An Exploratory Analysis of Changes in Circulating Plasma Protein Profiles Following Image-Guided Ablation of Renal Tumours Provides Evidence for Effects on Multiple Biological Processes
Abstract
:Simple Summary
Abstract
1. Introduction
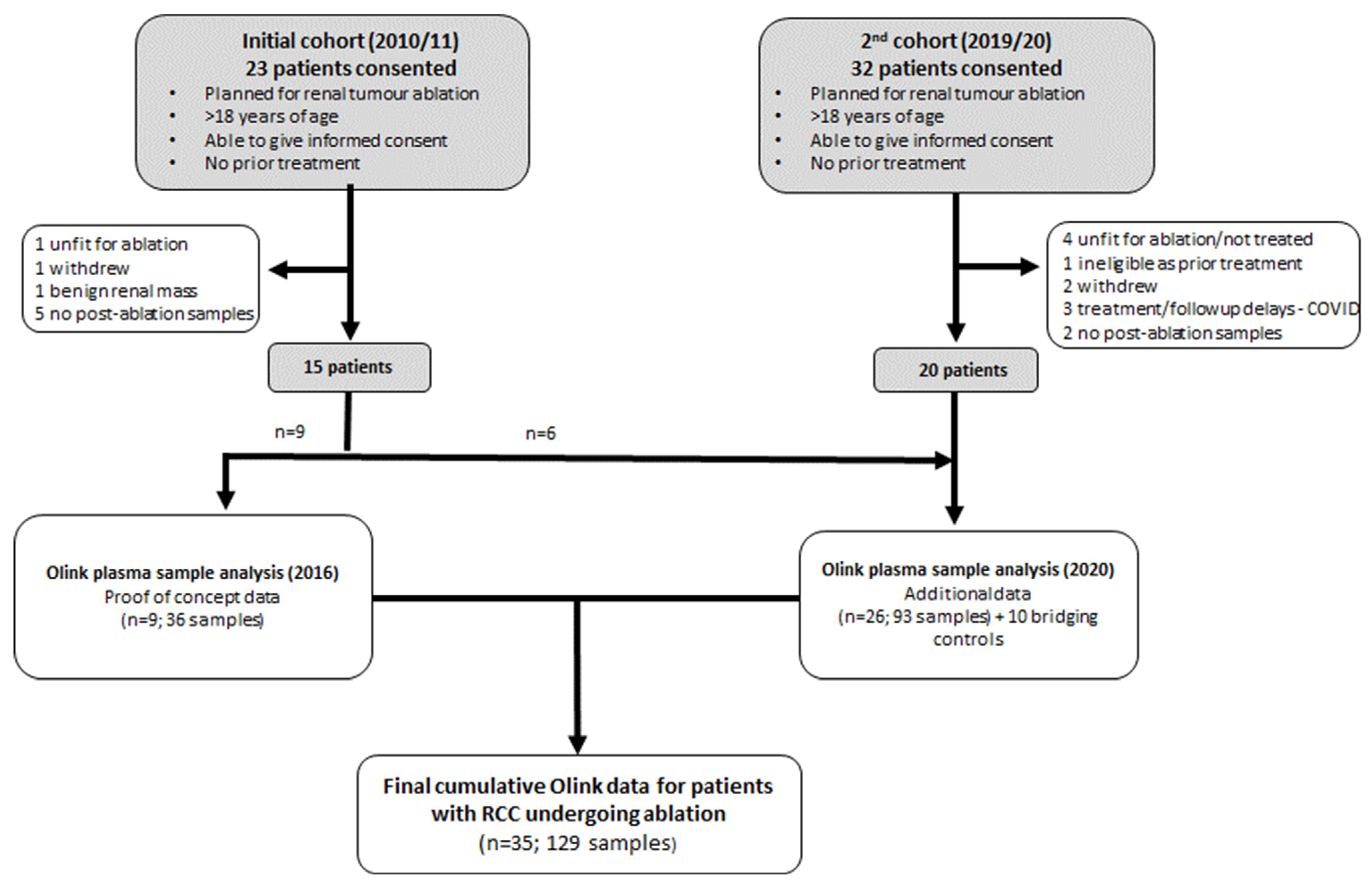
2. Materials and Methods
2.1. Study Design
2.2. IGA
2.3. Blood Sample Collection and Processing
2.4. Proteomic Analysis of Circulating Plasma Proteins
2.5. Statistical and Bioinformatic Analysis
3. Results
3.1. Patient Characteristics and Treatment
3.2. Baseline Intra-Individual Variability of Plasma Proteins and Comparison with Healthy Controls
3.3. Plasma Protein Changes Following CRYO
3.4. Plasma Protein Changes Following RFA or MWA
3.5. Functional Characteristics of Significantly Changing Proteins Post-CRYO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RFA | radiofrequency ablation; |
| CRYO | cryoablation; |
| MWA | microwave ablation; |
| RCC | renal cell carcinoma; |
| TKI | tyrosine kinase inhibitor; |
| ICI | immune checkpoint inhibitor; |
| IGA | image-guided ablation; |
| TP | timepoint; |
| FDR | false discovery rate; |
| DAMP | danger-associated molecular pattern |
References
- Kim, H.S.; Chapiro, J.; Geschwind, J.-F.H. From the guest editor: Interventional oncology: The fourth pillar of oncology. Cancer J. 2016, 22, 363–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, S.; Uzzo, R.G.; Allaf, M.E.; Bass, E.B.; Cadeddu, J.A.; Chang, A.; Clark, P.E.; Davis, B.J.; Derweesh, I.H.; Giambarresi, L.; et al. Renal mass and localized renal cancer: A.U.A. guideline. J. Urol. 2017, 198, 520–529. [Google Scholar] [CrossRef] [Green Version]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclerq, N.; Bex, A.; Khoo, V.; Grunwald, V.; Gillessen, S.; Horwich, A. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef] [Green Version]
- Shakeri, S.; Raman, S.S. Percutaneous thermal ablation for treatment of T1a renal cell carcinomas. Radiol. Clin. N. Am. 2020, 58, 981–993. [Google Scholar] [CrossRef]
- Andrews, J.R.; Atwell, T.; Schmit, G.; Lohse, C.M.; Kurup, A.N.; Weisbrod, A.; Callstrom, M.R.; Cheville, J.C.; Boorjian, S.A.; Leibovich, B.C.; et al. Oncologic outcomes following partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur. Urol. 2019, 76, 244–251. [Google Scholar] [CrossRef]
- Bhagavatula, S.K.; Tuncali, K.; Shyn, P.B.; Levesque, V.M.; Chang, S.L.; Silverman, S.G. Percutaneous CT- and MRI-guided cryoablation of cT1 renal cell carcinoma: Intermediate- to long-term outcomes in 307 patients. Radiology 2020, 296, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.A.; Bakouny, Z.; Hirsch, L.; Flippot, R.; Van Allen, E.M.; Wu, C.J.; Choueiri, T.K. Beyond conventional immune-checkpoint inhibition—novel immunotherapies for renal cell carcinoma. Nat. Rev. Clin. Oncol. 