Physiological Biomarkers Assessed by Low-Tech Exercise Tests Predict Complications and Overall Survival in Patients Undergoing Pneumonectomy Due to Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
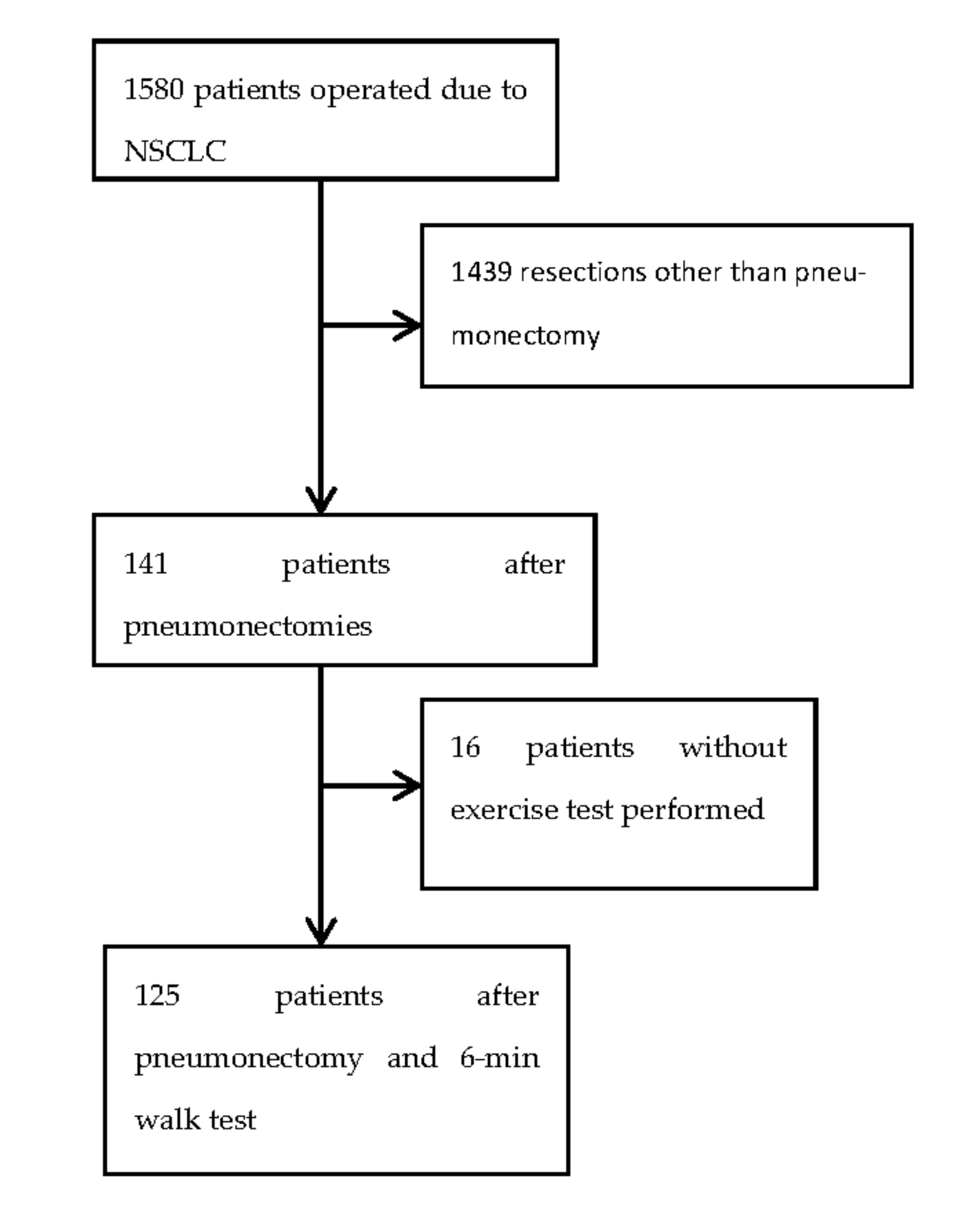
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [Green Version]
- De Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A.; Rocco, G.; Szanto, Z.; Thomas, P.; Falcoz, P.E. Morbidity and mortality of lobectomy or pneumonectomy after neoadjuvant treatment: An analysis from the ESTS database. Eur. J. Cardiothorac. Surg. 2020, 57, 740–746. [Google Scholar] [CrossRef]
- Pagès, P.B.; Mordant, P.; Renaud, S.; Brouchet, L.; Thomas, P.A.; Dahan, M.; Bernard, A.; Epithor Project (French Society of Thoracic and Cardiovascular Surgery). Sleeve lobectomy may provide better outcomes than pneumonectomy for non-small cell lung cancer. A decade in a nationwide study. J. Thorac. Cardiovasc. Surg. 2017, 153, 184–195. [Google Scholar] [CrossRef] [Green Version]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Brunelli, A.; Charloux, A.; Bolliger, C.T.; Rocco, G.; Sculier, J.P.; Varela, G.; Licker, M.; Ferguson, M.K.; Faivre-Finn, C.; Huber, R.M.; et al. European Respiratory Society and European Society of Thoracic Surgeons joint task force on fitness for radical therapy. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur. Respir. J. 2009, 34, 17–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunelli, A.; Kim, A.W.; Berger, K.I.; Addrizzo-Harris, D.J. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e166S–e190S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marjanski, T.; Badocha, M.; Wnuk, D.; Dziedzic, R.; Ostrowski, M.; Sawicka, W.; Rzyman, W. Result of the 6-min walk test is an independent prognostic factor of surgically treated non-small-cell lung cancer. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 368–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marjanski, T.; Wnuk, D.; Bosakowski, D.; Szmuda, T.; Sawicka, W.; Rzyman, W. Patients who do not reach a distance of 500 m during the 6-min walk test have an increased risk of postoperative complications and prolonged hospital stay after lobectomy. Eur. J. Cardiothorac. Surg. 2015, 47, e213–e219. [Google Scholar] [CrossRef] [Green Version]
- Holden, D.A.; Rice, T.W.; Stelmach, K.; Meeker, D.P. Exercise testing, 6-min walk, and stair climb in the evaluation of patients at high risk for pulmonary resection. Chest 1992, 102, 1774–1779. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Pierce, R.J.; Copland, J.M.; Sharpe, K.; Barter, C.E. Preoperative risk evaluation for lung cancer resection: Predicted postoperative product as a predictor of surgical mortality. Am. J. Respir. Crit. Care Med. 1994, 150, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.F.; Souza, H.C.; Miranda, A.P.; Cipriano, F.G.; Gastaldi, A.C. Performance in the 6-minute walk test and postoperative pulmonary complications in pulmonary surgery: An observational study. Braz. J. Phys. Ther. 2016, 20, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Wesolowski, S.; Orlowski, T.M.; Kram, M. The 6-min walk test in the functional evaluation of patients with lung cancer qualified for lobectomy. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 559–564. [Google Scholar] [CrossRef]
