1H-NMR Plasma Lipoproteins Profile Analysis Reveals Lipid Metabolism Alterations in HER2-Positive Breast Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Population
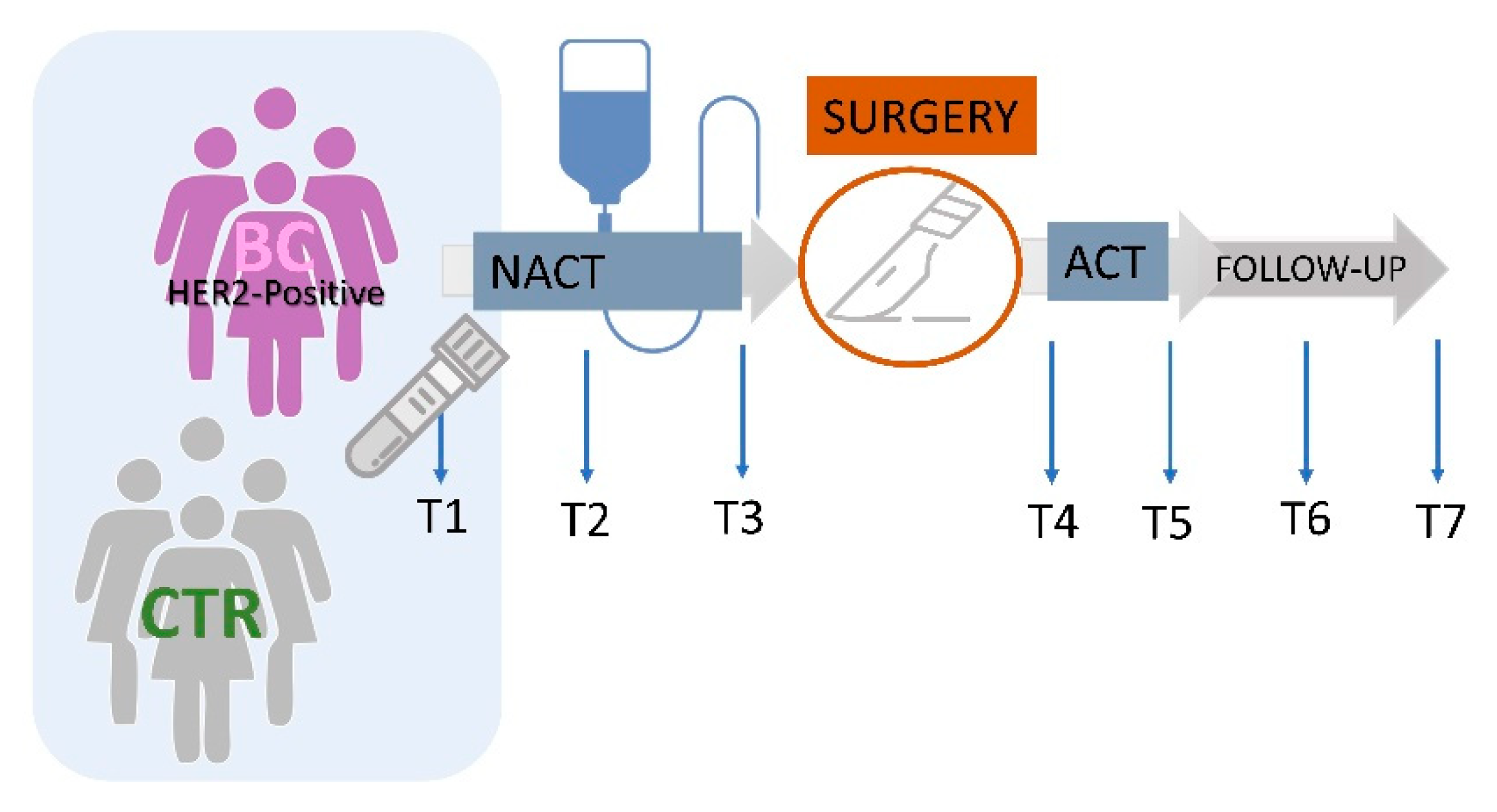
2.2. Study Design
2.3. Sample Collection
2.4. 1H-NMR Lipoproteins Analyses
2.5. Statistical Analyses
3. Results
3.1. Study Populations’ Characteristics
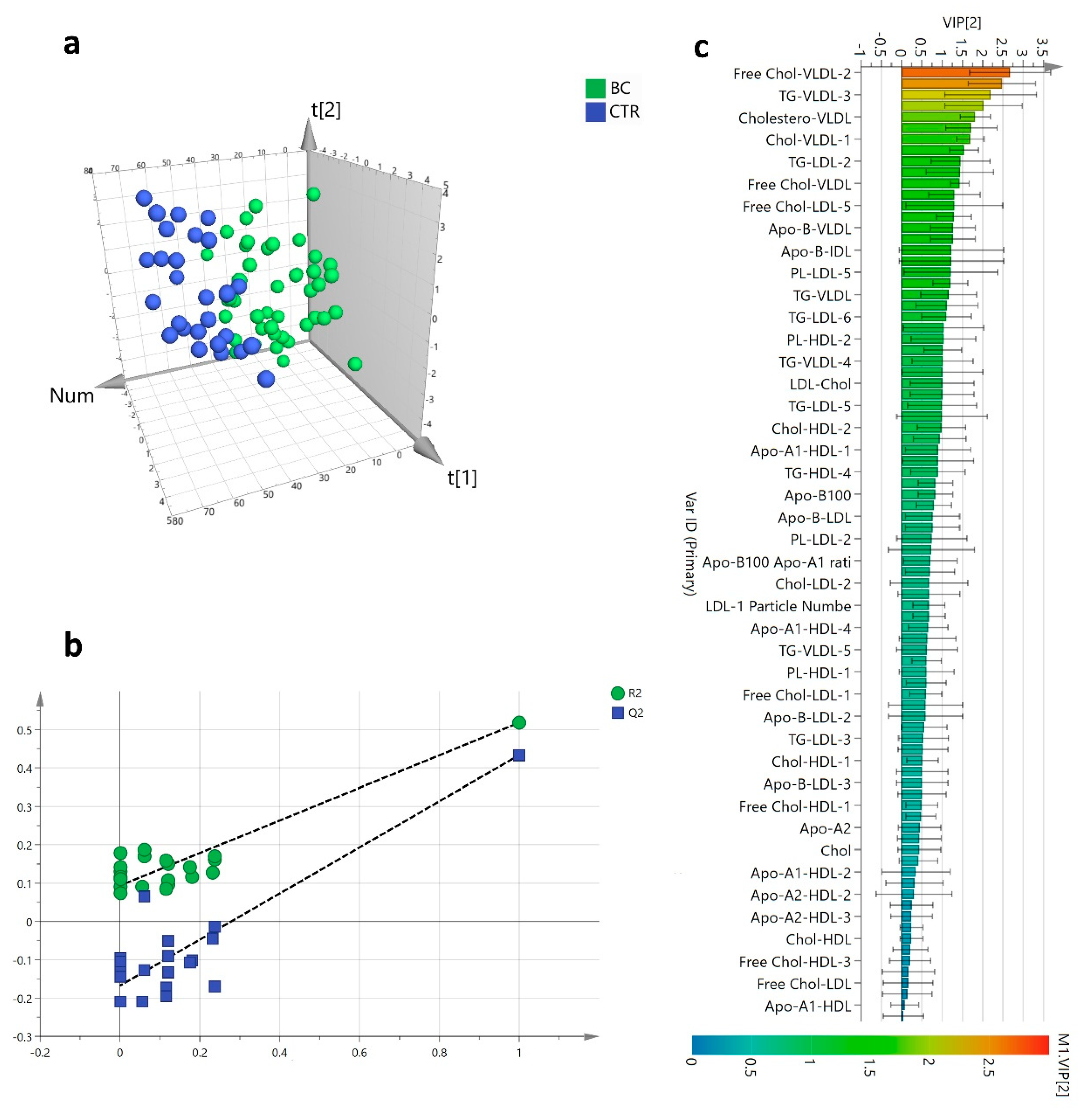
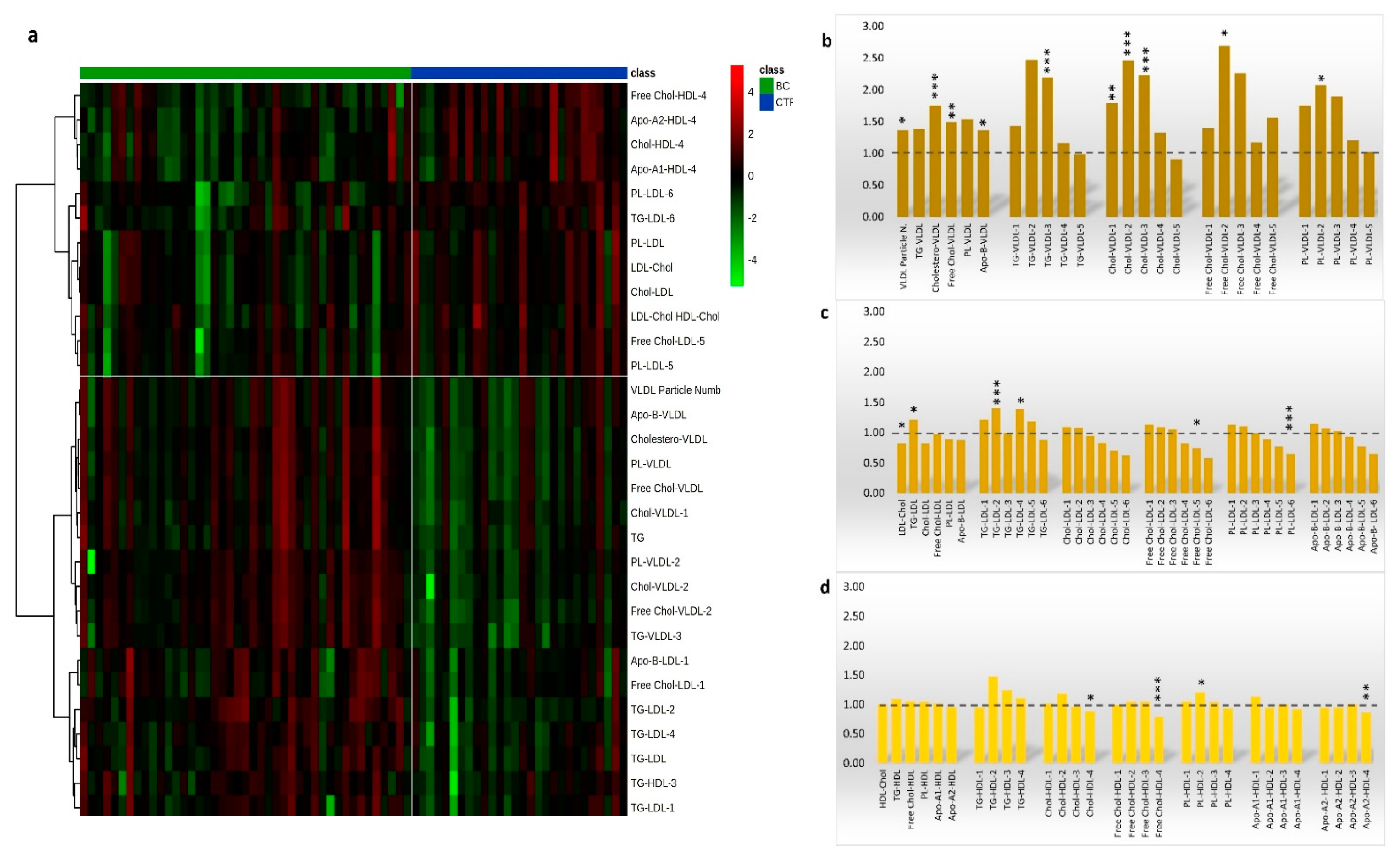
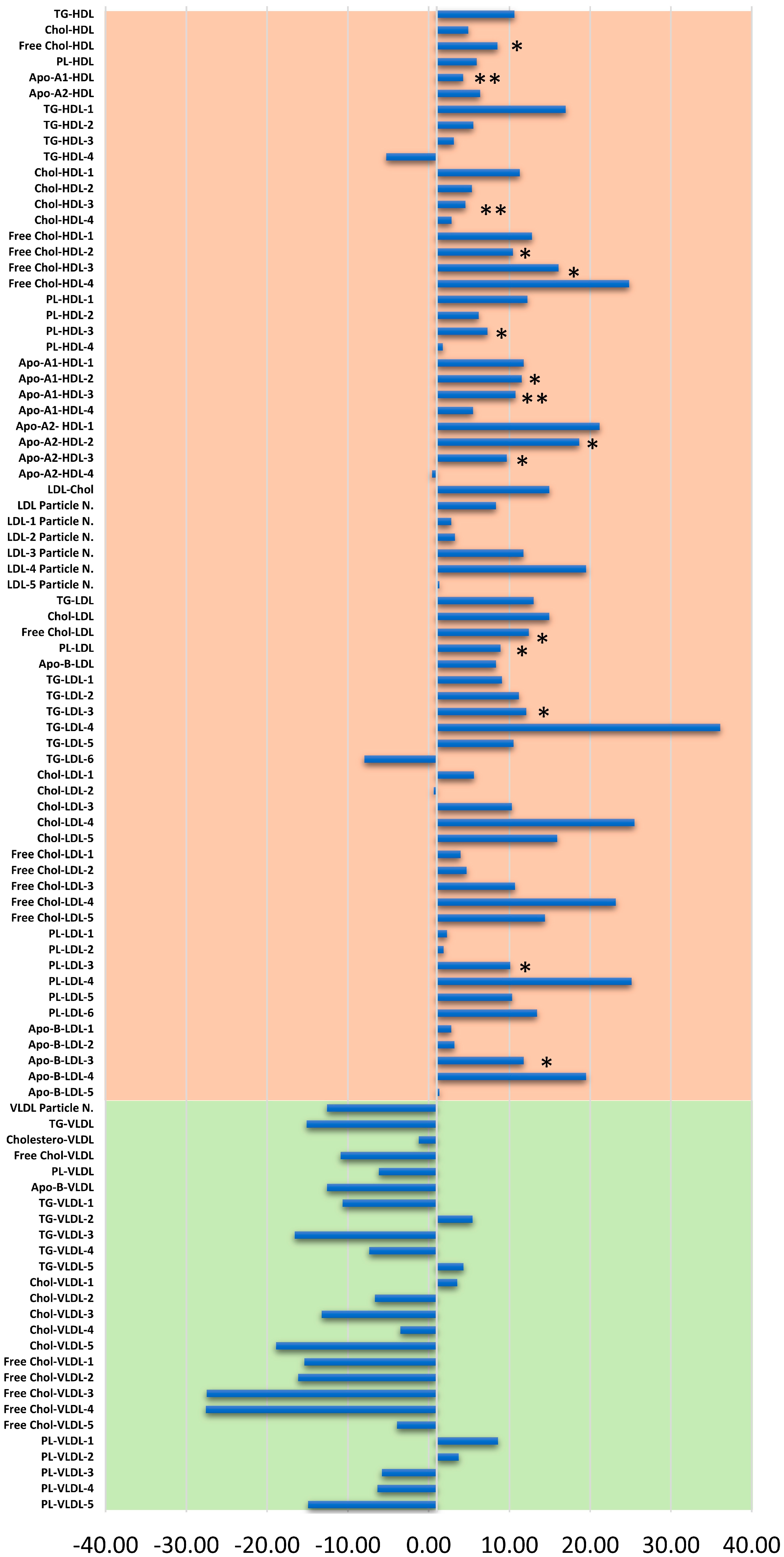
3.2. Lipoproteins Subfractions Profile in HER2-Positive BC Patients vs. CTR Group
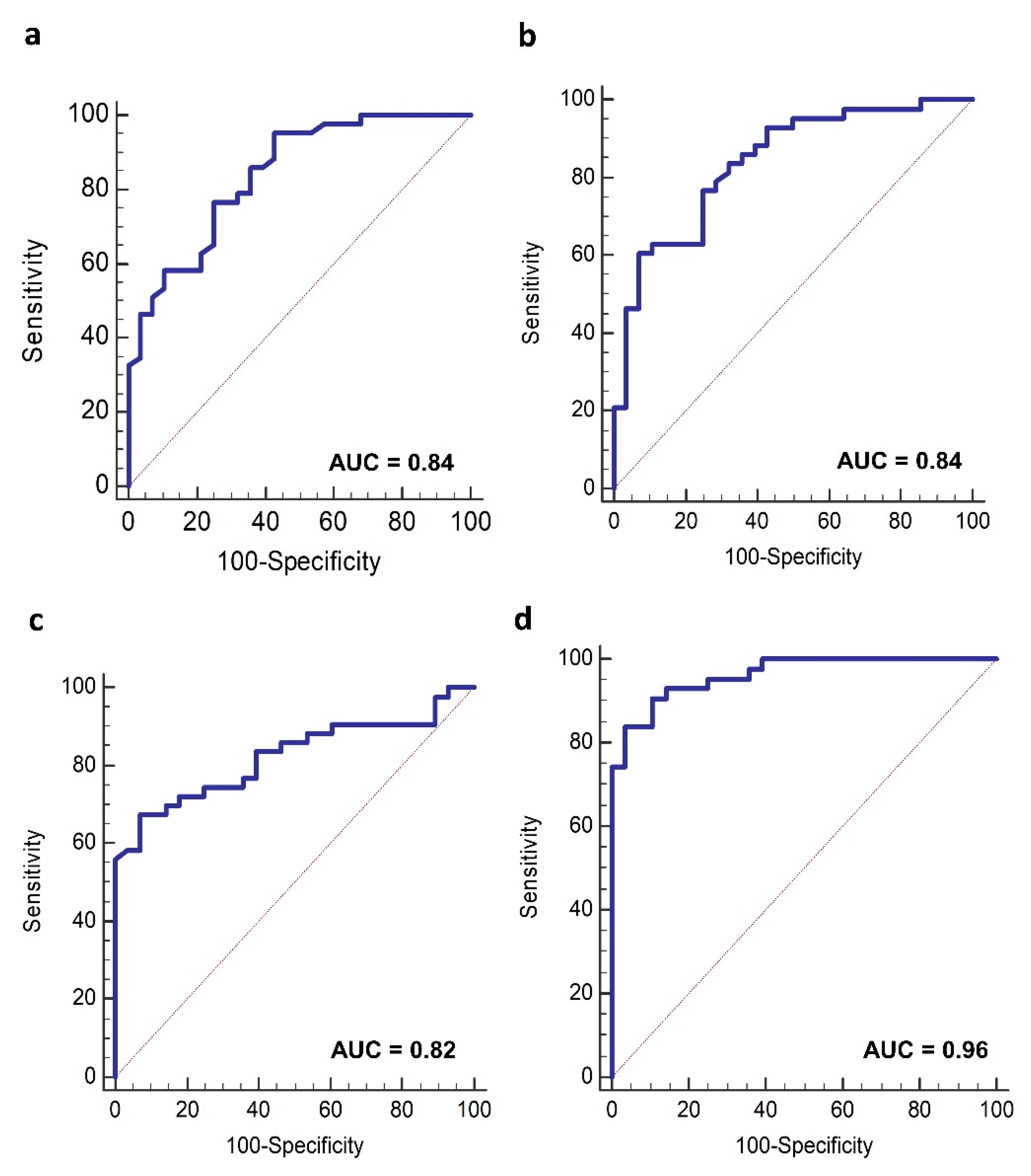
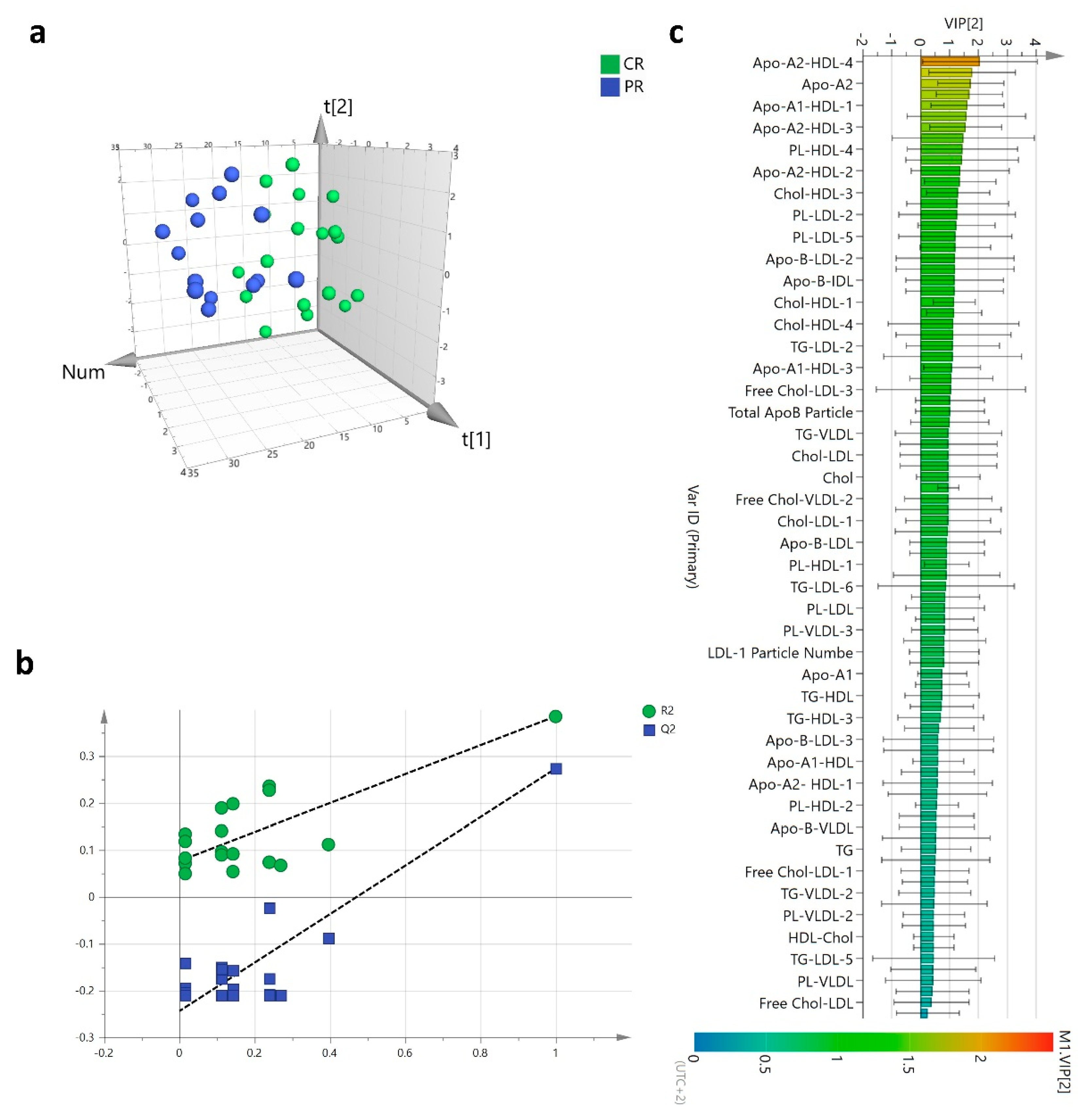
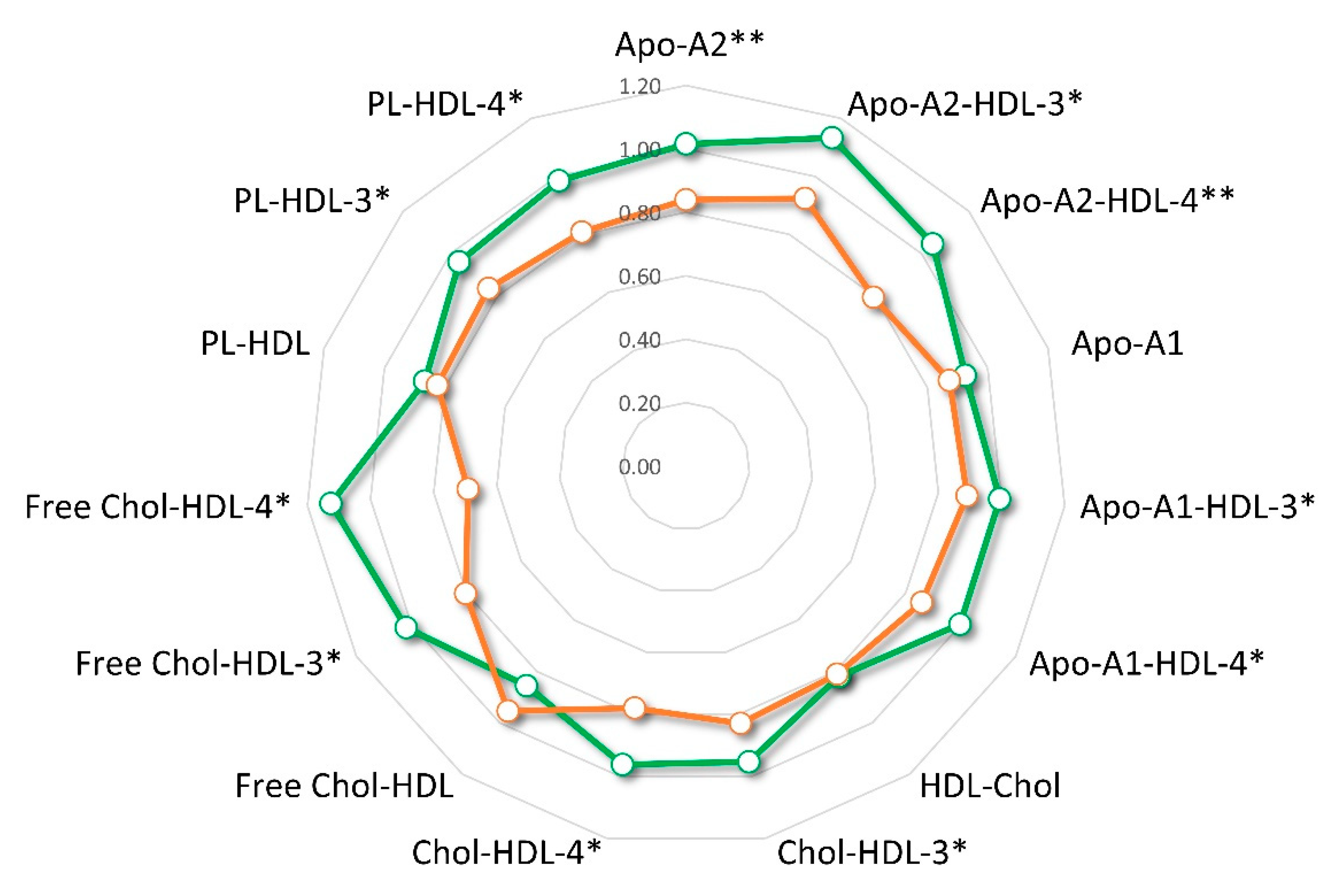
3.3. Lipoproteins Profile and HER2-Positive BC Diagnosis
3.4. Lipoproteins Profiles Time Changes
3.5. Lipoproteins Changes and Clinical Outcome
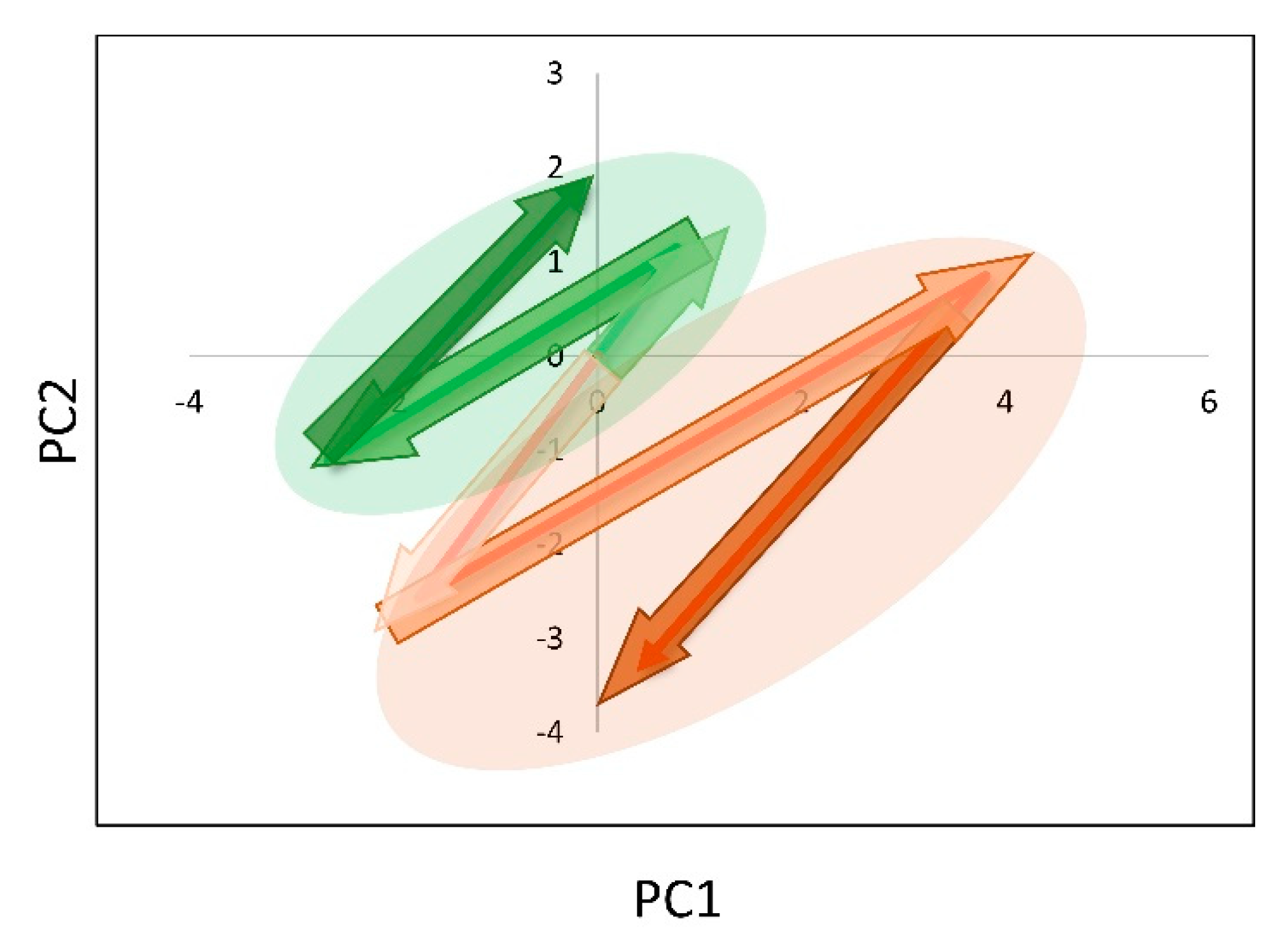
3.6. Plasma Lipoproteins Trajectories and Recurrence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, C.; Geng, F.; Cheng, X.; Guo, D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun. 2018, 38, 27. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef] [Green Version]
- Carbonetti, G.; Wilpshaar, T.; Kroonen, J.; Studholme, K.; Converso, C.; D’Oelsnitz, S.; Kaczocha, M. FABP5 coordinates lipid signaling that promotes prostate cancer metastasis. Sci. Rep. 2019, 9, 18944. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Lu, M.; Shi, J.; Gong, Z.; Hua, L.; Li, Q.; Lim, B.; Zhang, X.H.-F.; Chen, X.; Li, S.; et al. Lung mesenchymal cells elicit lipid storage in neutrophils that fuel breast cancer lung metastasis. Nat. Immunol. 2020, 21, 1444–1455. [Google Scholar] [CrossRef]
- Song, L.; Liu, Z.; Hu, H.-H.; Yang, Y.; Li, T.Y.; Lin, Z.-Z.; Ye, J.; Chen, J.; Huang, X.; Liu, D.-T.; et al. Proto-oncogene Src links lipogenesis via lipin-1 to breast cancer malignancy. Nat. Commun. 2020, 11, 5842. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Lee, M.-Y.; Park, S.W.; Moon, J.-S.; Koh, Y.-K.; Ahn, Y.H.; Park, B.-W.; Kim, K.-S. Up-regulation of Acetyl-CoA Carboxylase α and Fatty Acid Synthase by Human Epidermal Growth Factor Receptor 2 at the Translational Level in Breast Cancer Cells. J. Biol. Chem. 2007, 282, 26122–26131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaban, S.; Shearer, R.F.; Lee, L.S.; Van Geldermalsen, M.; Schreuder, M.; Shtein, H.C.; Cairns, R.; Thomas, K.C.; Fazakerley, D.; Grewal, T.; et al. Adipocyte lipolysis links obesity to breast cancer growth: Adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 2017, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kuemmerle, N.B.; Rysman, E.; Lombardo, P.S.; Flanagan, A.J.; Lipe, B.C.; Wells, W.A.; Pettus, J.R.; Froehlich, H.M.; Memoli, V.A.; Morganelli, P.M.; et al. Lipoprotein Lipase Links Dietary Fat to Solid Tumor Cell Proliferation. Mol. Cancer Ther. 2011, 10, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Lupien, L.; Bloch, K.; Dehairs, J.; Traphagen, N.A.; Feng, W.; Davis, W.L.; Dennis, T.; Swinnen, J.V.; Wells, W.A.; Smits, N.C.; et al. Endocytosis of very low-density lipoproteins: An unexpected mechanism for lipid acquisition by breast cancer cells. J. Lipid Res. 2020, 61, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W.; Innerarity, T.L.; Rall, S.C.; Weisgraber, K.H. Plasma lipoproteins: Apolipoprotein structure and function. J. Lipid Res. 1984, 25, 1277–1294. [Google Scholar] [CrossRef]
- Ramasamy, I. Recent advances in physiological lipoprotein metabolism. Clin. Chem. Lab. Med. 2014, 52, 1695–1727. [Google Scholar] [CrossRef]
- Anastasius, M.; Luquain-Costaz, C.; Kockx, M.; Jessup, W.; Kritharides, L. A critical appraisal of the measurement of serum ‘cholesterol efflux capacity’ and its use as surrogate marker of risk of cardiovascular disease. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2018, 1863, 1257–1273. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Nicholls, S.; Rye, K.-A.; Anantharamaiah, G.M.; Navab, M.; Fogelman, A.M. Antiinflammatory Properties of HDL. Circ. Res. 2004, 95, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Hunjadi, M.; Lamina, C.; Kahler, P.; Bernscherer, T.; Viikari, J.; Lehtimäki, T.; Kähönen, M.; Hurme, M.; Juonala, M.; Taittonen, L.; et al. HDL cholesterol efflux capacity is inversely associated with subclinical cardiovascular risk markers in young adults: The cardiovascular risk in Young Finns study. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Kontush, A. HDL particle number and size as predictors of cardiovascular disease. Front. Pharmacol. 2015, 6, 218. [Google Scholar] [CrossRef] [Green Version]
- Kontush, A.; Chapman, M.J. Antiatherogenic function of HDL particle subpopulations: Focus on antioxidative activities. Curr. Opin. Lipidol. 2010, 21, 312–318. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Barnett, J.; Fong, L.G. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1990, 1044, 275–283. [Google Scholar] [CrossRef]
- Bruno, D.S.; White, P.S.; Thompson, C.L.; Adebisi, M.; Berger, N.A. Lipid profile and breast cancer characteristics: A retrospective correlational study. J. Clin. Oncol. 2015, 33, e12659. [Google Scholar] [CrossRef]
- Carr, B.I.; Giannelli, G.; Guerra, V.; Giannini, E.G.; Farinati, F.; Rapaccini, G.L.; Di Marco, M.; Zoli, M.; Caturelli, E.; Masotto, A.; et al. Plasma cholesterol and lipoprotein levels in relation to tumor aggressiveness and survival in HCC patients. Int. J. Biol. Markers 2018, 33, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Flote, V.G.; Vettukattil, R.; Bathen, T.F.; Egeland, T.; McTiernan, A.; Frydenberg, H.; Husøy, A.; Finstad, S.E.; Lømo, J.; Garred, Ø.; et al. Lipoprotein subfractions by nuclear magnetic resonance are associated with tumor characteristics in breast cancer. Lipids Health Dis. 2016, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.M.; Kang, D.; Guallar, E.; Yu, J.; Lee, J.E.; Kim, S.W.; Nam, S.J.; Cho, J.; Lee, S.K. Impact of Serum Lipid on Breast Cancer Recurrence. J. Clin. Med. 2020, 9, 2846. [Google Scholar] [CrossRef]
