [101,102]. In NK/T-cell lymphomas, EBV latency II viral proteins LMP1, LMP2 and EBNA1 are less immunogenic and susceptible to immune surveillance [101]. Production of active autologous EBV-specific CTL targeting these latency type II EBV-positive tumours would require the use of adenoviral vector transduced dendritic cells expressing LMP1 and 2, and EBV-transformed lymphoblastoid cell lines as antigen-presenting cells to activate and expand EBV-antigen specific CTL [102]. In a recent phase II study of 47 patients with R/R NK/T-cell lymphoma, autologous EBV-specific CTL was successfully generated in 32 cases, with 15 patients subsequently infused with the products [103]. Ten of them had active lymphoma at the time of treatment and received a median of four doses of EBV-specific CTL. The ORR was 50%, with a CR rate of 30%. The median PFS was 12.3 months [103]. No grade 3 or 4 adverse events were observed. Cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome were not observed. The high manufacturing failure rate (32%) and the long turnaround time (approximately 25 days), however, limit the clinical utility of this treatment approach.
12.3. Novel Agents
Chidamide is an oral selective histone deacetylase (HDAC) inhibitor of HDAC1, 2, 3, and 10 [104]. In two studies with 49 cases of R/R NK/T-cell lymphomas [105,106], salvage treatment with chidamide resulted in an ORR of 18% (CR: 6%). Alisertib, a selective aurora A kinase inhibitor, had been examined in five patients of R/R NK/T-cell lymphomas [107,108], with only one case (20%) achieving a partial response. Other drugs approved in the treatment of peripheral T-cell lymphoma, including romidepsin and pralatrexate [109], had been used in too few cases of NK/T-cell lymphoma for their efficacy to be determined.
13. Future Treatment Strategies
Novel treatment approaches are needed for advanced-stage and R/R NK/T-cell lymphoma because outcome with current treatment remains poor. Combination of strategies with different mechanisms of action may improve outcome. In a phase Ib/II trial of chidamide combined with sintilimab, data reported in abstract form showed that, of 45 patients with R/R NK/T-cell lymphomas, 36 cases were evaluable, with an ORR of 58.3% and a CR rate of 44.4% [110], which appeared to be better than that of either agent alone in other studies. An immune checkpoint blockade may also work synergistically with conventional chemotherapy or cellular therapies. With the promising data on anti-PD-1 or anti-PD-L1, other immune blockade therapies are also worth studying. LAG3 and TIM-3 are expressed in more than 90% of NK/T-cell lymphomas, suggesting that antibodies against these T-cell inhibitory molecules might be potential therapeutic agents [111]. Furthermore, advances in the understanding of the tumour biology of NK/T-cell lymphoma have identified putative driver molecules or pathways such as the JAK-STAT pathway as potential druggable targets for therapeutic intervention.
14. Conclusions
NK/T-cell lymphomas are rare malignancies with specific clinicopathologic features and treatment strategies. Anthracycline-containing regimens for conventional lymphomas are ineffective. Non-anthracycline regimens containing asparaginase are the standard. Stage I/II lymphomas are treated with combined radiotherapy and chemotherapy. Stage III/IV lymphomas are treated with chemotherapy. Novel approaches including checkpoint inhibitors are needed for relapsed/refractory lymphomas.
Author Contributions
E.T.: conceived, wrote, and approved the manuscript; Y.-L.K.: conceived, wrote, and approved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
There are no funding sources to acknowledge.
Conflicts of Interest
There are no conflict of interests to declare.
References
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Geiger, T.L.; Sun, J.C. Development and maturation of natural killer cells. Curr. Opin. Immunol. 2016, 39, 82–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quatrini, L.; Della Chiesa, M.; Sivori, S.; Mingari, M.C.; Pende, D.; Moretta, L. Human NK cells, their receptors and function. Eur. J. Immunol. 2021, 51, 1566–1579. [Google Scholar] [CrossRef]
