How Can We Treat Vulvar Carcinoma in Pregnancy? A Systematic Review of the Literature
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
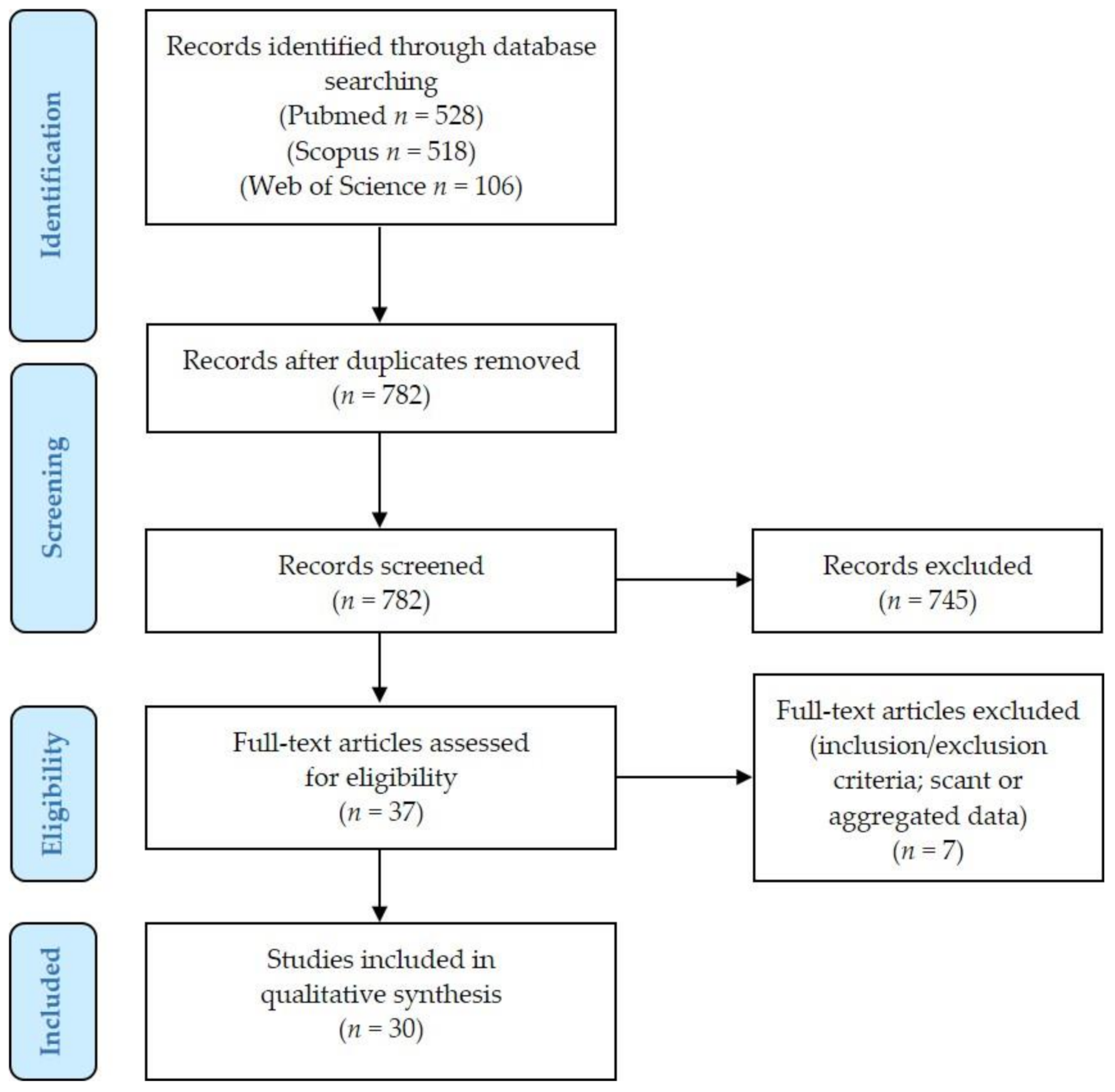
2.1. Literature Review Results and Details of Excluded Cases
2.2. Age and Race
2.3. Primary Tumor (T)
2.4. Clinical Presentation
- Pre-conceptional evidence of a vulvar lesion (4/37 cases, 11%). The vulvar lesion was evident 1 [15], some [17], 12 [28], and 15 months [25] before conception, respectively. However, the diagnosis of VSCC was made during the 1st trimester (1 case: 2nd month) [28] or even during the 2nd trimester of pregnancy (3 cases: 19 GW [17]; 16 GW [25]; 5th month [15]). A tumor was diagnosed as a condyloma 6 months before conception, while subsequent biopsy at 29 GW revealed a VSCC [25]: this discrepancy may be due to misdiagnosis of the first biopsy or malignant transformation during pregnancy. Another VSCC was diagnosed during the 5th month of pregnancy in the site where a small itchy lesion of unknown diagnosis had been excised 1 month before conception [15]. In the remaining 2 cases, it was unclear if the lesion was underestimated by clinicians or not presented to the gynecologists’ attention [17,28].
- Discovery during pregnancy without pre-conceptional evidence of vulvar lesions (25/37 cases, 68%). The VSCCs were identified during the 1st (2/25 cases, 8%) [14,35], 2nd (14/25 cases, 56%) [13,16,18,23,24,28,29,31,32,33,36,37,38,39], or 3rd trimester of pregnancy (9/25 cases, 36%) [11,12,19,21,26,30,31,36,41].
