Molecular and Metabolic Mechanisms Underlying Selective 5-Aminolevulinic Acid-Induced Fluorescence in Gliomas
Abstract
Simple Summary
Abstract
1. Introduction
2. 5-ALA Development and History
3. Mechanism of 5-ALA Fluorescence
4. Metabolic Activity
4.1. Membrane Transport
4.2. Blood–Brain Barrier
4.3. Tumor Microenvironmental Factors
5. Photodynamic Therapy
5.1. Cytotoxic Mechanisms
5.2. 5-ALA PDT Immune Effects
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| LGG | low-grade glioma |
| HGG | high-grade glioma |
| GBM | glioblastoma multiforme |
| EOR | extent of resection |
| 5-ALA | 5-aminolevulinic acid |
| 2-HG | R-2-hydroxyglutarate |
| α-KG | α-ketoglutarate |
| PpIX | protoporphyrin IX |
| BBB | blood–brain barrier |
| FECH | ferrochelatase |
| PBGD | porphobilinogen-deaminase |
| CPOX | coproporphyrinogen oxidase |
| PBGS | Porphobilinogen synthase |
| EGFR | epidermal growth factor receptor |
| PDT | photodynamic therapy |
| PEPT-2 | proton-dependent peptide transporter 2 |
| ROS | reactive oxygen species |
| ABCG2 | ATP-binding cassette transporter G2 |
| ABCB6 | ATP-binding cassette transporter B6 |
| TNFα | tumor necrosis factor alpha |
| IFNγ | interferon gamma |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| MRI | magnetic resonance imaging |
| TCA | tricarboxylic acid |
| CNS | Central nervous system |
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- McGirt, M.J.; Chaichana, K.L.; Gathinji, M.; Attenello, F.J.; Than, K.; Olivi, A.; Weingart, J.D.; Brem, H.; Quiñones-Hinojosa, A. Independent association of extent of resection with survival in patients with malignant brain astrocytoma: Clinical article. J. Neurosurg. 2009, 110, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas: Clinical article. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Stummer, W.; Reulen, H.J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 2008, 62, 564–574. [Google Scholar] [CrossRef]
- Stummer, W.; Van Den Bent, M.J.; Westphal, M. Cytoreductive surgery of glioblastoma as the key to successful adjuvant therapies: New arguments in an old discussion. Acta Neurochir. (Wien.) 2011, 153, 1211–1218. [Google Scholar] [CrossRef]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non-Contrast-Enhanced Tumor with Survival Within Molecular Subgroups of Patients with Newly Diagnosed Glioblastoma. JAMA Oncol. 2020, 6, 495–503. [Google Scholar] [CrossRef]
- Xia, L.; Fang, C.; Chen, G.; Sun, C. Relationship between the extent of resection and the survival of patients with low-grade gliomas: A systematic review and meta-analysis. BMC Cancer 2018, 18, 48. [Google Scholar] [CrossRef]
- Shaw, E.; Arusell, R.; Scheithauer, B.; O’Fallon, J.; O’Neill, B.; Dinapoli, R.; Nelson, D.; Earle, J.; Jones, C.; Cascino, T.; et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: Initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group Study. J. Clin. Oncol. 2002, 20, 2267–2276. [Google Scholar] [CrossRef]
- Claes, A.; Idema, A.J.; Wesseling, P. Diffuse glioma growth: A guerilla war. Acta Neuropathol. 2007, 114, 443–458. [Google Scholar] [CrossRef]
- Jaber, M.; Ewelt, C.; Wölfer, J.; Brokinkel, B.; Thomas, C.; Hasselblatt, M.; Grauer, O.; Stummer, W. Is Visible Aminolevulinic Acid-Induced Fluorescence an Independent Biomarker for Prognosis in Histologically Confirmed (World Health Organization 2016) Low-Grade Gliomas? Neurosurgery 2018, 84, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Tonn, J.C.; Goetz, C.; Ullrich, W.; Stepp, H.; Bink, A.; Pietsch, T.; Pichlmeier, U. 5-Aminolevulinic acid-derived tumor fluorescence: The diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery 2014, 74, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Kiesel, B.; Millesi, M.; Woehrer, A.; Furtner, J.; Bavand, A.; Roetzer, T.; Mischkulnig, M.; Wolfsberger, S.; Preusser, M.; Knosp, E.; et al. 5-ALA-induced fluorescence as a marker for diagnostic tissue in stereotactic biopsies of intracranial lymphomas: Experience in 41 patients. Neurosurg. Focus 2018, 44. [Google Scholar] [CrossRef] [PubMed]
- Kamp, M.A.; Fischer, I.; Bühner, J.; Turowski, B.; Cornelius, J.F.; Steiger, H.J.; Rapp, M.; Slotty, P.J.; Sabel, M. 5-ALA fluorescence of cerebral metastases and its impact for the local-in-brain progression. Oncotarget 2016, 7, 66776–66789. [Google Scholar] [CrossRef]
- Kitada, M.; Ohsaki, Y.; Yasuda, S.; Abe, M.; Takahashi, N.; Okazaki, S.; Ishibashi, K.; Hayashi, S. Photodynamic diagnosis of visceral pleural invasion of lung cancer with a combination of 5-aminolevulinic acid and autofluorescence observation systems. Photodiagnosis Photodyn. Ther. 2017, 20, 10–15. [Google Scholar] [CrossRef]