- Tse, E.; Kwong, Y.L. The diagnosis and management of NK/T-cell lymphomas. J. Hematol. Oncol. 2017, 10, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chim, C.S.; Ooi, G.C.; Shek, T.W.; Liang, R.; Kwong, Y.L. Lethal midline granuloma revisited: Nasal T/Natural-killer cell lymphoma. J. Clin. Oncol. 1999, 17, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.L.; Jaffe, E.S.; Stein, H.; Banks, P.M.; Chan, J.K.; Cleary, M.L.; Delsol, G.; De Wolf-Peeters, C.; Falini, B.; Gatter, K.C.; et al. A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood 1994, 84, 1361–1392. [Google Scholar] [CrossRef] [Green Version]
- Pongpruttipan, T.; Sukpanichnant, S.; Assanasen, T.; Wannakrairot, P.; Boonsakan, P.; Kanoksil, W.; Kayasut, K.; Mitarnun, W.; Khuhapinant, A.; Bunworasate, U.; et al. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and αβ, γδ, and αβ/γδ T-cell origin: A comprehensive clinicopathologic and phenotypic study. Am. J. Surg. Pathol. 2012, 36, 481–499. [Google Scholar] [CrossRef]
- Chan, J.K.; Quintanilla-Martinez, L.; Ferry, J.A. Extranodal NK/T-cell lymphoma, nasal type. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Eds.; International Agency for Research on Cancer: Lyon, France, 2017; pp. 368–371. [Google Scholar]
- Tse, E.; Kwong, Y.L. How I treat NK/T-cell lymphomas. Blood 2013, 121, 4997–5005. [Google Scholar] [CrossRef] [Green Version]
- Nicolae, A.; Ganapathi, K.A.; Pham, T.H.; Xi, L.; Torres-Cabala, C.A.; Nanaji, N.M.; Zha, H.D.; Fan, Z.; Irwin, S.; Pittaluga, S.; et al. EBV-negative Aggressive NK-cell Leukemia/Lymphoma: Clinical, Pathologic, and Genetic Features. Am. J. Surg. Pathol. 2017, 41, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Xia, Y.; Feng, L.N.; Chen, J.R.; Li, H.M.; Cui, J.; Cai, Q.Q.; Sim, K.S.; Nairismägi, M.L.; Laurensia, Y.; et al. Genetic risk of extranodal natural killer T-cell lymphoma: A genome-wide association study. Lancet Oncol. 2016, 17, 1240–1247. [Google Scholar] [CrossRef]
- Lin, G.W.; Xu, C.; Chen, K.; Huang, H.Q.; Chen, J.; Song, B.; Chan, J.K.C.; Li, W.; Liu, W.; Shih, L.Y.; et al. International NKTCL Working Group. Genetic risk of extranodal natural killer T-cell lymphoma: A genome-wide association study in multiple populations. Lancet Oncol. 2020, 21, 306–316. [Google Scholar] [CrossRef]
- Zhernakova, A.; Festen, E.M.; Franke, L.; Trynka, G.; van Diemen, C.C.; Monsuur, A.J.; Bevova, M.; Nijmeijer, R.M.; van’t Slot, R.; Heijmans, R.; et al. Genetic analysis of innate immunity in Crohn’s disease and ulcerative colitis identifies two susceptibility loci harboring CARD9 and IL18RAP. Am. J. Hum. Genet. 2008, 82, 1202–1210. [Google Scholar] [CrossRef] [Green Version]
- Raychaudhuri, S.; Sandor, C.; Stahl, E.A.; Freudenberg, J.; Lee, H.S.; Jia, X.; Alfredsson, L.; Padyukov, L.; Klareskog, L.; Worthington, J.; et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat. Genet. 2012, 44, 291–296. [Google Scholar] [CrossRef]
- Münz, C. Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat. Rev. Microbiol. 2019, 17, 691–700. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.F.; Chan, J.K.; Kwong, Y.L. Identification of del(6)(q21q25) as a recurring chromosomal abnormality in putative NK cell lymphoma/leukaemia. Br. J. Haematol. 1997, 98, 922–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siu, L.L.; Wong, K.F.; Chan, J.K.; Kwong, Y.L. Comparative genomic hybridization analysis of natural killer cell lymphoma/leukemia. Recognition of consistent patterns of genetic alterations. Am. J. Pathol. 1999, 155, 1419–1425. [Google Scholar] [CrossRef]
- Siu, L.L.; Chan, V.; Chan, J.K.; Kwong, Y.L. Consistent patterns of allelic loss in natural killer cell lymphoma. Am. J. Pathol. 2000, 157, 1803–1809. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; de Reyniès, A.; de Leval, L.; Ghazi, B.; Martin-Garcia, N.; Travert, M.; Bosq, J.; Brière, J.; Petit, B.; Thomas, E.; et al. Gene expression profiling identifies emerging oncogenic pathways operating in extranodal NK/T-cell lymphoma, nasal type. Blood 2010, 115, 1226–1237. [Google Scholar] [CrossRef]
