Molecular Pathology of Human Papilloma Virus-Negative Cervical Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Human Papillomavirus and Cervical Cancer
1.2. Definition of HPV-Independence in Cervical Cancer
- Very low viral load in latent HPV infections [8];
| Histological Type | HPV Positivity (%) | References |
|---|---|---|
| Squamous cell carcinoma | 87–100 | [8] |
| Usual type endocervical adenocarcinoma | 72–100 | [34,41,42] |
| Mucinous adenocarcinoma | 83–100 | [42] |
| Gastric-type adenocarcinoma | 0 | [41] |
| Clear cell adenocarcinoma | 0–28 | [34,41,43] |
| Mesonephric adenocarcinoma | 0 | [42] |
| Endometrioid adenocarcinoma | 0–27 | [34,41,42] |
| Serous adenocarcinoma | 0–30 | [34,41,44] |
| Adenosquamous carcinoma | 81–86 | [8,34,41] |
| Adenoid basal carcinoma | 80–100 | [45,46] |
| Carcinosarcoma | 100 | [47] |
| Neuroendocrine carcinoma | 86–100 | [48,49] |
1.3. WHO Histopathological Classification of Cervical Cancers Based on HPV Infection Status
2. Pathology and Genetics of HPV-Independent Cervical Cancer
2.1. HPV-Independent SqCC
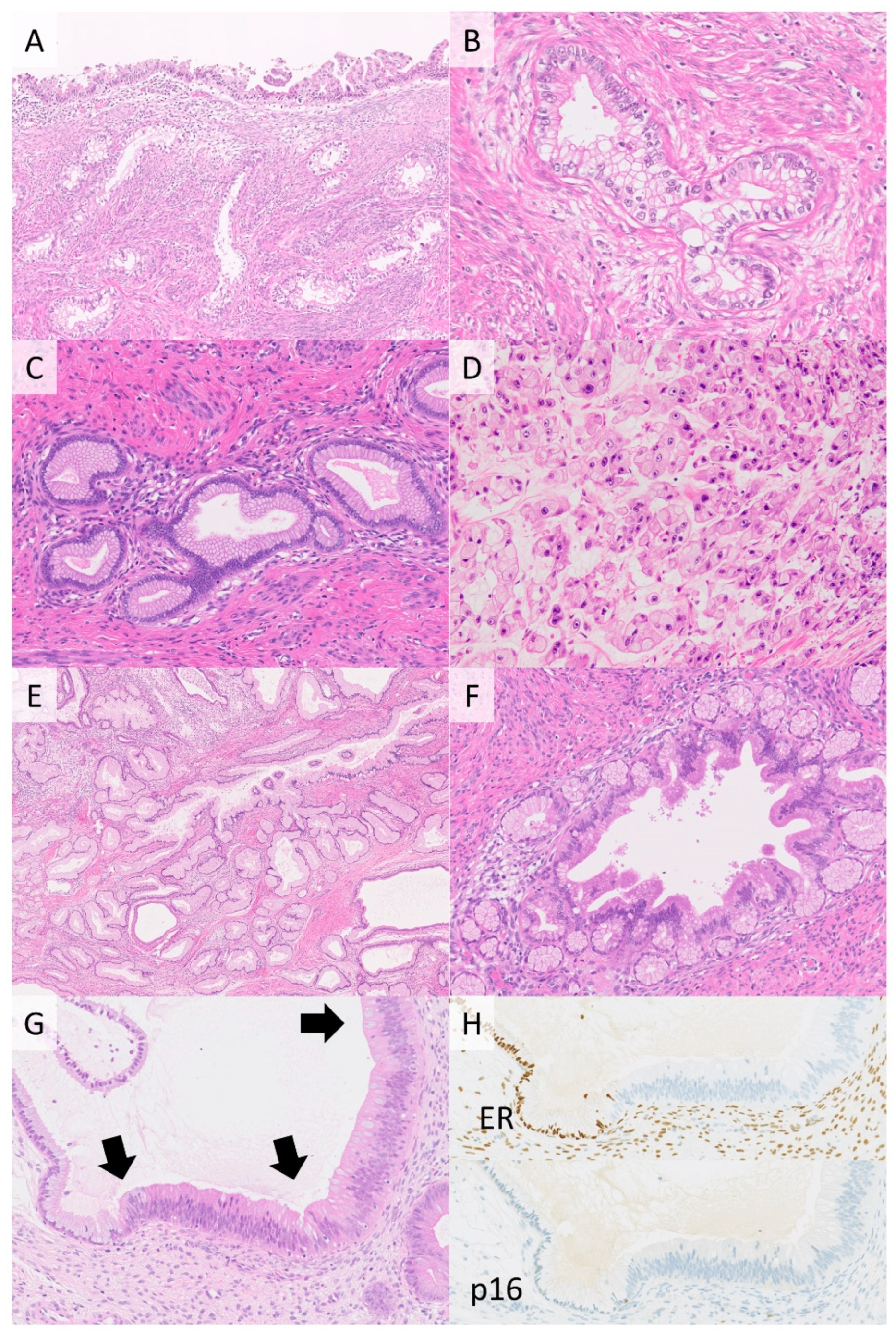
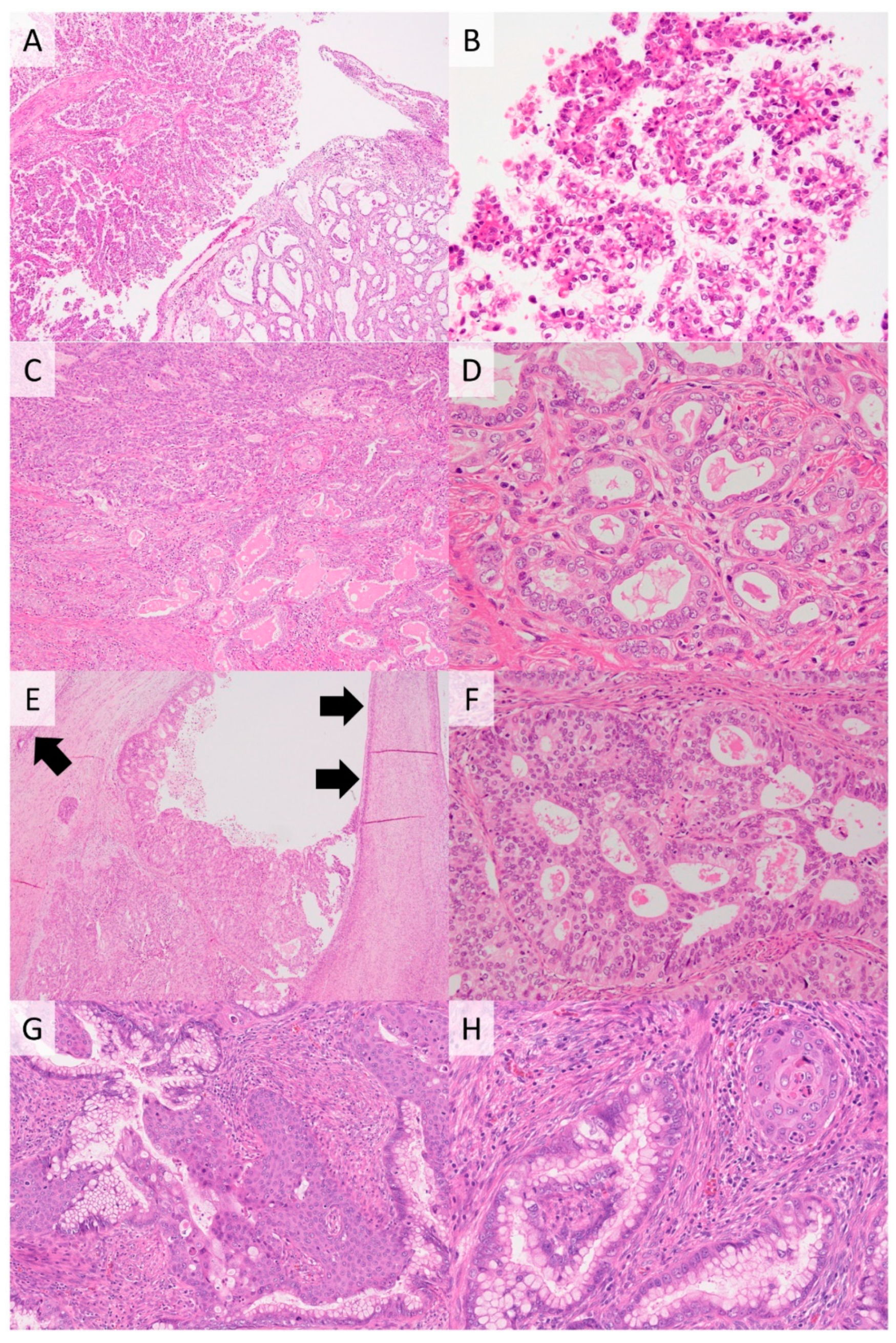
2.2. Gastric-Type Adenocarcinoma
2.3. Clear Cell Carcinoma
2.4. Mesonephric Carcinoma
2.5. Endometrioid Carcinoma
2.6. Serous Carcinoma
2.7. HPV-Independent Adenosquamous Carcinoma
2.8. HPV-Independent Neuroendocrine Carcinoma
3. Animal Models of Cervical Cancer and Their Uses
4. Future Directions and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Woodman, C.B.J.; Collins, S.I.; Young, L. The natural history of cervical HPV infection: Unresolved issues. Nat. Rev. Cancer 2007, 7, 11–22. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Bernard, H.-U.; Burk, R.D.; Chen, Z.; Van Doorslaer, K.; Hausen, H.Z.; de Villiers, E.-M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010, 401, 70–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.F.; Peto, J.; Meijer, C.J.L.M.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Geraets, D.; Alemany, L.; Guimera, N.; de Sanjose, S.; de Koning, M.; Molijn, A.; Jenkins, D.; Bosch, X.; Quint, W.; on behalf of the RIS HPV TT Study Group. Detection of rare and possibly carcinogenic human papillomavirus genotypes as single infections in invasive cervical cancer. J. Pathol. 2012, 228, 534–543. [Google Scholar] [CrossRef]
- Katki, H.A.; Kinney, W.K.; Fetterman, B.; Lorey, T.; Poitras, N.E.; Cheung, L.; Demuth, F.; Schiffman, M.; Wacholder, S.; Castle, P.E. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: A population-based study in routine clinical practice. Lancet Oncol. 2011, 12, 663–672. [Google Scholar] [CrossRef] [Green Version]
- Drolet, M.; Bénard, É.; Pérez, N.; Brisson, M.; HPV Vaccination Impact Study Group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef] [Green Version]
- Arbyn, M.; Xu, L.; Simoens, C.; Martin-Hirsch, P.P. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst. Rev. 2018, 5, CD009069. [Google Scholar] [CrossRef]
- Nicolás, I.; Marimon, L.; Barnadas, E.; Saco, A.; Rodríguez-Carunchio, L.; Fusté, P.; Martí, C.; Rodriguez-Trujillo, A.; Torne, A.; Del Pino, M.; et al. HPV-negative tumors of the uterine cervix. Mod. Pathol. 2019, 32, 1189–1196. [Google Scholar] [CrossRef] [Green Version]
- Van der Marel, J.; van Baars, R.; Quint, W.G.; Berkhof, J.; del Pino, M.; Torne, A.; Ordi, J.; Wentzensen, N.; Schiffman, M.; van de Sandt, M.M.; et al. The impact of human papillomavirus genotype on colposcopic appearance: A cross-sectional analysis. BJOG 2014, 121, 1117–1126. [Google Scholar] [CrossRef]
- Cuzick, J.; Cadman, L.; Mesher, D.; Austin, J.; Ashdown-Barr, L.; Ho, L.; Terry, G.; Liddle, S.; Wright, C.; Lyons, D.; et al. Comparing the performance of six human papillomavirus tests in a screening population. Br. J. Cancer 2013, 108, 908–913. [Google Scholar] [CrossRef]
- Sitarz, K.; Szostek, S. Food and drug administration—Approved molecular methods for detecting human papillomavirus infection. Ginekol. Polska 2019, 90, 104–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, A.M.; Dirks, D.C.; Poulter, M.D.; Mills, S.E.; Stoler, M.H. HR-HPV E6/E7 mRNA In Situ Hybridization: Validation against PCR, DNA In Situ Hybridization, and p16 Immunohistochemistry in 102 Samples of Cervical, Vulvar, Anal, and Head and Neck Neoplasia. Am. J. Surg. Pathol. 2017, 41, 607–615. [Google Scholar] [CrossRef]
- Zheng, R.; Heller, D.S. High-Risk Human Papillomavirus Identification in Precancerous Cervical Intraepithelial Lesions. J. Low. Genit. Tract Dis. 2020, 24, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.M.; Smith, J.S.; Plummer, M.; Muñoz, N.; Franceschi, S. Human papillomavirus types in invasive cervical cancer worldwide: A meta-analysis. Br. J. Cancer 2003, 88, 63–73. [Google Scholar] [CrossRef]
- Guan, P.; Howell-Jones, R.; Li, N.; Bruni, L.; De Sanjosé, S.; Franceschi, S.; Clifford, G.M. Human papillomavirus types in 115,789 HPV-positive women: A meta-analysis from cervical infection to cancer. Int. J. Cancer 2012, 131, 2349–2359. [Google Scholar] [CrossRef]
- Blatt, A.J.; Kennedy, R.; Luff, R.D.; Austin, R.M.; Rabin, D.S. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015, 123, 282–288. [Google Scholar] [CrossRef]
- Petry, K.U.; Liebrich, C.; Luyten, A.; Zander, M.; Iftner, T. Surgical staging identified false HPV-negative cases in a large series of invasive cervical cancers. Papillomavirus Res. 2017, 4, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Franceschi, S.; Howell-Jones, R.; Snijders, P.J.; Clifford, G.M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int. J. Cancer 2010, 128, 927–935. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef]
- Pirog, E.C.; Park, K.J.; Kiyokawa, T.; Zhang, X.; Chen, W.; Jenkins, D.; Quint, W. Gastric-type Adenocarcinoma of the Cervix: Tumor with Wide Range of Histologic Appearances. Adv. Anat. Pathol. 2019, 26, 1–12. [Google Scholar] [CrossRef]
- Stolnicu, S.; Barsan, I.; Hoang, L.; Patel, P.; Terinte, C.; Pesci, A. International Endocervical Adenocarcinoma Criteria and Clas-sification (IECC): A New Pathogenetic Classification for Invasive Adenocarcinomas of the Endocervix. Am. J. Surg. Pathol. 2018, 42, 214–226. [Google Scholar] [CrossRef]