- Ishizuka, M.; Abe, F.; Sano, Y.; Takahashi, K.; Inoue, K.; Nakajima, M.; Kohda, T.; Komatsu, N.; Ogura, S.I.; Tanaka, T. Novel development of 5-aminolevurinic acid (ALA) in cancer diagnoses and therapy. Int. Immunopharmacol. 2011, 11, 358–365. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339. [Google Scholar] [CrossRef]
- Markwardt, N.A.; Haj-Hosseini, N.; Hollnburger, B.; Stepp, H.; Zelenkov, P.; Rühm, A. 405 nm versus 633 nm for protoporphyrin IX excitation in fluorescence-guided stereotactic biopsy of brain tumors. J. Biophotonics 2016, 9, 901–912. [Google Scholar] [CrossRef]
- Kriegmair, M.; Baumgartner, R.; Knüchel, R.; Stepp, H.; Hofstädter, F.; Hofstetter, A. Detection of early bladder cancer by 5-aminolevulinic acid induced porphyrin fluorescence. J. Urol. 1996, 155, 105–110. [Google Scholar] [CrossRef]
- Fritsch, C.; Becker-Wegerich, P.M.; Schulte, K.W.; Neuse, W.; Lehmann, P.; Ruzicka, T.; Goerz, G. Photodynamische therapie und mamillenplastik eines grossflachigen rumpfhautbasalioms der mama. effektive kombinationstherapie unter photodynamischer diagnostik. Hautarzt 1996, 47, 438–442. [Google Scholar] [CrossRef]
- Regula, J.; MacRobert, A.J.; Gorchein, A.; Buonaccorsi, G.A.; Thorpe, S.M.; Spencer, G.M.; Hatfield, A.R.W.; Bown, S.G. Photosensitisation and photodynamic therapy of oesophageal, duodenal, and colorectal tumours using 5 aminolaevulinic acid induced protoporphyrin IX–a pilot study. Gut 1995, 36, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Liu, W.; Cheng, X.; Wang, J.; Wang, Q.; Qi, Q. A new strategy for production of 5-aminolevulinic acid in recombinant Corynebacterium glutamicum with high yield. Appl. Environ. Microbiol. 2016, 82, 2709–2717. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Stocker, S.; Wagner, S.; Stepp, H.; Fritsch, C.; Goetz, C.; Goetz, A.E.; Kiefmann, R.; Reulen, H.J. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 1998, 42, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Tonn, J.C.; Mehdorn, H.M.; Nestler, U.; Franz, K.; Goetz, C.; Bink, A.; Pichlmeier, U. Counterbalancing risks and gains from extended resections in malignant glioma surgery: A supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study: Clinical article. J. Neurosurg. 2011, 114, 613–623. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Stummer, W. 5-ALA and FDA approval for glioma surgery. J. Neurooncol. 2019, 141, 479–486. [Google Scholar] [CrossRef]
- Nabavi, A.; Thurm, H.; Zountsas, B.; Pietsch, T.; Lanfermann, H.; Pichlmeier, U.; Mehdorn, M. Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: A phase II study. Neurosurgery 2009, 65, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Ren, M.; Wick, W.; Abrey, L.; Das, A.; Jin, J.; Reardon, D.A. Progression-free survival as a surrogate endpoint for overall survival in glioblastoma: A literature-based meta-analysis from 91 trials. Neuro. Oncol. 2014, 16, 696–706. [Google Scholar] [CrossRef]
- Drug Approval Package: Gleolan (Aminolevulinic Acid Hydrochloride). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208630Orig1s000TOC.cfm (accessed on 10 November 2020).
- Grant, W.E.; MacRobert, A.; Bown, S.G.; Hopper, C.; Speight, P.M. Photodynamic therapy of oral cancer: Photosensitisation with systemic aminolaevulinic acid. Lancet 1993, 342, 147–148. [Google Scholar] [CrossRef]
- Berlin, N.I.; Neuberger, A.; Scott, J.J. The metabolism of δ-aminolaevulic acid. 1. Normal pathways, studied with the aid of 15N. Biochem. J. 1956, 64, 80–90. [Google Scholar] [CrossRef]
- Kennedy, J.C.; Pottier, R.H. New trends in photobiology. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J. Photochem. Photobiol. B Biol. 1992, 14, 275–292. [Google Scholar] [CrossRef]
- Stepp, H.; Stummer, W. Delineating normal from diseased brain by aminolevulinic acid-induced fluorescence. In Optical Methods and Instrumentation in Brain Imaging and Therapy; Springer: New York, NY, USA, 2013; pp. 173–205. ISBN 9781461449782. [Google Scholar]
- Fratz, E.J.; Hunter, G.A.; Ferreira, G.C. Expression of murine 5-aminolevulinate synthase variants causes protoporphyrin IX accumulation and light-induced mammalian cell death. PLoS ONE 2014, 9, e93078. [Google Scholar] [CrossRef] [PubMed]
- Krieg, R.C.; Messmann, H.; Rauch, J.; Seeger, S.; Knuechel, R. Metabolic Characterization of Tumor Cell–specific Protoporphyrin IX Accumulation After Exposure to 5-Aminolevulinic Acid in Human Colonic Cells. Photochem. Photobiol. 2002, 76, 518–525. [Google Scholar] [CrossRef]
- Krieg, R.C.; Fickweiler, S.; Wolfbeis, O.S.; Knuechel, R. Cell-type Specific Protoporphyrin IX Metabolism in Human Bladder Cancer in vitro. Photochem. Photobiol. 2000, 72, 226–233. [Google Scholar] [CrossRef]