2021, 18, 199–214. [Google Scholar] [CrossRef]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Kim, D.; Erinjeri, J.P. Postablation immune microenvironment: Synergy between interventional oncology and immuno-oncology. Semin. Intervent. Radiol. 2019, 36, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Baust, J.G.; Snyder, K.K.; Santucci, K.L.; Robilotto, A.T.; Van Buskirk, R.G.; Baust, J.M. Cryoablation: Physical and molecular basis with putative immunological consequences. Int. J. Hyperth. 2019, 36, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Abdo, J.; Cornell, D.L.; Mittal, S.K.; Agrawal, D.K. Immunotherapy plus cryotherapy: Potential augmented abscopal effect for advanced cancers. Front. Oncol. 2018, 8, 85. [Google Scholar] [CrossRef]
- Yakkala, C.; Denys, A.; Kandalaft, L.; Duran, R. Cryoablation and immunotherapy of cancer. Curr. Opin. Biotechnol. 2020, 65, 60–64. [Google Scholar] [CrossRef]
- Slovak, R.; Ludwig, J.M.; Gettinger, S.N.; Herbst, R.S.; Kim, H.S. Immuno-thermal ablations—Boosting the anticancer immune response. J. Immunother. Cancer 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Qi, H.; Chen, S.; Cao, F.; Xie, L.; Wu, Y.; Ma, W.; Song, Z.; Yuan, H.; Zhang, T.; et al. Cryoablation combined with transarterial infusion of pembrolizumab (CATAP) for liver metastases of melanoma: An ambispective, proof-of-concept cohort study. Cancer Immunol. Immunother. 2020, 69, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Leppelmann, K.S.; Mooradian, M.J.; Ganguli, S.; Uppot, R.N.; Yamada, K.; Irani, Z.; Wehrenberg-Klee, E.P.; Zubiri, L.; Reynolds, K.L.; Arellano, R.S.; et al. Thermal ablation, embolization, and selective internal radiation therapy combined with Checkpoint inhibitor cancer immunotherapy: Safety analysis. J. Vasc. Interv. Radiol. 2021, 32, 187–195. [Google Scholar] [CrossRef]
- Thakur, A.; Littrup, P.; Paul, E.N.; Adam, B.; Heilbrun, L.K.; Lum, L.G. Induction of specific cellular and humoral responses against renal cell carcinoma after combination therapy with cryoablation and granulocyte-macrophage colony stimulating factor: A pilot study. J. Immunother. 2011, 34, 457–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, T.; Iwasaki, T.; Uemura, M.; Nagahara, A.; Higashihara, H.; Osuga, K.; Ikeda, Y.; Kiyotani, K.; Park, J.