Radiodermatitis and Fibrosis in the Context of Breast Radiation Therapy: A Critical Review
Abstract
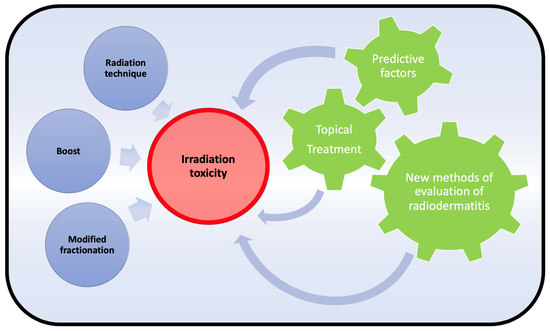
:Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
3.1. Pathophysiology
3.1.1. Radiodermatitis
3.1.2. Fibrosis
3.2. Radiation Techniques (3DCRT, IMRT, IORT, PBI/APBI)
| Authors | No. of Patients | Cancer Location | Radiation Dose (Gy) | No. of Fractions | Boost Dose (Gy) | Irradiation Modality | Radiodermatitis ≥ Grade 2 | Fibrosis ≥ Grade 2 | Cosmetic Satisfaction ≥ Good |
|---|---|---|---|---|---|---|---|---|---|
| Pignol et al. [13] | 330 | breast cancer | 50 | 25 | 16 | 3D vs. IMRT | 47.8% vs. 31.2% (p = 0.02) | NA | NA |
| Krug et al. [14] | 446 | breast cancer | 50.4 | 28 | 16 | 3D vs. IMRT | 29.1% vs. 20.1% (p = 0.02) | NA | NA |
| Hörner-Rieber et al. [15] | 502 | breast cancer | 50.4 | 28 | 14–16 | 3D vs. IMRT | NA | 10.4% vs. 11.5% (p = 0.27) | 77.5% vs. 77.3% (p = 0.33) |
| Askoxylakis et al. [16] | 502 | breast cancer | 50.4 | 28 | 14–16 | 3D vs. IMRT | 47.8% vs. 31.2% (p < 0.05) | NA | NA |
| Lee et al. [19] | 216 | breast cancer | 50.4 | 28 | 9.8 | IMRT vs. Tomo | 16% vs. 2.4% (p = 0.02) | 1.7% vs. 2.4% (p = 0.57) | NA |
| Joseph et al. [20] | 177 | breast cancer | 50 | 25 | NA | IMRT vs. Tomo | 33% vs. 11% (p < 0.001) | 75% vs. 67% (p = 0.13) | NA |
| McCormick et al. [23] | 2232 | breast cancer | 40–56 vs. 20 | NA | NA | WBRT vs. PBI | NA | NA | NA |
| Falco et al. [25] | 150 | breast cancer | 46–50 vs. 20 | 23–25 vs. 1 | NA | IORT vs. IORT + WBRT | NA | 1.4% vs. 23% (p < 0.001) | NA |
| Key et al. [26] | 41 | breast cancer | 46–50.4 vs. 20 | 23–28 vs. 1 | NA | IORT vs. IORT + WBRT | NA | 2.4% vs. 43.3% (p < 0.001) | 67.3% vs. NA |
| Sperk et al. [27] | 305 | breast cancer | 46–50 vs. 20 | 23–25 vs. 1 | NA | IORT vs. IORT + WBRT | NA | 5.9% vs. 37.5% (p < 0.001) | NA |
| Kraus et al. [28] | 73 | breast cancer | 46 + 20 | 23 + 1 | NA | IORT vs. IORT + WBRT | NA | 25% | >90% |
3.3. Roles of Fractionation and Boost
| Author | No. of Patients | Cancer Location | Radiation Dose (Gy) and No. of Fractions (*) | Boost Dose (Gy) | Radiodermatitis ≥ Grade 2 | Fibrosis ≥ Grade 2 | Cosmetic Satisfaction ≥ Good |
|---|---|---|---|---|---|---|---|
| Offersen et al. [35] | 1854 | breast cancer | 50 (25) vs. 40 (15) | NA | NA | 13% vs. 11% (p < 0.029) | 90% vs. 91% (p < 0.48) |
| Wang et al. [37] | 729 | breast cancer | 50 (25) vs. 40 (15) | 10 vs. 8.7 | 7.4% vs. 3% (p < 0.019) | 8.2% vs. 7.9% (p < 0.69) | 88.7% vs. 89% (p < 0.39) |
| Wang et al. [36] | 810 | breast cancer | 50 (25) vs. 40 (15) | NA | 8% vs. 3% (p < 0.0001) | 0% vs. 1% (p < 0.67) | NA |
| Bartelink et al. [34] | 5318 | breast cancer | 50 (25) | 16 vs. 0 | NA | 4.4% vs. 1.6% (p < 0.0001) | NA |
| Bartelink et al. [33] | 2657 | breast cancer | 50 (25) | 16 vs. 0 | NA | 5.2% vs. 1.8% (p < 0.0001) | NA |
| Palumbo et al. [39] | 218 | breast cancer | 42.4 (16) | 10.6–13.25 | 18.8% | 2.3% | NA |
