Current Roles of PET/CT in Thymic Epithelial Tumours: Which Evidences and Which Prospects? A Pictorial Review
Abstract
Simple Summary
Abstract
1. Introduction
- Which role has PET for diagnosis, staging, response prediction to treatment and long-term prognosis today?
- Which are the actual criticisms and future perspectives in this field?
2. The Role of 18F-FDG PET/CT in Thymic Epithelial Tumours
2.1. PET/CT to Distinguish Thymic Hiperplasia from Thymic Epithelial Tumours
18F-FDG PET May Be Useful to Distinguish Thymic Hiperplasia from Thymic Epithelial Tumours Is Integrated with Anatomical Consideration and Spatial 18F-FDG Uptake Distribution
2.2. PET/CT to Stage Thymic Epithelial Tumours
PET/CT Permits a Discrete Stage Prediction in TETs
- for distant localizations or lymph node involvement assessment; and
- for invasive thymoma identification.
2.3. PET/CT to Predict the Grade of Malignancy of Thymic Epithelial Tumours
PET/TC Predicts Ina Such Reliable Manner the Grade of Malignancy in TETs, Especially Considering a Low Grade vs. High Grade Thymoma or Thymic Carcinoma vs. Thymoma
2.4. PET/CT to Predict Pathological Response after Induction Therapy
The Knowledge of Pathologic Response Is Still Limited, but PET/CT Showed Interesting and Promising Results in This Field
2.5. PET/CT to Predict Prognosis
PET/CT Results Seem to Correlate with Prognosis Prediction in TET after Surgery and after Radio-Chemio Therapy, Even If Further Studies Are Needed to Validate These Data
3. Future Perspectives
3.1. Other PET/CT Radiopharmaceuticals in Thymic Epithelial Tumours
3.2. Future Perspectives
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riedel, R.F.; Burfeind, W.R., Jr. Thymoma: Benign appearance, malignant potential. Oncologist 2006, 11, 887–894. [Google Scholar] [CrossRef]
- Girard, N. Thymic epithelial tumours: From basic principles to individualized treatment strategies. Eur. Respir. Rev. 2013, 22, 75–87. [Google Scholar] [CrossRef] [PubMed]
- de Jong, W.K.; Blaauwgeers, J.L.; Schaapveld, M.; Timens, W.; Klinkenberg, T.J.; Groen, H.J. Thymic epithelial tumours: A population-based study of the incidence, diagnostic procedures and therapy. Eur. J. Cancer. 2008, 44, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Rosai, J.; Sobin, L.H. Histological Typing of Tumors of Thymus. International Histological Classification of Tumors, 2nd ed.; Springer: New York, NY, USA, 1999. [Google Scholar]
- Travis, W.D.; Brambilla, E.; Mueller-Hermelink, H.K.; Harris, C.C. WHO Classification of Tumors. Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart; IARC Press: Lyon, France, 2004. [Google Scholar]
- Kondo, K.; Yoshizawa, K.; Tsuyuguchi, M.; Kimura, S.; Sumitomo, M.; Morita, J.; Miyoshi, T.; Sakiyama, S.; Mukai, K.; Monden, Y. WHO histologic classification is a prognostic indicator in thymoma. Ann. Thorac. Surg. 2004, 77, 1183–1188. [Google Scholar] [CrossRef]
- Lococo, F.; Cafarotti, S.; Cesario, A.; Dall’Armi, V.; Cusumano, G.; Lauriola, L.; Frederic, M.; Evoli, A.; Margaritora, S.; Granone, P. Prognostic grading after complete resection for thymic malignancies. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2882–2891. [Google Scholar] [PubMed]