- Yang, H.-H.; Chen, X.-F.; Hu, W.; Lv, D.-Q.; Ding, W.-J.; Tang, L.-J.; Jiang, J.-J.; Ye, M.-H. Lipoprotein(a) level and its association with tumor stage in male patients with primary lung cancer. Clin. Chem. Lab. Med. 2009, 47, 452–457. [Google Scholar] [CrossRef]
- Ghahremanfard, F.; Mirmohammadkhani, M.; Shahnazari, B.; Gholami, G.; Mehdizadeh, J. The Valuable Role of Measuring Serum Lipid Profile in Cancer Progression. Oman Med. J. 2015, 30, 353–357. [Google Scholar] [CrossRef]
- Kumie, G.; Melak, T.; Baynes, H.W. The Association of Serum Lipid Levels with Breast Cancer Risks Among Women with Breast Cancer at Felege Hiwot Comprehensive Specialized Hospital, Northwest Ethiopia. Breast Cancer Targets Ther. 2020, 12, 279–287. [Google Scholar] [CrossRef]
- Probert, F.; Ruiz-Rodado, V.; Vruchte, D.T.; Nicoli, E.-R.; Claridge, T.D.W.; Wassif, C.A.; Farhat, N.; Porter, F.D.; Platt, F.; Grootveld, M. NMR analysis reveals significant differences in the plasma metabolic profiles of Niemann Pick C1 patients, heterozygous carriers, and healthy controls. Sci. Rep. 2017, 7, 6320. [Google Scholar] [CrossRef] [PubMed]
- Raittinen, P.; Niemistö, K.; Pennanen, E.; Syvälä, H.; Auriola, S.; Riikonen, J.; Lehtimäki, T.; Ilmonen, P.; Murtola, T. Circulatory and prostatic tissue lipidomic profiles shifts after high-dose atorvastatin use in men with prostate cancer. Sci. Rep. 2020, 10, 12016. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Liu, Z.; Zhao, Z.; Zhang, X.; Tao, S.; Yuan, B.; Zhang, J.; Wang, D.; Liu, Q.; Ding, Y. Emerging roles of low-density lipoprotein in the development and treatment of breast cancer. Lipids Health Dis. 2019, 18, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.-W.; Lo, Y.-H.; Chen, C.-H.; Lin, C.-Y.; Tsai, C.-H.; Chen, P.-J.; Yang, Y.-F.; Wang, C.-H.; Tan, C.-H.; Hou, M.-F.; et al. VLDL and LDL, but not HDL, promote breast cancer cell proliferation, metastasis and angiogenesis. Cancer Lett. 2017, 388, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Michalaki, V.; Koutroulis, G.; Syrigos, K.; Piperi, C.; Kalofoutis, A. Evaluation of serum lipids and high-density lipoprotein subfractions (HDL2, HDL3) in postmenopausal patients with breast cancer. Mol. Cell. Biochem. 2005, 268, 19–24. [Google Scholar] [CrossRef]
- Otvos, J.D.; Jeyarajah, E.J.; Cromwell, W. Measurement issues related to lipoprotein heterogeneity. Am. J. Cardiol. 2002, 90, 22–29. [Google Scholar] [CrossRef]