- Küçük, C.; Hu, X.; Iqbal, J.; Gaulard, P.; Klinkebiel, D.; Cornish, A.; Dave, B.J.; Chan, W.C. PRDM1 is a tumor suppressor gene in natural killer cell malignancies. Proc. Natl. Acad. Sci. USA 2011, 108, 20119–20124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karube, K.; Nakagawa, M.; Tsuzuki, S.; Takeuchi, I.; Honma, K.; Nakashima, Y.; Shimizu, N.; Ko, Y.H.; Morishima, Y.; Ohshima, K.; et al. Identification of FOXO3 and PRDM1 as tumor-suppressor gene candidates in NK-cell neoplasms by genomic and functional analyses. Blood 2011, 118, 3195–3204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.W.; Guo, T.; Shen, L.; Wong, K.Y.; Tao, Q.; Choi, W.W.; Au-Yeung, R.K.; Chan, Y.P.; Wong, M.L.; Tang, J.C.; et al. Receptor-type tyrosine-protein phosphatase κ directly targets STAT3 activation for tumor suppression in nasal NK/T-cell lymphoma. Blood 2015, 125, 1589–1600. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Ng, S.B.; Tay, J.L.; Lin, B.; Koh, T.L.; Tan, J.; Selvarajan, V.; Liu, S.C.; Bi, C.; Wang, S.; et al. EZH2 overexpression in natural killer/T-cell lymphoma confers growth advantage independently of histone methyltransferase activity. Blood 2013, 121, 4512–4520. [Google Scholar] [CrossRef] [Green Version]
- Selvarajan, V.; Osato, M.; Nah, G.S.S.; Yan, J.; Chung, T.H.; Voon, D.C.; Ito, Y.; Ham, M.F.; Salto-Tellez, M.; Shimizu, N.; et al. RUNX3 is oncogenic in natural killer/T-cell lymphoma and is transcriptionally regulated by MYC. Leukemia 2017, 31, 2219–2227. [Google Scholar] [CrossRef]
- Iqbal, J.; Wilcox, R.; Naushad, H.; Rohr, J.; Heavican, T.B.; Wang, C.; Bouska, A.; Fu, K.; Chan, W.C.; Vose, J.M. Genomic signatures in T-cell lymphoma: How can these improve precision in diagnosis and inform prognosis? Blood Rev. 2016, 30, 89–100. [Google Scholar] [CrossRef]
- Ng, S.B.; Selvarajan, V.; Huang, G.; Zhou, J.; Feldman, A.L.; Law, M.; Kwong, Y.L.; Shimizu, N.; Kagami, Y.; Aozasa, K.; et al. Activated oncogenic pathways and therapeutic targets in extranodal nasal-type NK/T cell lymphoma revealed by gene expression profiling. J. Pathol. 2011, 223, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Koo, G.C.; Tan, S.Y.; Tang, T.; Poon, S.L.; Allen, G.E.; Tan, L.; Chong, S.C.; Ong, W.S.; Tay, K.; Tao, M.; et al. Janus kinase 3-activating mutations identified in natural killer/T-cell lymphoma. Cancer Discov. 2012, 2, 591–597. [Google Scholar] [CrossRef] [Green Version]
- Küçük, C.; Jiang, B.; Hu, X.; Zhang, W.; Chan, J.K.; Xiao, W.; Lack, N.; Alkan, C.; Williams, J.C.; Avery, K.N.; et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from γδ-T or NK cells. Nat. Commun. 2015, 6, 6025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Park, H.Y.; Kang, S.Y.; Kim, S.J.; Hwang, J.; Lee, S.; Kwak, S.H.; Park, K.S.; Yoo, H.Y.; Kim, W.S.; et al. Genetic alterations of JAK/STAT cascade and histone modification in extranodal NK/T-cell lymphoma nasal type. Oncotarget 2015, 6, 17764–17776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Gu, Z.H.; Yan, Z.X.; Zhao, X.; Xie, Y.Y.; Zhang, Z.G.; Pan, C.M.; Hu, Y.; Cai, C.P.; Dong, Y.; et al. Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat. Genet. 2015, 47, 1061–1066. [Google Scholar] [CrossRef]
- Küçük, C.; Hu, X.; Jiang, B.; Klinkebiel, D.; Geng, H.; Gong, Q.; Bouska, A.; Iqbal, J.; Gaulard, P.; McKeithan, T.W.; et al. Global promoter methylation analysis reveals novel candidate tumor suppressor genes in natural killer cell lymphoma. Clin. Cancer Res. 2015, 21, 1699–1711. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.B.; Yan, J.; Huang, G.; Selvarajan, V.; Tay, J.L.; Lin, B.; Bi, C.; Tan, J.; Kwong, Y.L.; Shimizu, N.; et al. Dysregulated microRNAs affect pathways and targets of biologic relevance in nasal-type natural killer/T-cell lymphoma. Blood 2011, 118, 4919–4929. [Google Scholar] [CrossRef]
- Chen, B.J.; Chapuy, B.; Ouyang, J.; Sun, H.H.; Roemer, M.G.; Xu, M.L.; Yu, H.; Fletcher, C.D.; Freeman, G.J.; Shipp, M.A.; et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin. Cancer Res. 2013, 19, 3462–3473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tse, E.; Au-Yeung, R.; Kwong, Y.L. Recent advances in the diagnosis and treatment of natural killer/T-cell lymphomas. Expert Rev. Hematol. 2019, 12, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.W.; Wang, H.; Zhang, W.W.; Wang, J.H.; Liu, W.J.; Xia, Z.J.; Huang, H.Q.; Jiang, W.Q.; Zhang, Y.J.; Wang, L. PD-L1 is upregulated by EBV-driven LMP1 through NF-kappaB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J. Hematol. Oncol. 2016, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, M.R.; Rodig, S.; Juszczynski, P.; Ouyang, J.; Sinha, P.; O’Donnell, E.; Neuberg, D.; Shipp, M.A. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: Implications for targeted therapy. Clin. Cancer Res. 2012, 18, 1611–1618. [Google Scholar] [CrossRef] [Green Version]
