Intra-Tumoral Pharmacokinetics of Pazopanib in Combination with Radiotherapy in Patients with Non-Metastatic Soft-Tissue Sarcoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Study Treatment
2.4. Pharmacokinetics
2.5. Efficacy Endpoint
2.6. Statistics
3. Results
3.1. Patient Characteristics
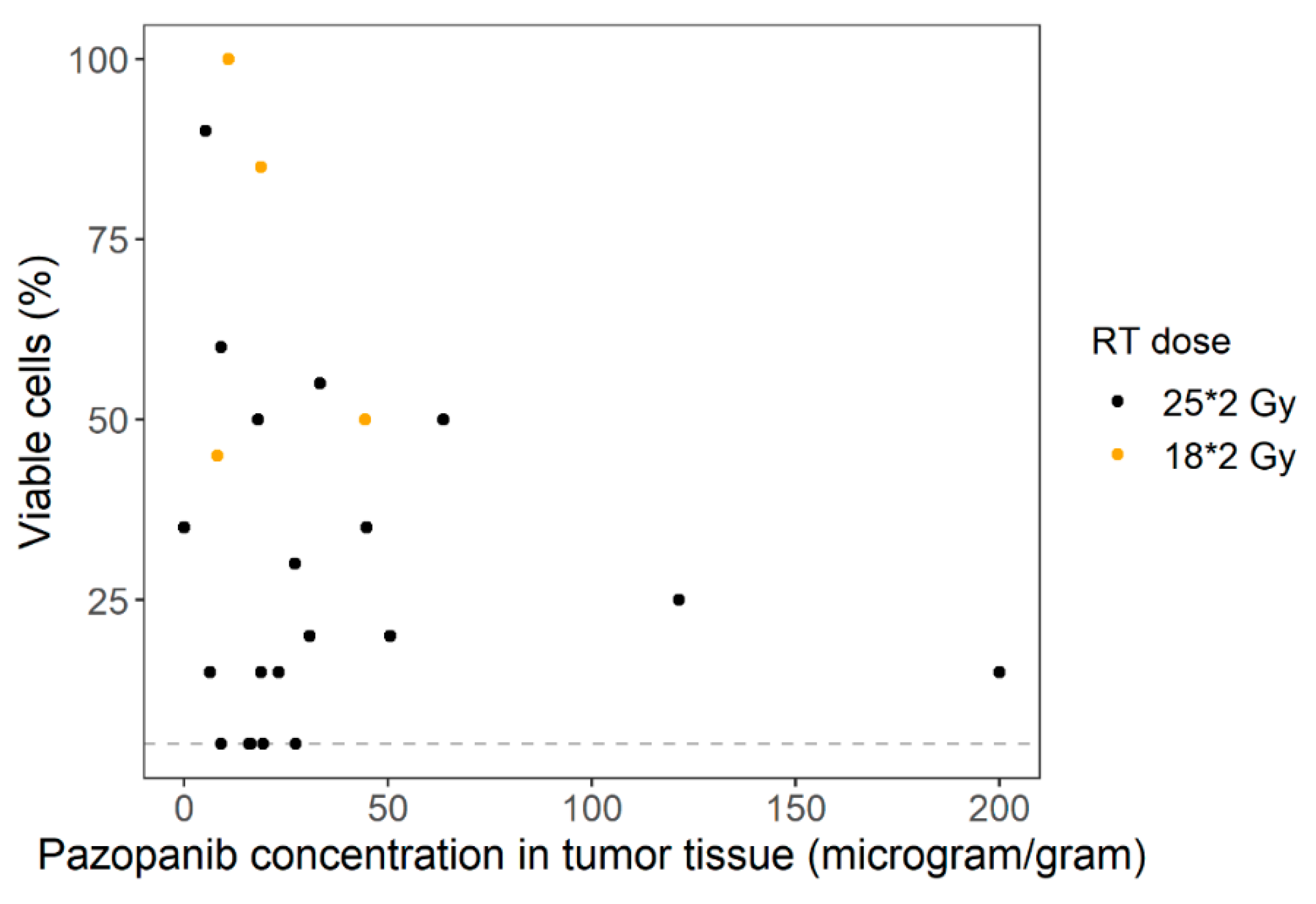