- Tjalma, W.A.; Weyler, J.J.; Bogers, J.J.; Pollefliet, C.; Baay, M.; Goovaerts, G.C.; Vermorken, J.B.; van Dam, P.A.; van Marck, E.A.; Buytaert, P.M. The importance of biological factors (bcl-2, bax, p53, PCNA, MI, HPV and angiogenesis) in invasive cervical cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 97, 223–230. [Google Scholar] [CrossRef]
- Tjalma, W.; Trinh, X.; Rosenlund, M.; Makar, A.; Kridelka, F.; Rosillon, D.; Van Dam, P.; De Souza, S.C.; Holl, K.; Simon, P.; et al. A cross-sectional, multicentre, epidemiological study on human papillomavirus (HPV) type distribution in adult women diagnosed with invasive cervical cancer in Belgium. Facts Views Vis. ObGyn 2015, 7, 101–108. [Google Scholar] [PubMed]
- Akagi, K.; Li, J.; Broutian, T.R.; Padilla-Nash, H.; Xiao, W.; Jiang, B.; Rocco, J.W.; Teknos, T.N.; Kumar, B.; Wangsa, D.; et al. Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability. Genome Res. 2013, 24, 185–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tjalma, W.A.; Depuydt, C.E. Cervical cancer screening: Which HPV test should be used—L1 or E6/E7? Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Banister, C.E.; Liu, C.; Pirisi, L.; Creek, K.E.; Buckhaults, P.J. Identification and characterization of HPV-independent cervical cancers. Oncotarget 2017, 8, 13375–13386. [Google Scholar] [CrossRef] [Green Version]
- Tsakogiannis, D.; Gartzonika, C.; Levidiotou-Stefanou, S.; Markoulatos, P. Molecular approaches for HPV genotyping and HPV-DNA physical status. Expert Rev. Mol. Med. 2017, 19, e1. [Google Scholar] [CrossRef]
- González-Bosquet, E.; Muñoz, A.; Suñol, M.; Lailla, J.M. Cervical cancer and low-risk HPV; a case report. Eur. J. Gynaecol. Oncol. 2006, 27, 193–194. [Google Scholar]
- Guimera, N.; Lloveras, B.; Lindeman, J.; Alemany, L.; van de Sandt, M.; Alejo, M.; Hernandez-Suarez, G.; Bravo, I.G.; Molijn, A.; Jenkins, D.; et al. The occasional role of low-risk human papil-lomaviruses 6, 11, 42, 44, and 70 in anogenital carcinoma defined by laser capture microdissection/PCR methodology: Results from a global study. Am. J. Surg. Pathol. 2013, 37, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Guimera, N.; Lloveras, B.; Alemany, L.; Iljazovic, E.; Shin, H.-R.; Jung-Il, S.; José, F.X.B.; Jenkins, D.; Bosch, F.X.; Quint, W. Laser capture microdissection shows HPV11 as both a causal and a coincidental infection in cervical cancer specimens with multiple HPV types. Histopathology 2013, 63, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Pirog, E.C.; on behalf of the RIS HPV TT study group; Lloveras, B.; Molijn, A.; Tous, S.; Guimerà, N.; Alejo, M.; Clavero, O.; Klaustermeier, J.; Jenkins, D.; et al. HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod. Pathol. 2014, 27, 1559–1567. [Google Scholar] [CrossRef] [Green Version]
- Poljak, M.; Kocjan, B.J.; Oštrbenk, A.; Seme, K. Commercially available molecular tests for human papillomaviruses (HPV): 2015 update. J. Clin. Virol. 2016, 76, S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Guo, J.; Sheng, Y.; Wu, G.; Zhao, Y. Human Papillomavirus-Negative Cervical Cancer: A Comprehensive Review. Front. Oncol. 2021, 10, 3007. [Google Scholar] [CrossRef]
- Koliopoulos, G.; Nyaga, V.N.; Santesso, N.; Bryant, A.; Martin-Hirsch, P.P.; Mustafa, R.A.; Schünemann, H.; Paraskevaidis, E.; Arbyn, M. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst. Rev. 2017, 8, CD008587. [Google Scholar] [CrossRef] [PubMed]
- Hopenhayn, C.; Christian, A.; Christian, W.J.; Watson, M.; Unger, E.; Lynch, C.F.; Peters, E.; Wilkinson, E.J.; Huang, Y.; Copeland, G.; et al. Prevalence of Human Papillomavirus Types in Invasive Cervical Cancers From 7 US Cancer Registries Before Vaccine Introduction. J. Low. Genit. Tract Dis. 2014, 18, 182–189. [Google Scholar] [CrossRef]
- Mazur, M.T.; Hsueh, S.; Gersell, D.J. Metastases to the female genital tract: Analysis of 325 cases. Cancer 1984, 53, 1978–1984. [Google Scholar] [CrossRef]
- Lemoine, N.R.; Hall, P.A. Epithelial tumors metastatic to the uterine cervix. A study of 33 cases and review of the literature. Cancer 1986, 57, 2002–2005. [Google Scholar] [CrossRef]
- Holl, K.; Nowakowski, A.M.; Powell, N.; McCluggage, W.G.; Pirog, E.C.; De Souza, S.C.; Tjalma, W.A.; Rosenlund, M.; Fiander, A.; Sánchez, M.C.; et al. Human papillomavirus prevalence and type-distribution in cervical glandular neoplasias: Results from a European multinational epidemiological study. Int. J. Cancer 2015, 137, 2858–2868. [Google Scholar] [CrossRef] [Green Version]
- Pirog, E.C. Cervical Adenocarcinoma: Diagnosis of Human Papillomavirus–Positive and Human Papillomavirus–Negative Tumors. Arch. Pathol. Lab. Med. 2017, 141, 1653–1667. [Google Scholar] [CrossRef] [Green Version]
- Ueno, S.; Sudo, T.; Oka, N.; Wakahashi, S.; Yamaguchi, S.; Fujiwara, K.; Mikami, Y.; Nishimura, R. Absence of Human Papillomavirus Infection and Activation of PI3K-AKT Pathway in Cervical Clear Cell Carcinoma. Int. J. Gynecol. Cancer 2013, 23, 1084–1091. [Google Scholar] [CrossRef]
- Jenkins, D.; Molijn, A.; Kazem, S.; Pirog, E.C.; Alemany, L.; de Sanjosé, S.; Dinjens, W.; Quint, W. Molecular and pathological basis of HPV -negative cervical adenocarcinoma seen in a global study. Int. J. Cancer 2020, 147, 2526–2536. [Google Scholar] [CrossRef] [PubMed]
- Kerdraon, O.; Cornélius, A.; Farine, M.-O.; Boulanger, L.; Wacrenier, A. Adenoid basal hyperplasia of the uterine cervix: A lesion of reserve cell type, distinct from adenoid basal carcinoma. Hum. Pathol. 2012, 43, 2255–2265. [Google Scholar] [CrossRef] [PubMed]
- Parwani, A.V.; Sehdev, A.E.S.; Kurman, R.J.; Ronnett, B.M. Cervical adenoid basal tumors comprised of adenoid basal epithelioma associated with various types of invasive carcinoma: Clinicopathologic features, human papillomavirus DNA detection, and P16 expression. Hum. Pathol. 2005, 36, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Grayson, W.; Taylor, L.F.; Cooper, K. Carcinosarcoma of the uterine cervix: A report of eight cases with immunohistochemical analysis and evaluation of human papillomavirus status. Am. J. Surg. Pathol. 2001, 25, 338–347. [Google Scholar] [CrossRef]