2.5. Risk Factors
2.6. Pap Smear
2.7. Biopsy
2.8. Fine Needle Aspiration Cytology of Lymph Nodes
2.9. Timing of Treatment
- Spontaneous vaginal delivery probably delaying surgery (1/14 cases, 7%): labor occurred 1 month after presentation [36]
- Misdiagnosis/underestimation by clinicians/patients (4/14 cases, 29%). Clinicians prescribed typical ointments and antibiotics for a vulvar lesion noted during the 3rd month of pregnancy: the correct diagnosis was made after delivery [14]. A lesion that was evident during the 2nd trimester of pregnancy was not biopsied until 3 months after spontaneous vaginal delivery [31]. In 1 case, it took to the clinicians 5 weeks (33–38 GW) to understand the tumor nature of the vulvar lesion and perform a vulvar biopsy: the diagnosis was low-grade squamous intraepithelial lesion (L-SIL; vulvar intraepithelial neoplasia, VIN1) + H-SIL (VIN2). An elective CS was performed at 38 GW; unfortunately, the patient and her family were non-compliant: the lesion grew and extended in the meanwhile [26]. A woman presented at 16 GW with a vulvar lesion and she was referred to the nearby district hospital for further investigations; however, she defaulted and presented instead during labor at term [32].
2.10. Surgery
- Partial vulvectomy (4/37 cases, 11%): during pregnancy in all cases (15 GW to 4 months before delivery) [18,24,37]. In 1 case, 2 partial vulvectomies were performed (15 and 22 GW) [24]. Complications: lymphocele (10 days after surgery) (1 case) [24]; right inguinal abscess, right lymphocele, and perineal mycosis after the first partial vulvectomy [24].
- Radical vulvectomy (22/37 cases, 60%):
- (a)
- Ten cases were performed during pregnancy (17–29 GW, mean 28) [23,28,29,30,33,34,35,36,39]. Complications: persistent watery vaginal discharge (intra-amniotic instillation of methylene blue ruled out rupture of membranes) (1 case) [30]; bilateral groin seromas after 12 days (resolved after aspiration and pressure dressing) (1 case) [28]; pain and swelling of left leg for a right inguinal wound seroma after 18 days (treated with drainage and bed rest), and cellulitis of left leg at 37 GW (treated with antibiotics) (1 case) [29]; initial vulvar edema [23].
- (b)
- Twelve cases were performed after delivery [18,19,21,26,27,31,32,35,36,37] (1–12 weeks postpartum, mean 7 weeks). Complications: fever (39.4 °C) on 2nd postoperative day [26]; hematoma in the right groin wound (drainage on 2nd postoperative day) [27]; necrosis of distal flaps, requiring surgical debridement [31].
2.11. Sentinel Lymph Node
2.12. Lymph Node Sampling/Lymphadenectomy
2.13. Chemotherapy
2.14. Radiotherapy
2.15. Pathological Examination and Stage
2.16. HPV Status and Cancer Precursors
2.17. Follow-Up Data
2.18. Pregnancy Course
3. Discussion
4. Materials and Methods
- Population: patients with a diagnosis of VSCC during pregnancy;
- Intervention: any type of treatment, including surgery, chemotherapy, radiotherapy, or observational treatment;
- Comparison: no comparisons are expected;
- Outcomes: (1) patient’s status at last follow-up: no evidence of disease, alive with disease, dead of disease; (2) pregnancy outcome: healthy baby; stillborn.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO Classification of Tumours Editorial Board. Female Genital Tumours, 5th ed.; IARCC: Lyon, France, 2020; pp. 419–449. [Google Scholar]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. Vulva. In AJCC Cancer Staging Manual, 8th ed.; Springer: Cham, Switzerland, 2017; pp. 633–640. [Google Scholar]
- National Cancer Institute. Surveillance, Epidemiology, and End Results Programs. Available online: https://seer.cancer.gov/statfacts/html/vulva.html (accessed on 5 December 2020).
- Korenaga, T.-R.K.; Tewari, K.S. Gynecologic cancer in pregnancy. Gynecol. Oncol. 2020, 157, 799–809. [Google Scholar] [CrossRef]
- Amant, F.; Berveiller, P.; Boere, I.; Cardonick, E.; Fruscio, R.; Fumagalli, M.; Halaska, M.; Hasenburg, A.; Johansson, A.; Lambertini, M.; et al. Gynecologic cancers in pregnancy: Guidelines based on a third international consensus meeting. Ann. Oncol. 2019, 30, 1601–1612. [Google Scholar] [CrossRef] [Green Version]