-H.; Nonomura, N.; et al. Characterization of the cryoablation-induced immune response in kidney cancer patients. Oncoimmunology 2017, 6, e1326441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.; Xu, K.; Liang, S.; Wang, X.; Liang, Y.; Zhang, M.; Chen, J.; Niu, L. Prospective study of percutaneous cryoablation combined with allogenic NK cell immunotherapy for advanced renal cell cancer. Immunol. Lett. 2017, 184, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Erinjeri, J.P.; Fine, G.C.; Adema, G.J.; Ahmed, M.; Chapiro, J.; den Brok, M.; Duran, R.; Hunt, S.J.; Johnson, D.T.; Ricke, J.; et al. Immunotherapy and the interventional oncologist: Challenges and opportunities—A society of interventional oncology white paper. Radiology 2019, 292, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Söderlund, S.; Christiansson, L.; Persson, I.; Hjorth-Hansen, H.; Richter, J.; Simonsson, B.; Mustjoki, S.; Olsson-Stromberg, U.; Loskog, A. Plasma proteomics in CML patients before and after initiation of tyrosine kinase inhibitor therapy reveals induced Th1 immunity and loss of angiogenic stimuli. Leuk. Res. 2016, 50, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Babačić, H.; Lehtiö, J.; Pico de Coaña, Y.; Pernemalm, M.; Eriksson, H. In-depth plasma proteomics reveals increase in circulating PD-1 during anti-PD-1 immunotherapy in patients with metastatic cutaneous melanoma. J. Immunother. Cancer 2020, 8, e000204. [Google Scholar] [CrossRef] [PubMed]
- Assarsson, E.; Lundberg, M.; Holmquist, G.; Björkesten, J.; Thorsen, S.B.; Ekman, D.; Eriksson, A.; Dickens, E.R.; Ohlsson, S.; Edfeldt, G.; et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE 2014, 9, e95192. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. GGPLOT2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2020, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, R. Natural history and therapy of metastatic renal cell carcinoma. The Role of Interleukin-2. Cancer 1997, 80, 1198–1220. [Google Scholar] [CrossRef] [Green Version]
- Posadas, E.M.; Limvorasak, S.; Figlin, R.A. Targeted therapies for renal cell carcinoma. Nat. Rev. Nephrol. 2017, 13, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Rassy, E.; Flippot, R.; Albiges, L. Tyrosine kinase inhibitors and immunotherapy combinations in renal cell carcinoma. Ther. Adv. Med. Oncol. 2020, 12, 1758835920907504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turajlic, S.; Litchfield, K.; Xu, H.; Rosenthal, R.; McGranahan, N.; Reading, J.L.; Wang, Y.N.S.; Rowan, A.; Kanu, N.; Al Bakir, M.; et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: A pan-cancer analysis. Lancet Oncol. 2017, 18, 1009–1020. [Google Scholar] [CrossRef] [Green Version]
- Campbell, M.T.; Matin, S.F.; Tam, A.L.; Sheth, R.A.; Ahrar, K.; Tidwell, R.S.; Rao, P.; Karam, J.A.; Wood, C.G.; Tannir, N.M.; et al. Pilot study of tremelimumab with and without cryoablation in patients with metastatic renal. Nat. Commun. 2021, 12, 6375. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Buque Martinez, A.