- Albain, K.S.; Swann, R.S.; Rusch, V.W.; Turrisi, A.T., 3rd; Shepherd, F.A.; Smith, C.; Chen, Y.; Livingston, R.B.; Feins, R.H.; Gandara, D.R.; et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: A phase III randomised controlled trial. Lancet 2009, 374, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.D.; Caso, R.; Tan, K.S.; Dycoco, J.; Adusumilli, P.S.; Bains, M.S.; Downey, R.J.; Huang, J.; Isbell, J.M.; Molena, D.; et al. Propensity-matched Analysis Demonstrates Long-term Risk of Respiratory and Cardiac Mortality After Pneumonectomy Compared With Lobectomy for Lung Cancer. Ann. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society; American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A.; Sabbatini, A.; Xiume’, F.; Borri, A.; Salati, M.; Marasco, R.D.; Fianchini, A. Inability to perform maximal stair climbing test before lung resection: A propensity score analysis on early outcome. Eur. J. Cardiothorac. Surg. 2005, 27, 367–372. [Google Scholar] [CrossRef]
- Epstein, S.K.; Faling, L.J.; Daly, B.D.; Celli, B.R. Inability to perform bicycle ergometry predicts increased morbidity and mortality after lung resection. Chest 1995, 107, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Falcoz, P.E.; Conti, M.; Brouchet, L.; Chocron, S.; Puyraveau, M.; Mercier, M.; Etievent, J.P.; Dahan, M. The Thoracic Surgery Scoring System (Thoracoscore): Risk model for in-hospital death in 15,183 patients requiring thoracic surgery. J. Thorac. Cardiovasc. Surg. 2007, 133, 325–332. [Google Scholar] [CrossRef]
- Bernard, A.; Rivera, C.; Pages, P.B.; Falcoz, P.E.; Vicaut, E.; Dahan, M. Risk model of in-hospital mortality after pulmonary resection for cancer: A national database of the French Society of Thoracic and Cardiovascular Surgery (Epithor). J. Thorac. Cardiovasc. Surg. 2011, 141, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Depypere, L.P.; Daddi, N.; Gooseman, M.R.; Batirel, H.F.; Brunelli, A. The impact of coronavirus disease 2019 on the practice of thoracic oncology surgery: A survey of members of the European Society of Thoracic Surgeons (ESTS). Eur. J. Cardiothorac. Surg. 2020, 58, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Mazzone, P.J.; Ries, A.L.; Malhotra, A.; Fuster, M. The Utility of Exercise Testing in Patients with Lung Cancer. J. Thorac. Oncol. 2016, 11, 1397–1410. [Google Scholar] [CrossRef] [Green Version]