- Eskander, R.N.; Elvin, J.; Gay, L.; Ross, J.S.; Miller, V.A.; Kurzrock, R. Unique Genomic Landscape of High-Grade Neuroendocrine Cervical Carcinoma: Implications for Rethinking Current Treatment Paradigms. JCO Precis. Oncol. 2020, 4, 972–987. [Google Scholar] [CrossRef]
- Takayanagi, D.; Hirose, S.; Kuno, I.; Asami, Y.; Murakami, N.; Matsuda, M.; Shimada, Y.; Sunami, K.; Komatsu, M.; Hamamoto, R.; et al. Comparative Analysis of Genetic Alterations, HPV-Status, and PD-L1 Expression in Neuroendocrine Carcinomas of the Cervix. Cancers 2021, 13, 1215. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Female Genital Tumours, 5th ed.; WHO Classification of Tumours: Geneva, Switzerland, 2020; Volume 4. [Google Scholar]
- Abu-Rustum, N.R.; Yashar, C.M.; Bean, S.; Bradley, K.; Campos, S.M.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; Damast, S.; et al. NCCN Guidelines Insights: Cervical Cancer, Version 1.2020. J. Natl. Compr. Canc. Netw. 2020, 18, 660–666. [Google Scholar] [CrossRef]
- Raspollini, M.R.; Lax, S.F.; McCluggage, W.G. The central role of the pathologist in the management of patients with cervical cancer: ESGO/ESTRO/ESP guidelines. Virchows Archiv 2018, 473, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Talia, K.L.; Arora, R.; McCluggage, W.G. Precursor Lesions of Cervical Clear Cell Carcinoma: Evidence for Origin From Tubo-Endometrial Metaplasia. Int. J. Gynecol. Pathol. 2021. [Google Scholar] [CrossRef]
- Kim, H.; Yoon, N.; Woo, H.Y.; Lee, E.-J.; Do, S.-I.; Na, K.; Kim, H.-S. Atypical Mesonephric Hyperplasia of the Uterus Harbors Pathogenic Mutation of Kirsten Rat Sarcoma 2 Viral Oncogene Homolog (KRAS) and Gain of Chromosome 1q. Cancer Genom.-Proteom. 2020, 17, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Maddox, W.A. Adenocarcinoma arising within cervical endometriosis and invading the adjacent vagina. Am. J. Obstet. Gynecol. 1971, 110, 1015–1017. [Google Scholar] [CrossRef]
- Rodríguez-Carunchio, L.; Soveral, I.; Steenbergen, R.; Torné, A.; Martinez, S.; Fusté, P.; Pahisa, J.; Marimon, L.; Ordi, J.; Del Pino, M. HPV-negative carcinoma of the uterine cervix: A distinct type of cervical cancer with poor prognosis. BJOG Int. J. Obstet. Gynaecol. 2014, 122, 119–127. [Google Scholar] [CrossRef]
- Pilch, H.; Gunzel, S.; Schaffer, U.; Tanner, B.; Brockerhoff, P.; Maeurer, M.; Hockel, M.; Hommel, G.; Knapstein, P.G. The presence of HPV DNA in cervical cancer: Correlation with clinico-pathologic parameters and prognostic significance: 10 years experience at the Department of Obstetrics and Gynecology of the Mainz University. Int. J. Gynecol. Cancer 2001, 11, 39–48. [Google Scholar] [CrossRef]
- Riou, P.; Favre, M.; Jeannel, D.; Bourhis, J.; Le Doussal, V.; Orth, G. Association between poor prognosis in early-stage invasive cervical carcinomas and non-detection of HPV DNA. Lancet 1990, 335, 1171–1174. [Google Scholar] [CrossRef]
- De Araújo Catão Zampronha, R.; Freitas-Junior, R.; Murta, E.F.; Michelin, M.A.; Barbaresco, A.A.; Adad, S.J.; de Oliveira, A.M.; Rassi, A.B.; Oton, G.J.B. Human papillomavirus types 16 and 18 and the prognosis of patients with stage I cervical cancer. Clinics 2013, 68, 809–814. [Google Scholar] [CrossRef]
- Feng, D.; Xu, H.; Li, X.; Wei, Y.; Jiang, H.; Xu, H.; Luo, A.; Zhou, F. An association analysis between mitochondrial DNA content, G10398A polymorphism, HPV infection, and the prognosis of cervical cancer in the Chinese Han population. Tumor Biol. 2015, 37, 5599–5607. [Google Scholar] [CrossRef]
- Ojesina, A.I.; Lichtenstein, L.; Freeman, S.S.; Pedamallu, C.S.; Imaz-Rosshandler, I.; Pugh, T.J.; Cherniack, A.D.; Ambrogio, L.; Cibulskis, K.; Bertelsen, B.; et al. Landscape of genomic alterations in cervical carcinomas. Nature 2014, 506, 371–375. [Google Scholar] [CrossRef]
- Scholl, S.; Popovic, M.; de la Rochefordiere, A.; Girard, E.; Dureau, S.; Mandic, A.; Koprivsek, K.; Samet, N.; Craina, M.; Margan, M.; et al. Clinical and genetic landscape of treatment naive cervical cancer: Alterations in PIK3CA and in epigenetic modulators associated with sub-optimal outcome. EBioMedicine 2019, 43, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Qian, Z.; Gong, Y.; Wang, Y.; Guan, Y.; Han, Y.; Yi, X.; Huang, W.; Ji, L.; Xu, J.; et al. Comprehensive genomic variation profiling of cervical intraepithelial neoplasia and cervical cancer identifies potential targets for cervical cancer early warning. J. Med. Genet. 2019, 56, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zammataro, L.; Lopez, S.; Bellone, S.; Pettinella, F.; Bonazzoli, E.; Perrone, E.; Zhao, S.; Menderes, G.; Altwerger, G.; Han, C.; et al. Whole-exome sequencing of cervical carcinomas identifies activating ERBB2 and PIK3CA mutations as targets for combination therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 22730–22736. [Google Scholar] [CrossRef]
- Gagliardi, A.; Porter, V.L.; Zong, Z.; Bowlby, R.; Titmuss, E.; Namirembe, C.; Griner, N.B.; Petrello, H.; Bowen, J.; Chan, S.K.; et al. Analysis of Ugandan cervical carcinomas identifies human papillomavirus clade–specific epigenome and transcriptome landscapes. Nat. Genet. 2020, 52, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y. Integrated genomic charac-terization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [PubMed] [Green Version]
- Goodman, A.; Zukerberg, L.R.; Rice, L.W.; Fuller, A.F.; Young, R.H.; Scully, R.E. Squamous Cell Carcinoma of the Endometrium: A Report of Eight Cases and a Review of the Literature. Gynecol. Oncol. 1996, 61, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kokka, F.; Singh, N.; Faruqi, A.; Gibbon, K.; Rosenthal, A. Is Differentiated Vulval Intraepithelial Neoplasia the Precursor Lesion of Human Papillomavirus-Negative Vulval Squamous Cell Carcinoma? Int. J. Gynecol. Cancer 2011, 21, 1297–1305. [Google Scholar] [CrossRef]