- Okumura, M.; Ohta, M.; Tateyama, H.; Nakagawa, K.; Matsumura, A.; Maeda, H.; Tada, H.; Eimoto, T.; Matsuda, H.; Masaoka, A. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: A clinical study of 273 patients. Cancer 2002, 94, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Marchevsky, A.M.; Gupta, R.; McKenna, R.J.; Wick, M.; Moran, C.; Zakowski, M.F.; Suster, S. Evidence-based pathology and the pathologic evaluation of thymomas: The World Health Organization classification can be simplified into only 3 categories other than thymic carcinoma. Cancer 2008, 112, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Koppitz, H.; Rockstroh, J.K.; Schüller, H.; Standop, J.; Skowasch, D.; Müller-Hermelink, H.K.; Schmidt-Wolf, I.G. State-of-the-art classification and multimodality treatment of malignant thymoma. Cancer Treat. Rev. 2012, 38, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Falkson, C.B.; Bezjak, A.; Darling, G.; Gregg, R.; Malthaner, R.; Maziak, D.E.; Yu, E.; Smith, C.A.; McNair, S.; Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care; et al. The management of thymoma: A systematic review and practice guideline. J. Thorac. Oncol. 2009, 4, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Seong, Y.W.; Kang, C.H.; Choi, J.W.; Kim, H.S.; Jeon, J.H.; Park, I.K.; Kim, Y.T. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: Its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur. J. Cardiothorac. Surg. 2014, 45, e68–e73. [Google Scholar] [CrossRef]
- Takahashi, K.; Al-Janabi, N.J. Computed tomography and magnetic resonance imaging of mediastinal tumors. J. Magn. Reson. Imaging 2010, 32, 1325–1339. [Google Scholar] [CrossRef]
- Restrepo, C.S.; Pandit, M.; Rojas, I.C.; Villamil, M.A.; Gordillo, H.; Lemos, D.; Mastrogiovanni, L.; Diethelm, L. Imaging findings of expansile lesions of the thymus. Curr. Probl. Diagn. Radiol. 2005, 34, 22–34. [Google Scholar] [CrossRef]
- Tomiyama, N.; Johkoh, T.; Mihara, N.; Honda, O.; Kozuka, T.; Koyama, M.; Hamada, S.; Okumura, M.; Ohta, M.; Eimoto, T.; et al. Using the World Health Organization classification of thymic epithelial neoplasms to describe CT findings. Am. J. Roentgenol. 2002, 179, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Sadohara, J.; Fujimoto, K.; Müller, N.L.; Kato, S.; Takamori, S.; Ohkuma, K.; Terasaki, H.; Hayabuchi, N. Thymic epithelial tumors: Comparison of CT and MR imaging findings of low-risk thymomas, high-risk thymomas, and thymic carcinomas. Eur. J. Radiol. 2006, 60, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Characterization of thymic lesions with F-18 FDG PET-CT: An emphasis on epithelial tumors. Nucl. Med. Commun. 2011, 32, 554–562. [Google Scholar] [CrossRef]
- Treglia, G.; Spitilli, M.G.; Calcagni, M.L.; Giordano, A. The role of Nuclear Medicine in the management of thymomas. Ann. Ital. Chir. 2007, 78, 371–374. [Google Scholar]
- Treglia, G.; Cason, E.; Fagioli, G. Recent applications of nuclear medicine in diagnostics (first part). Ital. J. Med. 2010, 4, 84–91. [Google Scholar] [CrossRef]
- Priola, A.M.; Priola, S.M. Imaging of thymus in myasthenia gravis: From thymic hyperplasia to thymic tumor. Clin. Radiol. 2014, 69, e230–e245. [Google Scholar] [CrossRef] [PubMed]
- El-Bawab, H.; Al-Sugair, A.A.; Rafay, M.; Hajjar, W.; Mahdy, M.; Al-Kattan, K. Role of flourine-18 fluorodeoxyglucose positron emission tomography in thymic pathology. Eur. J. Cardiothorac. Surg. 2007, 31, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Singhal, A.; Kumar, A.; Bal, C.; Malhotra, A.; Kumar, R. Evaluation of thymic tumors with 18F-FDG PET-CT: A pictorial review. Acta Radiol. 2013, 54, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Shimomura, H.; Mutoh, T.; Saito, R.; Goto, R.; Yamada, T.; Notsuda, H.; Matsuda, Y.; Noda, M.; Sakurada, A.; et al. Positron emission tomography/computed tomography as a clinical diagnostic tool for anterior mediastinal tumors. Surg. Today 2019, 49, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Girard, N.; Ruffini, E.; Marx, A.; Faivre-Finn, C.; Peters, S.; on Behalf of the ESMO Guidelines Committee. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), v40–v55. [Google Scholar] [CrossRef]
- Available online: www.nccn.org/professionals/physician_gls/ (accessed on 1 September 2021).