| Pealinck et al. [40] | 167 | breast cancer | 40.05 (15) | 10–14.88 | 45% vs. 27% (p = 0.037) | NA | NA |
| Brunt et al. [41] | 189 | breast cancer | 40 (15) vs. 27 (5) vs. 26 (5) | NA | 51% vs. 29% vs. 36% | NA | NA |
| Murray et al. [32] | 4096 | breast cancer | 40 (15) vs. 27 (5) vs. 26 (5) | 10–16 | NA | 4% vs. 7.4% vs. 5.6% | 70.3% vs. 69.6% vs. 73.3% |
3.4. Predictive Factors (Genetic, Environmental, Epigenetic)
3.5. Treatment and Adjuvant Techniques (Creams, Dressings)
3.6. New Methods of Evaluation of Radiodermatitis (RILA, Ultrasound, Spectrometry)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tubiana, M.; Eschwège, F. Conformal Radiotherapy and Intensity-Modulated Radiotherapy--Clinical Data. Acta Oncol. 2000, 39, 555–567. [Google Scholar] [CrossRef]
- Fourquet, A.; Campana, F.; Rosenwald, J.C.; Vilcoq, J.R. Breast Irradiation in the Lateral Decubitus Position: Technique of the Institut Curie. Radiother. Oncol. 1991, 22, 261–265. [Google Scholar] [CrossRef]
- Bergom, C.; Currey, A.; Desai, N.; Tai, A.; Strauss, J.B. Deep Inspiration Breath Hold: Techniques and Advantages for Cardiac Sparing During Breast Cancer Irradiation. Front. Oncol. 2018, 8, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandeli, C.; Smyth, L.M.L.; David, S.; See, A.W. Dose Reduction to Organs at Risk with Deep-Inspiration Breath-Hold during Right Breast Radiotherapy: A Treatment Planning Study. Radiat. Oncol. 2019, 14, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dueck, A.C.; Mendoza, T.R.; Mitchell, S.A.; Reeve, B.B.; Castro, K.M.; Rogak, L.J.; Atkinson, T.M.; Bennett, A.V.; Denicoff, A.M.; O’Mara, A.M. Validity and Reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015, 1, 1051–1059. [Google Scholar] [CrossRef]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity Criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef]
- Savarese, D.M. Common Terminology Criteria for Adverse Events; UpToDate Waltham: Waltham, MA, USA, 2013. [Google Scholar]
- Hymes, S.R.; Strom, E.A.; Fife, C. Radiation Dermatitis: Clinical Presentation, Pathophysiology, and Treatment 2006. J. Am. Acad. Dermatol. 2006, 54, 28–46. [Google Scholar] [CrossRef]
- Singh, M.; Alavi, A.; Wong, R.; Akita, S. Radiodermatitis: A Review of Our Current Understanding. Am. J. Clin. Dermatol. 2016, 17, 277–292. [Google Scholar] [CrossRef]
- Wang, B.; Wei, J.; Meng, L.; Wang, H.; Qu, C.; Chen, X.; Xin, Y.; Jiang, X. Advances in Pathogenic Mechanisms and Management of Radiation-Induced Fibrosis. Biomed. Pharmacother. 2020, 121, 109560. [Google Scholar] [CrossRef]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M. Radiation-Induced Fibrosis: Mechanisms and Implications for Therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1985–1994. [Google Scholar] [CrossRef] [Green Version]
- Hennequin, C.; Barillot, I.; Azria, D.; Belkacémi, Y.; Bollet, M.; Chauvet, B.; Cowen, D.; Cutuli, B.; Fourquet, A.; Hannoun-Lévi, J.M.; et al. Radiotherapy of breast cancer. Cancer Radiother. 2016, 20 (Suppl. l), S139–S146. [Google Scholar] [CrossRef] [PubMed]