- Oonk, M.H.; Planchamp, F.; Baldwin, P.; Bidzinski, M.; Brännström, M.; Landoni, F.; Mahner, S.; Mahantshetty, U.; Mirza, M.; Petersen, C.; et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer. Int. J. Gynecol. Cancer 2017, 27, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.H.; Underwood, P.B.; Rozier, J.C.; Putney, F.W. Genital malignancy in pregnancy. Am. J. Obstet. Gynecol. 1977, 129, 536–542. [Google Scholar] [CrossRef]
- Shafeek, M.A.; Osman, M.I.; Hussein, M.A. Carcinoma of the vulva arising in condylomata acuminata. Obstet. Gynecol. 1979, 54, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, S.; Tsuji, K.; Nakano, R. A case of vulvar carcinoma of 30-year-old pregnant woman. J. Wakayama Medical Soc. 1982, 33, 205–208. [Google Scholar]
- Carter, J.; Carlson, J.; Fowler, J.; Hartenbach, E.; Adcock, L.; Carson, L.; Twiggs, L.B. Invasive Vulvar Tumors in Young Women—A Disease of the Immunosuppressed? Gynecol. Oncol. 1993, 51, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Gitsham, S.; Rösch, M.; Fink, T.; Scharf, J.-P. Verkanntes verhornendes Vulvakarzinom bei 40- jähriger Schwangeren. Geburtshilfe Frauenheilkd. 2020, 80, e122. [Google Scholar]
- Metke, F.; Bösken, E.; Pixberg, M.; Bräuer, A.; Kies, P.; Kiesel, L.; Lellé, R.; Witteler, R. EP1176 Treatment of early stage vulvar carcinoma in a pregnant woman: A case report. ePoster 2019, 29, A608. [Google Scholar] [CrossRef]
- Lecointre, L.; Gaudineau, A.; Hild, C.; Sananes, N.; Langer, B. Squamous cell carcinoma of the vulva and pregnancy: Tough choices. Gynecol. Obstes. Fertil. 2015, 43, 625–627. (In French) [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Zamiri-Akhlaghi, A.; Hassanpoor-Moghaddam, M.; Shahidsales, S. Vulvar Carcinoma in Pregnant Women Aged Less than 40 Years: Case Report. Iran. J. Cancer Prev. 2014, 7, 175–178. [Google Scholar] [PubMed]
- Idi, Y.I.; Muteya, M.M.; Ngama, C.K.; Mwazaz, R.M.; Makinko, A.I.; Kaj, F.M.; Tambwe, A.M.; Mukalenge, F.C. Epidermoid carcinoma of the vulva during twin pregnancy: Apropos of a case from the Lubumbashi university clinics. Pan. Afr. Med. J. 2013, 2, 70. (In French) [Google Scholar] [CrossRef]
- Pariyar, J.; Shrestha, B.; Rauniyar, B.P.; Regmi, S.C.; Shrestha, J.; Jha, A.K.; Shrestha, S. Cancer with pregnancy in a cancer hospital. J. Nepal Health Res. Counc. 2012, 10, 224–228. [Google Scholar]
- Nijman, T.; Schutter, E.; Amant, F. Sentinel node procedure in vulvar carcinoma during pregnancy: A case report. Gynecol. Oncol. Case Rep. 2012, 2, 63–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parva, M.; Miroshnichenko, G.; Holtz, D.O.; Dunton, C.J. Invasive Vulvar Cancer in Pregnancy. J. Low. Genit. Tract. Dis. 2009, 13, 264–268. [Google Scholar] [CrossRef]
- Keskin, N.; Iyibozkurt, A.C.; Topuz, S.; Salihoğlu, Y.; Bengisu, E.; Berkman, S. Invasive squamous carcinoma of the vulva in women aged less than 40 years: Report of two cases and a third case diagnosed during pregnancy. Eur. J. Gynaecol. Oncol. 2008, 29, 399–401. [Google Scholar] [PubMed]
- Ghosh, T.; Banfield, P. Carcinoma of the vulva in pregnancy. J. Obstet. Gynaecol. 2004, 24, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Modares, G.M.; Hasanzadeh, M.; Behtash, N. Vulvar carcinoma in pregnancy: A case report. Med. J. Islam. Repub. Iran. 2005, 19, 185–187. [Google Scholar]
- Alexander-Sefre, F.; Siddiqui, N.; Weerasekera, D.S. Gynaecological malignancy in pregnancy. J. Obstet. Gynaecol. 2005, 25, 327–329. [Google Scholar] [CrossRef]
- Ogunleye, D.; Lewin, S.N.; Huettner, P.; Herzog, T.J. Recurrent vulvar carcinoma in pregnancy. Gynecol. Oncol. 2004, 95, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Couvreux-Dif, D.; Lhommé, C.; Querleu, D.; Castaigne, D.; Verhaeghe, Y. Cancer of the vulva and pregnancy: Two cases and review of the literature. J. Gynecol. Obstet. Biol. Reprod. (Paris) 2003, 32, 46–50. (In French) [Google Scholar]
- Olayemi, O.; Aimakhu, C.O.; Omigbodun, A.O.; Ogunbiyi, J.O.; Udoh, I.J. Vulval carcinoma in pregnancy. J. Obstet. Gynaecol. 2002, 22, 441–442. [Google Scholar] [CrossRef]
- Bakour, S.H.; Jaleel, H.; Weaver, J.B.; Kehoe, S.; Radcliffe, K.W. Vulvar Carcinoma Presenting during Pregnancy, Associated with Recurrent Bone Marrow Hypoplasia: A Case Report and Literature Review. Gynecol. Oncol. 2002, 87, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.S.; Cracchiolo, B.; Hameed, M.; May, T. Pregnancy-associated invasive squamous cell carcinoma of the vulva in a 28-year-old, HIV-negative woman. A case report. J. Reprod. Med. 2000, 45, 659–661. [Google Scholar] [PubMed]