- Moser, B.; Fadel, E.; Fabre, D.; Keshavjee, S.; de Perrot, M.; Thomas, P.; Brioude, G.; Van Raemdonck, D.; Viskens, S.; Lang-Lazdunski, L.; et al. Surgical therapy of thymic tumours with pleural involvement: An ESTS Thymic Working Group Project. Eur. J. Cardiothorac. Surg. 2017, 52, 346–355. [Google Scholar] [CrossRef]
- Leuzzi, G.; Rocco, G.; Ruffini, E.; Sperduti, I.; Detterbeck, F.; Weder, W.; Venuta, F.; Van Raemdonck, D.; Thomas, P.; ESTS Thymic Working Group; et al. Multimodality therapy for locally advanced thymomas: A propensity score–matched cohort study from the European Society of Thoracic Surgeons database. J. Thorac. Cardiovasc. Surg. 2016, 151, 47–57. [Google Scholar] [CrossRef]
- Sasaki, M.; Kuwabara, Y.; Ichiya, Y.; Akashi, Y.; Yoshida, T.; Nakagawa, M.; Murayama, S.; Masuda, K. Differential diagnosis of thymic tumors using a combination of 11C-methionine PET and FDG-PET. J. Nucl. Med. 1999, 40, 1595–1601. [Google Scholar]
- Lee, J.; Cho, Y.S.; Kim, J.; Shim, Y.M.; Lee, K.-H.; Choi, J.Y. Prognostic Significance of Metabolic Parameters by 18F-FDG PET/CT in Thymic Epithelial Tumors. Cancers 2021, 13, 712. [Google Scholar] [CrossRef]
- Ito, T.; Suzuki, H.; Sakairi, Y.; Wada, H.; Nakajima, T.; Yoshino, I. 18F-FDG-PET/CT predicts grade of malignancy and invasive potential of thymic epithelial tumors. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Matsuno, Y.; Noguchi, M.; Mukai, K.; Asamura, H.; Goya, T.; Shimosato, Y. A review of 79 thymomas: Modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol. Int. 1994, 44, 359–367. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Stratton, K.; Giroux, D.; Asamura, H.; Crowley, J.; Falkson, C.; Filosso, P.L.; Frazier, A.A.; Giaccone, G.; Huang, J.; et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: Proposal for an evidence-based stage classification system for the forthcoming(8th)edition of the TNM classification of malignant tumors. J. Thorac. Oncol. 2014, 9 (Suppl. S2), S65–S72. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, I.; Oda, M.; Takizawa, M.; Waseda, R.; Nakajima, K.; Kawano, M.; Mochizuki, T.; Ikeda, H.; Watanabe, G. Usefulness of Fluorine-18 Fluorodeoxyglucose-Positron Emission Tomography in Management Strategy for Thymic Epithelial Tumors. Ann. Thorac. Surg. 2013, 95, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Taniguchi, T.; Ishikawa, Y.; Kawaguchi, K.; Fukui, T.; Kato, K.; Matsuo, K.; Yokoi, K. The utility of [18F]-fluorodeoxyglucose positron emission tomography-computed tomography in thymic epithelial tumours. Eur. J. Cardio-Thoracic Surg. 2012, 42, e152–e156. [Google Scholar] [CrossRef] [PubMed]