- Pignol, J.-P.; Olivotto, I.; Rakovitch, E.; Gardner, S.; Sixel, K.; Beckham, W.; Vu, T.T.T.; Truong, P.; Ackerman, I.; Paszat, L. A Multicenter Randomized Trial of Breast Intensity-Modulated Radiation Therapy to Reduce Acute Radiation Dermatitis. J. Clin. Oncol. 2008, 26, 2085–2092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krug, D.; Köder, C.; Häfner, M.F.; Arians, N.; Harrabi, S.B.; Koerber, S.A.; Forster, T.; Schlampp, I.; Sohn, C.; Heil, J.; et al. Acute Toxicity of Normofractionated Intensity Modulated Radiotherapy with Simultaneous Integrated Boost Compared to Three-Dimensional Conformal Radiotherapy with Sequential Boost in the Adjuvant Treatment of Breast Cancer. Radiat. Oncol. 2020, 15, 235. [Google Scholar] [CrossRef] [PubMed]
- Hörner-Rieber, J.; Forster, T.; Hommertgen, A.; Haefner, M.F.; Arians, N.; König, L.; Harrabi, S.B.; Schlampp, I.; Weykamp, F.; Lischalk, J.W.; et al. Intensity Modulated Radiation Therapy (IMRT) With Simultaneously Integrated Boost Shortens Treatment Time and Is Noninferior to Conventional Radiation Therapy Followed by Sequential Boost in Adjuvant Breast Cancer Treatment: Results of a Large Randomized Phase III Trial (IMRT-MC2 Trial). Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1311–1324. [Google Scholar] [CrossRef]
- Askoxylakis, V.; Jensen, A.D.; Häfner, M.F.; Fetzner, L.; Sterzing, F.; Heil, J.; Sohn, C.; Hüsing, J.; Tiefenbacher, U.; Wenz, F.; et al. Simultaneous Integrated Boost for Adjuvant Treatment of Breast Cancer--Intensity Modulated vs. Conventional Radiotherapy: The IMRT-MC2 Trial. BMC Cancer 2011, 11, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LENT SOMA Tables. Radiother. Oncol. 1995, 35, 17–60. [CrossRef]
- Lauche, O.; Kirova, Y.M.; Fenoglietto, P.; Costa, E.; Lemanski, C.; Bourgier, C.; Riou, O.; Tiberi, D.; Campana, F.; Fourquet, A.; et al. Helical Tomotherapy and Volumetric Modulated Arc Therapy: New Therapeutic Arms in the Breast Cancer Radiotherapy. World J. Radiol. 2016, 8, 735–742. [Google Scholar] [CrossRef]
- Lee, H.-H.; Chen, C.-H.; Luo, K.-H.; Chuang, H.-Y.; Huang, C.-J.; Cheng, Y.-K.; Chen, F.; Kuo, S.-H.; Huang, M.-Y. Five-Year Survival Outcomes of Intensity-Modulated Radiotherapy with Simultaneous Integrated Boost (IMRT-SIB) Using Forward IMRT or Tomotherapy for Breast Cancer. Sci. Rep. 2020, 10, 4342. [Google Scholar] [CrossRef]
- Joseph, K.; Vos, L.J.; Gabos, Z.; Pervez, N.; Chafe, S.; Tankel, K.; Warkentin, H.; Ghosh, S.; Amanie, J.; Powell, K.; et al. Skin Toxicity in Early Breast Cancer Patients Treated with Field-In-Field Breast Intensity-Modulated Radiotherapy versus Helical Inverse Breast Intensity-Modulated Radiotherapy: Results of a Phase III Randomised Controlled Trial. Clin. Oncol. (R. Coll. Radiol.) 2021, 33, 30–39. [Google Scholar] [CrossRef]
- Hickey, B.E.; Lehman, M.; Francis, D.P.; See, A.M. Partial Breast Irradiation for Early Breast Cancer. Cochrane Database Syst. Rev. 2016, CD007077. [Google Scholar] [CrossRef]