- Song, T.L.; Nairismägi, M.L.; Laurensia, Y.; Lim, J.Q.; Tan, J.; Li, Z.M.; Pang, W.L.; Kizhakeyil, A.; Wijaya, G.C.; Huang, D.C.; et al. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood 2018, 132, 1146–1158. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.; Cui, B.W.; Wang, N.; Dai, Y.T.; Zhang, H.; Wang, C.F.; Zhong, H.J.; Cheng, S.; Ou-Yang, B.S.; Hu, Y.; et al. Genomic and Transcriptomic Characterization of Natural Killer T Cell Lymphoma. Cancer Cell 2020, 37, 403–419. [Google Scholar] [CrossRef]
- Takata, K.; Hong, M.E.; Sitthinamsuwan, P.; Loong, F.; Tan, S.Y.; Liau, J.Y.; Hsieh, P.P.; Ng, S.B.; Yang, S.F.; Pongpruttipan, T.; et al. Primary cutaneous NK/T-cell lymphoma, nasal type and CD56-positive peripheral T-cell lymphoma: A cellular lineage and clinicopathologic study of 60 patients from Asia. Am. J. Surg. Pathol. 2015, 39, 1–12. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, H.; Yamaguchi, M.; Sohn, I.; Yoon, S.E.; Byeon, S.; Hur, J.Y.; Koh, Y.; Yoon, S.S.; Kim, E.J.; et al. Prediction and prevention of central nervous system relapse in patients with extranodal natural killer/T-cell lymphoma. Blood 2020, 136, 2548–2556. [Google Scholar] [CrossRef]
- Chan, J.K.; Jaffe, E.S.; Ko, Y.H. Aggressive NK-Cell Leukaemia. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Arber, D.A., Hasserjian, R.P., Le Beau, M.M., Eds.; International Agency for Research on Cancer: Lyon, France, 2017; pp. 353–354. [Google Scholar]
- Tse, E.; Kwong, Y.L. Practical management of natural killer/T-cell lymphoma. Curr. Opin. Oncol. 2012, 24, 480–486. [Google Scholar] [CrossRef]
- Facchetti, F.; Cigognetti, M.; Fisogni, S.; Rossi, G.; Lonardi, S.; Vermi, W. Neoplasms derived from plasmacytoid dendritic cells. Mod. Pathol. 2016, 29, 98–111. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, K.; Yokoyama, M.; Ishizawa, S.; Terui, Y.; Nomura, K.; Marutsuka, K.; Nunomura, M.; Fukushima, N.; Yagyuu, T.; Nakamine, H.; et al. Lymphomatoid gastropathy: A distinct clinicopathologic entity of self-limited pseudomalignant NK-cell proliferation. Blood 2010, 116, 5631–5637. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, A.; Pittaluga, S.; Beck, P.L.; Wilson, W.H.; Ferry, J.A.; Jaffe, E.S. NK-cell enteropathy: A benign NK-cell lymphoproliferative disease mimicking intestinal lymphoma: Clinicopathologic features and follow-up in a unique case series. Blood 2011, 117, 1447–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, H.; Kwong, Y.L. EBV viral Loads in diagnosis, monitoring, and response assessment. Front. Oncol. 2019, 9, 62. [Google Scholar] [CrossRef] [Green Version]
- Kanakry, J.A.; Hegde, A.M.; Durand, C.M.; Massie, A.B.; Greer, A.E.; Ambinder, R.F.; Valsamakis, A. The clinical significance of EBV DNA in the plasma and peripheral blood mononuclear cells of patients with or without EBV diseases. Blood 2016, 127, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Au, W.Y.; Pang, A.; Choy, C.; Chim, C.S.; Kwong, Y.L. Quantification of circulating Epstein-Barr virus (EBV) DNA in the diagnosis and monitoring of natural killer cell and EBV-positive lymphomas in immunocompetent patients. Blood 2004, 104, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Kwong, Y.L.; Pang, A.W.; Leung, A.Y.; Chim, C.S.; Tse, E. Quantification of circulating Epstein-Barr virus DNA in NK/T-cell lymphoma treated with the SMILE protocol: Diagnostic and prognostic significance. Leukemia 2014, 28, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Choi, J.Y.; Hyun, S.H.; Ki, C.S.; Oh, D.; Ahn, Y.C.; Ko, Y.H.; Choi, S.; Jung, S.H.; Khong, P.L.; et al. Asia Lymphoma Study Group. Risk stratification on the basis of Deauville score on PET-CT and the presence of Epstein-Barr virus DNA after completion of primary treatment for extranodal natural killer/T-cell lymphoma, nasal type: A multicentre, retrospective analysis. Lancet Haematol. 2015, 2, e66–e74. [Google Scholar] [PubMed]
- Khong, P.L.; Pang, C.B.; Liang, R.; Kwong, Y.L.; Au, W.Y. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann. Hematol. 2008, 87, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Au, W.Y.; Wong, C.Y.; Liang, R.; Leung, A.Y.; Kwong, Y.L.; Khong, P.L. Metabolic activity measured by F-18 FDG PET in natural killer-cell lymphoma compared to aggressive B- and T-cell lymphomas. Clin. Nucl. Med. 2010, 35, 571–575. [Google Scholar] [CrossRef]