- Jones, L.W.; Hornsby, W.E.; Goetzinger, A.; Forbes, L.M.; Sherrard, E.L.; Quist, M.; Lane, A.T.; West, M.; Eves, N.D.; Gradison, M.; et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer 2012, 76, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Kasymjanova, G.; Correa, J.A.; Kreisman, H.; Dajczman, E.; Pepe, C.; Dobson, S.; Lajeunesse, L.; Sharma, R.; Small, D. Prognostic value of the six-minute walk in advanced non-small cell lung cancer. J. Thorac. Oncol. 2009, 4, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Pompili, C.; McLennan Battleday, F.; Chia, W.L.; Chaudhuri, N.; Kefaloyannis, E.; Milton, R.; Papagiannopoulos, K.; Tcherveniakov, P.; Brunelli, A. Poor preoperative quality of life predicts prolonged hospital stay after VATS lobectomy for lung cancer. Eur. J. Cardiothorac. Surg. 2020, 59, 116–121. [Google Scholar] [CrossRef]
- Pompili, C.; Salati, M.; Refai, M.; Berardi, R.; Onofri, A.; Mazzanti, P.; Brunelli, A. Preoperative quality of life predicts survival following pulmonary resection in stage I non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2013, 43, 905–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awdeh, H.; Kassak, K.; Sfeir, P.; Hatoum, H.; Bitar, H.; Husari, A. The SF-36 and 6-Minute Walk Test are Significant Predictors of Complications after Major Surgery. World J. Surg. 2015, 39, 1406–1412. [Google Scholar] [CrossRef]
- Dall’Olio, F.G.; Maggio, I.; Massucci, M.; Mollica, V.; Fragomeno, B.; Ardizzoni, A. ECOG performance status ≥2 as a prognostic factor in patients with advanced non small cell lung cancer treated with immune checkpoint inhibitors-A systematic review and meta-analysis of real world data. Lung Cancer 2020, 145, 95–104. [Google Scholar] [CrossRef]
- Kong, S.; Shin, S.; Lee, J.K.; Lee, G.; Kang, D.; Cho, J.; Kim, H.K.; Zo, J.I.; Shim, Y.M.; Park, H.Y.; et al. Association between Sarcopenia and Physical Function among Preoperative Lung Cancer Patients. J. Pers. Med. 2020, 10, 166. [Google Scholar] [CrossRef] [PubMed]



| Clinical Characteristics | Prevalence |
|---|---|
| Tobacco smoking habit | 88% |
| Median pack-years | 32 |
| Gender | 82% male |
| Side | 82% left |
| Median age | 63 |
| FEV1 | 2.17 |
| FEV1% | 71% |
| FVC | 3.04 |
| FVC% | 80% |
| 30-day mortality | 0% |
| 90-day mortality | 12% |
| First-year mortality | 18% |
| Rate of complications | 25% |
| Median hospital stay [days] | 8 |
| Clinical Feature | 6MWD ≤ 500 M n = 39 Patients | 6MWD >500 M n = 86 Patients | p Value |
|---|---|---|---|
| Smoking habit | 36 (92%) | 73 (84%) | 0.250 |
| Pack-years | 34.5 ± 18.5 | 30.5 ± 21.3 | 0.128 |
| Male gender | 28 (72%) | 63 (73%) | 0.865 |
| Age | 64.9 ± 7.5 | 62.0 ± 7.0 | 0.080 |
| AACCI | 4.0 ± 1.6 | 2.9 ± 1.3 | 0.003 |
| Spirometry | |||
| FEV1 | 2.2 ± 0.7 | 2.3 ± 0.7 | 0.880 |
| FEV1% | 84.0% ± 20.9 | 80.9 ± 19.3 | 0.638 |
| FVC | 3.3 ± 0.9 | 3.0 ± 0.7 | 0.395 |
| FVC% | 91.7% ± 15.9 | 93.8 ± 21.1 | 0.541 |
| 6MWT result | |||
| 6MWD | 427.7 ± 75.8 | 572.4 ± 42.9 | <0.001 |
| 6MWD% | 87.3 ± 21.5 | 107.2 ± 16.3 | 0.006 |
| 7th TNM | |||
| pIA | 2 (5%) | 6 (7%) | 1.000 |