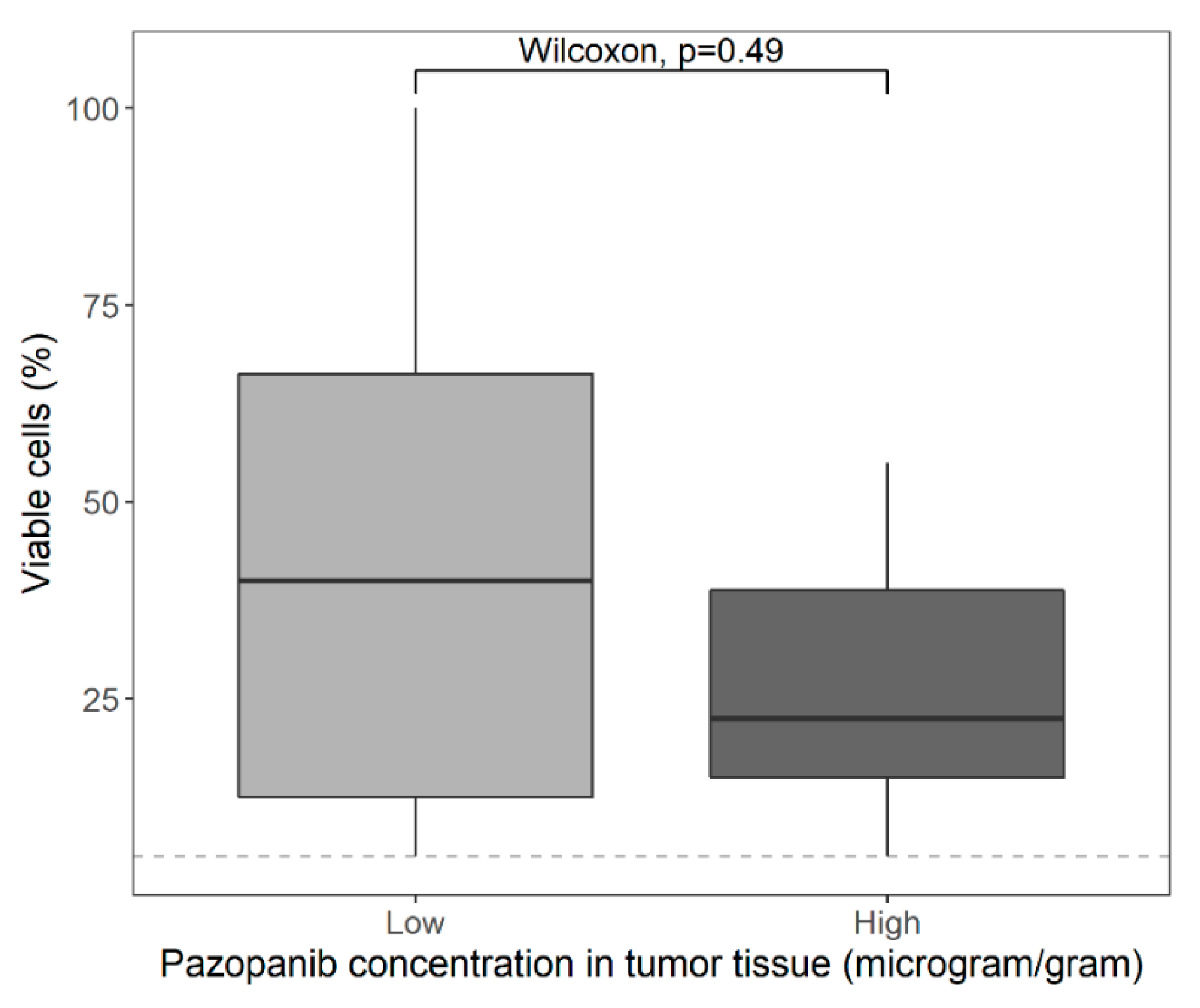
3.2. Pharmacokinetics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Medicines Agency Committee for Medicinal Products for Human Use (CHMP) Pazopanib European Public Assessment Report. Available online: https://www.ema.europa.eu/en/documents/product-information/votrient-epar-product-information_en.pdf (accessed on 1 April 2020).