- Bertolaccini, L.; Viti, A.; Lanzi, E.; Fortunato, M.; Chauvie, S.; Bianchi, A.; Terzi, A. 18Fluorine-fluorodeoxyglucose positron emission tomography/computed tomography total glycolytic volume in thymic epithelial neoplasms evaluation: A reproducible image biomarker. Gen. Thorac. Cardiovasc. Surg. 2014, 62, 228–233. [Google Scholar] [CrossRef]
- Endo, M.; Nakagawa, K.; Ohde, Y.; Okumura, T.; Kondo, H.; Igawa, S.; Nakamura, Y.; Tsuya, A.; Murakami, H.; Takahashi, T.; et al. Utility of 18F-FDG PET for differentiating the grade of malignancy in thymic epithelial tumors. Lung Cancer 2008, 61, 350–355. [Google Scholar] [CrossRef] [PubMed]
- El-Bawab, H.Y.; Abouzied, M.M.; Rafay, M.A.; Hajjar, W.M.; Saleh, W.M.; Alkattan, K.M. Clinical use of combined positron emission tomography and computed tomography in thymoma recurrence. Interact. Cardiovasc. Thorac. Surg. 2010, 11, 395–399. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J. Nucl. Med. 2009, 50 (Suppl. 1), 122S–150S. [Google Scholar] [CrossRef]
- Kaira, K.; Sunaga, N.; Ishizuka, T.; Shimizu, K.; Yamamoto, N. The role of [18F]fluorodeoxyglucose positron emission tomography in thymic epithelial tumors. Cancer Imaging 2011, 11, 195–201. [Google Scholar] [CrossRef]
- Viti, A.; Terzi, A.; Bianchi, A.; Bertolaccini, L. Is a positron emission tomography-computed tomography scan useful in the staging of thymic epithelial neoplasms? Interact. Cardiovasc. Thorac. Surg. 2014, 19, 129–134. [Google Scholar] [CrossRef][Green Version]
- Eguchi, T.; Yoshida, K.; Hamanaka, K.; Shiina, T.; Koizumi, T.; Kawakami, S.; Oguchi, K.; Amano, J. Utility of 18F-fluorodeoxyglucose positron emission tomography for distinguishing between the histological types of early stage thymic epithelial tumours. Eur. J. Cardio.-Thoracic. Surg. 2011, 41, 1059–1062. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Treglia, G.; Sadeghi, R.; Giovanella, L.; Cafarotti, S.; Filosso, P.L.; Lococo, F. Is 18F-FDG PET useful in predicting the WHO grade of malignancy in thymic epithelial tumors? A meta-analysis. Lung Cancer 2014, 86, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Sadeghi, R. Meta-analyses and systematic reviews on PET and PET/CT in oncology: The state of the art. Clin. Transl. Imaging 2013, 1, 73–75. [Google Scholar] [CrossRef][Green Version]