- Lehman, M.; Hickey, B.E.; Francis, D.P.; See, A.M. Partial Breast Irradiation for Early Breast Cancer. Cochrane Database Syst. Rev. 2014, CD007077. [Google Scholar] [CrossRef] [Green Version]
- McCormick, B. Partial Breast Radiation for Early-Stage Breast Cancer. Curr. Opin. Obstet. Gynecol. 2012, 24, 31–37. [Google Scholar] [CrossRef]
- Viani, G.A.; Arruda, C.V.; Faustino, A.C.; De Fendi, L.I. Partial-Breast Irradiation versus Whole-Breast Radiotherapy for Early Breast Cancer: A Systematic Review and Update Meta-Analysis. Brachytherapy 2020, 19, 491–498. [Google Scholar] [CrossRef]
- Falco, M.; Masojć, B.; Rolla, M.; Czekała, A.; Milchert-Leszczyńska, M.; Pietruszewska, J.; Lewocki, M. Analysis of Breast Cosmetic Effects 3 Years after Breast-Conserving Surgery and Intraoperative Radiotherapy with and without Adjuvant Whole Breast Irradiation. Breast J. 2020, 26, 882–887. [Google Scholar] [CrossRef]
- Key, S.; Miglierini, P.; Dupré, P.-F.; Guilbert, S.; Lucia, A.-S.; Abgral, R.; Conan-Charlet, V.; Uguen, A.; Pradier, O.; Schick, U. Cosmetic Outcome and Chronic Breast Toxicity After Intraoperative Radiation Therapy (IORT) as a Single Modality or as a Boost Using the Intrabeam® Device: A Prospective Study. Ann. Surg. Oncol. 2017, 24, 2547–2555. [Google Scholar] [CrossRef]
- Sperk, E.; Welzel, G.; Keller, A.; Kraus-Tiefenbacher, U.; Gerhardt, A.; Sütterlin, M.; Wenz, F. Late Radiation Toxicity after Intraoperative Radiotherapy (IORT) for Breast Cancer: Results from the Randomized Phase III Trial TARGIT A. Breast Cancer Res. Treat. 2012, 135, 253–260. [Google Scholar] [CrossRef]
- Kraus-Tiefenbacher, U.; Bauer, L.; Scheda, A.; Fleckenstein, K.; Keller, A.; Herskind, C.; Steil, V.; Melchert, F.; Wenz, F. Long-Term Toxicity of an Intraoperative Radiotherapy Boost Using Low Energy X-Rays during Breast-Conserving Surgery. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 377–381. [Google Scholar] [CrossRef]
- Bronsart, E.; Dureau, S.; Xu, H.P.; Bazire, L.; Chilles, A.; Costa, E.; Logerot, C.; Falcou, M.-C.; Campana, F.; Berger, F.; et al. Whole Breast Radiotherapy in the Lateral Isocentric Lateral Decubitus Position: Long-Term Efficacy and Toxicity Results. Radiother. Oncol. 2017, 124, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Meattini, I.; Poortmans, P.; Kirova, Y.; Saieva, C.; Visani, L.; Salvestrini, V.; Kim, J.; Jung, W.; Olmetto, E.; Mariotti, M.; et al. Hypofractionated Whole Breast Irradiation after Conservative Surgery for Patients Aged Less than 60 Years: A Multi-Centre Comparative Study. Acta Oncol. 2020, 59, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Valle, L.F.; Agarwal, S.; Bickel, K.E.; Herchek, H.A.; Nalepinski, D.C.; Kapadia, N.S. Hypofractionated Whole Breast Radiotherapy in Breast Conservation for Early-Stage Breast Cancer: A Systematic Review and Meta-Analysis of Randomized Trials. Breast Cancer Res. Treat. 2017, 162, 409–417. [Google Scholar] [CrossRef]