- Medeiros, F.; Nascimento, A.F.; Crum, C.P. Early vulvar squamous neoplasia: Advances in classification, diagnosis, and differential diagnosis. Adv. Anat. Pathol. 2005, 12, 20–26. [Google Scholar] [CrossRef]
- Watkins, J.C.; Howitt, B.E.; Horowitz, N.S.; Ritterhouse, L.L.; Dong, F.; Macconaill, L.E.; Garcia, E.; Lindeman, N.I.; Lee, L.J.; Berkowitz, R.S.; et al. Differentiated exophytic vulvar intraepithelial lesions are genetically distinct from keratinizing squamous cell carcinomas and contain mutations in PIK3CA. Mod. Pathol. 2017, 30, 448–458. [Google Scholar] [CrossRef] [Green Version]
- Nooij, L.S.; Ter Haar, N.T.; Ruano, D.; Rakislova, N.; Van Wezel, T.; Smit, V.T.; Trimbos, B.J.; Ordi, J.; Van Poelgeest, M.I.; Bosse, T. Genomic Characterization of Vulvar (Pre)cancers Identifies Distinct Molecular Subtypes with Prognostic Significance. Clin. Cancer Res. 2017, 23, 6781–6789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- 73. Ruiz, F.J.; Sundaresan, A.; Zhang, J.; Pedamallu, C.S.; Halle, M.K.; Srinivasasainagendra, V.; Zhang, J.; Muhammad, N.; Stan-ley, J.; Markovina, S.; et al. Genomic Characterization and Therapeutic Targeting of HPV Undetected Cervical Carcinomas. Cancers 2021, 13, 4551. [Google Scholar] [CrossRef]
- Selenica, P.; Alemar, B.; Matrai, C.; Talia, K.L.; Veras, E.; Hussein, Y.; Oliva, E.; Beets-Tan, R.G.H.; Mikami, Y.; McCluggage, W.G.; et al. Massively parallel sequencing analysis of 68 gastric-type cervical adenocarcinomas reveals mutations in cell cycle-related genes and potentially targetable mutations. Mod. Pathol. 2021, 34, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Kim, S.W.; Kim, S.; Kim, H.-S.; Lee, J.-Y.; Kim, Y.T.; Cho, N.H. Genetic characteristics of gastric-type mucinous carcinoma of the uterine cervix. Mod. Pathol. 2021, 34, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Shi, J.; Zhang, X.; Kong, F.; Liu, L.; Dong, X.; Wang, K.; Shen, D. Comprehensive genomic profiling and prognostic analysis of cervical gastric-type mucinous adenocarcinoma. Virchows Archiv 2021, 479, 893–903. [Google Scholar] [CrossRef]
- Garg, S.; Nagaria, T.S.; Clarke, B.; Freedman, O.; Khan, Z.; Schwock, J.; Bernardini, M.Q.; Oza, A.; Han, K.; Smith, A.C.; et al. Molecular characterization of gastric-type endocervical adenocarcinoma using next-generation sequencing. Mod. Pathol. 2019, 32, 1823–1833. [Google Scholar] [CrossRef]
- Hodgson, A.; Howitt, B.E.; Park, K.J.; Lindeman, N.; Nucci, M.R.; Parra-Herran, C. Genomic Characterization of HPV-related and Gastric-type Endocervical Adenocarcinoma: Correlation with Subtype and Clinical Behavior. Int. J. Gynecol. Pathol. 2020, 39, 578–586. [Google Scholar] [CrossRef]
- Lee, E.; Lindeman, N.I.; Matulonis, U.A.; Konstantinopoulos, P.A. POLE-mutated clear cell cervical cancer associated with in-utero diethylstilbestrol exposure. Gynecol. Oncol. Rep. 2019, 28, 15–17. [Google Scholar] [CrossRef]
- Mirkovic, J.; Sholl, L.M.; Garcia, E.; Lindeman, N.I.E.; Macconaill, L.; Hirsch, M.S.; Cin, P.D.; Gorman, M.; A. Barletta, J.; Nucci, M.R.; et al. Targeted genomic profiling reveals recurrent KRAS mutations and gain of chromosome 1q in mesonephric carcinomas of the female genital tract. Mod. Pathol. 2015, 28, 1504–1514. [Google Scholar] [CrossRef] [Green Version]
- Montalvo, N.; Redrobán, L.; Galarza, D. Mesonephric adenocarcinoma of the cervix: A case report with a three-year follow-up, lung metastases, and next-generation sequencing analysis. Diagn. Pathol. 2019, 14, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalcanti, M.S.; Schultheis, A.M.; Ho, C.; Wang, L.; DeLair, D.F.; Weigelt, B.; Gardner, G.; Lichtman, S.M.; Hameed, M.; Park, K.J. Mixed Mesonephric Adenocarcinoma and High-grade Neuroendocrine Carcinoma of the Uterine Cervix: Case Description of a Previously Unreported Entity with In-sights Into Its Molecular Pathogenesis. Int. J. Gynecol. Pathol. 2017, 36, 76–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikami, Y.; Hata, S.; Melamed, J.; Fujiwara, K.; Manabe, T. Lobular endocervical glandular hyperplasia is a metaplastic process with a pyloric gland phenotype. Histopathology 2001, 39, 364–372. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G. New developments in endocervical glandular lesions. Histopathology 2012, 62, 138–160. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Kiyokawa, T.; Soslow, R.A.; Lamb, C.A.; Oliva, E.; Zivanovic, O.; Juretzka, M.M.; Pirog, E.C. Unusual endocervical adenocarcinomas: An im-munohistochemical analysis with molecular detection of human papillomavirus. Am. J. Surg. Pathol. 2011, 35, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Kusanagi, Y.; Kojima, A.; Mikami, Y.; Kiyokawa, T.; Sudo, T.; Yamaguchi, S.; Nishimura, R. Absence of High-Risk Human Papillomavirus (HPV) Detection in Endocervical Adenocarcinoma with Gastric Morphology and Phenotype. Am. J. Pathol. 2010, 177, 2169–2175. [Google Scholar] [CrossRef]
- Carleton, C.; Hoang, L.; Sah, S.; Kiyokawa, T.; Karamurzin, Y.S.; Talia, K.L.; Park, K.J.; McCluggage, W.G. A Detailed Immunohistochemical Analysis of a Large Series of Cervical and Vaginal Gastric-type Adenocarcinomas. Am. J. Surg. Pathol. 2016, 40, 636–644. [Google Scholar] [CrossRef] [Green Version]
- Mikami, Y.; McCluggage, W.G. Endocervical glandular lesions exhibiting gastric differentiation: An emerging spectrum of benign, premalignant, and malignant lesions. Adv. Anat. Pathol. 2013, 20, 227–237. [Google Scholar] [CrossRef]
- Karamurzin, Y.S.; Kiyokawa, T.; Parkash, V.; Jotwani, A.R.; Patel, P.; Pike, M.C.; Soslow, R.A.; Park, K.J. Gastric-type Endocervical Adenocarcinoma: An Aggressive Tumor with Unusual Metastatic Patterns and Poor Prognosis. Am. J. Surg. Pathol. 2015, 39, 1449–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, A.; Shimada, M.; Mikami, Y.; Nagao, S.; Takeshima, N.; Sugiyama, T.; Teramoto, N.; Kiyokawa, T.; Kigawa, J.; Nishimura, R. Chemoresistance of Gastric-Type Mucinous Carcinoma of the Uterine Cervix: A Study of the Sankai Gynecology Study Group. Int. J. Gynecol. Cancer 2018, 28, 99–106. [Google Scholar] [CrossRef]