- Zhao, J.; Wang, H.; Li, Q. Value of 18F-FDG PET/computed tomography in predicting the simplified WHO grade of malignancy in thymic epithelial tumors. Nucl. Med. Commun. 2020, 41, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, T.; Tsuchiya, K.; Nakamura, K.; Tomita, M. Fluorodeoxyglucose Positron Emission Tomography Can Provide Useful Information for Differentiating Thymic Epithelial Tumors. Thorac. Cardiovasc. Surg. 2017, 66, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Cho, A.; Bae, M.K.; Lee, C.Y.; Kim, D.J.; Chung, K.Y. Value of 18F-FDG PET/CT for Predicting the World Health Organization Malignant Grade of Thymic Epithelial Tumors. Clin. Nucl. Med. 2016, 41, 15–20. [Google Scholar] [CrossRef]
- Shinya, T.; Tanaka, T.; Soh, J.; Matsushita, T.; Sato, S.; Toyooka, S.; Yoshino, T.; Miyoshi, S.; Kanazawa, S. Diagnostic Value of Dual-time-point F-18 FDG PET/CT and Chest CT for the Prediction of Thymic Epithelial Neoplasms. Acta Med. Okayama. 2017, 71, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Kim, H.S.; Cho, Y.S.; Sun, J.-M.; Ahn, J.S.; Park, K.; Kim, B.-T.; Ahn, M.-J.; Lee, K.-H. Value of volume-based early metabolic response in patients with unresectable thymic epithelial tumor. Lung Cancer 2016, 100, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Mena, E.; Kurdziel, K.; Venzon, D.; Khozin, S.; Berman, A.W.; Choyke, P.; Szabo, E.; Rajan, A.; Giaccone, G. 18F-Fluorodeoxyglucose Positron Emission Tomography in the Management of Patients with Thymic Epithelial Tumors. Clin. Cancer Res. 2013, 19, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Fukui, T.; Okasaka, T.; Kawaguchi, K.; Nakamura, S.; Hakiri, S.; Ozeki, N.; Sugiyama, T.; Kato, K.; Yokoi, K. The Role of 18F-fluorodeoxyglucose Positron Emission Tomography-Computed Tomography for Predicting Pathologic Response After Induction Therapy for Thymic Epithelial Tumors. World J. Surg. 2017, 63, 670–1833. [Google Scholar] [CrossRef] [PubMed]
- Korst, R.J.; Bezjak, A.; Blackmon, S.; Choi, N.; Fidias, P.; Liu, G.; Marx, A.; Wright, C.; Mock, S.; Rutledge, J.R.; et al. Neoadjuvant chemoradiotherapy for locally advanced thymic tumors: A phase II, multi-institutional clinical trial. J. Thorac. Cardiovasc. Surg. 2014, 147, 36–46.e1. [Google Scholar] [CrossRef]
- Marom, E.M. Imaging Thymoma. J. Thorac. Oncol. 2010, 5, S296–S303. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, H.O.; Kim, J.S.; Moon, D.H.; Kim, Y.H.; Kim, D.K.; Park, S.-I.; Park, Y.S.; Ryu, J.