- Murray Brunt, A.; Haviland, J.S.; Wheatley, D.A.; Sydenham, M.A.; Alhasso, A.; Bloomfield, D.J.; Chan, C.; Churn, M.; Cleator, S.; Coles, C.E.; et al. Hypofractionated Breast Radiotherapy for 1 Week versus 3 Weeks (FAST-Forward): 5-Year Efficacy and Late Normal Tissue Effects Results from a Multicentre, Non-Inferiority, Randomised, Phase 3 Trial. Lancet 2020, 395, 1613–1626. [Google Scholar] [CrossRef]
- Bartelink, H.; Horiot, J.-C.; Poortmans, P.M.; Struikmans, H.; Van den Bogaert, W.; Fourquet, A.; Jager, J.J.; Hoogenraad, W.J.; Oei, S.B.; Wárlám-Rodenhuis, C.C.; et al. Impact of a Higher Radiation Dose on Local Control and Survival in Breast-Conserving Therapy of Early Breast Cancer: 10-Year Results of the Randomized Boost versus No Boost EORTC 22881-10882 Trial. J. Clin. Oncol. 2007, 25, 3259–3265. [Google Scholar] [CrossRef]
- Bartelink, H.; Maingon, P.; Poortmans, P.; Weltens, C.; Fourquet, A.; Jager, J.; Schinagl, D.; Oei, B.; Rodenhuis, C.; Horiot, J.-C.; et al. Whole-Breast Irradiation with or without a Boost for Patients Treated with Breast-Conserving Surgery for Early Breast Cancer: 20-Year Follow-up of a Randomised Phase 3 Trial. Lancet Oncol. 2015, 16, 47–56. [Google Scholar] [CrossRef]
- Offersen, B.V.; Alsner, J.; Nielsen, H.M.; Jakobsen, E.H.; Nielsen, M.H.; Krause, M.; Stenbygaard, L.; Mjaaland, I.; Schreiber, A.; Kasti, U.-M.; et al. Hypofractionated Versus Standard Fractionated Radiotherapy in Patients with Early Breast Cancer or Ductal Carcinoma In Situ in a Randomized Phase III Trial: The DBCG HYPO Trial. J. Clin. Oncol. 2020, 38, 3615–3625. [Google Scholar] [CrossRef]
- Wang, S.-L.; Fang, H.; Song, Y.-W.; Wang, W.-H.; Hu, C.; Liu, Y.-P.; Jin, J.; Liu, X.-F.; Yu, Z.-H.; Ren, H.; et al. Hypofractionated versus Conventional Fractionated Postmastectomy Radiotherapy for Patients with High-Risk Breast Cancer: A Randomised, Non-Inferiority, Open-Label, Phase 3 Trial. Lancet Oncol. 2019, 20, 352–360. [Google Scholar] [CrossRef]
- Wang, S.-L.; Fang, H.; Hu, C.; Song, Y.-W.; Wang, W.-H.; Jin, J.; Liu, Y.-P.; Ren, H.; Liu, J.; Li, G.-F.; et al. Hypofractionated Versus Conventional Fractionated Radiotherapy After Breast-Conserving Surgery in the Modern Treatment Era: A Multicenter, Randomized Controlled Trial from China. J. Clin. Oncol. 2020, 38, 3604–3614. [Google Scholar] [CrossRef]
- Hamilton, D.G.; Bale, R.; Jones, C.; Fitzgerald, E.; Khor, R.; Knight, K.; Wasiak, J. Impact of Tumour Bed Boost Integration on Acute and Late Toxicity in Patients with Breast Cancer: A Systematic Review. Breast 2016, 27, 126–135. [Google Scholar] [CrossRef]