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, L. Complexity of danger: The diverse nature of damage-associated molecular patterns. J. Biol. Chem. 2014, 289, 35237–35245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, T.; Liu, P.; Zhou, Y.; Liu, K.; Yang, L.; Moritz, R.L.; Yan, W.; Xu, L.X. Interleukin-6 induced “acute” phenotypic microenvironment promotes Th1 anti-tumour immunity in cryo-thermal therapy revealed by shotgun and parallel reaction monitoring proteomics. Theranostics 2016, 6, 773–794. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Q.; Muktiali, M.; Ren, B.; Hu, Y.; Li, D.; Li, Z.; Li, D.; Xie, Y.; Tao, M.; et al. Effect of microwave ablation treatment of hepatic malignancies on serum cytokine levels. BMC Cancer 2020, 20, 812. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.C.; van Hillegersberg, R.; Schoots, I.G.; Levi, M.; Beek, J.F.; Crezee, H.; van Gulik, T.M. Cryoablation induces greater inflammatory and coagulative responses than radiofrequency ablation or laser induced thermotherapy in a rat liver model. Surgery 2010, 147, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Erinjeri, J.P.; Thomas, C.T.; Samoilia, A.; Fleisher, M.; Gonen, M.; Sofocleous, C.T.; Thornton, R.H.; Siegelbaum, R.H.; Covey, A.M.; Brody, L.A.; et al. Image-guided thermal ablation of tumours increases the plasma level of Interleukin-6 and Interleukin-10. J. Vasc. Interv. Radiol. 2013, 24, 1105–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McArthur, H.L.; Diab, A.; Page, D.B.; Yuan, J.; Solomon, S.B.; Sacchini, V.; Cornstock, C.; Durack, J.C.; Maybody, M.; Sung, J.; et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling. Clin. Cancer Res. 2016, 22, 5729–5737. [Google Scholar] [CrossRef] [Green Version]
- Enroth, S.; Enroth, S.B.; Johansson, A.; Gyllensten, U. Effect of genetic and environmental factors on protein biomarkers for common non-communicable disease and use of Personally Normalized Plama Protein Profiles (PNPPP). Biomarker 2015, 20, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Sabel, M.S.; Su, G.; Griffith, K.A.; Chang, A.E. Rate of freeze alters the immunologic response after cryoablation of breast cancer. Ann. Surg. Oncol. 2010, 17, 1187–1193. [Google Scholar] [CrossRef]
- Deiner, S.; Baxter, M.G.; Mincer, J.S.; Sano, M.; Hall, J.; Mohammed, I.; O’Bryant, S.; Zetterberg, H.; Blennow, K.; Eckenhoff, R. Human plasma biomarker responses to inhalational general anaesthesia without surgery. Br. J. Anaeth. 2020, 125, 282–290. [Google Scholar] [CrossRef]
- Gyllensten, U.; Enroth, S.B.; Stalberg, K.; Sundfeldt, K.; Enroth, S. Preoperative fasting and general anaesthesia alter the plasma proteome. Cancers 2020, 12, 2439. [Google Scholar] [CrossRef] [PubMed]