- Fukuhara, H.; Inoue, K.; Kurabayashi, A.; Furihata, M.; Fujita, H.; Utsumi, K.; Sasaki, J.; Shuin, T. The inhibition of ferrochelatase enhances 5-aminolevulinic acid-based photodynamic action for prostate cancer. Photodiagnosis Photodyn. Ther. 2013, 10, 399–409. [Google Scholar] [CrossRef]
- Navone, N.M.; Polo, C.F.; Frisardi, A.L.; Andrade, N.E.; Alcira, A.M. Heme biosynthesis in human breast cancer-mimetic “in vitro” studies and some heme enzymic activity levels. Int. J. Biochem. 1990, 22, 1407–1411. [Google Scholar] [CrossRef]
- Hinnen, P.; De Rooij, F.W.M.; Van Velthuysen, M.L.F.; Edixhoven, A.; Van Hillegersberg, R.; Tilanus, H.W.; Wilson, J.H.P.; Siersema, P.D. Biochemical basis of 5-aminolaevulinic acid-induced protoporphyrin IX accumulation: A study in patients with (pre)malignant lesions of the oesophagus. Br. J. Cancer 1998, 78, 679–682. [Google Scholar] [CrossRef]
- Schauder, A.; Feuerstein, T.; Malik, Z. The centrality of PBGD expression levels on ALA-PDT efficacy. Photochem. Photobiol. Sci. 2011, 10, 1310–1317. [Google Scholar] [CrossRef]
- Auer, R.N.; Maestro, R.F.D.; Anderson, R. A Simple and Reproducible Experimental in Vivo Glioma Model. Can. J. Neurol. Sci./J. Can. Sci. Neurol. 1981, 8, 325–331. [Google Scholar] [CrossRef]
- Greenbaum, L.; Gozlan, Y.; Schwartz, D.; Katcoff, D.J.; Malik, Z. Nuclear distribution of porphobilinogen deaminase (PBGD) in glioma cells: A regulatory role in cancer transformation? Br. J. Cancer 2002, 86, 1006–1011. [Google Scholar] [CrossRef]
- Greenbaum, L.; Katcoff, D.J.; Dou, H.; Gozlan, Y.; Malik, Z. A porphobilinogen deaminase (PBGD) Ran-binding protein interaction is implicated in nuclear trafficking of PBGD in differentiating glioma cells. Oncogene 2003, 22, 5221–5228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pustogarov, N.; Panteleev, D.; Goryaynov, S.A.; Ryabova, A.V.; Rybalkina, E.Y.; Revishchin, A.; Potapov, A.A.; Pavlova, G. Hiding in the Shadows: CPOX Expression and 5-ALA Induced Fluorescence in Human Glioma Cells. Mol. Neurobiol. 2017, 54, 5699–5708. [Google Scholar] [CrossRef]
- Stepp, H.; Stummer, W. 5-ALA in the management of malignant glioma. Lasers Surg. Med. 2018, 50, 399–419. [Google Scholar] [CrossRef] [PubMed]
- Briel-Pump, A.; Beez, T.; Ebbert, L.; Remke, M.; Weinhold, S.; Sabel, M.C.; Sorg, R.V. Accumulation of protoporphyrin IX in medulloblastoma cell lines and sensitivity to subsequent photodynamic treatment. J. Photochem. Photobiol. B Biol. 2018, 189, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Anholt, H.; Bech, O.; Moan, J. The influence of iron chelators on the accumulation of protoporphyrin IX in 5-aminolaevulinic acid-treated cells. Br. J. Cancer 1996, 74, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tabu, K.; Hagiya, Y.; Sugiyama, Y.; Kokubu, Y.; Murota, Y.; Ogura, S.I.; Taga, T. Enhancement of 5-aminolevulinic acid-based fluorescence detection of side population-defined glioma stem cells by iron chelation. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Blake, E.; Allen, J.; Curnow, A. An in vitro comparison of the effects of the iron-chelating agents, CP94 and dexrazoxane, on protoporphyrin IX accumulation for photodynamic therapy and/or fluorescence guided resection. Photochem. Photobiol. 2011, 87, 1419–1426. [Google Scholar] [CrossRef]
- Blake, E.; Curnow, A. The hydroxypyridinone iron chelator CP94 can enhance PpIX-induced PDT of cultured human glioma cells. Photochem. Photobiol. 2010, 86, 1154–1160. [Google Scholar] [CrossRef]
- Sinha, A.K.; Anand, S.; Ortel, B.J.; Chang, Y.; Mai, Z.; Hasan, T.; Maytin, E.V. Methotrexate used in combination with aminolaevulinic acid for photodynamic killing of prostate cancer cells. Br. J. Cancer 2006, 95, 485–495. [Google Scholar] [CrossRef]
- Park, N.I.; Guilhamon, P.; Desai, K.; McAdam, R.F.; Langille, E.; O’Connor, M.; Lan, X.; Whetstone, H.; Coutinho, F.J.; Vanner, R.J.; et al. ASCL1 Reorganizes Chromatin to Direct Neuronal Fate and Suppress Tumorigenicity of Glioblastoma Stem Cells. Cell Stem Cell 2017, 21, 209–224.e7. [Google Scholar] [CrossRef]
- Kim, J.E.; Cho, H.R.; Xu, W.J.; Kim, J.Y.; Kim, S.K.; Kim, S.K.; Park, S.H.; Kim, H.; Lee, S.H.; Choi, S.H.; et al. Mechanism for enhanced 5-aminolevulinic acid fluorescence in isocitrate dehydrogenase 1 mutant malignant gliomas. Oncotarget 2015, 6, 20266–20277. [Google Scholar] [CrossRef] [PubMed]