- Palumbo, I.; Mariucci, C.; Falcinelli, L.; Perrucci, E.; Lancellotta, V.; Podlesko, A.M.; Marcantonini, M.; Saldi, S.; Bini, V.; Aristei, C. Hypofractionated Whole Breast Radiotherapy with or without Hypofractionated Boost in Early Stage Breast Cancer Patients: A Mono-Institutional Analysis of Skin and Subcutaneous Toxicity. Breast Cancer 2019, 26, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Paelinck, L.; Gulyban, A.; Lakosi, F.; Vercauteren, T.; De Gersem, W.; Speleers, B.; Monten, C.; Mulliez, T.; Berkovic, P.; van Greveling, A.; et al. Does an Integrated Boost Increase Acute Toxicity in Prone Hypofractionated Breast Irradiation? A Randomized Controlled Trial. Radiother. Oncol. 2017, 122, 30–36. [Google Scholar] [CrossRef]
- Brunt, A.M.; Wheatley, D.; Yarnold, J.; Somaiah, N.; Kelly, S.; Harnett, A.; Coles, C.; Goodman, A.; Bahl, A.; Churn, M.; et al. Acute Skin Toxicity Associated with a 1-Week Schedule of Whole Breast Radiotherapy Compared with a Standard 3-Week Regimen Delivered in the UK FAST-Forward Trial. Radiother. Oncol. 2016, 120, 114–118. [Google Scholar] [CrossRef] [Green Version]
- De Santis, M.C.; Bonfantini, F.; Di Salvo, F.; Dispinzieri, M.; Mantero, E.; Soncini, F.; Baili, P.; Sant, M.; Bianchi, G.; Maggi, C.; et al. Factors Influencing Acute and Late Toxicity in the Era of Adjuvant Hypofractionated Breast Radiotherapy. Breast 2016, 29, 90–95. [Google Scholar] [CrossRef]
- Kraus-Tiefenbacher, U.; Sfintizky, A.; Welzel, G.; Simeonova, A.; Sperk, E.; Siebenlist, K.; Mai, S.; Wenz, F. Factors of Influence on Acute Skin Toxicity of Breast Cancer Patients Treated with Standard Three-Dimensional Conformal Radiotherapy (3D-CRT) after Breast Conserving Surgery (BCS). Radiat. Oncol. 2012, 7, 217. [Google Scholar] [CrossRef] [Green Version]
- Lilla, C.; Ambrosone, C.B.; Kropp, S.; Helmbold, I.; Schmezer, P.; von Fournier, D.; Haase, W.; Sautter-Bihl, M.-L.; Wenz, F.; Chang-Claude, J. Predictive Factors for Late Normal Tissue Complications Following Radiotherapy for Breast Cancer. Breast Cancer Res. Treat. 2007, 106, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, P.J.A.M.; van Werkhoven, E.; Bartelink, H.; Fourquet, A.; Lemanski, C.; van Loon, J.; Maduro, J.H.; Russell, N.S.; Scheijmans, L.J.E.E.; Schinagl, D.A.X.; et al. Predictors for Poor Cosmetic Outcome in Patients with Early Stage Breast Cancer Treated with Breast Conserving Therapy: Results of the Young Boost Trial. Radiother. Oncol. 2018, 128, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Sharp, L.; Johansson, H.; Hatschek, T.; Bergenmar, M. Smoking as an Independent Risk Factor for Severe Skin Reactions Due to Adjuvant Radiotherapy for Breast Cancer. Breast 2013, 22, 634–638. [Google Scholar] [CrossRef]
- Männle, H.; Momm, F.; Münstedt, K. Vitamin D and Selenium Blood Levels and Acute Skin Toxicity during Radiotherapy for Breast Cancer. Complement. Ther. Med. 2020, 49, 102291. [Google Scholar] [CrossRef] [PubMed]
- Borger, J.H.; Kemperman, H.; Smitt, H.S.; Hart, A.; van Dongen, J.; Lebesque, J.; Bartelink, H. Dose and Volume Effects on Fibrosis after Breast Conservation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 1994, 30, 1073–1081. [Google Scholar] [CrossRef]