- Kojima, A.; Mikami, Y.; Sudo, T.; Yamaguchi, S.; Kusanagi, Y.; Ito, M.; Nishimura, R. Gastric Morphology and Immunophenotype Predict Poor Outcome in Mucinous Adenocarcinoma of the Uterine Cervix. Am. J. Surg. Pathol. 2007, 31, 664–672. [Google Scholar] [CrossRef]
- Fulmer, C.G.; Hoda, R.S.; Pirog, E.C.; Park, K.J.; Holcomb, K. Cytomorphology of Gastric-Type Cervical Adenocarcinoma on a ThinPrep Pap Test: Report of a p16-Positive Tumor Case. Diagn. Cytopathol. 2016, 44, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Ohishi, Y.; Kaku, T.; Aman, M.; Imamura, H.; Yasutake, N. Endocervical Adenocarcinoma with Morphologic Fea-tures of Both Usual and Gastric Types: Clinicopathologic and Immunohistochemical Analyses and High-risk HPV Detection by In Situ Hybridization. Am. J. Surg. Pathol. 2017, 41, 696–705. [Google Scholar] [CrossRef]
- Stewart, C.J.R.; Frost, F.; Leake, R.; Mohan, G.R.; Tan, J. Foamy gland changes in gastric-type endocervical neoplasia. Pathology 2015, 47, 653–658. [Google Scholar] [CrossRef]
- Giardiello, F.M.; Brensinger, J.D.; Tersmette, A.C.; Goodman, S.N.; Petersen, G.M.; Booker, S.V.; Cruz–Correa, M.; Offerhaus, J.A. Very high risk of cancer in familial Peutz–Jeghers syndrome. Gastroenterol. 2000, 119, 1447–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuragaki, C.; Enomoto, T.; Ueno, Y.; Sun, H.; Fujita, M.; Nakashima, R.; Ueda, Y.; Wada, H.; Murata, Y.; Toki, T.; et al. Mutations in the STK11 Gene Characterize Minimal Deviation Adenocarcinoma of the Uterine Cervix. Lab. Investig. 2003, 83, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nucci, M.R.; Clement, P.B.; Young, R.H. Lobular endocervical glandular hyperplasia, not otherwise specified: A clinicopathologic analysis of thirteen cases of a distinctive pseudoneoplastic lesion and comparison with fourteen cases of adenoma malignum. Am. J. Surg. Pathol. 1999, 23, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Kiyokawa, T.; Hata, S.; Fujiwara, K.; Moriya, T.; Sasano, H.; Manabe, T.; Akahira, J.-I.; Ito, K.; Tase, T.; et al. Gastrointestinal immunophenotype in adenocarcinomas of the uterine cervix and related glandular lesions: A possible link between lobular endocervical glandular hyperplasia/pyloric gland metaplasia and ‘adenoma malignum’. Mod. Pathol. 2004, 17, 962–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, C.-T.; Lin, M.-C.; Kuo, K.-T.; Wang, T.-H.; Mao, T.-L. Gastric-type adenocarcinoma in situ of uterine cervix: Cytological and histopathological features of two cases. Virchows Archiv 2016, 469, 351–356. [Google Scholar] [CrossRef]
- Talia, K.L.; Stewart, C.J.R.; Howitt, B.E.; Nucci, M.R.; McCluggage, W.G. HPV-negative Gastric Type Adenocarcinoma In Situ of the Cervix: A Spectrum of Rare Lesions Exhibiting Gastric and Intestinal Differentiation. Am. J. Surg. Pathol. 2017, 41, 1023–1033. [Google Scholar] [CrossRef]
- Talia, K.L.; McCluggage, W.G. The developing spectrum of gastric-type cervical glandular lesions. Pathology 2018, 50, 122–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanselaar, A.; van Loosbroek, M.; Schuurbiers, O.; Helmerhorst, T.; Bulten, J.; Bernhelm, J. Clear cell adenocarcinoma of the vagina and cervix. An update of the central Netherlands registry showing twin age incidence peaks. Cancer 1997, 79, 2229–2236. [Google Scholar] [CrossRef] [Green Version]
- Boyd, J.; Takahashi, H.; Waggoner, S.E.; Jones, L.A.; Hajek, R.A.; Wharton, J.T.; Liu, F.-S.; Fujino, T.; Barrett, J.C.; McLachlan, J.A. Molecular genetic analysis of clear cell adenocarcinomas of the vagina and cervix associated and unassociated with di-ethylstilbestrol exposure in utero. Cancer 1996, 77, 507–513. [Google Scholar] [CrossRef]
- Mills, A.M.; Liou, S.; Kong, C.S.; Longacre, T.A. Are Women with Endocervical Adenocarcinoma at Risk for Lynch Syndrome? Evaluation of 101 Cases Including Unusual Subtypes and Lower Uterine Segment Tumors. Int. J. Gynecol. Pathol. 2012, 31, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nakayama, K.; Minamoto, T.; Ishibashi, T.; Ohnishi, K.; Yamashita, H.; Ono, R.; Sasamori, H.; Razia, S.; Hossain, M.M.; et al. Lynch Syndrome-Related Clear Cell Carcinoma of the Cervix: A Case Report. Int. J. Mol. Sci. 2018, 19, 979. [Google Scholar] [CrossRef] [Green Version]
- Suryawanshi, S.; Huang, X.; Elishaev, E.; Budiu, R.A.; Zhang, L.; Kim, S.; Donnellan, N.; Mantia-Smaldone, G.; Ma, T.; Tseng, G.; et al. Complement Pathway Is Frequently Altered in Endometriosis and Endometriosis-Associated Ovarian Cancer. Clin. Cancer Res. 2014, 20, 6163–6174. [Google Scholar] [CrossRef] [Green Version]
- Kenny, S.L.; McBride, H.A.; Jamison, J.; McCluggage, W.G. Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-beta. Am. J. Surg. Pathol. 2012, 36, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Silver, S.A.; Devouassoux-Shisheboran, M.; Mezzetti, T.P.; Tavassoli, F.A. Mesonephric adenocarcinomas of the uterine cervix: A study of 11 cases with immunohistochemical findings. Am. J. Surg. Pathol. 2001, 25, 379–387. [Google Scholar] [CrossRef]
- Pirog, E.C.; Kleter, B.; Olgac, S.; Bobkiewicz, P.; Lindeman, J.; Quint, W.G.; Richart, R.M.; Isacson, C. Prevalence of Human Papillomavirus DNA in Different Histological Subtypes of Cervical Adenocarcinoma. Am. J. Pathol. 2000, 157, 1055–1062. [Google Scholar] [CrossRef] [Green Version]
- Mirkovic, J.; Schoolmeester, J.K.; Campbell, F.; Miron, A.; Nucci, M.R.; Howitt, B. Cervical mesonephric hyperplasia lacks KRAS/NRAS mutations. Histopathology 2017, 71, 1003–1005. [Google Scholar] [CrossRef]
- Skala, S.L.; Gregg, P.A.; Orr, J.W.; Udager, A.M.; Brown, N.A.; Cho, K.R. Cervical Mesonephric Adenocarcinoma With Novel FGFR2 Mutation. Int. J. Gynecol. Pathol. 2020, 39, 452–455. [Google Scholar] [CrossRef]
- Hodgson, A.; Park, K.J. Cervical Adenocarcinomas: A Heterogeneous Group of Tumors With Variable Etiologies and Clinical Outcomes. Arch. Pathol. Lab. Med. 2019, 143, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Kurman, R.J.; Carcangiu, M.L.; Young, R.H.; Herrington, C.S. WHO Classification of Tumours of Female Reproductive Organs; International Agency for Research on Cancer: Lyon, France, 2014.