- Shiffman, D.; Louie, J.Z.; Caulfield, M.P.; Nilsson, P.M.; Devlin, J.J.; Melander, O. LDL subfractions are associated with incident cardiovascular disease in the Malmö Prevention Project Study. Atherosclerosis 2017, 263, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Soedamah-Muthu, S.S.; Chang, Y.-F.; Otvos, J.; Evans, R.W.; Orchard, T. Lipoprotein subclass measurements by nuclear magnetic resonance spectroscopy improve the prediction of coronary artery disease in Type 1 Diabetes. A prospective report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetology 2003, 46, 674–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, M.J.; Goldstein, S.; Lagrange, D.; Laplaud, P.M. A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J. Lipid Res. 1981, 22, 339–358. [Google Scholar] [CrossRef]
- Potts, J.; Fisher, R.; Humphreys, S.; Gibbons, G.; Frayn, K. Separation of lipoprotein fractions by ultracentrifugation: Investigation of analytical recovery with sequential flotation and density gradient procedures. Clin. Chim. Acta 1994, 230, 215–220. [Google Scholar] [CrossRef]
- Redgrave, T.; Roberts, D.; West, C. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal. Biochem. 1975, 65, 42–49. [Google Scholar] [CrossRef]
- Aru, V.; Lam, C.; Khakimov, B.; Hoefsloot, H.C.; Zwanenburg, G.; Lind, M.V.; Schäfer, H.; van Duynhoven, J.; Jacobs, D.M.; Smilde, A.K.; et al. Quantification of lipoprotein profiles by nuclear magnetic resonance spectroscopy and multivariate data analysis. TrAC Trends Anal. Chem. 2017, 94, 210–219. [Google Scholar] [CrossRef]
- Balder, Y.; Vignoli, A.; Tenori, L.; Luchinat, C.; Saccenti, E. Exploration of Blood Lipoprotein and Lipid Fraction Profiles in Healthy Subjects through Integrated Univariate, Multivariate, and Network Analysis Reveals Association of Lipase Activity and Cholesterol Esterification with Sex and Age. Metabolites 2021, 11, 326. [Google Scholar] [CrossRef]
- Jiménez, B.; Holmes, E.; Heude, C.; Tolson, R.F.; Harvey, N.; Lodge, S.L.; Chetwynd, A.J.; Cannet, C.; Fang, F.; Pearce, J.T.M.; et al. Quantitative Lipoprotein Subclass and Low Molecular Weight Metabolite Analysis in Human Serum and Plasma by 1H NMR Spectroscopy in a Multilaboratory Trial. Anal. Chem. 2018, 90, 11962–11971. [Google Scholar] [CrossRef]
- Monsonis Centelles, S.; Hoefsloot, H.C.J.; Khakimov, B.; Ebrahimi, P.; Lind, M.V.; Kristensen, M.; de Roo, N.; Jacobs, D.M.; van Duynhoven, J.; Cannet, C.; et al. Toward Reliable Lipoprotein Particle Predictions from NMR Spectra of Human Blood: An Interlaboratory Ring Test. Anal. Chem. 2017, 89, 8004–8012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, D.; Gao, Y.; Chen, J.; Huang, Y.; Zhang, Z.; Xu, F. Time-resolved metabolomics analysis of individual differences during the early stage of lipopolysaccharide-treated rats. Sci. Rep. 2016, 6, 34136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-J.; Hou, M.-F.; Tsai, S.-M.; Wu, S.-H.; Hou, L.; Ma, H.; Shann, T.-Y.; Tsai, L.-Y. The association between lipid profiles and breast cancer among Taiwanese women. Clin. Chem. Lab. Med. 2007, 45, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.A.; Islam, F.; Prova, M.T.; Khatun, M.; Sharmin, I.; Islam, K.M.; Hassan, K.; Khan, A.S.; Rahman, M.M. Association of hyperlipidemia with breast cancer in Bangladeshi women. Lipids Health Dis. 2021, 20, 52. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Z.-L.; Wu, Y.-T.; Wu, H.; Dai, W.; Arshad, B.; Xu, Z.; Li, H.; Wu, K.-N.; Kong, L.-Q. Status of lipid and lipoprotein in female breast cancer patients at initial diagnosis and during chemotherapy. Lipids Health Dis. 2018, 17, 91. [Google Scholar] [CrossRef] [Green Version]