- Schaeffeler, E.; Buttner, F.; Reustle, A.; Klumpp, V.; Winter, S.; Rausch, S.; Fisel, P.; Hennenlotter, J.; Kruck, S.; Stenzl, A.; et al. Metabolic and lipidomic reprogramming in renal cell carcinoma subtypes reflects regions of tumor origin. Eur. Urol. Focus 2019, 5, 608–618. [Google Scholar] [CrossRef] [Green Version]
- Melana, J.P.; Mignolli, F.; Stoyanoff, T.; Aguirre, M.V.; Balboa, M.A.; Balsinde, J.; Rodriguez, J.P. The Hypoxic Microenvironment Induces Stearoyl-CoA Desaturase-1 overexpression and lipidomic profile changes in clear cell renal cell carcinoma. Cancers 2021, 13, 2962. [Google Scholar] [CrossRef]
- Shimizu, T. Lipid mediators in health and disease: Enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 123–150. [Google Scholar] [CrossRef] [Green Version]
- Leuti, A.; Fazio, D.; Fava, M.; Piccoli, A.; Oddi, S.; Maccarrone, M. Bioactive lipids, inflammation and chronic diseases. Adv. Drug Deliv. Rev. 2020, 159, 133–169. [Google Scholar] [CrossRef]






| Parameter | Value |
|---|---|
| Age median (range) | 70 (38–86) |
| Sex | Male (22) Female (13) |
| Treatment Type | |
| CRYO | 25 (71.4%) |
| RFA | 8 (22.9%) |
| MWA | 2 (5.7%) |
| Tumour Size (cm); median (range) | |
| Histopathology | |
| Clear cell RCC | 25 (71.4%) |
| Papillary RCC | 4 (11.4%) |
| Chromophobe RCC | 3 (8.5%) |
| Xp11.2/TFE3 translocation RCC | 1 (2.9%) |
| Unclassified RCC | 1 (2.9%) |
| Insufficient sampling | 1 (2.9%) |
| Protein | Uniprot Accession Number | TP3 Protein Result as % of TP2 Baseline; Median (Range) | Number of Patients with Increase | Number of Patients with Increase > IQR of Baseline Variability | p Value | q Value (FDR) |
|---|---|---|---|---|---|---|
| Interleukin-6 (IL-6) | P05231 ** | 518.7 (172.1–2917) | 24 (100%) | 24 (100%) | <0.001 | <0.001 |
| Interleukin-10 (IL-10) | P22301 | 199.6 (79.6–721) | 22 (91.7%) | 22 (91.7%) | <0.001 | <0.001 |
| C-C motif chemokine 23 (CCL23) | P55773 | 192.3 (94.4–338.6) | 23 (95.8%) | 22 (91.7%) | <0.001 | <0.001 |
| Heme oxygenase 1 (HO-1) | P09601 | 140.9 (99.6–407.1) | 23 (95.8%) | 21 (87.5%) | <0.001 | <0.001 |
| Endothelial cell-specific molecule 1 (ESM-1) | Q9NQ30 | 177.7 (87.1–720.9) | 22 (91.7%) | 21 (87.5%) | <0.001 | <0.001 |
| Decorin (DCN) | P07585 | 116.8 (86.1–175.1) | 20 (83.3%) | 20 (83.3%) | <0.001 | <0.001 |
| Interleukin-15 (IL-15) | P40933 * | 154.2 (77.3–229.1) | 16 (80%) | 16 (80%) | <0.001 | <0.001 |
| Carbonic anhydrase-IX (CA-IX) | Q16790 ** | 147.6 (76.9–473.2) | 21 (87.5%) | 19 (79.2%) | <0.001 | <0.001 |
| Macrophage colony stimulating factor 1 (CSF-1/M-CSF) | P09603 | 116.8 (99.4–237.5) | 22 (91.7%) | 18 (75%) | <0.001 | <0.001 |
| Tumour necrosis factor ligand superfamily member 14 (TNFSF14) | O43557 | 154.1 (49.1–360.4) | 19 (79.2%) | 18 (75%) | <0.001 | 0.002 |
| Syndecan-1 (SYND1) | P18827 | 174.3 (82.2–515.6) | 22 (91.7%) | 18 (75%) | <0.001 | <0.001 |