- Badur, M.G.; Muthusamy, T.; Parker, S.J.; Ma, S.; McBrayer, S.K.; Cordes, T.; Magana, J.H.; Guan, K.L.; Metallo, C.M. Oncogenic R132 IDH1 Mutations Limit NADPH for De Novo Lipogenesis through (D)2-Hydroxyglutarate Production in Fibrosarcoma Cells. Cell Rep. 2018, 25, 1018–1026.e4. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.E.; Kim, Y.H.; Hwang, T.; Kim, S.K.; Xu, W.J.; Shin, J.Y.; Kim, J.I.; Choi, H.; Kim, H.C.; et al. Glutaminase 2 expression is associated with regional heterogeneity of 5-aminolevulinic acid fluorescence in glioblastoma. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Yang, X.; Li, W.; Palasuberniam, P.; Myers, K.A.; Wang, C.; Chen, B. Effects of Silencing Heme Biosynthesis Enzymes on 5-Aminolevulinic Acid-mediated Protoporphyrin IX Fluorescence and Photodynamic Therapy. Photochem. Photobiol. 2015, 91, 923–930. [Google Scholar] [CrossRef]
- Ogino, T.; Kobuchi, H.; Munetomo, K.; Fujita, H.; Yamamoto, M.; Utsumi, T.; Inoue, K.; Shuin, T.; Sasaki, J.; Inoue, M.; et al. Serum-dependent export of protoporphyrin IX by ATP-binding cassette transporter G2 in T24 cells. Mol. Cell. Biochem. 2011, 358, 297–307. [Google Scholar] [CrossRef]
- Mesenhöller, M.; Matthews, E.K. A key role for the mitochondrial benzodiazepine receptor in cellular photosensitisation with δ-aminolaevulinic acid. Eur. J. Pharmacol. 2000, 406, 171–180. [Google Scholar] [CrossRef]
- Bisland, S.K.; Goebel, E.A.; Hassanali, N.S.; Johnson, C.; Wilson, B.C. Increased expression of mitochondrial benzodiazepine receptors following low-level light treatment facilitates enhanced protoporphyrin IX production in glioma-derived cells in vitro. Lasers Surg. Med. 2007, 39, 678–684. [Google Scholar] [CrossRef]
- Oberstadt, M.C.; Bien-Möller, S.; Weitmann, K.; Herzog, S.; Hentschel, K.; Rimmbach, C.; Vogelgesang, S.; Balz, E.; Fink, M.; Michael, H.; et al. Epigenetic modulation of the drug resistance genes MGMT, ABCB1 and ABCG2 in glioblastoma multiforme. BMC Cancer 2013, 13. [Google Scholar] [CrossRef]
- Kawai, N.; Hirohashi, Y.; Ebihara, Y.; Saito, T.; Murai, A.; Saito, T.; Shirosaki, T.; Kubo, T.; Nakatsugawa, M.; Kanaseki, T.; et al. ABCG2 expression is related to low 5-ALA photodynamic diagnosis (PDD) efficacy and cancer stem cell phenotype, and suppression of ABCG2 improves the efficacy of PDD. PLoS ONE 2019, 14, e216503. [Google Scholar] [CrossRef]
- Zhao, S.G.; Chen, X.F.; Wang, L.G.; Yang, G.; Han, D.Y.; Teng, L.; Yang, M.C.; Wang, D.Y.; Shi, C.; Liu, Y.H.; et al. Increased expression of ABCB6 enhances protoporphyrin ix accumulation and photodynamic effect in human glioma. Ann. Surg. Oncol. 2013, 20, 4379–4388. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.O.; Piffaretti, D.; Marchi, F.; Burgio, F.; Faia-Torres, A.B.; Paganetti, P.; Pinton, S.; Pieles, U.; Reinert, M. Epithelial growth factor receptor expression influences 5-ALA induced glioblastoma fluorescence. J. Neurooncol. 2017, 133, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Wyld, L.; Burn, J.L.; Reed, M.W.R.; Brown, N.J. Factors affecting aminolaevulinic acid-induced generation of protoporphyrin IX. Br. J. Cancer 1997, 76, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Collaud, S.; Juzeniene, A.; Moan, J.; Lange, N. On the selectivity of 5-aminolevulinic acid-induced protoporphyrin IX formation. Curr. Med. Chem. Anti-Cancer Agents 2004, 4, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Novotny, A.; Xiang, J.; Stummer, W.; Teuscher, N.S.; Smith, D.E.; Keep, R.F. Mechanisms of 5-aminolevulinic acid uptake at the choroid plexus. J. Neurochem. 2000, 75, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Utsuki, S.; Oka, H.; Sato, S.; Suzuki, S.; Shimizu, S.; Tanaka, S.; Fujii, K. Possibility of using laser spectroscopy for the intraoperative detection of nonfluorescing brain tumors and the boundaries of brain tumor infiltrates: Technical note. J. Neurosurg. 2006, 104, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Ennis, S.R.; Novotny, A.; Xiang, J.; Shakui, P.; Masada, T.; Stummer, W.; Smith, D.E.; Keep, R.F. Transport of 5-aminolevulinic acid between blood and brain. Brain Res. 2003, 959, 226–234. [Google Scholar] [CrossRef]
- García, S.C.; Moretti, M.B.; Garay, M.V.R.; Batlle, A. δ-Aminolevulinic acid transport through blood-brain barrier. Gen. Pharmacol. 1998, 31, 579–582. [Google Scholar] [CrossRef]
- Malakoutikhah, M.; Pradesh, R.; Teixidó, M.; Giralt, E. N-Methyl Phenylalanine-Rich peptides as highly versatile blood-brain barrier shuttles. J. Med. Chem. 2010, 53, 2354–2363. [Google Scholar] [CrossRef]
- Terr, L.; Weiner, L.P. An autoradiographic study of δ-aminolevulinic acid uptake by mouse brain. Exp. Neurol. 1983, 79, 564–568. [Google Scholar] [CrossRef]