-S. 18F-FDG PET/CT is Useful for Pretreatment Assessment of the Histopathologic Type of Thymic Epithelial Tumors. Nucl. Med. Mol. Imaging 2010, 44, 177–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, A.; Regmi, S.K.; Dutta, R.; Kumar, R.; Gupta, S.D.; Das, P.; Halanaik, D.; Jindal, T. Characterization of thymic masses using 18F-FDG PET-CT. Ann. Nucl. Med. 2009, 23, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Seki, N.; Sakamoto, S.; Karube, Y.; Oyaizu, T.; Ishihama, H.; Chida, M. 18F-fluorodeoxyglucose positron emission tomography for evaluation of thymic epithelial tumors: Utility for World Health Organization classification and predicting recurrence-free survival. Ann. Nucl. Med. 2014, 28, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Calabria, F.; D’Auria, S.; Sannino, P.; Schillaci, O. A case of thymoma detected by 18F-choline positron emission tomography/computed tomography. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 602. [Google Scholar] [CrossRef][Green Version]
- Fallanca, F.; Picchio, M.; Spinapolice, E.G.; Ugolini, C.; Proietti, A.; Messa, C. Imaging of a Thymoma Incidentally Detected by C-11 Choline PET/CT. Clin. Nucl. Med. 2011, 36, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Takesh, M.; Adams, S. Imaging Comparison between 18F-FDG-PET/CT and 18F-Flouroethyl Choline PET/CT in Rare Case of Thymus Carcinoma Exhibiting a Positive Choline Uptake. Case Rep. Oncol. Med. 2013, 2013, 464396. [Google Scholar] [CrossRef] [PubMed]
- Bricaire, L.; Richard, C.; Gauthé, M.; Cochand-Priollet, B.; Gaujoux, S. False-Positive Results in 18F-Fluorocholine PET/CT for a Thymoma in Workup of a Hereditary Primary Hyperparathyroidism. Clin. Nucl. Med. 2018, 43, e151–e153. [Google Scholar] [CrossRef]
- Shibata, H.; Nomori, H.; Uno, K.; Sakaguchi, K.; Nakashima, R.; Iyama, K.; Tomiyoshi, K.; Kaji, M.; Goya, T.; Suzuki, T.; et al. 18F-fluorodeoxyglucose and11C-acetate positron emission tomography are useful modalities for diagnosing the histologic type of thymoma. Cancer 2009, 115, 2531–2538. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Nomori, H.; Watanabe, K.; Naruke, T.; Suemasu, K.; Kosaka, N.; Uno, K. Positive Imaging of Thymoma by 11C-Acetate Positron Emission Tomography. Ann. Thorac. Surg. 2006, 81, 1132–1134. [Google Scholar] [CrossRef]
- Sakurai, H.; Kaji, M.; Suemasu, K. Thymoma of the Middle Mediastinum: 11C-Acetate Positron Emission Tomography Imaging. Ann. Thorac. Surg. 2009, 87, 1271–1274. [Google Scholar] [CrossRef]
- Huang, H.L.; Dharmawan, A.R.; Tan, C.J.; Thang, S.P. A rare case of thymoma first detected on gallium-68 PSMA PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2148–2149. [Google Scholar] [CrossRef]