- Nowak, C.; Ärnlöv, J. A Mendelian randomization study of the effects of blood lipids on breast cancer risk. Nat. Commun. 2018, 9, 3957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucharska-Newton, A.M.; Rosamond, W.D.; Mink, P.J.; Alberg, A.J.; Shahar, E.; Folsom, A.R. HDL-Cholesterol and Incidence of Breast Cancer in the ARIC Cohort Study. Ann. Epidemiol. 2008, 18, 671–677. [Google Scholar] [CrossRef]
- Li, X.; Tang, H.; Wang, J.; Xie, X.; Liu, P.; Kong, Y.; Ye, F.; Shuang, Z.; Xie, Z.; Xie, X. The effect of preoperative serum triglycerides and high-density lipoprotein-cholesterol levels on the prognosis of breast cancer. Breast 2017, 32, 1–6. [Google Scholar] [CrossRef]
- Zhou, P.; Li, B.; Liu, B.; Chen, T.; Xiao, J. Prognostic role of serum total cholesterol and high-density lipoprotein cholesterol in cancer survivors: A systematic review and meta-analysis. Clin. Chim. Acta 2018, 477, 94–104. [Google Scholar] [CrossRef]
- Garcia-Estevez, L.; Moreno-Bueno, G. Updating the role of obesity and cholesterol in breast cancer. Breast Cancer Res. 2019, 21, 35. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Park, S.K.; Han, W.; Kim, D.-H.; Hong, Y.-C.; Ha, E.H.; Ahn, S.-H.; Noh, D.-Y.; Kang, D.; Yoo, K.-Y. Serum High-Density Lipoprotein Cholesterol and Breast Cancer Risk by Menopausal Status, Body Mass Index, and Hormonal Receptor in Korea. Cancer Epidemiol. Biomark. Prev. 2009, 18, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Sharma, A. Serum cholesterol levels in carcinoma breast. Indian J. Med. Res. 1991, 94, 193–196. [Google Scholar] [PubMed]
- Howlader, N.; Altekruse, S.F.; Li, C.I.; Chen, V.W.; Clarke, C.A.; Ries, L.A.; Cronin, K.A. US Incidence of Breast Cancer Subtypes Defined by Joint Hormone Receptor and HER2 Status. J. Natl. Cancer Inst. 2014, 106, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.-I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/MTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.R.; Pan, T.; Pant, D.K.; Shih, N.N.C.; Chen, Y.; Harvey, K.L.; Solomon, A.; Lieberman, D.; Morrissette, J.J.D.; Soucier-Ernst, D.; et al. Genomic Landscape of Metastatic Breast Cancer Identifies Preferentially Dysregulated Pathways and Targets. J. Clin. Investig. 2020, 130, 4252–4265. [Google Scholar] [CrossRef] [PubMed]
- Holloway, R.; Marignani, P. Targeting mTOR and Glycolysis in HER2-Positive Breast Cancer. Cancers 2021, 13, 2922. [Google Scholar] [CrossRef]