- Khong, P.L.; Huang, B.; Lee, E.Y.; Chan, W.K.; Kwong, Y.L. Midtreatment ¹⁸F-FDG PET/CT Scan for Early Response Assessment of SMILE Therapy in Natural Killer/T-Cell Lymphoma: A Prospective Study from a Single Center. J. Nucl. Med. 2014, 55, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Kita, K.; Miwa, H.; Nishii, K.; Oka, K.; Ohno, T.; Shirakawa, S.; Fukumoto, M. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer 1995, 76, 2351–2356. [Google Scholar] [CrossRef]
- Ando, M.; Sugimoto, K.; Kitoh, T.; Sasaki, M.; Mukai, K.; Ando, J.; Egashira, M.; Schuster, S.M.; Oshimi, K. Selective apoptosis of natural killer-cell tumours by l-asparaginase. Br. J. Haematol. 2005, 130, 860–868. [Google Scholar] [CrossRef]
- Chim, C.S.; Ma, S.Y.; Au, W.Y.; Choy, C.; Lie, A.K.; Liang, R.; Yau, C.C.; Kwong, Y.L. Primary nasal natural killer cell lymphoma: Long-term treatment outcome and relationship with the International Prognostic Index. Blood 2004, 103, 216–221. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Suh, C.; Park, Y.H.; Ko, Y.H.; Bang, S.M.; Lee, J.H.; Lee, D.H.; Huh, J.; Oh, S.Y.; Kwon, H.C.; et al. Extranodal natural killer T-cell lymphoma, nasal-type: A prognostic model from a retrospective multicenter study. J. Clin. Oncol. 2006, 24, 612–618. [Google Scholar] [CrossRef]
- Kim, S.J.; Yoon, D.H.; Jaccard, A.; Chng, W.J.; Lim, S.T.; Hong, H.; Park, Y.; Chang, K.M.; Maeda, Y.; Ishida, F.; et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: A multicentre, retrospective analysis. Lancet Oncol. 2016, 17, 389–400. [Google Scholar] [CrossRef]
- Tse, E.; Kwong, Y.L. Nasal NK/T-cell lymphoma: RT, CT, or both. Blood 2015, 126, 1400–1401. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Tobinai, K.; Oguchi, M.; Ishizuka, N.; Kobayashi, Y.; Isobe, Y.; Ishizawa, K.; Maseki, N.; Itoh, K.; Usui, N.; et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOGJ0211. J Clin. Oncol. 2009, 27, 5594–5600. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Suzuki, R.; Oguchi, M.; Asano, N.; Amaki, J.; Akiba, T.; Maeda, T.; Itasaka, S.; Kubota, N.; Saito, T.; et al. Treatments and Outcomes of Patients with Extranodal Natural Killer/T-Cell Lymphoma Diagnosed Between 2000 and 2013: A Cooperative Study in Japan. J. Clin. Oncol. 2017, 35, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, K.; Kim, B.S.; Kim, C.Y.; Suh, C.; Huh, J.; Lee, S.-W.; Kim, J.S.; Cho, J.; Lee, G.-W.; et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma study. J. Clin. Oncol. 2009, 27, 6027–6032. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Yang, D.H.; Kim, J.S.; Kwak, J.Y.; Eom, H.S.; Hong, D.S.; Won, J.H.; Lee, J.H.; Yoon, D.H.; Cho, J.; et al. Concurrent chemoradiotherapy followed by L-asparaginase-containing chemotherapy, VIDL, for localized nasal extranodal NK/T cell lymphoma: CISL08-01 phase II study. Ann. Hematol. 2014, 93, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Kwong, Y.L.; Kim, S.J.; Tse, E.; Oh, S.Y.; Kwak, J.Y.; Eom, H.S.; Do, Y.R.; Mun, Y.C.; Lee, S.R.; Shin, H.J.; et al. Sequential chemotherapy/radiotherapy was comparable with concurrent chemoradiotherapy for stage I/II NK/T-cell lymphoma. Ann. Oncol. 2018, 29, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Kwong, Y.L.; Kim, W.S.; Lim, S.T.; Kim, S.J.; Tang, T.; Tse, E.; Leung, A.Y.; Chim, C.S. SMILE for natural killer/T-cell lymphoma: Analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood 2012, 120, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Z.H.; Chen, X.Q.; Li, Y.J.; Wang, K.F.; Xia, Y.F.; Xia, Z.J. First-line combination of gemcitabine, oxaliplatin, and L-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer 2013, 119, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Z.H.; Chen, X.Q.; Wang, K.F.; Huang, H.Q.; Xia, Z.J. First-line combination of GELOX followed by radiation therapy for patients with stage IE/IIE ENKTL: An updated analysis with long-term follow-up. Oncol. Lett. 2015, 10, 1036–1040. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Wu, P.; Li, L.; Zhang, Z.H. Effectiveness of pegaspargase, gemcitabine, and oxaliplatin (P-GEMOX) chemotherapy combined with radiotherapy in newly diagnosed, stage IE to IIE, nasal-type, extranodal natural killer/T-cell lymphoma. Hematology 2017, 22, 320–329. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.P.; Xiong, J.; Cheng, S.; Zhao, X.; Wang, C.F.; Cai, G.; Zhong, H.J.; Huang, H.Y.; Chen, J.Y.; Zhao, W.L. A Phase II Study of Methotrexate, Etoposide, Dexamethasone and Pegaspargase Sandwiched with Radiotherapy in the Treatment of Newly Diagnosed, Stage IE to IIE Extranodal Natural-Killer/T-Cell Lymphoma, Nasal-Type. EBioMedicine 2017, 25, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Qi, S.N.; Yang, Y.; Song, Y.Q.; Wang, Y.; He, X.; Hu, C.; Zhang, L.L.; Wu, G.; Qu, B.L.; Qian, L.T.; et al. First-line non-anthracycline-based chemotherapy for extranodal nasal-type NK/T-cell lymphoma: A retrospective analysis from the CLCG. Blood Adv. 2020, 4, 3141–3153. [Google Scholar] [CrossRef]
- Zheng, X.; He, X.; Yang, Y.; Liu, X.; Zhang, L.L.; Qu, B.L.; Zhong, Q.Z.; Qian, L.T.; Hou, X.R.; Qiao, X.Y.; et al. Association of improved overall survival with decreased distant metastasis following asparaginase-based chemotherapy and radiotherapy for intermediate- and high-risk early-stage extranodal nasal-type NK/T-cell lymphoma: A CLCG study. ESMO Open 2021, 6, 100206. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Y.; Cao, J.Z.; Zhang, Y.J.; Xu, L.M.; Yuan, Z.Y.; Wu, J.X.; Wang, W.; Wu, T.; Lu, B.; et al. Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: Analysis from a multicenter study. Blood 2015, 126, 1424–1432. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.W.; Wu, J.X.; Wu, T.; Zhu, S.Y.; Shi, M.; Su, H.; Wang, Y.; He, X.; Xu, L.M.; Yuan, Z.Y.; et al. Radiotherapy is essential after complete response to asparaginase-containing chemotherapy in early-stage extranodal nasal-type NK/T-cell lymphoma: A multicenter study from the China Lymphoma Collaborative Group (CLCG). Radiother. Oncol. 2018, 129, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cao, J.Z.; Lan, S.M.; Wu, J.X.; Wu, T.; Zhu, S.Y.; Qian, L.T.; Hou, X.R.; Zhang, F.Q.; Zhang, Y.J.; et al. Association of Improved Locoregional Control With Prolonged Survival in Early-Stage Extranodal Nasal-Type Natural Killer/T-Cell Lymphoma. JAMA Oncol. 2017, 3, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Vargo, J.A.; Patel, A.; Glaser, S.M.; Balasubramani, G.K.; Farah, R.J.; Marks, S.M.; Beriwal, S. The impact of the omission or inadequate dosing of radiotherapy in extranodal natural killer T-cell lymphoma, nasal type, in the United States. Cancer 2017, 123, 3176–3185. [Google Scholar] [CrossRef]
- Qi, S.N.; Li, Y.X.; Specht, L.; Oguchi, M.; Tsang, R.; Ng, A.; Suh, C.O.; Ricardi, U.; Mac Manus, M.; Dabaja, B.; et al. Modern Radiation Therapy for Extranodal Nasal-Type NK/T-cell Lymphoma: Risk-Adapted Therapy, Target Volume, and Dose Guidelines from the International Lymphoma Radiation Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1064–1081. [Google Scholar] [CrossRef]
- Wu, T.; Yang, Y.; Zhu, S.Y.; Shi, M.; Su, H.; Wang, Y.; He, X.; Xu, L.M.; Yuan, Z.Y.; Zhang, L.L.; et al. Risk-adapted survival benefit of IMRT in early-stage NKTCL: A multicenter study from the China Lymphoma Collaborative Group. Blood Adv. 2018, 2, 2369–2377. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Kwong, Y.L.; Kim, W.S.; Maeda, Y.; Hashimoto, C.; Suh, C.; Izutsu, K.; Ishida, F.; Isobe, Y.; Sueoka, E.; et al. Phase II study of SMILE chemotherapy for newly diagnosed stage, I.V.; relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: The NK-Cell Tumor Study Group study. J. Clin. Oncol. 2011, 29, 4410–4416. [Google Scholar] [CrossRef]
- Jaccard, A.; Gachard, N.; Marin, B.; Rogez, S.; Audrain, M.; Suarez, F.; Tilly, H.; Morschhauser, F.; Thieblemont, C.; Ysebaert, L.; et al. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood 2011, 117, 1834–1839. [Google Scholar] [CrossRef]
- Ding, H.; Chang, J.; Liu, L.G.; Hu, D.; Zhang, W.H.; Yan, Y.; Ma, L.Y.; Li, Z.C.; Ma, Y.J.; Hao, S.G.; et al. High-dose methotrexate, etoposide, dexamethasone and pegaspargase (MEDA) combination chemotherapy is effective for advanced and relapsed/refractory extranodal natural killer/T cell lymphoma: A retrospective study. Int. J. Hematol. 2015, 102, 181–187. [Google Scholar] [CrossRef]