- Farolfi, A.; Ceci, F.; Graziani, T.; Lambertini, A.; Cervati, V.; Basilico, G.L.; Biraghi, T.; Briganti, A.; Castellucci, P.; Fanti, S. Incidental Detection of Basaloid Thymic Carcinoma With 68Ga-PSMA-11 PET/CT in a Patient With Recurrent Prostate Cancer. Clin. Genitourin. Cancer 2018, 16, e497–e499. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Thapa, P.; Parghane, R. 177Lu-DOTATATE peptide receptor radionuclide therapy in metastatic or advanced and inoperable primary neuroendocrine tumors of rare sites. World J. Nucl. Med. 2017, 16, 223–228. [Google Scholar] [CrossRef]
- Treglia, G.; Sadeghi, R.; Annunziata, S.; Lococo, F.; Cafarotti, S.; Prior, J.; Bertagna, F.; Ceriani, L.; Giovanella, L. Diagnostic performance of Fluorine-18-Fluorodeoxyglucose positron emission tomography in the assessment of pleural abnormalities in cancer patients: A systematic review and a meta-analysis. Lung Cancer 2014, 83, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nakajo, M.; Jinguji, M.; Shinaji, T.; Nakajo, M.; Aoki, M.; Tani, A.; Sato, M.; Yoshiura, T. Texture analysis of 18F-FDG PET/CT for grading thymic epithelial tumours: Usefulness of combining SUV and texture parameters. Br. J. Radiol. 2018, 91, 20170546. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2019, 60, 386–392. [Google Scholar] [CrossRef] [PubMed]
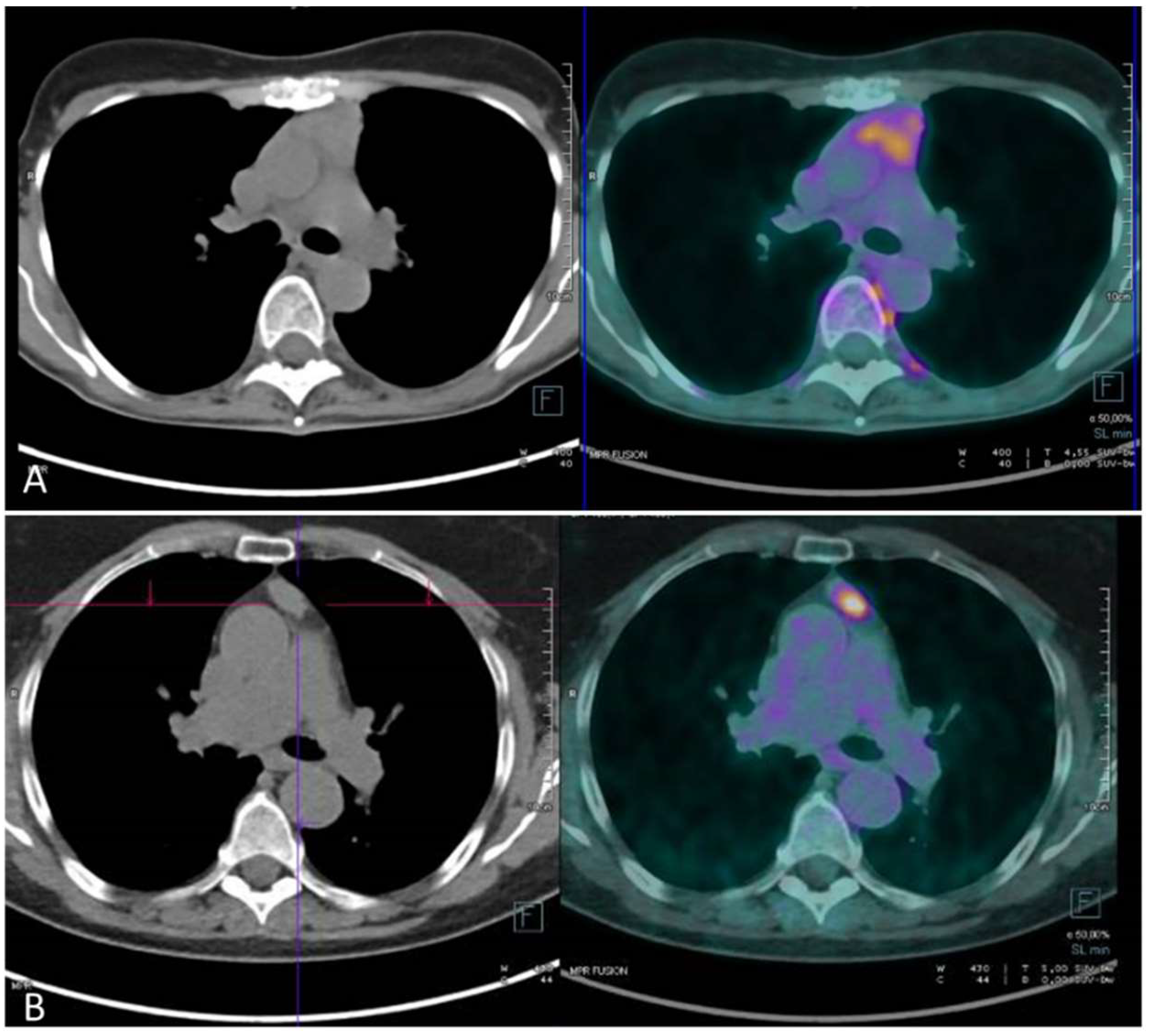
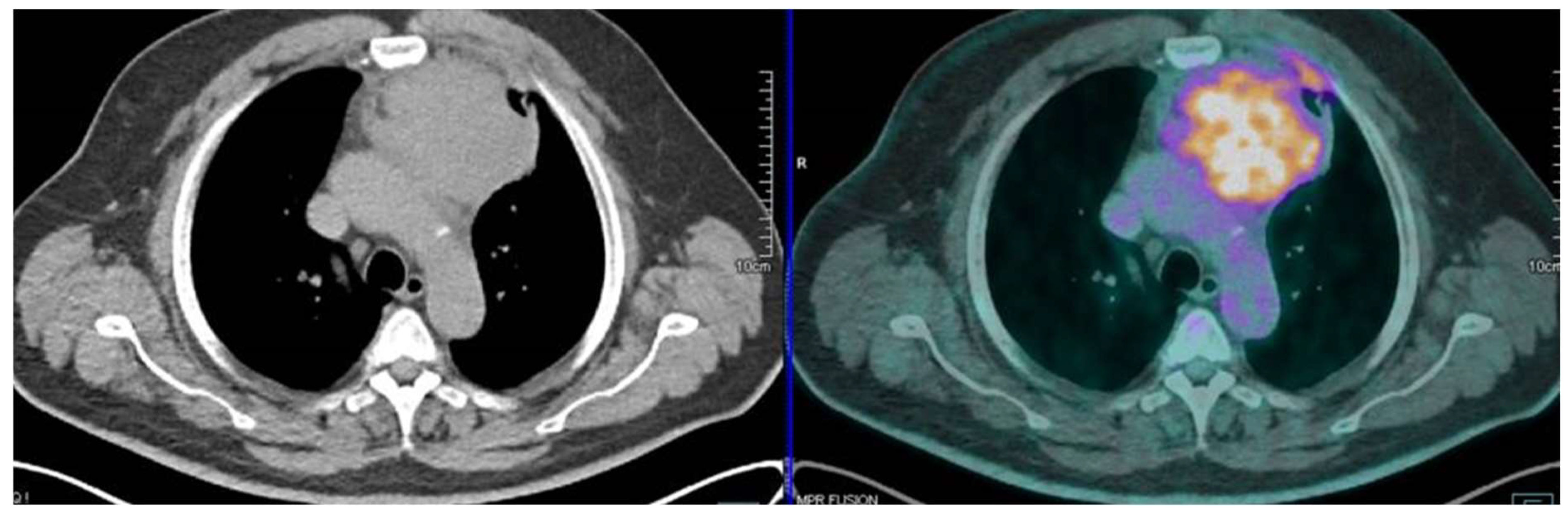
| Study | Number of Patients | Masaoka Stage and 18F FDG SUV Max | Tnm Stage and 18F FDG SUV Max | Histology and 18F FDG SUV Max |
|---|---|---|---|---|
| Sasaki (1999) | 29 | Thymica carcinoma: 7.2 ± 2.9 Invasive thymoma: 3.8 ± 1.3 non-invasive thymoma: 3.0 ± 1.0 thymic cysts: 0.9 (p < 0.01) | ||
| Matsumoto (2013) | 39 | Masaoka stage I: 3.0 ± 1.1 Masaoka stage II: 3.6 ± 1.4 Masaoka stage III: 5.3 ± 3.4 Masaoka stage IV: 8.3 ± 4.0 | A: 2.3 AB: 2.3 ± 0.8 B1: 3.7 ± 1.5 B2: 4.1 ± 1.4 B3: 4.9 ± 1.5 C: 12.1 ± 3.2 C vs. other histology p < 0.0001 | |
| Bertolaccini (2014) | 23 | Significant SUV T/M ratio Masaoka stage I: 2.31 ± 1.06 Masaoka stage II: 2.15 ± 1.27 Masaoka stage III: 2.69 ± 0.62 Masaoka stage IV: 2.53 (ρ = 0.555); no statistical correlation with MTV Masaoka stage I: 6.50 ± 3.69 Masaoka stage II: 5.40 ± 2.71 Masaoka stage III: 9.22 ± 1.86 Masaoka stage IV: 4.2 (ρ = 0.185), no statistical correlation with TGV Masaoka stage I: 233.7 ± 234.61 Masaoka stage II: 144.14 ± 276.18 Masaoka stage III: 431.80 ± 343.31 Masaoka stage IV: 55 (ρ = 0.199) | TGV and WHO classification: Low risk thymoma 99.12 ± 125.98 High risk thymoma 645.83 ± 159.87 (q = 0.897) SUV T/M ratio and WHO classification: Low risk thymoma 1.91 ± 0.45 High risk thymoma 3.73 ± 0.95 (q = 0.873). | |