- Olivo, M.; Wilson, C.B. Mapping ALA-induced PPIX fluorescence in normal brain and brain tumour using confocal fluorescence microscopy. Int. J. Oncol. 2004, 25, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.J.S.; Ardon, H.; Schroeteler, J.; Klingenhöfer, M.; Holling, M.; Wölfer, J.; Fischer, B.; Stummer, W.; Ewelt, C. Aquaporin-4 in glioma and metastatic tissues harboring 5-aminolevulinic acid-induced porphyrin fluorescence. Clin. Neurol. Neurosurg. 2013, 115, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Paredes, S.; Batlle, A.M. Tumour-localizing properties of porphyrins. In vivo studies using free and liposome encapsulated aminolevulinic acid. Comp. Biochem. Physiol. Part. B Biochem. 1992, 102, 433–436. [Google Scholar] [CrossRef]
- Teng, L.; Nakada, M.; Zhao, S.G.; Endo, Y.; Furuyama, N.; Nambu, E.; Pyko, I.V.; Hayashi, Y.; Hamada, J.I. Silencing of ferrochelatase enhances 5-aminolevulinic acid-based fluorescence and photodynamic therapy efficacy. Br. J. Cancer 2011, 104, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Van Hillegersberg, R.; Van Den Berg, J.W.O.; Kort, W.J.; Terpstra, O.T.; Wilson, J.H.P. Selective accumulation of endogenously produced porphyrins in a liver metastasis model in rats. Gastroenterology 1992, 103, 647–651. [Google Scholar] [CrossRef]
- Hirschberg, H.; Sun, C.H.; Tromberg, B.J.; Yeh, A.T.; Madsen, S.J. Enhanced cytotoxic effects of 5-aminolevulinic acid-mediated photodynamic therapy by concurrent hyperthermia in glioma spheroids. J. Neurooncol. 2004, 70, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.J.; Niu, C.; Foltz, W.; Chen, Y.; Sidorova-Darmos, E.; Eubanks, J.H.; Lilge, L. ALA-PpIX mediated photodynamic therapy of malignant gliomas augmented by hypothermia. PLoS ONE 2017, 12, e181654. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Sommer, S. Oxygen Dependence of the Photosensitizing Effect of Hematoporphyrin Derivative in NHIK 3025 Cells. Cancer Res. 1985, 45, 1608–1610. [Google Scholar]
- Albert, I.; Hefti, M.; Luginbuehl, V. Physiological oxygen concentration alters glioma cell malignancy and responsiveness to photodynamic therapy in vitro. Neurol. Res. 2014, 36, 1001–1010. [Google Scholar] [CrossRef]
- Arbizu, J.; Tejada, S.; Marti-Climent, J.M.; Diez-Valle, R.; Prieto, E.; Quincoces, G.; Vigil, C.; Idoate, M.A.; Zubieta, J.L.; Peñuelas, I.; et al. Quantitative volumetric analysis of gliomas with sequential MRI and 11C-methionine PET assessment: Patterns of integration in therapy planning. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 771–781. [Google Scholar] [CrossRef]
- Roessler, K.; Becherer, A.; Donat, M.; Cejna, M.; Zachenhofer, I. Intraoperative tissue fluorescence using 5- aminolevolinic acid (5-ALA) is more sensitive than contrast MRI or amino acid positron emission tomography (18F-FET PET) in glioblastoma surgery. Neurol. Res. 2012, 34, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.A.; Keep, R.F.; Smith, D.E. Role and relevance of PEPT2 in drug disposition, dynamics, and toxicity. Drug Metab. Pharmacokinet. 2008, 23, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.; Batlle, A.; Di Venosa, G.; MacRobert, A.J.; Battah, S.; Daniel, H.; Casas, A. Study of the mechanisms of uptake of 5-aminolevulinic acid derivatives by PEPT1 and PEPT2 transporters as a tool to improve photodynamic therapy of tumours. Int. J. Biochem. Cell Biol. 2006, 38, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Stan, A.C. PepT2 transporter protein expression in human neoplastic glial cells and mediation of fluorescently tagged dipeptide derivative β-Ala-Lys-N ε-7-amino-4-methyl-coumarin-3-acetic acid accumulation: Laboratory investigation. J. Neurosurg. 2010, 112, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Yamaguchi, S.; Ishi, Y.; Terasaka, S.; Kobayashi, H.; Motegi, H.; Hatanaka, K.C.; Houkin, K. Identification of PEPT2 as an important candidate molecule in 5-ALA-mediated fluorescence-guided surgery in WHO grade II/III gliomas. J. Neurooncol. 2019, 143, 197–206. [Google Scholar] [CrossRef]
- Novotny, A.; Stummer, W. 5-Aminolevulinic acid and the blood-brain barrier–A review. Med. Laser Appl. 2003, 18, 36–40. [Google Scholar] [CrossRef]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro. Oncol. 2018, 20, 184–191. [Google Scholar] [CrossRef]
- Agarwal, S.; Sane, R.; Oberoi, R.; Ohlfest, J.R.; Elmquist, W.F. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev. Mol. Med. 2011, 13, e17. [Google Scholar] [CrossRef]
- Plate, K.H.; Scholz, A.; Dumont, D.J. Tumor angiogenesis and anti-angiogenic therapy in malignant gliomas revisited. Acta Neuropathol. 2012, 124, 763–775. [Google Scholar] [CrossRef]