- Wang, J.H.; Wang, L.; Liu, C.C.; Xia, Z.J.; Huang, H.Q.; Lin, T.Y.; Jiang, W.Q.; Lu, Y. Analysis of the efficacy and safety of a combined gemcitabine, oxaliplatin and pegaspargase regimen for NK/T-cell lymphoma. Oncotarget 2016, 7, 35412–35422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Cui, Y.; Sun, Z.; Zhang, L.; Li, L.; Wang, X.; Wu, J.; Fu, X.; Ma, W.; Zhang, X.; et al. DDGP versus SMILE in Newly Diagnosed Advanced Natural Killer/T-Cell Lymphoma: A Randomized Controlled, Multicenter, Open-label Study in China. Clin. Cancer Res. 2016, 22, 5223–5228. [Google Scholar] [CrossRef] [Green Version]
- Au, W.Y.; Lie, A.K.; Liang, R.; Kwong, Y.L.; Yau, C.C.; Cheung, M.M.; Ngan, K.C.; Lau, W.H.; Wong, K.H.; Yiu, H.Y.; et al. Autologous stem cell transplantation for nasal NK/T-cell lymphoma: A progress report on its value. Ann. Oncol. 2003, 14, 1673–1676. [Google Scholar] [CrossRef]
- Yhim, H.Y.; Kim, J.S.; Mun, Y.C.; Moon, J.H.; Chae, Y.S.; Park, Y.; Jo, J.C.; Kim, S.J.; Yoon, D.H.; Cheong, J.W.; et al. Consortium for Improving Survival of Lymphoma Study. Clinical Outcomes and Prognostic Factors of Up-Front Autologous Stem Cell Transplantation in Patients with Extranodal Natural Killer/T Cell Lymphoma. Biol. Blood Marrow Transplant. 2015, 21, 1597–1604. [Google Scholar] [CrossRef] [Green Version]
- Song, G.Y.; Yoon, D.H.; Suh, C.; Moon, J.H.; Baek, D.W.; Kim, J.S.; Lee, G.W.; Yi, J.H.; Park, Y.; Jung, K.S.; et al. Open-label, single arm, multicenter phase II study of VIDL induction chemotherapy followed by upfront autologous stem cell transplantation in patients with advanced stage extranodal NK/T-cell lymphoma. Bone Marrow Transplant. 2021, 56, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Tse, E.; Chan, T.S.; Koh, L.P.; Chng, W.J.; Kim, W.S.; Tang, T.; Lim, S.T.; Lie, A.K.; Kwong, Y.L. Allogeneic haematopoietic SCT for natural killer/T-cell lymphoma: A multicentre analysis from the Asia Lymphoma Study Group. Bone Marrow Transplant. 2014, 49, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Kanate, A.S.; DiGilio, A.; Ahn, K.W.; Al Malki, M.; Jacobsen, E.; Steinberg, A.; Hamerschlak, N.; Kharfan-Dabaja, M.; Salit, R.; Ball, E.; et al. Allogeneic haematopoietic cell transplantation for extranodal natural killer/T-cell lymphoma, nasal type: A CIBMTR analysis. Br. J. Haematol. 2018, 182, 916–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.H.; Hong, J.Y.; Lim, S.T.; Hong, H.; Arnoud, J.; Zhao, W.; Yoon, D.H.; Tang, T.; Cho, J.; Park, S.; et al. Beyond first-line non-anthracycline-based chemotherapy for extranodal NK/T-cell lymphoma: Clinical outcome and current perspectives on salvage therapy for patients after first relapse and progression of disease. Ann. Oncol. 2017, 28, 2199–2205. [Google Scholar] [CrossRef]
- Jo, J.C.; Kim, M.; Choi, Y.; Kim, H.J.; Kim, J.E.; Chae, S.W.; Kim, H.; Cha, H.J. Expression of programmed cell death 1 and programmed cell death ligand 1 in extranodal NK/T-cell lymphoma, nasal type. Ann. Hematol. 2017, 96, 25–31. [Google Scholar] [CrossRef]
- Kwong, Y.L.; Chan, T.S.Y.; Tan, D.; Kim, S.J.; Poon, L.M.; Mow, B.; Khong, P.L.; Loong, F.; Au-Yeung, R.; Iqbal, J.; et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood 2017, 129, 2437–2442. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cheng, Y.; Zhang, M.; Yan, J.; Li, L.; Fu, X.; Zhang, X.; Chang, Y.; Sun, Z.; Yu, H.; et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J. Hematol. Oncol. 2018, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.Q.; Huang, D.; Tang, T.; Tan, D.; Laurensia, Y.; Peng, R.J.; Wong, E.K.Y.; Cheah, D.M.Z.; Chia, B.K.H.; Iqbal, J.; et al. Whole-genome sequencing identifies responders to Pembrolizumab in relapse/refractory natural-killer/T cell lymphoma. Leukemia 2020, 34, 3413–3419. [Google Scholar] [CrossRef]
- Chan, T.S.Y.; Li, J.; Loong, F.; Khong, P.L.; Tse, E.; Kwong, Y.L. PD1 blockade with low-dose nivolumab in NK/T cell lymphoma failing L-asparaginase: Efficacy and safety. Ann. Hematol. 2018, 97, 193–196. [Google Scholar] [CrossRef]
- Tao, R.; Fan, L.; Song, Y.; Hu, Y.; Zhang, W.; Wang, Y.; Xu, W.; Li, J. Sintilimab for relapsed/refractory extranodal NK/T cell lymphoma: A multicenter, single-arm, phase 2 trial (ORIENT-4). Signal Transduct. Target. Ther. 2021, 6, 365. [Google Scholar] [CrossRef]