| Watanabe (2018) | 63 | Masaoka stage I: 3.0 ± 1.2 Masaoka stage II: 3.8 ± 1.6 Masaoka stage III: 4.4 ± 1.2 Masaoka stage IV: 4.8 ± 1.0 | T1a: 3.2 ± 1.3 T1b: 5.4 ± 2.0 T2: 5.1 ± 0.9 T3: 4.6 ± 1.1 T3 and T1b thymomas had significantly higher SUVmax than T1a | A: 3.1 ± 1.8 AB: 3.4 ± 1.5 B1: 3.8 ± 1.5 B2: 3.7 ± 1.6 B3: 4.3 ± 1.0 No significant difference |
| ITO (2020) | 56 | Masaoka stage I-II: 4.52 ± 2.66 Masaoka stage III-IV: 7.73 ± 2.83 (p < 0.01) Cut off: 5.6 (sensitivity 0.89, specificity 0.78) | T1a: 4.45 ± 2.06 T1b: 4.9 ± 2.0 T2: 7.12 ± 2.69 T3: 8.31 ± 2.57 T4: 9.79 ± 7.48 T3 vs. T1a (p < 0.01) | Thymic carcinoma vs. high-grade thymoma: 9.09 ± 3.34 vs. 6.01 ± 2.78 (p < 0.01), Cut-off 7.40 (sensitivity 0.72, specificity 0.79) high-grade thymoma vs. low-grade thymoma: 6.01 ± 2.78 vs. 4.06 ± 1.86 (p < 0.01) Cut-off 5.40 (sensitivity 0.61, specificity 0.85 |
| LEE (2021) | 83 | Masaoka stage I: 6.6 ± 4.8 Masaoka stage II: 4.9 ± 1.8 Masaoka stage III: 8.9 ± 5.7 Masaoka stage IV: 10.5 ± 6.7 (p = 0.001) | Low risk thymomas 3.98 ± 2.59 high risk thymoma 5.63 ± 2.89 thymic carcinoma 10.42 ± 3.53 p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lococo, F.; Chiappetta, M.; Triumbari, E.K.A.; Evangelista, J.; Congedo, M.T.; Pizzuto, D.A.; Brascia, D.; Marulli, G.; Annunziata, S.; Margaritora, S. Current Roles of PET/CT in Thymic Epithelial Tumours: Which Evidences and Which Prospects? A Pictorial Review. Cancers 2021, 13, 6091. https://doi.org/10.3390/cancers13236091
Lococo F, Chiappetta M, Triumbari EKA, Evangelista J, Congedo MT, Pizzuto DA, Brascia D, Marulli G, Annunziata S, Margaritora S. Current Roles of PET/CT in Thymic Epithelial Tumours: Which Evidences and Which Prospects? A Pictorial Review. Cancers. 2021; 13(23):6091. https://doi.org/10.3390/cancers13236091
Chicago/Turabian StyleLococo, Filippo, Marco Chiappetta, Elizabeth Katherine Anna Triumbari, Jessica Evangelista, Maria Teresa Congedo, Daniele Antonio Pizzuto, Debora Brascia, Giuseppe Marulli, Salvatore Annunziata, and Stefano Margaritora. 2021. "Current Roles of PET/CT in Thymic Epithelial Tumours: Which Evidences and Which Prospects? A Pictorial Review" Cancers 13, no. 23: 6091. https://doi.org/10.3390/cancers13236091
APA StyleLococo, F., Chiappetta, M., Triumbari, E. K. A., Evangelista, J., Congedo, M. T., Pizzuto, D. A., Brascia, D., Marulli, G., Annunziata, S., & Margaritora, S. (2021). Current Roles of PET/CT in Thymic Epithelial Tumours: Which Evidences and Which Prospects? A Pictorial Review. Cancers, 13(23), 6091. https://doi.org/10.3390/cancers13236091