- Uehara, T.; Yoshida, H.; Kondo, A.; Kato, T. A case of cervical adenocarcinoma arising from endometriosis in the absence of human papilloma virus infection. J. Obstet. Gynaecol. Res. 2019, 46, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Getz, G.; Gabriel, S.B.; Cibulskis, K.; Lander, E.; Sivachenko, A.; Sougnez, C.; Lawrence, M. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 7447. [Google Scholar]
- Parra-Herran, C.; Lerner-Ellis, J.; Xu, B.; Khalouei, S.; Bassiouny, D.; Cesari, M.; Ismiil, N.; Nofech-Mozes, S. Molecular-based classification algorithm for endometrial carcinoma categorizes ovarian endometrioid carcinoma into prognostically significant groups. Mod. Pathol. 2017, 30, 1748–1759. [Google Scholar] [CrossRef]
- Park, K.J. Cervical adenocarcinoma: Integration of HPV status, pattern of invasion, morphology and molecular markers into classification. Histopathology 2019, 76, 112–127. [Google Scholar] [CrossRef]
- Wong, R.W.-C.; Ng, J.H.Y.; Han, K.C.; Leung, Y.P.; Shek, C.M.; Cheung, K.N.; Choi, C.K.M.; Tse, K.Y.; Ip, P.P.C. Cervical carcinomas with serous-like papillary and micropapillary components: Illustrating the heterogeneity of primary cervical carcinomas. Mod. Pathol. 2021, 34, 207–221. [Google Scholar] [CrossRef]
- Gilks, C.B.; Clement, P.B. Papillary serous adenocarcinoma of the uterine cervix: A report of three cases. Mod. Pathol. 1992, 5, 5. [Google Scholar]
- Zhou, C.; Gilks, C.B.; Hayes, M.; Clement, P.B. Papillary serous carcinoma of the uterine cervix: A clinicopathologic study of 17 cases. Am. J. Surg. Pathol. 1998, 22, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Casey, L.; Singh, N. Metastases to the ovary arising from endometrial, cervical and fallopian tube cancer: Recent advances. Histopathology 2019, 76, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Ushigusa, T.; Yoshida, H.; Kuno, I.; Kojima, N.; Ishikawa, M.; Kato, T. Characteristics and prognostic significance of incidentally detected cancer cells in uterine specimens of patients with pelvic high-grade serous carcinoma. Cytopathol. 2019, 31, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Fader, A.N.; Roque, D.M.; Siegel, E.; Buza, N.; Hui, P.; Abdelghany, O.; Chambers, S.K.; Secord, A.A.; Havrilesky, L.; O’Malley, D.M.; et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Versus Carboplatin-Paclitaxel-Trastuzumab in Uterine Serous Carcinomas That Overexpress Human Epidermal Growth Factor Receptor 2/neu. J. Clin. Oncol. 2018, 36, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Ceccaldi, R.; Shapiro, G.I.; D’Andrea, A.D. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov. 2015, 5, 1137–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tew, W.P.; Lacchetti, C.; Ellis, A.; Maxian, K.; Banerjee, S.; Bookman, M.; Jones, M.B.; Lee, J.-M.; Lheureux, S.; Liu, J.F.; et al. PARP Inhibitors in the Management of Ovarian Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 3468–3493. [Google Scholar] [CrossRef]
- Randall, M.E.; Constable, W.C.; Hahn, S.S.; Kim, J.-A.; Mills, S.E. Results of the radiotherapeutic management of carcinoma of the cervix with emphasis on the influence of histologic classification. Cancer 1988, 62, 48–53. [Google Scholar] [CrossRef]
- Stolnicu, S.; Hoang, L.; Hanko-Bauer, O.; Barsan, I.; Terinte, C.; Pesci, A.; Aviel-Ronen, S.; Kiyokawa, T.; Alvarado-Cabrero, I.; Oliva, E.; et al. Cervical adenosquamous carcinoma: Detailed analysis of morphology, immunohistochemical profile, and clinical outcomes in 59 cases. Mod. Pathol. 2018, 32, 269–279. [Google Scholar] [CrossRef]
- Yoshida, H.; Naka, T.; Kobayashi-Kato, M.; Kikkawa, N.; Tanase, Y.; Uno, M.; Ishikawa, M.; Kato, T. Gastric-type cervical adenocarcinoma with squamous differentiation: Buried in adenosquamous carcinomas? Virchows Archiv 2021, 479, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Salvo, G.; Gonzalez Martin, A.; Gonzales, N.R.; Frumovitz, M. Updates and management algorithm for neuroendocrine tumors of the uterine cervix. Int. J. Gynecol. Cancer 2019, 29, 986–995. [Google Scholar] [CrossRef]
- Alejo, M.; Alemany, L.; Clavero, O.; Quiros, B.; Vighi, S.; Seoud, M.; Cheng-Yang, C.; Garland, S.M.; Juanpere, N.; Lloreta, J.; et al. Contribution of Human papillomavirus in neuroendocrine tumors from a series of 10,575 invasive cervical cancer cases. Papillomavirus Res. 2018, 5, 134–142. [Google Scholar] [CrossRef]
- Ambros, R.A.; Park, J.S.; Shah, K.V.; Kurman, R.J. Evaluation of histologic, morphometric, and immunohistochemical criteria in the differential diagnosis of small cell carcinomas of the cervix with particular reference to human papillomavirus types 16 and 18. Mod. Pathol. 1991, 4, 586–593. [Google Scholar] [PubMed]
- Ilett, E.E.; Langer, S.W.; Olsen, I.H.; Federspiel, B.; Kjaer, A.; Knigge, U. Neuroendocrine Carcinomas of the Gastroenteropancreatic System: A Comprehensive Review. Diagnostics 2015, 5, 119–176. [Google Scholar] [CrossRef] [Green Version]
- Basturk, O.; Tang, L.; Hruban, R.H.; Adsay, V.; Yang, Z.; Krasinskas, A.M.; Vakiani, E.; La Rosa, S.; Jang, K.-T.; Frankel, W.L.; et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: A clinicopathologic analysis of 44 cases. Am. J. Surg. Pathol. 2014, 38, 437–447. [Google Scholar] [CrossRef]
- Pocrnich, C.E.; Ramalingam, P.; Euscher, E.D.; Malpica, A. Neuroendocrine Carcinoma of the Endometrium: A Clinicopathologic Study of 25 Cases. Am. J. Surg. Pathol. 2016, 40, 577–586. [Google Scholar] [CrossRef]
- Doorbar, J. Model systems of human papillomavirus-associated disease. J. Pathol. 2015, 238, 166–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spurgeon, M.E.; Uberoi, A.; McGregor, S.M.; Wei, T.; Ward-Shaw, E.; Lambert, P.F. A Novel In Vivo Infection Model To Study Papillomavirus-Mediated Disease of the Female Reproductive Tract. mBio 2019, 10, e00180-19. [Google Scholar] [CrossRef] [Green Version]
- Brake, T.; Lambert, P.F. Estrogen contributes to the onset, persistence, and malignant progression of cervical cancer in a human papillomavirus-transgenic mouse model. Proc. Natl. Acad. Sci. USA 2005, 102, 2490–2495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, R.R.; Duensing, S.; Brake, T.; Münger, K.; Lambert, P.F.; Arbeit, J.M. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003, 63, 63. [Google Scholar]