- Kim, S.J.; Lim, J.Q.; Laurensia, Y.; Cho, J.; Yoon, S.E.; Lee, J.Y.; Ryu, K.J.; Ko, Y.H.; Koh, Y.; Cho, D.; et al. Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: An open-label phase 2 study. Blood 2020, 136, 2754–2763. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Li, P.F.; Lu, Y.; Xia, Z.J.; Huang, H.Q.; Zhang, Y.J. CD38 expression predicts poor prognosis and might be a potential therapy target in extranodal NK/T cell lymphoma, nasal type. Ann. Hematol. 2015, 94, 1381–1388. [Google Scholar] [CrossRef]
- Hari, P.; Raj, R.V.; Olteanu, H. Targeting CD38 in Refractory Extranodal Natural Killer Cell-T-Cell Lymphoma. N. Engl. J. Med. 2016, 375, 1501–1502. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, J.; Yao, M.; Kim, T.M.; Yoon, D.H.; Cho, S.G.; Eom, H.S.; Lim, S.T.; Yeh, S.P.; Song, Y.; et al. Daratumumab monotherapy for patients with relapsed or refractory natural killer/T-cell lymphoma, nasal type: An open-label, single-arm, multicenter, phase 2 study. J. Hematol. Oncol. 2021, 14, 25. [Google Scholar] [CrossRef]
- Kim, H.K.; Moon, S.M.; Moon, J.H.; Park, J.E.; Byeon, S.; Kim, W.S. Complete remission in CD30-positive refractory extranodal NK/T-cell lymphoma with brentuximab vedotin. Blood Res. 2015, 50, 254–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poon, L.M.; Kwong, Y.L. Complete remission of refractory disseminated NK/T cell lymphoma with brentuximab vedotin and bendamustine. Ann. Hematol. 2016, 95, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Tse, E.; Kwong, Y.L. Epstein Barr virus-associated lymphoproliferative diseases: The virus as a therapeutic target. Exp. Mol. Med. 2015, 47, e136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollard, C.M.; Gottschalk, S.; Torrano, V.; Diouf, O.; Ku, S.; Hazrat, Y.; Carrum, G.; Ramos, C.; Fayad, L.; Shpall, E.J.; et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J. Clin. Oncol. 2014, 32, 798–808. [Google Scholar] [CrossRef]
- Kim, W.S.; Oki, Y.; Kim, S.J.; Yoon, S.E.; Ardeshna, K.M.; Lin, Y.; Ruan, J.; Porcu, P.; Brammer, J.E.; Jacobsen, E.D.; et al. Autologous EBV-specific T cell treatment results in sustained responses in patients with advanced extranodal NK/T lymphoma: Results of a multicenter study. Ann. Hematol. 2021, 100, 2529–2539. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.S.; Tse, E.; Kwong, Y.L. Chidamide in the treatment of peripheral T-cell lymphoma. Onco Targets 2017, 10, 347–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Dong, M.; Hong, X.; Zhang, W.; Feng, J.; Zhu, J.; Yu, L.; Ke, X.; Huang, H.; Shen, Z.; et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann. Oncol 2015, 26, 1766–1771. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Jia, B.; Xu, W.; Li, W.; Liu, T.; Liu, P.; Zhao, W.; Zhang, H.; Sun, X.; Yang, H.; et al. Chidamide in relapsed or refractory peripheral T cell lymphoma: A multicenter real-world study in China. J. Hematol. Oncol. 2017, 10, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, P.M.; Li, H.; Spier, C.; Mahadevan, D.; LeBlanc, M.; Ul Haq, M.; Huber, B.D.; Flowers, C.R.; Wagner-Johnston, N.D.; Horwitz, S.M.; et al. Phase II Intergroup Trial of Alisertib in Relapsed and Refractory Peripheral T-Cell Lymphoma and Transformed Mycosis Fungoides: SWOG 1108. J. Clin. Oncol. 2015, 33, 2399–2404. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, K.; Kim, T.M.; Lin, C.C.; Thye, L.S.; Chng, W.J.; Ma, B.; Chen, M.H.; Zhou, X.; Liu, H.; Kelly, V.; et al. Phase 1 study of the investigational Aurora A kinase inhibitor alisertib (MLN8237) in East Asian cancer patients: Pharmacokinetics and recommended phase 2 dose. Investig. New Drugs 2015, 33, 942–953. [Google Scholar] [CrossRef]
- Chan, T.S.; Kwong, Y.L.; Tse, E. Novel therapeutic agents for T-cell lymphomas. Discov. Med. 2013, 16, 27–35. [Google Scholar]
- Gao, Y.; Huang, H.Q.; Wang, X.; Bai, B.; Zhang, L.; Xiao, Y.; Liu, X.; Li, W.; Xu, W.; Feng, R.; et al. Anti-PD-1 Antibody (Sintilimab) Plus Histone Deacetylase Inhibitor (Chidamide) for the Treatment of Refractory or Relapsed Extranodal Natural Killer/T Cell Lymphoma, Nasal Type (r/r-ENKTL): Preliminary Results from a Prospective, Multicenter, Single-Arm, Phase Ib/II Trial (SCENT). Blood 2020, 136 (Suppl. 1), 39–40. [Google Scholar]
- Feng, Y.; Zhong, M.; Liu, Y.; Wang, L.; Tang, Y. Expression of TIM-3 and LAG-3 in extranodal NK/T cell lymphoma, nasal type. Histol. Histopathol. 2018, 33, 307–315. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).