- Provenzale, J.M.; Wang, G.R.; Brenner, T.; Petrella, J.R.; Sorensen, A.G. Comparison of permeability in high-grade and low-grade brain tumors using dynamic susceptibility contrast MR imaging. Am. J. Roentgenol. 2002, 178, 711–716. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Kang, H.; Zhang, Y.; Liang, H.; Wang, S.; Zhang, W. Glioma grading by microvascular permeability parameters derived from dynamic contrast-enhanced MRI and intratumoral susceptibility signal on susceptibility weighted imaging. Cancer Imaging 2015, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Goryaynov, S.A.; Widhalm, G.; Goldberg, M.F.; Chelushkin, D.; Spallone, A.; Chernyshov, K.A.; Ryzhova, M.; Pavlova, G.; Revischin, A.; Shishkina, L.; et al. The role of 5-ALA in low-grade gliomas and the influence of antiepileptic drugs on intraoperative fluorescence. Front. Oncol. 2019, 9, 423. [Google Scholar] [CrossRef]
- Valdés, P.A.; Jacobs, V.; Harris, B.T.; Wilson, B.C.; Leblond, F.; Paulsen, K.D.; Roberts, D.W. Quantitative fluorescence using 5-aminolevulinic acid-induced protoporphyrin IX biomarker as a surgical adjunct in low-grade glioma surgery. J. Neurosurg. 2015, 123, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Widhalm, G.; Kiesel, B.; Woehrer, A.; Traub-Weidinger, T.; Preusser, M.; Marosi, C.; Prayer, D.; Hainfellner, J.A.; Knosp, E.; Wolfsberger, S. 5-Aminolevulinic Acid Induced Fluorescence Is a Powerful Intraoperative Marker for Precise Histopathological Grading of Gliomas with Non-Significant Contrast-Enhancement. PLoS ONE 2013, 8, e76988. [Google Scholar] [CrossRef] [PubMed]
- Widhalm, G.; Olson, J.; Weller, J.; Bravo, J.; Han, S.J.; Phillips, J.; Hervey-Jumper, S.L.; Chang, S.M.; Roberts, D.W.; Berger, M.S. The value of visible 5-ALA fluorescence and quantitative protoporphyrin IX analysis for improved surgery of suspected low-grade gliomas. J. Neurosurg. 2020, 133, 79–88. [Google Scholar] [CrossRef]
- Valdés, P.A.; Kim, A.; Leblond, F.; Conde, O.M.; Harris, B.T.; Paulsen, K.D.; Wilson, B.C.; Roberts, D.W. Combined fluorescence and reflectance spectroscopy for in vivo quantification of cancer biomarkers in low- and high-grade glioma surgery. J. Biomed. Opt. 2011, 16, 116007. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, R.; Katayama, Y.; Watanabe, T.; Yoshino, A.; Fukushima, T.; Sakatani, K. Quantitative spectroscopic analysis of 5-aminolevulinic acid-induced protoporphyrin IX fluorescence intensity in diffusely infiltrating astrocytomas. Neurol. Med. Chir. 2007, 47, 53–57. [Google Scholar] [CrossRef]
- Anderson, P.M.; Desnick, R.J. Purification and properties of delta-aminolevulinate dehydrase from human erythrocytes. J. Biol. Chem. 1979, 254, 6924–6930. [Google Scholar] [CrossRef]
- Stefanadis, C.; Chrysochoou, C.; Markou, D.; Petraki, K.; Panagiotakos, D.B.; Fasoulakis, C.; Kyriakidis, A.; Papadimitriou, C.; Toutouzas, P.K. Increased temperature of malignant urinary bladder tumors in vivo: The application of a new method based on a catheter technique. J. Clin. Oncol. 2001, 19, 676–681. [Google Scholar] [CrossRef]
- Niu, C.J.; Fisher, C.; Scheffler, K.; Wan, R.; Maleki, H.; Liu, H.; Sun, Y.; Simmons, C.A.A.; Birngruber, R.; Lilge, L. Polyacrylamide gel substrates that simulate the mechanical stiffness of normal and malignant neuronal tissues increase protoporphyin IX synthesis in glioma cells. J. Biomed. Opt. 2015, 20, 098002. [Google Scholar] [CrossRef]
- Keyal, U.; Bhatta, A.K.; Wang, X.L. Photodynamic therapy for the treatment of different severity of acne: A systematic review. Photodiagnosis Photodyn. Ther. 2016, 14, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Lipson, R.L.; Baldes, E.J.; Olsen, A.M. The use of a derivative of hematoporphyrin in tumor detection. J. Natl. Cancer Inst. 1961, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yanovsky, R.L.; Bartenstein, D.W.; Rogers, G.S.; Isakoff, S.J.; Chen, S.T. Photodynamic therapy for solid tumors: A review of the literature. Photodermatol. Photoimmunol. Photomed. 2019, 35, 295–303. [Google Scholar] [CrossRef]
- Garretson, C.; Taub, A.F. Photodynamic Therapy and Inflammatory Disorders. In Photodynamic Therapy in Dermatology; Springer: New York, NY, USA, 2011; pp. 105–122. [Google Scholar]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L. Photodynamic combinational therapy in cancer treatment. JBUON 2018, 23, 561–567. [Google Scholar]
- Schipmann, S.; Müther, M.; Stögbauer, L.; Zimmer, S.; Brokinkel, B.; Holling, M.; Grauer, O.; Suero Molina, E.; Warneke, N.; Stummer, W. Combination of ALA-induced fluorescence-guided resection and intraoperative open photodynamic therapy for recurrent glioblastoma: Case series on a promising dual strategy for local tumor control. J. Neurosurg. 2020, 1–11. [Google Scholar] [CrossRef]