- Collette, S.; Collette, L.; Budiharto, T.; Horiot, J.-C.; Poortmans, P.M.; Struikmans, H.; Van den Bogaert, W.; Fourquet, A.; Jager, J.J.; Hoogenraad, W.; et al. Predictors of the Risk of Fibrosis at 10 Years after Breast Conserving Therapy for Early Breast Cancer: A Study Based on the EORTC Trial 22881-10882 “Boost versus No Boost”. Eur. J. Cancer 2008, 44, 2587–2599. [Google Scholar] [CrossRef]
- Kuptsova, N.; Chang-Claude, J.; Kropp, S.; Helmbold, I.; Schmezer, P.; von Fournier, D.; Haase, W.; Sautter-Bihl, M.L.; Wenz, F.; Onel, K.; et al. Genetic Predictors of Long-Term Toxicities after Radiation Therapy for Breast Cancer. Int. J. Cancer 2008, 122, 1333–1339. [Google Scholar] [CrossRef]
- Shanley, S.; McReynolds, K.; Ardern-Jones, A.; Ahern, R.; Fernando, I.; Yarnold, J.; Evans, G.; Eccles, D.; Hodgson, S.; Ashley, S.; et al. Late Toxicity Is Not Increased in BRCA1/BRCA2 Mutation Carriers Undergoing Breast Radiotherapy in the United Kingdom. Clin. Cancer Res. 2006, 12, 7025–7032. [Google Scholar] [CrossRef] [Green Version]
- Terrazzino, S.; Cargnin, S.; Deantonio, L.; Pisani, C.; Masini, L.; Canonico, P.L.; Genazzani, A.A.; Krengli, M. Impact of ATM Rs1801516 on Late Skin Reactions of Radiotherapy for Breast Cancer: Evidences from a Cohort Study and a Trial Sequential Meta-Analysis. PLoS ONE 2019, 14, e0225685. [Google Scholar] [CrossRef]
- Lazzari, G.; Buono, G.; Zannino, B.; Silvano, G. Breast Cancer Adjuvant Radiotherapy in BRCA1/2, TP53, ATM Genes Mutations: Are There Solved Issues? Breast Cancer 2021, 13, 299–310. [Google Scholar] [CrossRef]
- Chargari, C.; Fromantin, I.; Kirova, Y.M. Importance of local skin treatments during radiotherapy for prevention and treatment of radio-induced epithelitis. Cancer Radiother. 2009, 13, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Kirova, Y.M.; Fromantin, I.; De Rycke, Y.; Fourquet, A.; Morvan, E.; Padiglione, S.; Falcou, M.-C.; Campana, F.; Bollet, M.A. Can We Decrease the Skin Reaction in Breast Cancer Patients Using Hyaluronic Acid during Radiation Therapy? Results of Phase III Randomised Trial. Radiother. Oncol. 2011, 100, 205–209. [Google Scholar] [CrossRef]
- Rosenthal, A.; Israilevich, R.; Moy, R. Management of Acute Radiation Dermatitis: A Review of the Literature and Proposal for Treatment Algorithm. J. Am. Acad. Dermatol. 2019, 81, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Shao, X. Topical Agent Therapy for Prevention and Treatment of Radiodermatitis: A Meta-Analysis. Support. Care Cancer 2013, 21, 1025–1031. [Google Scholar] [CrossRef]
- Bazire, L.; Fromantin, I.; Diallo, A.; de la Lande, B.; Pernin, V.; Dendale, R.; Fourquet, A.; Savignoni, A.; Kirova, Y.M. Hydrosorb® versus Control (Water Based Spray) in the Management of Radio-Induced Skin Toxicity: Results of Multicentre Controlled Randomized Trial. Radiother. Oncol. 2015, 117, 229–233. [Google Scholar] [CrossRef]
- Azria, D.; Riou, O.; Castan, F.; Nguyen, T.D.; Peignaux, K.; Lemanski, C.; Lagrange, J.-L.; Kirova, Y.; Lartigau, E.; Belkacemi, Y.; et al. Radiation-Induced CD8 T-Lymphocyte Apoptosis as a Predictor of Breast Fibrosis after Radiotherapy: Results of the Prospective Multicenter French Trial. EBioMedicine 2015, 2, 1965–1973. [Google Scholar] [CrossRef] [Green Version]