- Gitsch, G.; Van Eijkeren, M.; Hacker, N.F. Surgical Therapy of Vulvar Cancer in Pregnancy. Gynecol. Oncol. 1995, 56, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Regan, M.; Rosenzweig, B. Vulvar Carcinoma in Pregnancy: A Case Report and Literature Review. Am. J. Perinatol. 1993, 10, 334–335. [Google Scholar] [CrossRef] [PubMed]
- Del Priore, G.; Schink, J.; Lurain, J. A two-step approach to the treatment of invasive vulvar cancer in pregnancy. Int. J. Gynecol. Obstet. 1992, 39, 335–336. [Google Scholar] [CrossRef]
- Moore, D.H.; Fowler, W.C.; Currie, J.L.; Walton, L.A. Squamous cell carcinoma of the vulva in pregnancy. Gynecol. Oncol. 1991, 41, 74–77. [Google Scholar] [CrossRef]
- Sivanesaratnam, V.; Pathmanathan, R. Carcinoma of the Vulva in Pregnancy: A Rare Occurrence. Asia Ocean. J. Obstet. Gynaecol. 2010, 16, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Robson, P.; Johnson, I.R. Carcinoma of the vulva in pregnancy. J. Obstet. Gynaecol. 1989, 10, 47–48. [Google Scholar] [CrossRef]
- Rahman, M.S.; Rahman, J.; Al-Sibai, M.H.; Al-Suleiman, S.A. Carcinoma of the vulva in pregnancy. Case report. BJOG Int. J. Obstet. Gynaecol. 1982, 89, 244–246. [Google Scholar] [CrossRef]
- Kempers, R.D.; E Symmonds, R. Invasive carcinoma of the vulva and pregnancy. Report of 2 cases. Obstet. Gynecol. 1965, 26, 749–751. [Google Scholar]
- Collins, C.G.; Barclay, D.L. Cancer of the vulva, and cancer of the vagina in pregnancy. Clin. Obstet. Gynecol. 1963, 6, 927–942. [Google Scholar] [CrossRef]
- Barber, H.R.; Brunschwig, A. Gynecologic cancer complicating pregnancy. Am. J. Obstet. Gynecol. 1963, 85, 156–164. [Google Scholar] [CrossRef]
- Gemmell, A.A.; Haines, M. Pregnancy following radical vulvectomy for carcinoma of the vulva. BJOG Int. J. Obstet. Gynaecol. 1960, 67, 199–207. [Google Scholar] [CrossRef] [PubMed]
- De Bruïne, T. Cancer of the Vulva and Pregnancy. Gynecol. Obstet. Investig. 1958, 146, 152–156. [Google Scholar] [CrossRef]
- Shannon, W.F.; Marting, E. Primary epidermoid carcinoma of the vulva complicating pregnancy. Am. J. Obstet. Gynecol. 1941, 41, 117–121. [Google Scholar] [CrossRef]
- Russell, P. Carcinoma of the vulva complicating pregnancy. Am. J. Obstet. Gynecol. 1940, 39, 873–875. [Google Scholar] [CrossRef]
- Matsuo, K.; Whitman, S.A.; Blake, E.A.; Conturie, C.L.; Ciccone, M.A.; Jung, C.E.; Takiuchi, T.; Nishimura, M. Feto-maternal outcome of pregnancy complicated by vulvar cancer: A systematic review of literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 216–223. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Vulvar Cancer (Squamous Cell Carcinoma). Version 2.2021—19 October 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/vulvar.pdf (accessed on 30 January 2021).
- European Society of Gynecological Oncology (ESGO) Guidelines. Vulvar Cancer Guidelines. Available online: https://guidelines.esgo.org/media/2016/08/ESGO-Vulvar-cancer-Complete-report-fxd2.pdf (accessed on 30 January 2021).
- Wong, R.W.-C.; Palicelli, A.; Hoang, L.; Singh, N. Interpretation of p16, p53 and mismatch repair protein immunohistochemistry in gynaecological neoplasia. Diagn. Histopathol. 2020, 26, 257–277. [Google Scholar] [CrossRef]
- Santandrea, G.; Piana, S.; Valli, R.; Zanelli, M.; Gasparini, E.; De Leo, A.; Mandato, V.; Palicelli, A. Immunohistochemical Biomarkers as a Surrogate of Molecular Analysis in Ovarian Carcinomas: A Review of the Literature. Diagnostics 2021, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Tessier-Cloutier, B.; Kortekaas, K.E.; Thompson, E.; Pors, J.; Chen, J.; Ho, J.; Prentice, L.M.; McConechy, M.K.; Chow, C.; Proctor, L.; et al. Major p53 immunohistochemical patterns in in situ and invasive squamous cell carcinomas of the vulva and correlation with TP53 mutation status. Mod. Pathol. 2020, 33, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, K.E.; Bastiaannet, E.; Van Doorn, H.C.; Steenwijk, P.J.D.V.V.; Ewing-Graham, P.C.; Creutzberg, C.L.; Akdeniz, K.; Nooij, L.S.; Van Der Burg, S.H.; Bosse, T.; et al. Vulvar cancer subclassification by HPV and p53 status results in three clinically distinct subtypes. Gynecol. Oncol. 2020, 159, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Bigby, S.M.; Eva, L.J.; Fong, K.L.; Jones, R.W. The Natural History of Vulvar Intraepithelial Neoplasia, Differentiated Type. Int. J. Gynecol. Pathol. 2016, 35, 574–584. [Google Scholar] [CrossRef]