- Johansson, A.; Faber, F.; Kniebühler, G.; Stepp, H.; Sroka, R.; Egensperger, R.; Beyer, W.; Kreth, F.W. Protoporphyrin IX fluorescence and photobleaching during interstitial photodynamic therapy of malignant gliomas for early treatment prognosis. Lasers Surg. Med. 2013, 45, 225–234. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Garvey, K.L.; Bouras, A.; Cramer, G.; Stepp, H.; Jesu Raj, J.G.; Bozec, D.; Busch, T.M.; Hadjipanayis, C.G. 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. J. Neurooncol. 2019, 141, 595–607. [Google Scholar]
- Kessel, D. Apoptosis, Paraptosis and Autophagy: Death and Survival Pathways Associated with Photodynamic Therapy. Photochem. Photobiol. 2019, 95, 119–125. [Google Scholar] [CrossRef]
- Karmakar, S.; Banik, N.L.; Patel, S.J.; Ray, S.K. 5-Aminolevulinic acid-based photodynamic therapy suppressed survival factors and activated proteases for apoptosis in human glioblastoma U87MG cells. Neurosci. Lett. 2007, 415, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Kajimoto, Y.; Shibata, M.A.; Miyoshi, N.; Ogawa, N.; Miyatake, S.I.; Otsuki, Y.; Kuroiwa, T. Massive apoptotic cell death of human glioma cells via a mitochondrial pathway following 5-aminolevulinic acid-mediated photodynamic therapy. J. Neurooncol. 2007, 83, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Sun, C.H.; Liaw, L.H.L.; Berns, M.W.; Nelson, J.S. In vitro and in vivo photosensitizing capabilities of 5-ALA versus Photofrin® in vascular endothelial cells. Lasers Surg. Med. 1999, 24, 178–186. [Google Scholar] [CrossRef]
- Ueta, K.; Yamamoto, J.; Tanaka, T.; Nakano, Y.; Kitagawa, T.; Nishizawa, S. 5-Aminolevulinic acid enhances mitochondrial stress upon ionizing irradiation exposure and increases delayed production of reactive oxygen species and cell death in glioma cells. Int. J. Mol. Med. 2017, 39, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Korbelik, M.; Sun, J.; Cecic, I. Photodynamic Therapy–Induced Cell Surface Expression and Release of Heat Shock Proteins: Relevance for Tumor Response. Cancer Res. 2005, 65, 1018–1026. [Google Scholar]
- Li, F.; Cheng, Y.; Lu, J.; Hu, R.; Wan, Q.; Feng, H. Photodynamic therapy boosts anti-glioma immunity in mice: A dependence on the activities of T cells and complement C3. J. Cell. Biochem. 2011, 112, 3035–3043. [Google Scholar] [CrossRef]
- Nakano, Y.; Kitagawa, T.; Osada, Y.; Tanaka, T.; Nishizawa, S.; Yamamoto, J. 5-Aminolevulinic Acid Suppresses Prostaglandin E2 Production by Murine Macrophages and Enhances Macrophage Cytotoxicity Against Glioma. World Neurosurg. 2019, 127, e669–e676. [Google Scholar] [CrossRef]
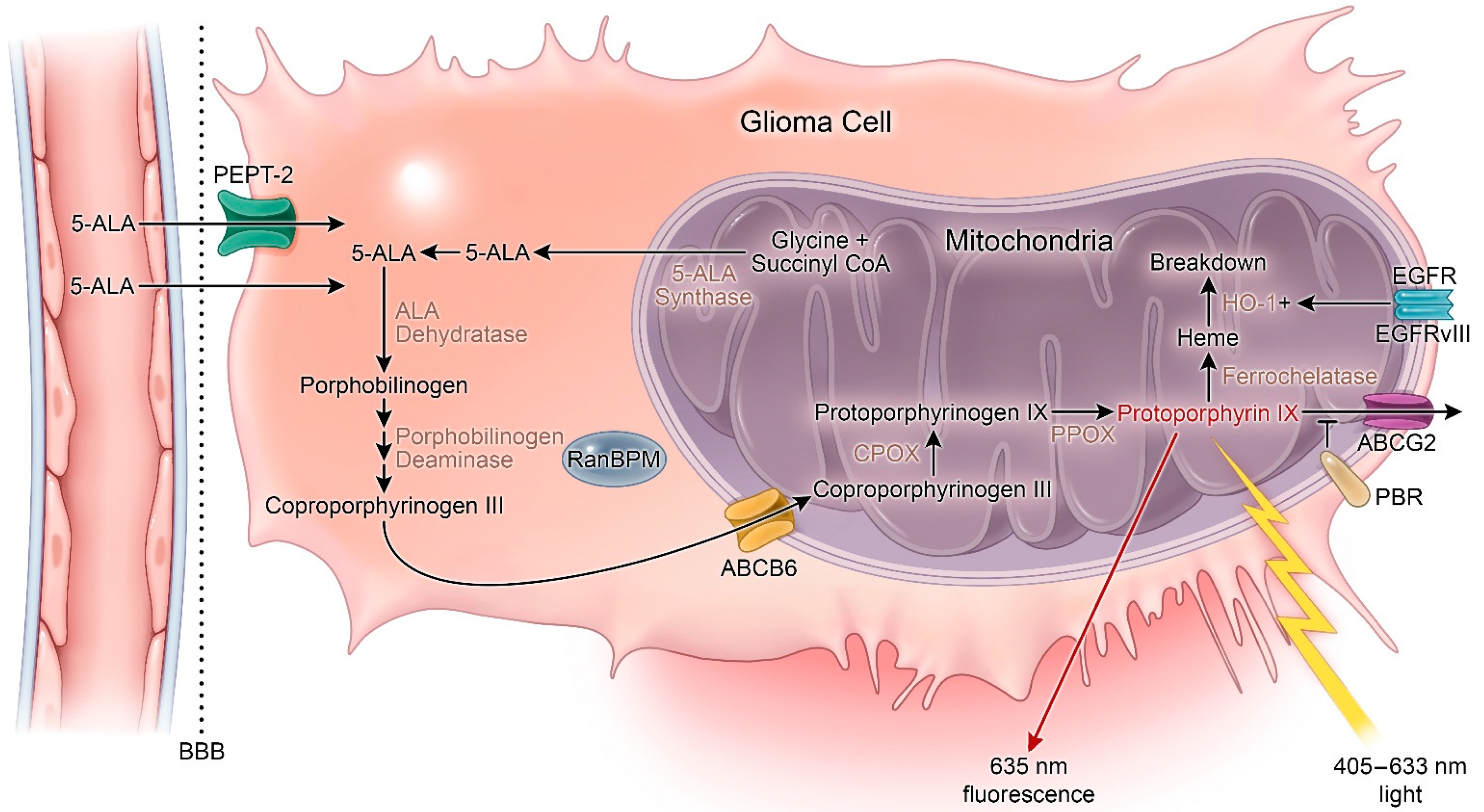
| Potential Target | Supporting Literature | Findings |
|---|---|---|
| Blood–brain barrier | Novotny et al., 2000 [67] | 5-ALA uptake into the choroid plexus occurs via a low pH-dependent uptake by PEPT2, and possibly a separate putative Na and HCO3-dependent mechanism |
| Utsuki et al., 2006 [68] | Small, but detectable accumulations of PpIX were found in cellular boundaries of diffuse astrocytoma treated with 5-ALA using laser spectroscopy intraoperatively | |
| Ennis et al., 2003 [69]; Garcia et al., 1998 [70]; Malakoutikhah et al., 2010 [71]; Terr et al., 1983 [72] | The intact BBB is relatively impermeable to 5-ALA | |
| Olivo and Wilson, 2004 [73] | 5-ALA can weakly penetrate the intact BBB leading to PpIX accumulation, but at markedly lower concentrations than areas without BBB such as circumventricular regions; PpIX accumulation is higher around tumor and inflamed brain tissue | |
| Molina et al., 2013 [74] | Aquaporin-4 expression was higher in fluorescent gliomas and metastatic tissues compared to non-fluorescent tissues | |
| Fukuda et al., 1992 [75] | Liposome encapsulated ALA administration in a mouse tumor model led to greater porphyrin accumulation compared to free ALA | |
| Membrane transporters | ||
| ABCB6 | Zhao et al., 2013 [63] | ABCB6 expression was elevated in human glioma cell lines which correlated with an increase in intracellular PpIX accumulation |
| ABCG2 | Kawai et al., 2019 [62] | ABCG2 expression was inversely related to 5-ALA positive staining in various non-CNS cancer cell lines; knockdown and inhibition of ABCG2 lead to increased 5-ALA cell staining |
| Ogino et al., 2011 [58] | ABCG2 mediates PpIX cellular efflux and prevents PpIX in select cancer cells; inhibition of ABCG2 increased PpIX accumulation | |
| Oberstadt et al., 2013 [61] | ABCG2 is variably expressed in GBM | |
| Peripheral benzodiazepine receptor (PBR) | Mesenholler et al., 2000 [59] | Competitive inhibition of the mitochondrial PBR lead to decreased PpIX accumulation in the mitochondria in a pancreatoma cell line; the PRB plays a role in PpIX translocation out of the mitochondrial membrane |