- Kumar, R.; Knick, V.B.; Rudolph, S.K.; Johnson, J.H.; Crosby, R.M.; Crouthamel, M.C.; Hopper, T.M.; Miller, C.G.; Harrington, L.E.; Onori, J.A.; et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol. Cancer Ther. 2007, 6, 2012–2021. [Google Scholar] [CrossRef] [Green Version]
- Hamberg, P.; Verweij, J.; Sleijfer, S. (Pre-)Clinical Pharmacology and Activity of Pazopanib, a Novel Multikinase Angiogenesis Inhibitor. Oncologist 2010, 15, 539–547. [Google Scholar] [CrossRef] [Green Version]
- Haas, R.L.M.; Gelderblom, H.; Sleijfer, S.; Van Boven, H.H.; Scholten, A.; Dewit, L.; Borst, G.; Van Der Hage, J.; Kerst, J.M.; Nout, R.A.; et al. A phase I study on the combination of neoadjuvant radiotherapy plus pazopanib in patients with locally advanced soft tissue sarcoma of the extremities. Acta Oncol. (Madr). 2015, 54, 1195–1201. [Google Scholar] [CrossRef] [Green Version]
- van Meekeren, M.; Bovee, J.V.M.G.; van Coevorden, F.; van Houdt, W.; Schrage, Y.; Koenen, A.M.; Miah, A.B.; Zaidi, S.; Hayes, A.J.; Thway, K.; et al. A phase II study on the neo-adjuvant combination of pazopanib and radiotherapy in patients with high-risk, localized soft tissue sarcoma. Acta Oncol. (Madr) 2021, 1–8. [Google Scholar] [CrossRef]
- Suttle, A.B.; Ball, H.A.; Molimard, M.; Hutson, T.E.; Carpenter, C.; Rajagopalan, D.; Lin, Y.; Swann, S.; Amado, R.; Pandite, L. Relationships between pazopanib exposure and clinical safety and efficacy in patients with advanced renal cell carcinoma. Br. J. Cancer 2014, 111, 1909–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verheijen, R.B.; Beijnen, J.H.; Schellens, J.H.M.; Huitema, A.D.R.; Steeghs, N. Clinical Pharmacokinetics and Pharmacodynamics of Pazopanib: Towards Optimized Dosing. Clin. Pharmacokinet. 2017, 56, 987–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerdijk, K.; Desar, I.M.E.; Steeghs, N.; van der Graaf, W.T.A.; van Erp, N.P. Imatinib, sunitinib and pazopanib: From flat-fixed dosing towards a pharmacokinetically guided personalized dose. Br. J. Clin. Pharmacol. 2020, 86, 258–273. [Google Scholar] [CrossRef] [Green Version]
- Verheijen, R.B.; Swart, L.E.; Beijnen, J.H.; Schellens, J.H.M.; Huitema, A.D.R.; Steeghs, N. Exposure-survival analyses of pazopanib in renal cell carcinoma and soft tissue sarcoma patients: Opportunities for dose optimization. Cancer Chemother. Pharmacol. 2017, 80, 1171–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartelink, I.H.; Jones, E.F.; Shahidi-Latham, S.K.; Lee, P.R.E.; Zheng, Y.; Vicini, P.; van ‘t Veer, L.; Wolf, D.; Iagaru, A.; Kroetz, D.L.; et al. Tumor Drug Penetration Measurements Could Be the Neglected Piece of the Personalized Cancer Treatment Puzzle. Clin. Pharmacol. Ther. 2019, 106, 148–163. [Google Scholar] [CrossRef] [Green Version]
- Haura, E.B.; Sommers, E.; Song, L.; Chiappori, A.; Becker, A. A pilot study of preoperative gefitinib for early-stage lung cancer to assess intratumor drug concentration and pathways mediating primary resistance. J. Thorac. Oncol. 2010, 5, 1806–1814. [Google Scholar] [CrossRef] [Green Version]
- Lankheet, N.A.G.; Schaake, E.E.; Burgers, S.A.; Van Pel, R.; Beijnen, J.H.; Huitema, A.D.R.; Klomp, H. Concentrations of erlotinib in tumor tissue and plasma in non-small-cell lung cancer patients after neoadjuvant therapy. Clin. Lung Cancer 2015, 16, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Labots, M.; Pham, T.V.; Honeywell, R.J.; Knol, J.C.; Beekhof, R.; de Goeij-De Haas, R.; Dekker, H.; Neerincx, M.; Piersma, S.R.; van der Mijn, J.C.; et al. Kinase inhibitor treatment of patients with advanced cancer results in high tumor drug concentrations and in specific alterations of the tumor phosphoproteome. Cancers (Basel) 2020, 12, 330. [Google Scholar] [CrossRef] [Green Version]