- Song, S.; Pitot, H.C.; Lambert, P.F. The human papillomavirus type 16 E6 gene alone is sufficient to induce carcinomas in trans-genic animals. J. Virol. 1999, 73, 5887–5893. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Lv, X.; Huang, C.; Angeletti, P.C.; Hua, G.; Dong, J.; Zhou, J.; Wang, Z.; Ma, B.; Chen, X.; et al. A Human Papillomavirus-Independent Cervical Cancer Animal Model Reveals Unconventional Mechanisms of Cervical Carcinogenesis. Cell Rep. 2019, 26, 2636–2650.e5. [Google Scholar] [CrossRef] [Green Version]
- Henkle, T.R.; Lam, B.; Kung, Y.J.; Lin, J.; Tseng, S.H.; Ferrall, L.; Xing, D.; Hung, C.-F.; Wu, T.-C. Development of a Novel Mouse Model of Spontaneous High-Risk HPVE6/E7-Expressing Carcinoma in the Cervicovaginal Tract. Cancer Res. 2021, 81, 4560–4569. [Google Scholar] [CrossRef]
- Tanaka, T.; Nishie, R.; Ueda, S.; Miyamoto, S.; Hashida, S.; Konishi, H.; Terada, S.; Kogata, Y.; Sasaki, H.; Tsunetoh, S.; et al. Patient-Derived Xenograft Models in Cervical Cancer: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 9369. [Google Scholar] [CrossRef]
- Strati, K.; Pitot, H.C.; Lambert, P.F. Identification of biomarkers that distinguish human papillomavirus (HPV)-positive versus HPV-negative head and neck cancers in a mouse model. Proc. Natl. Acad. Sci. USA 2006, 103, 14152–14157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bu, L.-L.; Li, Y.-C.; Yu, G.-T.; Liu, J.-F.; Deng, W.-W.; Zhang, W.-F.; Zhang, L.; Sun, Z.-J. Targeting phosphorylation of STAT3 delays tumor growth in HPV-negative anal squamous cell carcinoma mouse model. Sci. Rep. 2017, 7, 6629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Bayoumy, K.; Christensen, N.D.; Hu, J.; Viscidi, R.; Stairs, D.B.; Walter, V.; Chen, K.-M.; Sun, Y.-W.; Muscat, J.E.; Richie, J.P. An Integrated Approach for Preventing Oral Cavity and Oropharyngeal Cancers: Two Etiologies with Distinct and Shared Mechanisms of Carcinogenesis. Cancer Prev. Res. 2020, 13, 649–660. [Google Scholar] [CrossRef]
- Sarogni, P.; Mapanao, A.K.; Marchetti, S.; Kusmic, C.; Voliani, V. A Standard Protocol for the Production and Bioevaluation of Ethical In Vivo Models of HPV-Negative Head and Neck Squamous Cell Carcinoma. ACS Pharmacol. Transl. Sci. 2021, 4, 1227–1234. [Google Scholar] [CrossRef]
- Cibula, D.; Potter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie-Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaeco-logical Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Man-agement of Patients with Cervical Cancer. Virchows Arch. 2018, 472, 919–936. [Google Scholar] [CrossRef] [Green Version]
- Del Pino, M.; Rodriguez-Carunchio, L.; Alonso, I.; Torne, A.; Rodriguez, A.; Fuste, P.; Castillob, P.; Nonella, R.; Abu-Lhigaa, N.; Ordib, J. Clinical, colposcopic and pathological characteristics of cervical and vaginal high-grade lesions negative for HPV by Hybrid Capture 2. Gynecol. Oncol. 2011, 122, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Zheng, B.; Yin, F.; Zeng, Z.; Li, Z.; Griffith, C.C.; Luo, B.; Ding, X.; Zhou, X.; Zhao, C. Polymerase Chain Reaction Human Papillomavirus (HPV) Detection and HPV Genotyping in Invasive Cervical Cancers with Prior Negative HC2 Test Results. Am. J. Clin. Pathol. 2017, 147, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Tan, Y.; Zhu, L.-X.; Zhou, L.-N.; Zeng, P.; Liu, Q.; Chen, M.-B.; Tian, Y. Prognostic value of HPV DNA status in cervical cancer before treatment: A systematic review and meta-analysis. Oncotarget 2017, 8, 66352–66359. [Google Scholar] [CrossRef] [Green Version]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Cheng, X. Advances in diagnosis and treatment of metastatic cervical cancer. J. Gynecol. Oncol. 2016, 27, e43. [Google Scholar] [CrossRef] [Green Version]
- Gadducci, A.; Cosio, S. Pharmacological Treatment of Patients with Metastatic, Recurrent or Persistent Cervical Cancer Not Amenable by Surgery or Radiotherapy: State of Art and Perspectives of Clinical Research. Cancers 2020, 12, 2678. [Google Scholar] [CrossRef] [PubMed]
- Crowley, F.; O’Cearbhaill, R.; Collins, D. Exploiting somatic alterations as therapeutic targets in advanced and metastatic cervical cancer. Cancer Treat. Rev. 2021, 98, 102225. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Hoyos Usta, E.; Yañez, E.; et al. Pembrolizumab for Persistent, Recur-rent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef] [PubMed]


| Cause | Examples | Solution |
|---|---|---|
| False-negative HPV test results | ||
| Deletion of targeted HPV DNA fragments during host genome integration | Cases with L1 loss during host genome integration can appear HPV-negative in tests covering only L1 fragments | Select a standard HPV testing method covering E6/E7 regions |
| Very low viral load in latent HPV infections | Less than 0.1% of HPV-negative cases develop HSIL or cervical cancer within 3–5 years | It may not be a problem for clinically evident cancers. Use p16 IHC and/or HPV ISH for tissue samples |
| Undetectable cervical cancer caused by non-high-risk HPV | Approximately 1–2% of cervical cancers are associated with non-high-risk HPV infection | Non-high-risk HPV may be a coincidental multiple infection or causal carcinogen. Combining different types of HPV tests may help |
| Inadequate sampling and various pre-analytical factors associated with HPV testing | False negatives are often the result of poor quality, insufficient tumor cells, or inadequate specimen fixation | Quality control of sampling methods, specimen handling, and processing to prevent HPV DNA degradation |
| Non-cervical cancer misclassification | ||
| Cervical involvement of endometrial cancer | Endometrial endometrioid carcinoma with squamous differentiation misinterpreted as cervical adenosquamous carcinoma | IHC panel including p16, with HPV ISH for difficult cases |