- Menendez, J.A.; Lupu, R. Fatty Acid Synthase Regulates Estrogen Receptor-α Signaling in Breast Cancer Cells. Oncogenesis 2017, 6, e299. [Google Scholar] [CrossRef]
- Zhang, J.; Song, F.; Zhao, X.; Jiang, H.; Wu, X.; Wang, B.; Zhou, M.; Tian, M.; Shi, B.; Wang, H.; et al. EGFR Modulates Monounsaturated Fatty Acid Synthesis through Phosphorylation of SCD1 in Lung Cancer. Mol. Cancer 2017, 16, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, W.W.; Wilkins, O.; Bang, S.; Ung, M.; Li, J.; An, J.; del Genio, C.; Canfield, K.; DiRenzo, J.; Wells, W.; et al. CD36-Mediated Metabolic Rewiring of Breast Cancer Cells Promotes Resistance to HER2-Targeted Therapies. Cell Rep. 2019, 29, 3405–3420.e5. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, E.J.; Zelenko, Z.; Neel, B.A.; Antoniou, I.M.; Rajan, L.; Kase, N.; LeRoith, D. Elevated Tumor LDLR Expression Accelerates LDL Cholesterol-Mediated Breast Cancer Growth in Mouse Models of Hyperlipidemia. Oncogene 2017, 36, 6462–6471. [Google Scholar] [CrossRef] [Green Version]
- Zaoui, M.; Morel, M.; Ferrand, N.; Fellahi, S.; Bastard, J.-P.; Lamazière, A.; Larsen, A.K.; Béréziat, V.; Atlan, M.; Sabbah, M. Breast-Associated Adipocytes Secretome Induce Fatty Acid Uptake and Invasiveness in Breast Cancer Cells via CD36 Independently of Body Mass Index, Menopausal Status and Mammary Density. Cancers 2019, 11, 2012. [Google Scholar] [CrossRef] [Green Version]
- Catasus, L.; Gallardo, A.; Llorente-Cortes, V.; Escuin, D.; Muñoz, J.; Tibau, A.; Peiro, G.; Barnadas, A.; Lerma, E. Low-Density Lipoprotein Receptor-Related Protein 1 Is Associated with Proliferation and Invasiveness in Her-2/Neu and Triple-Negative Breast Carcinomas. Hum. Pathol. 2011, 42, 1581–1588. [Google Scholar] [CrossRef]
- Maubant, S.; Tahtouh, T.; Brisson, A.; Maire, V.; Némati, F.; Tesson, B.; Ye, M.; Rigaill, G.; Noizet, M.; Dumont, A.; et al. LRP5 Regulates the Expression of STK40, a New Potential Target in Triple-Negative Breast Cancers. Oncotarget 2018, 9, 22586–22604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, W.; Li, Y. Salinomycin Suppresses LRP6 Expression and Inhibits Both Wnt/β-Catenin and MTORC1 Signaling in Breast and Prostate Cancer Cells. J. Cell Biochem. 2014, 115, 1799–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, D.-N.; Chen, J.; Li, Z.; Yan, H.; Yin, Y.; Wo, D.; Zhang, J.; Ao, L.; Chen, B.; Ito, T.K.; et al. LRP5/6 directly bind to Frizzled and prevent Frizzled-regulated tumour metastasis. Nat. Commun. 2015, 6, 6906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debik, J.; Schaefer, H.; Andreassen, T.; Wang, F.; Fang, F.; Cannet, C.; Spraul, M.; Bathen, T.F.; Giskeødegård, G.F. Lipoprotein and Metabolite Associations to Breast Cancer Risk in the HUNT2 Study. medRxiv 2021. [Google Scholar] [CrossRef]
- Huang, J.; Li, L.; Lian, J.; Schauer, S.; Vesely, P.W.; Kratky, D.; Hoefler, G.; Lehner, R. Tumor-Induced Hyperlipidemia Contributes to Tumor Growth. Cell Rep. 2016, 15, 336–348. [Google Scholar] [CrossRef] [Green Version]