| pIB | 5 (13%) | 8 (9%) | 0.551 |
| pIIA | 10 (26%) | 25 (29%) | 0.692 |
| pIIB | 7 (18%) | 13 (15%) | 0.689 |
| pIIIA | 14 (36%) | 32 (37%) | 0.888 |
| pIIIIB | 0 | 2 (2%) | 0.849 |
| pIV | 1 (3%) | 0 | 0.684 |
| Histology | |||
| Squamous cell carcinoma | 25 (64%) | 50 (58%) | 0.582 |
| Adenocarcinoma | 11 (28%) | 17 (20%) | 0.294 |
| Other | 3 (8%) | 19 (22%) | 0.050 |
| Complications | All Patients n = 125 | ≤500 n = 39 | >500 n = 86 | p Value | 95% CI |
|---|---|---|---|---|---|
| Cardiovascular | 32 | 15 (38.4%) | 17 (19.8%) | 0.026 | 2.537 1.100–5.849 |
| Pulmonary | 44 | 14 (35.9%) | 30 (34.8%) | 0.912 | 1.045 0.474–2.304 |
| Cardiopulmonary | 63 | 23 (58.9%) | 40 (46.5%) | 0.197 | 1.653 0.769–3.556 |
| Atrial arrhytmia(as a part of cardiac complications) | 28 | 14 (35.9%) | 14 (16.3%) | 0.015 | 2.880 1.207–6.870 |
| 30-day mortality | 6 (4.8%) | 4 (10.3%) | 2 (2.3%) | 0.055 | 4.800 0.840–27.418 |
| 90-day mortality | 10 (8.0%) | 7 (17.9%) | 3 (3.5%) | 0.005 | 6.271 1.528–25.739 |
| 1-year mortality | 22 (17.6%) | 12 (30.7%) | 10 (11.6%) | 0.009 | 3.378 1.310–8.709 |
| Hospital stay | 8.0 | 7.5 | 0.180 |
| Clinical Feature | 30-Day Mortality | 90-Day Mortality | First Year Mortality | Long-Term Survival | Cardiac Complications | Pulmonary Complications |
|---|---|---|---|---|---|---|
| Side (left vs. right) | p = 0.247 | p = 0.596 | p = 0.976 | p = 0.302 | p = 0.565 | p = 0.122 |
| pTNM (I + II vs. III + IV) | p = 0.158 | p = 0.700 | p = 0.216 | p = 0.239 | p = 0.774 | p = 0.293 |
| 6MWT (≤500 m vs. >500 m) | - | p = 0.335 | p = 0.442 | p = 0.135 | p = 0.370 | p = 0.489 |
| Gender (female vs. male) | p = 0.637 | p = 0.566 | p = 0.838 | p = 0.239 | p = 0.068 | p = 0.184 |
| Age (≤63 vs. >63) | p = 0.219 | p = 0.467 | p = 0.656 | p = 0.289 | p = 0.167 | p = 0.425 |
| AACCI(0–3 vs. >3) | p = 0.906 | p = 0.863 | p = 0.606 | p = 0.769 | p = 0.185 | p = 0.286 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marjanski, T.; Wnuk, D.; Dziedzic, R.; Ostrowski, M.; Sawicka, W.; Marjanska, E.; Rzyman, W. Physiological Biomarkers Assessed by Low-Tech Exercise Tests Predict Complications and Overall Survival in Patients Undergoing Pneumonectomy Due to Lung Cancer. Cancers 2021, 13, 735. https://doi.org/10.3390/cancers13040735
Marjanski T, Wnuk D, Dziedzic R, Ostrowski M, Sawicka W, Marjanska E, Rzyman W. Physiological Biomarkers Assessed by Low-Tech Exercise Tests Predict Complications and Overall Survival in Patients Undergoing Pneumonectomy Due to Lung Cancer. Cancers. 2021; 13(4):735. https://doi.org/10.3390/cancers13040735
Chicago/Turabian StyleMarjanski, Tomasz, Damian Wnuk, Robert Dziedzic, Marcin Ostrowski, Wioletta Sawicka, Ewa Marjanska, and Witold Rzyman. 2021. "Physiological Biomarkers Assessed by Low-Tech Exercise Tests Predict Complications and Overall Survival in Patients Undergoing Pneumonectomy Due to Lung Cancer" Cancers 13, no. 4: 735. https://doi.org/10.3390/cancers13040735