| Metastasis to the uterine cervix | High-grade serous fallopian tube carcinoma implanted in the cervix or small cell lung carcinoma metastasized to the cervix | A careful review of clinical and radiological findings with IHC and/or HPV ISH |
| Invasive Carcinoma | Non-Invasive Lesion |
|---|---|
| Squamous tumors | |
| SqCC, HPV-associated | High-grade squamous intraepithelial lesion |
| SqCC, HPV-independent | |
| SqCC NOS | |
| Glandular tumors | |
| Adenocarcinoma NOS | Adenocarcinoma in situ NOS |
| Adenocarcinoma, HPV-associated | Adenocarcinoma in situ, HPV-associated |
| Usual type, villoglandular, intestinal type, signet-ring, iSMILE | |
| Adenocarcinoma, HPV-independent, gastric type | Adenocarcinoma in situ, HPV-independent: atypical LEGH, gastric type AIS |
| Adenocarcinoma, HPV-independent, clear cell type | Tubo-endometrioid metaplasia with atypia? [53] |
| Adenocarcinoma, HPV-independent, mesonephric type | Atypical mesonephric hyperplasia? [54] |
| Adenocarcinoma, HPV-independent, NOS | |
| Endometrioid adenocarcinoma NOS | Cervical endometriosis? [55] |
| Carcinosarcoma NOS | |
| Adenosquamous carcinoma | |
| Mucoepidermoid carcinoma | |
| Adenoid basal carcinoma | |
| Carcinoma, undifferentiated, NOS | |
| Neuroendocrine tumors * | |
| Small/large cell neuroendocrine carcinoma | |
| Combined small/large cell neuroendocrine carcinoma |
| Author/Year | Cases | SMGs |
|---|---|---|
| Ojesina 2014 [61] | n = 115 (WES) HPV-positive (96%) SqCC (n = 79), ADC (n = 24), ADSC (n = 7), Others (N = 5) | SqCC: PIK3CA (14%), EP300 (16%), FBXW7 (15%), PTEN (6%), STK11 (4%), HLA-B (9%), MAPK1 (8%), NFE2L2 (4%), TP53 (9%), ERBB2 (5%), ADC: ELF3 (13%), CBFB (8%), PIK3CA (16%), KRAS (8%) |
| TCGA 2017 [22] | n = 178 (core set, WES) HPV-positive (95%) SqCC (n = 144), ADC (n = 31), ADSC (n = 3) | SHKBP1 (2%), ERBB3 (6%), CASP8 (4%), HLA-A (8%), TGFBR2 (3%), PIK3CA (26%), EP300 (11%), FBXW7 (11%), HLA-B (6%), PTEN (8%), NFE2L2 (7%), ARID1A (7%), KRAS (6%), MAPK1 (5%) |
| Huang 2019 [63] | n = 102 (WES) HPV-positive (93%) SqCC (n = 93), ADC (n = 6), ADSC (n = 3) | PIK3CA (16.7%), FBXW7 (12.8%), MLL3 (7.8%), CASP8 (3.9%), FADD (3.9%) |
| Zammataro 2019 [65] | n = 69 (WES) HPV-positive (100%) SqCC (n = 44), ADC (n = 17), ADSC (n = 6) | PIK3CA (27.5%), STK11 (8.7%), HUWE1 (15.9%), FAT1 (15.9%), NIPBL (11.6%), EPPK1 (10%), BCORL1 (8.7%), FBXW7, SMARCA4, NUP98, KRAS (all 7.2%), SMAD4 (5.8%), MAPK1 (4.3%) |
| Gagliardi 2020 [66] | n = 118 (WGS) HPV-positive (100%), SqCC (n = 97), ADC (n = 8), ADSC (n = 10), Others (n = 3) | PIK3CA (35%), FAT1 (19%), KMT2D (14%), FBXW7 (10%), CASP8 (7%), SLC35G5 (7%), PCDHGA12 (6%), MAPK1 (5%), PSPC1 (5%), ZNF750 (4%), PCDHA9 (3%), ZC3H6 (3%) |
| Histological Type | N | Method | SNVindel | Structural Variants | Ref |
|---|---|---|---|---|---|
| HPV-negative squamous cell carcinoma | 4 | Whole exome sequencing (WES) | CHD8 (3/4), PIK3CA (2/4), LRP2 (2/4), COL7A1 (2/4), MTFR (2/4), PTEN (2/4) | No recurrent CNV | [22] |
| Gastric-type adenocarcinoma | 68 | MSK-IMPACT 410–468 genes | TP53 (28/68), CDKN2A (12/68), KRAS (12/68), STK11 (7/68), ERBB3 (7/68), GNAS (6/68), ERBB2 (6/68), SMAD4 (6/68), PIK3CA (5/68), ARID1A (4/68) | No recurrent CNV | [74] |
| 21 | Targeted sequencing 96 genes | TP53 (11/21), STK11 (4/21), HLA-B (4/21), PTPRS (4/21), FGFR4 (3/21), GNAS (2/21), BRCA2 (2/21), ELF3 (2/21), ERBB3 (2/21), KMT2D (2/21), SLX4 (2/21) | - | [75] | |
| 15 | YuanSu 450 panel 450 genes/39 fusions | TP53 (8/15), STK11 (5/15), CDKN2A (4/15), ARID1A (3/15), PTEN (3/15) | ERBB2, CDK12, MECOM, PRKC1 (a// 2/15) | [76] | |
| 14 | The Oncomine assay v3 161 genes | TP53 (7/14) MSH6 (6/14), CDKN2A/B (5/14), POLE (5/14), SLX4 (5/14), ARID1A (4/14), STK11 (4/14), BRCA2 (3/14), MSH2 (3/14) | MDM2 (2/14) | [77] | |
| 11 | Targeted sequencing 447 genes/60 fusions | KRAS (4/11), TP53 (5/11), and PIK3CA (2/11), STK11 (3/11), CDKN2A (3/11), ATM (2/11), NTRK3 (2/11) | No recurrent CNV | [78] | |
| 3 | Targeted sequencing 48 genes | p53 (2/3), CDKN2A (2/3), KRAS (1/3), AKT1 (1/3), STK11 (1/3) | - | [44] | |
| Clear cell carcinoma | 3 | Targeted sequencing 48 genes | TP53 (1/3), PIK3CA (1/3) | - | [44] |
| 1 | Targeted sequencing 447 genes/60 fusions | POLE P286R and 201 additional mutations, including in PIK3CA, ARID1A, and PTEN. | Not detected | [79] | |
| Mesonephric carcinoma | 13 | Targeted sequencing 413 genes/35 fusions | KRAS (9/13), ARID1A/B (7/13), BCOR/BCOL1 (5/13), SMARCA4 (2/13) | Gain of 1q (9/13) | [80] |
| 4 | OncomineComprehensive assay v1 143 genes/22 fusions | KRAS (4/4), PIK3CA (1/4) | Gain of 1q (4/4) | [54] | |
| 1 | FoundationOne CDx | KRAS, CTNNB1 | Gain of 1q | [81] | |
| Endometrioid carcinoma | 8 | Targeted sequencing 48 genes | PIK3CA (4/8), PTEN (4/8), CTNNB1 (3/8), FBXW7 (2/8), KRAS (1/8), AKT1 (1/8), TP53(1/8), MSI-H (1/8) | - | [44] |
| Serous carcinomoa | 6 | Targeted sequencing 48 genes | TP53 (4/6), KRAS (2/6), PIK3CA (1/6), PTEN (1/6) | - | [44] |
| Adenosquamous carcinoma | 3 | Targeted sequencing 50 genes | Not detected | - | [49] |
| 1 | WES | Not detected | Not detected | [22] | |
| Neuroendocrine carcinoma | 14 | Foundation Medicine 315 genes/19 fusions | PTEN (7/14), TP53 (6/14), PIK3CA (5/14), ARID1A (5/14), RB1 (5/14) | MYC (1/14) | [48] |
| Mixed mesonephric adenocarcinoma and neuroendocrine carcinoma | 1 | MSK-IMPACT 410–468 genes | U2AF1, GATA3, TP53, MST1R | MYCN, ISR2 | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, H.; Shiraishi, K.; Kato, T. Molecular Pathology of Human Papilloma Virus-Negative Cervical Cancers. Cancers 2021, 13, 6351. https://doi.org/10.3390/cancers13246351
Yoshida H, Shiraishi K, Kato T. Molecular Pathology of Human Papilloma Virus-Negative Cervical Cancers. Cancers. 2021; 13(24):6351. https://doi.org/10.3390/cancers13246351
Chicago/Turabian StyleYoshida, Hiroshi, Kouya Shiraishi, and Tomoyasu Kato. 2021. "Molecular Pathology of Human Papilloma Virus-Negative Cervical Cancers" Cancers 13, no. 24: 6351. https://doi.org/10.3390/cancers13246351
APA StyleYoshida, H., Shiraishi, K., & Kato, T. (2021). Molecular Pathology of Human Papilloma Virus-Negative Cervical Cancers. Cancers, 13(24), 6351. https://doi.org/10.3390/cancers13246351