- Ordi, J.; Alejo, M.; Fusté, V.; Lloveras, B.; Del Pino, M.; Alonso, I.; Torné, A. HPV-negative Vulvar Intraepithelial Neoplasia (VIN) with Basaloid Histologic Pattern. Am. J. Surg. Pathol. 2009, 33, 1659–1665. [Google Scholar] [CrossRef]
- Rakislova, N.; Alemany, L.; Clavero, O.; Del Pino, M.; Saco, A.; Quirós, B.; Lloveras, B.; Alejo, M.; Halec, G.; Quint, W.; et al. Differentiated Vulvar Intraepithelial Neoplasia-like and Lichen Sclerosus-like Lesions in HPV-associated Squamous Cell Carcinomas of the Vulva. Am. J. Surg. Pathol. 2018, 42, 828–835. [Google Scholar] [CrossRef] [Green Version]
- Watkins, J.C.; Yang, E.; Crum, C.P.; Herfs, M.; Gheit, T.; Tommasino, M.; Nucci, M.R. Classic Vulvar Intraepithelial Neoplasia with Superimposed Lichen Simplex Chronicus. Int. J. Gynecol. Pathol. 2019, 38, 175–182. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, C.; Surico, D.; Monga, G.; Palicelli, A. Pregnancy-related decidualization of subcutaneous endometriosis occurring in a post-caesarean section scar: Case study and review of the literature. Pathol.-Res. Pract. 2019, 215, 828–831. [Google Scholar] [CrossRef]
- Heijink, T.; Bogers, H.; Steensma, A. Endometriosis of the Bartholin gland: A case report and review of the literature. J. Med. Case Rep. 2020, 14, 1–4. [Google Scholar] [CrossRef]
- Rocconi, R.P.; Leath, C.A.; Johnson, W.M.; Barnes, M.N.; Conner, M.G. Primary lung large cell carcinoma metastatic to the vulva: A case report and review of the literature. Gynecol. Oncol. 2004, 94, 829–831. [Google Scholar] [CrossRef]
- Weiss, S.; Amit, A.; Schwartz, M.R.; Kaplan, A.L. Primary choriocarcinoma of the vulva. Int. J. Gynecol. Cancer 2001, 11, 251–254. [Google Scholar] [CrossRef]
- Kyriazi, M.A.; Carvounis, E.E.; Kitsou, M.; Arkadopoulos, N.; Nicolaidou, E.; Fotiou, S.; Smyrniotis, V. Myoepithelial Carcinoma of the Vulva Mimicking Bartholin Gland Abscess in a Pregnant Woman. Int. J. Gynecol. Pathol. 2010, 29, 501–504. [Google Scholar] [CrossRef]
- Copeland, L.J.; Sneige, N.; Gershenson, D.M.; Saul, P.B.; A Stringer, C.; Seski, J.C. Adenoid cystic carcinoma of Bartholin gland. Obstet. Gynecol. 1986, 67, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Wilson, J.M.; Bickel, D.A. Adenoid cystic carcinoma of the Bartholin gland and pregnancy. Am. J. Obstet. Gynecol. 1962, 83, 612–614. [Google Scholar] [CrossRef]
- Schweppe, K.-W.; Schlake, W. Adenokarzinom der Bartholinschen Drüse. Geburtshilfe Frauenheilkd. 1980, 40, 437–443. [Google Scholar] [CrossRef]
- Palicelli, A. What do we know about the cytological features of pure intraductal carcinomas of the salivary glands? Cytopathology 2019, 31, 185–192. [Google Scholar] [CrossRef]
- Palicelli, A. Intraductal carcinomas of the salivary glands: Systematic review and classification of 93 published cases. Acta Pathol. Microbiol. Immunol. Scand. 2019, 128, 191–200. [Google Scholar] [CrossRef]
- Palicelli, A.; Barbieri, P.; Mariani, N.; Re, P.; Galla, S.; Sorrentino, R.; Locatelli, F.; Salfi, N.; Valente, G. Unicystic high-grade intraductal carcinoma of the parotid gland: Cytological and histological description with clinic-pathologic review of the literature. Acta Pathol. Microbiol. Immunol. Scand. 2018, 126, 771–776. [Google Scholar] [CrossRef]
- Prisma Transparent Reporting of Systematic Reviews and Meta-Analyses. Available online: http://www.prisma-statement.org/ (accessed on 5 December 2020).
- Ardighieri, L.; Palicelli, A.; Ferrari, F.; Bugatti, M.; Drera, E.; Sartori, E.; Odicino, F. Endometrial Carcinomas with Intestinal-Type Metaplasia/Differentiation: Does Mismatch Repair System Defects Matter? Case Report and Systematic Review of the Literature. J. Clin. Med. 2020, 9, 2552. [Google Scholar] [CrossRef] [PubMed]
| Authors | Age | Site | Size (cm) | Gross Examination |
|---|---|---|---|---|
| Gitsham et al., 2020 [11] | 40 | NR | NR | NR |
| Metke et al., 2019 [12] | 39 | NR | NR | NR |
| Lecointre et al., 2015 [13] | 29 | Periclitoral, Lmi, LMA (mainly right) | 6.7 | Ulcerated |
| Hasanzadeh et al., 2014 [14] | 37 | Lmi, left | 3 | Ulcerated |
| Idi et al., 2013 [15] | 32 | LMA, right (site of previous excision) | 15 | Nodular, bleeding |
| Pariyar et al., 2012 [16] | 20 | NR | NR | NR |
| Nijman et al., 2012 [17] | 27 | Lmi, right | 1.2 | Painful, verrucous, ulcerated |
| Parva et al., 2009 [18] | 29 | Posterior fourchette | 1 | Raised, pigmented |