| Transforming growth factor-alpha (TGFA) | P01135 | 129.7 (81.2–488) | 19 (79.2%) | 18 (75%) | <0.001 | 0.002 |
| Hepatocyte growth factor (HGF) | P14210 ** | 145.0 (95.9–545.7) | 23 (95.8%) | 18 (75%) | <0.001 | <0.001 |
| WAP-four disulfide core domain protein 2 (WFDC2) | Q14508 | 130.4 (64.3–197) | 22 (91.7%) | 18 (75%) | <0.001 | <0.001 |
| Amphiregulin (AREG) | P15514 | 140.5 (71.7–373.1) | 21 (87.5%) | 18 (75%) | <0.001 | 0.002 |
| Protein S100-A11 (S100-A11) | P31949 | 126.5 (79.6–224.5) | 20 (83.3%) | 18 (75%) | <0.001 | <0.001 |
| Ephrin type-A receptor 2 (EPHA2) | P29317 | 114.1 (58.6–246.7) | 20 (83.3%) | 17 (70.8%) | <0.001 | 0.004 |
| Placenta growth factor (PlGF) | P49763 | 126.0 (79.6–235.7) | 20 (83.3%) | 17 (70.8%) | <0.001 | 0.001 |
| Fibroblast growth factor-binding protein 1 (FGFBP1) | Q14512 | 125.7 (76.1–706.6) | 18 (75%) | 17 (70.8%) | 0.001 | 0.005 |
| T cell surface glycoprotein CD5 (CD5) | P06127 | 118.8 (84.7–192.5) | 20 (83.3%) | 16 (66.7%) | <0.001 | <0.001 |
| Pleiotrophin (PTN) | P21246 | 207.2 (49.8–4195.3) | 19 (79.2%) | 16 (66.7%) | <0.001 | 0.002 |
| Tissue factor pathway inhibitor 2 (TFPI-2) | P48307 | 134.7 (47.5–290.1) | 19 (79.2%) | 16 (66.7%) | <0.001 | 0.004 |
| Galectin-1 (GAL-1) | P09382 | 111.8 (74.5–183.6) | 18 (75%) | 15 (62.5%) | 0.002 | 0.003 |
| R-spondin 3 (RSPO3) | Q9BXY4 | 133.3 (75.3–477.4) | 19 (79.2%) | 15 (62.5%) | <0.001 | 0.002 |
| Monocyte chemotactic protein 3 (MCP-3) | P80098 | 152.6 (52.9–330.5) | 19 (79.2%) | 14 (58.3%) | <0.001 | 0.001 |
| Interleukin-8 (IL-8) | P10145 | 154.1 (59.7–348.1) | 18 (75%) | 13 (54.2%) | <0.001 | 0.002 |
| Vascular endothelial growth factor-A (VEGF-A) | P15692 | 124.3 (80.6–230.6) | 18 (75%) | 12 (50%) | <0.001 | 0.002 |
| CD27 antigen (CD27) | P26842 ** | 110.5 (85.5–170.1) | 21 (87.5%) | 12 (50%) | <0.001 | <0.001 |
| Tumour necrosis factor ligand superfamily member 13 (TNFSF13) | O75888 | 112.5 (71.8–245.1) | 18 (75%) | 12 (50%) | 0.001 | 0.005 |
| Protein | Uniprot Accession Number | TP3 Protein Result as % of TP2 Baseline; Median (Range) | Number of Patients with Decrease | Number of Patients with Decrease > IQR of Baseline Variability | p Value | q Value (FDR) |
|---|---|---|---|---|---|---|
| TNF-related apoptosis inducing ligand (TRAIL) | P50591 * | 72.3 (45.1–132.5) | 23 (95.8%) | 22 (91.7%) | <0.001 | <0.001 |
| C-X-C motif chemokine 10 (CXCL10) | P02778 | 64.1 (42.3–186) | 21 (87.5%) | 20 (83.3%) | <0.001 | <0.001 |
| C-X-C motif chemokine 11 (CXCL11) | O14625 | 55.8 (2.8–155) | 23 (95.8%) | 20 (83.3%) | <0.001 | <0.001 |
| Interferon gamma (IFN-gamma) | P01579 | 55.0 (8.8–102.2) | 20 (83.3%) | 20 (83.3%) | <0.001 | <0.001 |
| Mothers against decapentaplegic homolog 5 (MAD homolog 5) | Q99717 | 83.0 (62.9–126.6) | 21 (87.5%) | 18 (75%) | <0.001 | <0.001 |