| Bisland et al., 2007 [60] | Induction of PBR in glioma cell lines with low-level light treatment increased PpIX accumulation in cells | |
| Epidermal growth factor receptor (EGFR) | Fontana et al., 2017 [64] | Co-expression of EGFR and EGFRvIII in GBM cell lines lead to activation of heme-oxygenase 1 (HO-1) and reduced cell fluorescence; inhibition of HO-1 restored fluorescence |
| Heme synthesis enzymes | ||
| Ferrochelatase | Teng et al., 2011 [76] | Ferrochelatase mRNA is downregulated in glioblastoma tissue; glioma cells treated with interference RNA showed enhanced PpIX fluorescence after 5-ALA exposure |
| Briel–Pump et al., 2018 [46] | Medulloblastoma cell lines treated with 5-ALA had increased PpIX enhancement associated with decreased ferrochelatase expression | |
| Fukuhara et al., 2013 [37] | Inhibition of ferrochelatase in human prostate cancer cell lines led to increased PpIX accumulation after 5-ALA treatment; in vivo experiments showed increased phototherapy-induced cell death after 5-ALA plus deferoxamine | |
| Krieg et al., 2000 [36] | Bladder carcinoma cell lines had decreased ferrochelatase and altered iron content which may be regulators of fluorescence after 5-ALA treatment | |
| Krieg et al., 2002 [35] | Human colon carcinoma cells showed higher fluorescence after 5-ALA treatment, higher porphobilinogen deaminase activity, and lower ferrochelatase activity compared to human fibroblasts | |
| Van Hillegersberg et al., 1992 [77] | Ferrochelatase activity is decreased 3-fold in a rat model of colon carcinoma liver metastases compared to normal liver cells | |
| Berg et al., 1996 [47] | Human adenocarcinoma and hamster fibroblast cell lines treated with iron chelators plus 5-ALA showed increased PpIX accumulation and inhibition of ferrochelatase activity | |
| Porphobilinogen deaminase (PGDB) | Greenbaum et al., 2002 [42] | A significant fraction of PGBD localizes to glioma cell nuclei following cellular differentiation |
| Greenbaum et al., 2003 [43] | In glioma cells PBGD interacts with RanBPM to induce cellular differentiation via interactions with chromatin | |
| ALA-dehydratase (ALAD) | Hinnen et al., 1998 [39]; Navone et al., 1990 [38] | ALA-dehydratase and other heme synthesis enzyme activities are upregulated in tumor cell lines compared to normal cells |
| Schauder et al., 2011 [40] | Down-regulation of either ALAD or PGDB lead to increased activity of the other enzyme and decreases in PpIX accumulation; both enzymes are important for 5-ALA induced phototherapy | |
| Environmental factors | ||
| Temperature | Hirschberg et al., 2004 [78] | Concurrent photodynamic therapy and hyperthermia (40–46 °C) produced a synergistic effect in inducing apoptosis in glioma spheroids |
| Fisher et al., 2017 [79] | Mild hypothermia (34 °C) in a rat glioma model increased PpIX accumulation and fluorescence, increased normal neuron survival after photodynamic therapy, and extended animal lifespan | |
| pH | Collaud et al., 2004 [66] | PpIX formation is maximized in a slightly alkaline, but physiological pH of 7.4 |
| Oxygen | Moan and Sommer, 1985 [80] | Decreased oxygen partial pressure reduced the efficacy of photodynamic therapy in tumor cell lines |
| Albert et al., 2014 [81] | 5-ALA-treated glioma cell lines in atmospheric oxygen conditions (pO2 = 19%) required less irradiation to kill compared to cell lines at physiological tumor conditions (pO2 = 9%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traylor, J.I.; Pernik, M.N.; Sternisha, A.C.; McBrayer, S.K.; Abdullah, K.G. Molecular and Metabolic Mechanisms Underlying Selective 5-Aminolevulinic Acid-Induced Fluorescence in Gliomas. Cancers 2021, 13, 580. https://doi.org/10.3390/cancers13030580
Traylor JI, Pernik MN, Sternisha AC, McBrayer SK, Abdullah KG. Molecular and Metabolic Mechanisms Underlying Selective 5-Aminolevulinic Acid-Induced Fluorescence in Gliomas. Cancers. 2021; 13(3):580. https://doi.org/10.3390/cancers13030580
Chicago/Turabian StyleTraylor, Jeffrey I., Mark N. Pernik, Alex C. Sternisha, Samuel K. McBrayer, and Kalil G. Abdullah. 2021. "Molecular and Metabolic Mechanisms Underlying Selective 5-Aminolevulinic Acid-Induced Fluorescence in Gliomas" Cancers 13, no. 3: 580. https://doi.org/10.3390/cancers13030580
APA StyleTraylor, J. I., Pernik, M. N., Sternisha, A. C., McBrayer, S. K., & Abdullah, K. G. (2021). Molecular and Metabolic Mechanisms Underlying Selective 5-Aminolevulinic Acid-Induced Fluorescence in Gliomas. Cancers, 13(3), 580. https://doi.org/10.3390/cancers13030580