| Keskin et al., 2008: case 3 [19] | 31 | LMA, right, extending to the clitoris | 3 | NR |
| Ghosh et al., 2004 [20] | 41 | (1) Lower part of right labia, eroding posterior fourchette, lower vagina and anal canal (2) LMA, right | (1) ~4 (2) 2 | (1) Solid, necrotic (2) Exophytic ulcerated |
| Modares Gilani et al., 2005 [21] | 28 | Clitoris, upper Lmi and LMA | 8 | Ulcerated, verrucous, necrotic |
| Alexander-Sefre et al., 1998 [22] | 18 | LMA, right (3 mm from the clitoris) | 1 | Ulcerated, white, firm, freely mobile plaque |
| Ogunleye et al., 2004 [23] | 36 | (1) Anterior to the left LMA (2) Posterior to the right of the clitoris (*) | (1) 3.5 (2) 0.3 | (1) Painful, tender (2) Tender, raised |
| Couvreux-Dif et al., 2003: case 1 [24] | 34 | Lmi, left anterior | 1 | Painful, raised |
| Couvreux-Dif et al., 2003: case 2 [24] | 31 | (1) Lmi, right (11 o’clock) (2) Lmi, left (*) | (1) 2 (2) NR | (1) Nodule; (2) NR |
| Olayemi et al., 2002 [25] | 29 | Whole vulva (more on the left part) | 6 | Cauliflower-like, exophytic |
| Bakour et al., 2002 [26] | 29 | Clitoris | 2 | Indurated ulcerated, then more edematous and necrotic. |
| Heller et al., 2000 [27] | 28 | Lmi, left | 4 | Painful, ulcerated |
| Gitsch et al., 1995: case 1 [28] | 29 | Bilateral Lmi, anterior and periclitoral (extension within 1 mm of the urethra) | 5 | Ulcerated |
| Gitsch et al., 1995: case 2 [28] | 35 | Multifocal: most vulva (especially posterior 2/3), fourchette | NR | Red-white papillary |
| Regan et al., 1993 [29] | 37 | LMA, left upper half | 2 | Ulcerated |
| Del Priore et al., 1992 [30] | 39 | Periclitoral | NR | Ulcerated |
| Moore et al., 1991: case 1 [31] | 24 | (1) Clitoris and LMA, upper left (2) LMA, lower right | (1) 5 (2) 1 | Abscess-like, raised |
| Moore et al., 1991: case 2 [31] | 35 | LMA, right lower third | 1 | NR |
| Sivanesaratnam et al., 1990 [32] | 28 | Periclitoral (then upper half of bilateral LMA, clitoris, lower mons pubis) | 2 | Fan-shaped, exophytic |
| Robson et al., 1989 [33] | 33 | LMA, left | NR | Ulcerated |
| Rahman et al., 1982 [34] | 28 | LMA, left upper third, involving the clitoris | 3 | Proliferative |
| Kempers et al., 1965: case 1 [35] | 25 | Posterior vulva | large | Fungating, ulcerated |
| Kempers et al., 1965: case 2 [35] | 32 | Posterior vulva and perineum | large | Fungating |
| Collins et al., 1963: case 3 [36] | 28 | Lmi, left (upper third) | 2 | Ulcerated |
| Collins et al., 1963: case 4 [36] | 26 | (1) Lmi, right (upper 2/3) (2) LMA, left | (1) 5 (2) small | (1) Exophytic (2) Ulcerated |
| Collins et al., 1963: case 5 [36] | 31 | LMA, right (middle third) | 4 | Flat, hard |
| Barber et al., 1963: case 3 [37] | 27 | LMA, bilateral | NR | NR |
| Gemmell et al., 1962: case 10 [38] | 30 | Clitoris | small | Ulcerated |
| De Bruine TLA, 1958 [39] | 32 | Clitoris, extending in all directions | 4 | Ulcerated |
| Shannon et al., 1941 [40] | 26 | LMA, right | 6 | Fungating, ulcerated |
| Russell et al., 1940 [41] | 17 | LMA, left | 4 | Raised |
| Authors | Grade | Stage (°) | HPV-Related Lesions | Inflammatory Conditions |
|---|---|---|---|---|
| Gitsham et al. [11] | G2 | IIIA | NR | NR |
| Metke et al. [12] | NR | IB | NR | NR |
| Lecointre et al. [13] | G3 | IVB | NR | Recurrent vulvar psoriasis |
| Hasanzadeh et al. [14] | NR | IB | NR | VH + CD (history) |
| Idi et al. [15] | G3 | IIIA | NR | NR |
| Pariyar et al. [16] | NR | III | NR | NR |
| Nijman et al. [17] | G1 | IB | no HPV | Lichen sclerosus |
| Parva et al. [18] | G1 | IB | H-SIL (VIN2) | Lichen sclerosus |
| Keskin et al.: case 3 [19] | G1 | IIIA | NR | NR |
| Ghosh et al. [20] | G3 | IVB(m) | NR | NR |
| Modares Gilani et al. [21] | G1 | IIIA | VCs/HPV (history) | NR |
| Alexander-Sefre et al. [22] | G1 | IA | No SIL | HLP + HVD |
| Ogunleye et al. [23] | G2 | IB(m) | H-SIL (VIN3) | Lichen sclerosus |
| Couvreux-Dif et al.: case 1 [24] | G1 | IA | NR | Lichen sclerosus |
| Couvreux-Dif et al.: case 2 [24] | G1 | IB(m) | VCs, H-SIL (bowenoid VIN) | NR |
| Olayemi et al. [25] | NR | IB (@) | VC (?) | NR |
| Bakour et al. [26] | G3 | IIIA | NR | NR |
| Heller et al. [27] | G2 | IIIA | VCs, H-SIL (cervix, vulva, anus) | NR |
| Gitsch et al.: case 1 [28] | G2 | IB | NR | NR |
| Gitsch et al.: case 2 [28] | NR | IIIA(m) | VCs/HPV, H-SIL (VIN3, cervix), VW | NR |
| Regan et al. [29] | G2 | IB | VCs, PBD (#) | NR (#) |
| Del Priore et al. [30] | G2 | IB | NR | Lichen sclerosus |