| Monocyte chemotactic protein 2 (MCP-2) | P80075 | 72.3 (45.1–146.4) | 22 (91.7%) | 17 (70.8%) | <0.001 | <0.001 |
| Monocyte chemotactic protein 4 (MCP-4) | Q99616 | 59.2 (13.5–146.5) | 23 (95.8%) | 17 (70.8%) | <0.001 | <0.001 |
| Fas ligand (FASL) | P48023 * | 80.2 (66–116.8) | 20 (83.3%) | 16 (66.7%) | <0.001 | 0.001 |
| Matrix metalloproteinase-12 (MMP-12) | P39900 | 77.9 (52.4–124.7) | 20 (83.3%) | 16 (66.7%) | <0.001 | <0.001 |
| Proto-oncogene tyrosine-protein kinase receptor Ret (RET) | P07949 | 79.2 (50.5–213.4) | 20 (83.3%) | 15 (62.5%) | 0.002 | 0.007 |
| Cathepsin L2 (CTSV) | O60911 | 77.5 (11.9–117.3) | 18 (75%) | 15 (62.5%) | <0.001 | 0.004 |
| Vascular endothelial growth factor receptor 2 (VEGFR-2) | P35968 | 91.4 (72.7–127.9) | 20 (83.3%) | 14 (58.3%) | 0.001 | 0.003 |
| Angiopoietin-1 (ANGPT-1) | Q15389 | 63.4 (23–199.7) | 19 (79.2%) | 14 (58.3%) | 0.003 | 0.005 |
| Platelet-derived growth factor subunit B (PDGF subunit B) | P01127 | 69.3 (32–208) | 20 (83.3%) | 12 (50%) | <0.001 | 0.002 |
| Natural killer cell receptor 2B4 (CD244) | Q9BZW8 | 84.4 (64.6–181.3) | 17 (70.8%) | 11 (45.8%) | 0.004 | 0.007 |
| Fibroblast growth factor 2 (FGF-2) | P09038 | 81.4 (1.8–200.2) | 18 (75%) | 11 (45.8%) | 0.001 | 0.003 |
| C-X-C motif chemokine 5 (CXCL5) | P42830 | 61.0 (15.7–358.5) | 20 (83.3%) | 9 (37.5%) | <0.001 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wah, T.M.; Zhong, J.; Wilson, M.; Vasudev, N.S.; Banks, R.E. An Exploratory Analysis of Changes in Circulating Plasma Protein Profiles Following Image-Guided Ablation of Renal Tumours Provides Evidence for Effects on Multiple Biological Processes. Cancers 2021, 13, 6037. https://doi.org/10.3390/cancers13236037
Wah TM, Zhong J, Wilson M, Vasudev NS, Banks RE. An Exploratory Analysis of Changes in Circulating Plasma Protein Profiles Following Image-Guided Ablation of Renal Tumours Provides Evidence for Effects on Multiple Biological Processes. Cancers. 2021; 13(23):6037. https://doi.org/10.3390/cancers13236037
Chicago/Turabian StyleWah, Tze Min, Jim Zhong, Michelle Wilson, Naveen S. Vasudev, and Rosamonde E. Banks. 2021. "An Exploratory Analysis of Changes in Circulating Plasma Protein Profiles Following Image-Guided Ablation of Renal Tumours Provides Evidence for Effects on Multiple Biological Processes" Cancers 13, no. 23: 6037. https://doi.org/10.3390/cancers13236037
APA StyleWah, T. M., Zhong, J., Wilson, M., Vasudev, N. S., & Banks, R. E. (2021). An Exploratory Analysis of Changes in Circulating Plasma Protein Profiles Following Image-Guided Ablation of Renal Tumours Provides Evidence for Effects on Multiple Biological Processes. Cancers, 13(23), 6037. https://doi.org/10.3390/cancers13236037