- Gumà, J.; Adriá-Cebrián, J.; Ruiz-Aguado, B.; Albacar, C.; Girona, J.; Rodríguez-Calvo, R.; Martínez-Micaelo, N.; Lam, E.W.F.; Masana, L.; Guaita-Esteruelas, S. Altered Serum Metabolic Profile Assessed by Advanced 1H-NMR in Breast Cancer Patients. Cancers 2021, 13, 4281. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, C.G.; Pournaras, S.; Vaslamatzis, M.; Avgerinos, A.; Raptis, S. Changes in Serum Lipids and Lipoproteins in Cancer Patients during Chemotherapy. Cancer Chemother. Pharmacol. 1992, 30, 412–416. [Google Scholar] [CrossRef]
- Sharma, M.; Tuaine, J.; McLaren, B.; Waters, D.L.; Black, K.; Jones, L.M.; McCormick, S.P.A. Chemotherapy Agents Alter Plasma Lipids in Breast Cancer Patients and Show Differential Effects on Lipid Metabolism Genes in Liver Cells. PLoS ONE 2016, 11, e0148049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, W.; Yao, Y.; Fan, G.; Zhou, Y.; Wu, M.; Xu, D.; Deng, Y. Changes in Lipid Profiles during and after (Neo)Adjuvant Chemotherapy in Women with Early-Stage Breast Cancer: A Retrospective Study. PLoS ONE 2019, 14, e0221866. [Google Scholar] [CrossRef]
- Basso, F.; Freeman, L.; Knapper, C.L.; Remaley, A.; Stonik, J.; Neufeld, E.B.; Tansey, T.; Amar, M.J.A.; Fruchart-Najib, J.; Duverger, N.; et al. Role of the Hepatic ABCA1 Transporter in Modulating Intrahepatic Cholesterol and Plasma HDL Cholesterol Concentrations. J. Lipid Res. 2003, 44, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan, A.M.; Oram, J.F. ABCA1 and ABCG1 or ABCG4 Act Sequentially to Remove Cellular Cholesterol and Generate Cholesterol-Rich HDL. J. Lipid Res. 2006, 47, 2433–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garner, B.; Waldeck, A.R.; Witting, P.K.; Rye, K.A.; Stocker, R. Oxidation of High Density Lipoproteins. II. Evidence for Direct Reduction of Lipid Hydroperoxides by Methionine Residues of Apolipoproteins AI and AII. J. Biol. Chem. 1998, 273, 6088–6095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panzenböck, U.; Stocker, R. Formation of Methionine Sulfoxide-Containing Specific Forms of Oxidized High-Density Lipoproteins. Biochim. Biophys. Acta 2005, 1703, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef]
- Sakuma, N.; Yoshikawa, M.; Hibino, T.; Ohte, N.; Kamiya, T.; Kunimatsu, M.; Kimura, G.; Inoue, M. HDL3 Exerts a More Powerful Antiperoxidative and Protective Effect against Peroxidative Modification of LDL than HDL2 Does. J. Nutr. Sci. Vitaminol. 2002, 48, 278–282. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, M.; Sakuma, N.; Hibino, T.; Sato, T.; Fujinami, T. HDL3 exerts more powerful anti-oxidative, protective effects against copper-catalyzed LDL oxidation than HDL2. Clin. Biochem. 1997, 30, 221–225. [Google Scholar] [CrossRef]
- Larbi, A.; Fortin, C.; Dupuis, G.; Berrougui, H.; Khalil, A.; Fulop, T. Immunomodulatory Role of High-Density Lipoproteins: Impact on Immunosenescence. AGE 2014, 36, 9712. [Google Scholar] [CrossRef] [Green Version]