| Moore et al.: case 1 [31] | G2 | IIIB (m) | H-SIL (VIN3) | NR |
| Moore et al.: case 2 [31] | G2 | IIIB | NR | NR |
| Sivanesaratnam et al. [32] | G1 (§) | IVB | NR | NR |
| Robson et al. [33] | G2 | I | NR | NR |
| Rahman et al. [34] | G1 | IB | NR (#) | NR (#) |
| Kempers et al.: case 1 [35] | G3 | IIIB | NR | NR |
| Kempers et al.: case 2 [35] | G1 | II | NR | NR |
| Collins et al.: case 3 [36] | NR | IA | NR | NR |
| Collins et al.: case 4 [36] | NR | IB(m) | VCs, vulvar H-SIL (CIS), PCs | NR |
| Collins et al.: case 5 [36] | NR | IB | NR | NR |
| Barber et al.: case 3 [37] | NR | I | NR | NR |
| Gemmell et al.: case 10 [38] | G1 | IA | NR (#) | NR (#) |
| De Bruine TLA [39] | NR | IB | NR | Severe kraurosis vulvae |
| Shannon et al. [40] | NR | IB | VCs | NR |
| Russell et al. [41] | NR | II | NR | NR |
| Authors | Recurrence | Follow-Up |
|---|---|---|
| Gitsham et al. [11] | Local (6 mo) (wide excision + left IF-LND + local RT) | AWD (6 mo) |
| Metke et al. [12] | No | NED (6 mo) |
| Lecointre et al. [13] | PD dp (vulva: 13 + 2 we ad; lungs: 5 mo ad) | DOD (cardiorespiratory decompensation for pulmonary MTS) (5 mo) |
| Hasanzadeh et al. [14] | No | NED (24 mo) |
| Idi et al. [15] | PD (right inguinal) (no treatment; lack of RT) | DOD (5 mo) |
| Pariyar et al. [16] | NR | NR |
| Nijman et al. [17] | No | NED (12 mo) |
| Parva et al. [18] | (1) Subcutis (6 mo) (excision with negative margins); (2) Subcutis (NR) (FNA + ChT/RT) | AWD (>6 mo) |
| Keskin et al.: case 3 [19] | Inguinal lymph nodes (36 mo) (salvage therapy) | DOD (48 mo) |
| Ghosh et al. [20] | PD (bilateral lower limb edema, enlarged groin nodes) (3 mo) (declined ChT) | DOD (11 mo) |
| Modares Gilani et al. [21] | No | NED (7 mo) |
| Alexander-Sefre et al. [22] | No | NED (4 mo) |
| Ogunleye et al. [23] | No (*) | NED (16 mo) |
| Couvreux-Dif et al.: case 1 [24] | No | NED (33 mo) |
| Couvreux-Dif et al.: case 2 [24] | No (*) | NED (19 mo) |
| Olayemi et al. [25] | PD (abdomen, anus, inguinal) (15 we ad) (*) (i.v. fluids, antibiotics, blood transfusions, suprapubic cystostomy) | DOD (sepsis) (4 mo) |
| Bakour et al. [26] | PD (new inguinal lesions) (after RT completion) (palliative) | DOD (9 mo) |
| Heller et al. [27] | NR | NED or AWD (lost at FU) |
| Gitsch et al.: case 1 [28] | No | NED (39 mo) |
| Gitsch et al.: case 2 [28] | Periclitoral maculo-papular eruption (23 mo) (biopsy + anterior superficial vulvectomy) (VIN3; VIN2 on surgical margin) | NED (28 mo) |
| Regan et al. [29] | NR | NED or AWD (lost at FU) |
| Del Priore et al. [30] | NR | NR |
| Moore et al.: case 1 [31] | No | NED (16 mo) |
| Moore et al.: case 2 [31] | Lumbar vertebrae, periaortic lymph nodes (16 mo) (palliative RT) | DOD (27 mo) |
| Sivanesaratnam et al. [32] | PD (NR) | DOD (sepsis) (2.5 mo) |
| Robson et al. [33] | No | NED (6 mo) |
| Rahman et al. [34] | No | NED (24 mo) |
| Kempers et al.: case 1 [35] | No | NED (96 mo) |
| Kempers et al.: case 2 [35] | No | NED (48 mo) |
| Collins et al.: case 3 [36] | No | NED (54 mo) |
| Collins et al.: case 4 [36] | Bilateral sides of introitus (12 mo) (wide excision of 5 vulvar and perineal areas; diagnosis: superficially-invading SCC, CIS, MAEH) Cervical stage 2 SCC (9 years; RT). Small vaginal areas of CIS (16 years) (wide excision) | NED (204 mo) |
| Collins et al.: case 5 [36] | No | NED (92 mo) |
| Barber et al.: case 3 [37] | No | NED (72 mo) |
| Gemmell et al.: case 10 [38] | No | NED (53 mo) |
| De Bruine TLA [39] | No | NED (50 mo) |
| Shannon et al. [40] | No | NED (17 mo) |
| Russell et al. [41] | No | NED (60 mo) |
| Authors. | Age | Pregnancy History | Time of Delivery | Delivery-Type | Relevant Events of Ongoing Pregnancy | Baby Weight (gr) |
|---|---|---|---|---|---|---|
| Gitsham et al. [11] | 40 | G3P2A1 | 37 GW + 2 d | CS | NR | NR |
| Metke et al. [12] | 39 | NR | NR | NR | NR | NR |
| Lecointre et al. [13] | 29 | G2P0A2 (#) | 29 GW + 1 d | CS (emergency for sepsis) | Sepsis (Pseudomonas aeruginosa) of vulvar origin, uncontrolled by antibiotics | 1296 |
| Hasanzadeh et al. [14] | 37 | G8P6 (§) | term | CS | NR | NR |
| Idi et al. [15] | 32 | G6P5 (1 EPr 3 years before) | NR | CS | NR | NR |
| Pariyar et al. [16] | 20 | G1P0 | NR | VA | NR | NR |
| Nijman et al. [17] | 27 | NR | 38 GW + 3 d | CS | Obstetrical reasons lead to CS | NR |
| Parva et al. [18] | 29 | G0P0 | 32 GW | CS (elective, after 1 course of steroids for lung maturation) | no | 1928 |