- Torok, S.; Rezeli, M.; Kelemen, O.; Vegvari, A.; Watanabe, K.; Sugihara, Y.; Tisza, A.; Marton, T.; Kovacs, I.; Tovari, J.; et al. Limited tumor tissue drug penetration contributes to primary resistance against angiogenesis inhibitors. Theranostics 2017, 7, 400–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbrink, M.; De Vries, N.; Rosing, H.; Huitema, A.D.R.; Nuijen, B.; Schellens, J.H.M.; Beijnen, J.H. Quantification of 11 therapeutic kinase inhibitors in human plasma for therapeutic drug monitoring using liquid chromatography coupled with tandem mass spectrometry. Ther. Drug Monit. 2016, 38, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, R.B.; Thijssen, B.; Rosing, H.; Schellens, J.H.M.; Nan, L.; Venekamp, N.; Beijnen, J.H.; Steeghs, N.; Huitema, A.D.R. Fast and Straightforward Method for the Quantification of Pazopanib in Human Plasma Using LC-MS/MS. Ther. Drug Monit. 2018, 40, 230–236. [Google Scholar] [CrossRef]
- Wang, Y.; Chia, Y.L.; Nedelman, J.; Schran, H.; Mahon, F.X.; Molimard, M. A therapeutic drug monitoring algorithm for refining the imatinib trough level obtained at different sampling times. Ther. Drug Monit. 2009, 31, 579–584. [Google Scholar] [CrossRef]
- Fletcher, C.; Hogendoorn, P.; Mertens, F.; Bridge, J. Who Classification of Tumours of Soft Tissue and Bone, 4th ed.; IARC Press: France, Lyon, 2013. [Google Scholar]
- Verheijen, R.B.; Yu, H.; Schellens, J.H.M.; Beijnen, J.H.; Steeghs, N.; Huitema, A.D.R. Practical Recommendations for Therapeutic Drug Monitoring of Kinase Inhibitors in Oncology. Clin. Pharmacol. Ther. 2017, 102, 765–776. [Google Scholar] [CrossRef]
- Lee, C.G.; Heijn, M.; Di Tomaso, E.; Griffon-Etienne, G.; Ancukiewicz, M.; Koike, C.; Park, K.R.; Ferrara, N.; Jain, R.K.; Suit, H.D.; et al. Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000, 60, 5565–5570. [Google Scholar]
- Wildiers, H.; Guetens, G.; De Boeck, G.; Verbeken, E.; Landuyt, B.; Landuyt, W.; De Bruijn, E.A.; Van Oosterom, A.T. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br. J. Cancer 2003, 88, 1979–1986. [Google Scholar] [CrossRef] [Green Version]
- Batchelor, T.T.; Gerstner, E.R.; Emblem, K.E.; Duda, D.G.; Kalpathy-Cramer, J.; Snuderl, M.; Ancukiewicz, M.; Polaskova, P.; Pinho, M.C.; Jennings, D.; et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc. Natl. Acad. Sci. USA 2013, 110, 19059–19064. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.K. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat. Med. 2001, 7, 987–989. [Google Scholar] [CrossRef]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the Vasculature for Treatment of Cancer and Other Diseases. Physiol. Rev. 2012, 91, 1071–1121. [Google Scholar] [CrossRef]
- Goedegebuure, R.S.A.; De Klerk, L.K.; Bass, A.J.; Derks, S.; Thijssen, V.L.J.L. Combining radiotherapy with anti-angiogenic therapy and immunotherapy; A therapeutic triad for cancer? Front. Immunol. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Kleibeuker, E.A.; Griffioen, A.W.; Verheul, H.M.; Slotman, B.J.; Thijssen, V.L. Combining angiogenesis inhibition and radiotherapy: A double-edged sword. Drug Resist. Updat. 2012, 15, 173–182. [Google Scholar] [CrossRef]
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Winkler, F.; Kozin, S.V.; Tong, R.T.; Chae, S.-S.; Booth, M.F.; Garkavtsev, I.; Xu, L.; Hicklin, D.J.; Fukumura, D.; di Tomaso, E.; et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation. Cancer Cell 2004, 6, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Dings, R.P.M.; Loren, M.; Heun, H.; McNiel, E.; Griffioen, A.W.; Mayo, K.H.; Griffin, R.J. Scheduling of Radiation with Angiogenesis Inhibitors Anginex and Avastin Improves Therapeutic Outcome via Vessel Normalization. Clin. Cancer Res. 2007, 13, 3395–3402. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, S.; Batra, S.; Saito, K.; Yasui, H.; Choudhuri, R.; Gadisetti, C.; Subramanian, S.; Devasahayam, N.; Munasinghe, J.P.; Mitchell, J.B.; et al. Antiangiogenic agent sunitinib transiently increases tumor oxygenation and suppresses cycling hypoxia. Cancer Res. 2011, 71, 6350–6359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledoux, P.; Kind, M.; Le Loarer, F.; Stoeckle, E.; Italiano, A.; Tirode, F.; Buy, X.; Crombé, A. Abnormal vascularization of soft-tissue sarcomas on conventional MRI: Diagnostic and prognostic values. Eur. J. Radiol. 2019, 117, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Mentzel, T.; Brown, L.F.; Dvorak, H.F.; Kuhnen, C.; Stiller, K.J.; Katenkamp, D.; Fletcher, C.D.M. The association between tumour progression and vascularity in myxofibrosarcoma and myxoid/round cell liposarcoma. Virchows Arch. 2001, 438, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Jarosz-Biej, M.; Smolarczyk, R.; Cichoń, T.; Kułach, N. Tumor microenvironment as a “game changer” in cancer radiotherapy. Int. J. Mol. Sci. 2019, 20, 3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Characteristic | n (%) or Median (Range) |
|---|---|
| Gender | |
| Male | 14 (58) |
| Female | 10 (42) |
| Age (years) | 57 (24–79) |
| Tumor histology | |
| Undifferentiated pleomorphic sarcoma | 9 (38) |
| Myxofibrosarcoma | 8 (33) |
| Spindle cell sarcoma (not otherwise specified) | 2 (8) |
| Myxoid liposarcoma | 1 (4) |
| Synovial sarcoma | 1 (4) |
| Spindle cell rhabdomyosarcoma | 1 (4) |
| Clear cell sarcoma | 1 (4) |
| Malignant peripheral nerve sheath tumor | 1 (4) |
| Pazopanib trough levels in plasma on day 22 (mg/L) | 34.8 (8.38–120.7) |
| Pazopanib concentrations in tumor tissue (µg/g) | |
| Total | 19.2 (0.149–200) |
| Undifferentiated pleomorphic sarcoma | 27.4 (0.149–121) |
| Myxofibrosarcoma | 18.9 (5.31–200) |
| Spindle cell sarcoma (not otherwise specified) | 36.4 (9.08–63.7) |
| Myxoid liposarcoma | 16.3 (N/A) |
| Synovial sarcoma | 27.3 (N/A) |
| Spindle cell rhabdomyosarcoma | 18.2 (N/A) |
| Clear cell sarcoma | 16.0 (N/A) |
| Malignant peripheral nerve sheath tumor | 11.0 (N/A) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molenaar-Kuijsten, L.; van Meekeren, M.; Verheijen, R.B.; Bovée, J.V.M.G.; Fiocco, M.; Thijssen, B.; Rosing, H.; Huitema, A.D.R.; Miah, A.B.; Gelderblom, H.; et al. Intra-Tumoral Pharmacokinetics of Pazopanib in Combination with Radiotherapy in Patients with Non-Metastatic Soft-Tissue Sarcoma. Cancers 2021, 13, 5780. https://doi.org/10.3390/cancers13225780
Molenaar-Kuijsten L, van Meekeren M, Verheijen RB, Bovée JVMG, Fiocco M, Thijssen B, Rosing H, Huitema ADR, Miah AB, Gelderblom H, et al. Intra-Tumoral Pharmacokinetics of Pazopanib in Combination with Radiotherapy in Patients with Non-Metastatic Soft-Tissue Sarcoma. Cancers. 2021; 13(22):5780. https://doi.org/10.3390/cancers13225780
Chicago/Turabian StyleMolenaar-Kuijsten, Laura, Milan van Meekeren, Remy B. Verheijen, Judith V. M. G. Bovée, Marta Fiocco, Bas Thijssen, Hilde Rosing, Alwin D. R. Huitema, Aisha B. Miah, Hans Gelderblom, and et al. 2021. "Intra-Tumoral Pharmacokinetics of Pazopanib in Combination with Radiotherapy in Patients with Non-Metastatic Soft-Tissue Sarcoma" Cancers 13, no. 22: 5780. https://doi.org/10.3390/cancers13225780
APA StyleMolenaar-Kuijsten, L., van Meekeren, M., Verheijen, R. B., Bovée, J. V. M. G., Fiocco, M., Thijssen, B., Rosing, H., Huitema, A. D. R., Miah, A. B., Gelderblom, H., Haas, R. L. M., & Steeghs, N. (2021). Intra-Tumoral Pharmacokinetics of Pazopanib in Combination with Radiotherapy in Patients with Non-Metastatic Soft-Tissue Sarcoma. Cancers, 13(22), 5780. https://doi.org/10.3390/cancers13225780