- Vilahur, G. High-Density Lipoprotein Benefits beyond the Cardiovascular System: A Potential Key Role for Modulating Acquired Immunity through Cholesterol Efflux. Cardiovasc. Res. 2017, 113, e51–e53. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-H.; Yuan, S.-G.; Peng, D.-Q.; Zhao, S.-P. HDL and ApoA-I Inhibit Antigen Presentation-Mediated T Cell Activation by Disrupting Lipid Rafts in Antigen Presenting Cells. Atherosclerosis 2012, 225, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Valabrega, G.; Montemurro, F.; Aglietta, M. Trastuzumab: Mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann. Oncol. 2007, 18, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Jain, D.; Yadav, R.; Naik, S.L.; Sharma, S.; Bahadur, A.K.; Sharma, B. Effect of cancer treatment modalities on serum lipids and lipoproteins among women with carcinoma of the breast. Indian J. Physiol. Pharmacol. 2001, 45, 337–344. [Google Scholar] [PubMed]
- Subramaniam, S.; Marar, T.; Devi, C.S. Studies on the Changes in Plasma Lipids and Lipoproteins in CMF Treated Breast Cancer Patients. Biochem. Int. 1991, 24, 1015–1024. [Google Scholar] [PubMed]

| Characteristics | BC (n = 43) |
|---|---|
| Age, years (Median, range) | 49 (23–70) |
| BMI, kg/m2 (Mean ± SD) | 25.0 ± 5.3 |
| Pre-menopause (n, %) | 25 (58.1) |
| Post-menopause (n, %) | 18 (41.9) |
| Stage (n, %) | |
| II | 33 (76.7) |
| III | 10 (23.3) |
| Ki67 (n, %) | |
| <20 | 20 (46.5) |
| ≥20 | 23 (53.5) |
| ER status (n, %) | |
| Positive | 22 (51.2) |
| Negative | 21 (48.8) |
| Pathological Response (n, %) * | |
| Complete | 18 (56.2) |
| Partial | 14 (43.8) |
| Recurrence (n, %) * | |
| Yes | 9 (28.1) |
| No | 23 (71.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corona, G.; Di Gregorio, E.; Vignoli, A.; Muraro, E.; Steffan, A.; Miolo, G. 1H-NMR Plasma Lipoproteins Profile Analysis Reveals Lipid Metabolism Alterations in HER2-Positive Breast Cancer Patients. Cancers 2021, 13, 5845. https://doi.org/10.3390/cancers13225845
Corona G, Di Gregorio E, Vignoli A, Muraro E, Steffan A, Miolo G. 1H-NMR Plasma Lipoproteins Profile Analysis Reveals Lipid Metabolism Alterations in HER2-Positive Breast Cancer Patients. Cancers. 2021; 13(22):5845. https://doi.org/10.3390/cancers13225845
Chicago/Turabian StyleCorona, Giuseppe, Emanuela Di Gregorio, Alessia Vignoli, Elena Muraro, Agostino Steffan, and Gianmaria Miolo. 2021. "1H-NMR Plasma Lipoproteins Profile Analysis Reveals Lipid Metabolism Alterations in HER2-Positive Breast Cancer Patients" Cancers 13, no. 22: 5845. https://doi.org/10.3390/cancers13225845
APA StyleCorona, G., Di Gregorio, E., Vignoli, A., Muraro, E., Steffan, A., & Miolo, G. (2021). 1H-NMR Plasma Lipoproteins Profile Analysis Reveals Lipid Metabolism Alterations in HER2-Positive Breast Cancer Patients. Cancers, 13(22), 5845. https://doi.org/10.3390/cancers13225845