| Keskin et al.: case 3 [19] | 31 | G3P2 | NR (>31) | CS | no | NR |
| Ghosh et al. [20] | 41 | G2P1(*) A1 | term + 7 d | CS (lower segment) | Prolonged pregnancy | 3320 |
| Modares Gilani et al. [21] | 28 | G6P5 | 36 GW | CS | no | 2800 |
| Alexander-Sefre et al. [22] | 18 | G0P0 | 29 GW | VA | (@) | 1000 |
| Ogunleye et al. [23] | 36 | G5P4 | 37 GW | VA (induced) | Subsequent focus of VSCC (34 GW) | NR |
| Couvreux-Dif et al.: case 1 [24] | 34 | G4P3 | 38 GW | VA + right lateral EP | no | 3750 |
| Couvreux-Dif et al.: case 2 [24] | 31 | G4P3 | 38 GW | CS | Subsequent focus of VSCC (22 GW, 2 weeks after surgery); VC (right labium minus, 27 GW); vulvar scars; abnormal fetal heartbeat; membrane rupture | 2300 |
| Olayemi et al. [25] | 29 | G2P2 (none alive) | 37 GW | CS (elective) | no | 2250 |
| Bakour et al. [26] | 29 | G1P1 (°) | 38 GW | CS (elective) | Uterine fibroids Small antepartum hemorrhage | NR |
| Heller et al. [27] | 28 | G5P5 | NR | CS | NR | NR |
| Gitsch et al.: case 1 [28] | 29 | G4P3 | 40 GW | CS | CS for vulvar scars | NR |
| Gitsch et al.: case 2 [28] | 35 | G4P3 | 35 GW | CS | NR | NR |
| Regan et al. [29] | 37 | G3P1A2 | 38 GW | VA + midline EP (no laceration or extension) | Chlamydia infection (treated) Cocaine and heroin abuse | 2780 |
| Del Priore et al. [30] | 39 | G8P4A4 | 38 GW | VA (#) + midline EP extended into 4th degree laceration | Suppurative vulvar cellulitis (intravenous antibiotics) (25–27 GW) | 3990 |
| Moore et al.: case 1 [31] | 24 | G0P0 | 36 GW | CS + I/E-I-LNS + right oophoropexy to right paracolic gutter | Amniocentesis: lecithin–sphingomyelin ratio >2.0; presence of phosphatidyl glycerol. | 2670 |
| Moore et al.: case 2 [31] | 35 | G9P6A3 | term | VA (#) | NR | NR |
| Sivanesaratnam et al. [32] | 28 | G2P1 | term | VA (#) | NR | 3500 |
| Robson et al. [33] | 33 | G0P0 | >24 GW | CS | no | NR |
| Rahman et al. [34] | 28 | G7P6 | term | VA + right mediolateral EP | no | 3400 |
| Kempers et al.: case 1 [35] | 25 | G4P4 | NR | VA | NR | NR |
| Kempers et al.: case 2 [35] | 32 | G7P6 | 37 GW | VA (#) + bilateral EPs | no | NR |
| Collins et al.: case 3 [36] | 28 | G8P6A2 | 37 GW | low-forceps under spinal anesthesia + PC | no | 2268 |
| Collins et al.: case 4 [36] | 26 | G4P2 | 8 mo | VA (#) | NR | NR |
| Collins et al.: case 5 [36] | 31 | G7P6 | NR | VA (#) + left mediolateral EP | Stillborn | NR |
| Barber et al.: case 3 [37] | 27 | G2P2 | NR | CS + completion surgery | NR | NR |
| Gemmell et al.: case 10 [38] | 30 | G0P0 | 9 mo | CS (hemorrhage) | Hypertension and proteinuria at 34 GW; hemorrhage at 36–37 GW | 2295 |
| De Bruine TLA [39] | 32 | G0P0 | 9 mo | CS (lower segment) | Progesterone therapy to protect pregnancy during surgery | 3490 |
| Shannon et al. [40] | 26 | G2P0A2 | 8 mo | CS | no | 2807 |
| Russell et al. [41] | 17 | G0P0 | 8 mo | CS | (ç) | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palicelli, A.; Giaccherini, L.; Zanelli, M.; Bonasoni, M.P.; Gelli, M.C.; Bisagni, A.; Zanetti, E.; De Marco, L.; Torricelli, F.; Manzotti, G.; et al. How Can We Treat Vulvar Carcinoma in Pregnancy? A Systematic Review of the Literature. Cancers 2021, 13, 836. https://doi.org/10.3390/cancers13040836
Palicelli A, Giaccherini L, Zanelli M, Bonasoni MP, Gelli MC, Bisagni A, Zanetti E, De Marco L, Torricelli F, Manzotti G, et al. How Can We Treat Vulvar Carcinoma in Pregnancy? A Systematic Review of the Literature. Cancers. 2021; 13(4):836. https://doi.org/10.3390/cancers13040836
Chicago/Turabian StylePalicelli, Andrea, Lucia Giaccherini, Magda Zanelli, Maria Paola Bonasoni, Maria Carolina Gelli, Alessandra Bisagni, Eleonora Zanetti, Loredana De Marco, Federica Torricelli, Gloria Manzotti, and et al. 2021. "How Can We Treat Vulvar Carcinoma in Pregnancy? A Systematic Review of the Literature" Cancers 13, no. 4: 836. https://doi.org/10.3390/cancers13040836
APA StylePalicelli, A., Giaccherini, L., Zanelli, M., Bonasoni, M. P., Gelli, M. C., Bisagni, A., Zanetti, E., De Marco, L., Torricelli, F., Manzotti, G., Gugnoni, M., D’Ippolito, G., Falbo, A. I., Sileo, F. G., Aguzzoli, L., Mastrofilippo, V., Bonacini, M., De Giorgi, F., Ricci, S., ... Mandato, V. D. (2021). How Can We Treat Vulvar Carcinoma in Pregnancy? A Systematic Review of the Literature. Cancers, 13(4), 836. https://doi.org/10.3390/cancers13040836