- Bourgier, C.; Castan, F.; Riou, O.; Nguyen, T.-D.; Peignaux, K.; Lemanski, C.; Lagrange, J.-L.; Kirova, Y.; Lartigau, E.; Belkacemi, Y.; et al. Impact of Adjuvant Hormonotherapy on Radiation-Induced Breast Fibrosis According to the Individual Radiosensitivity: Results of a Multicenter Prospective French Trial. Oncotarget 2018, 9, 15757–15765. [Google Scholar] [CrossRef]
- González Sanchis, A.; Brualla González, L.; Sánchez Carazo, J.L.; Gordo Partearroyo, J.C.; Esteve Martínez, A.; Vicedo González, A.; López Torrecilla, J.L. Evaluation of Acute Skin Toxicity in Breast Radiotherapy with a New Quantitative Approach. Radiother. Oncol. 2017, 122, 54–59. [Google Scholar] [CrossRef]
- Yoshida, E.J.; Chen, H.; Torres, M.A.; Curran, W.J.; Liu, T. Spectrophotometer and Ultrasound Evaluation of Late Toxicity Following Breast-Cancer Radiotherapy. Med. Phys. 2011, 38, 5747–5755. [Google Scholar] [CrossRef] [Green Version]
- Landoni, V.; Giordano, C.; Marsella, A.; Saracino, B.; Petrongari, M.; Ferraro, A.; Strigari, L.; Pinnarò, P. Evidence from a Breast Cancer Hypofractionated Schedule: Late Skin Toxicity Assessed by Ultrasound. J. Exp. Clin. Cancer Res. 2013, 32, 80. [Google Scholar] [CrossRef] [Green Version]
- Donovan, E.M.; Yarnold, J.R.; Adams, E.J.; Morgan, A.; Warrington, A.P.J.; Evans, P.M. An investigation into methods of IMRT planning applied to breast radiotherapy. Br. J. Radiol. 2008, 81, 311–322. [Google Scholar] [CrossRef]
- Mukesh, M.B.; Qian, W.; Wah Hak, C.C.; Wikkinson, J.S.; Barnett, G.C.; Moody, A.M.; Wilson, C.; Coles, C.E. The Cambridge Breast Intensity-modulated Radiotherapy Trial: Comparison of Clinician- versus Patient-reported Outcomes. Clin. Oncol. (R. Coll. Radiol.) 2016, 28, 354–364. [Google Scholar] [CrossRef] [Green Version]
- Coles, C.E.; Griffin, C.L.; Kirby, A.M.; Titley, J.; Agrawal, R.K.; Alhasso, A.; Bhattacharya, I.S.; Wilcox, M.; Yarnold, J.R.; Bliss, J.M.; et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 2017, 390, 1048–1060. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allali, S.; Kirova, Y. Radiodermatitis and Fibrosis in the Context of Breast Radiation Therapy: A Critical Review. Cancers 2021, 13, 5928. https://doi.org/10.3390/cancers13235928
Allali S, Kirova Y. Radiodermatitis and Fibrosis in the Context of Breast Radiation Therapy: A Critical Review. Cancers. 2021; 13(23):5928. https://doi.org/10.3390/cancers13235928
Chicago/Turabian StyleAllali, Sofiane, and Youlia Kirova. 2021. "Radiodermatitis and Fibrosis in the Context of Breast Radiation Therapy: A Critical Review" Cancers 13, no. 23: 5928. https://doi.org/10.3390/cancers13235928
APA StyleAllali, S., & Kirova, Y. (2021). Radiodermatitis and Fibrosis in the Context of Breast Radiation Therapy: A Critical Review. Cancers, 13(23), 5928. https://doi.org/10.3390/cancers13235928