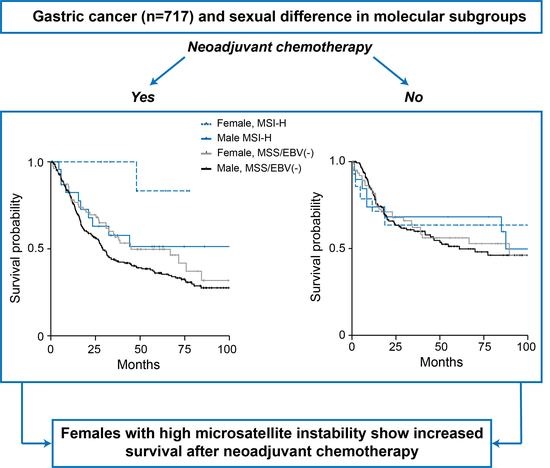
Sexual Difference Matters: Females with High Microsatellite Instability Show Increased Survival after Neoadjuvant Chemotherapy in Gastric Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Chemotherapy
2.3. Response Evaluation
2.4. Follow-Up and Overall Survival
2.5. Molecular Analysis
2.6. Statistical Analysis
3. Results
3.1. Sex/Age and Association with Patient Characteristics and Response to Chemotherapy
3.2. Sex/Age and Survival of the Patients
3.3. Sex/Age and Frequency of Molecular Subgroups and Association with Patient Characteristics and Response to Chemotherapy
3.4. Sex-Specificity of the Molecular Subgroups and Survival of the Patients
3.5. Multivariable Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van De Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ychou, M.; Boige, V.; Pignon, J.-P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.-M.; Saint-Aubert, B.; et al. Perioperative Chemotherapy Compared with Surgery Alone for Resectable Gastroesophageal Adenocarcinoma: An FNCLCC and FFCD Multicenter Phase III Trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Özdemir, B.C.; Csajka, C.; Dotto, G.P.; Wagner, A.D. Sex Differences in Efficacy and Toxicity of Systemic Treatments: An Undervalued Issue in the Era of Precision Oncology. J. Clin. Oncol. 2018, 36, 2680–2683. [Google Scholar] [CrossRef]
- Rutegård, M.; Shore, R.; Lu, Y.; Lagergren, P.; Lindblad, M. Sex differences in the incidence of gastrointestinal adenocarcinoma in Sweden 1970–2006. Eur. J. Cancer 2010, 46, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Chon, H.J.; Hyung, W.J.; Kim, C.; Park, S.; Kim, J.H.; Park, C.H.; Ahn, J.B.; Kim, H.; Chung, H.C.; Rha, S.Y.; et al. Differential Prognostic Implications of Gastric Signet Ring Cell Carcinoma: Stage Adjusted Analysis from a Single High-volume Center in Asia. Ann. Surg. 2017, 265, 946–953. [Google Scholar] [CrossRef] [Green Version]
- Feng, F.; Liu, J.; Wang, F.; Zheng, G.; Wang, Q.; Liu, S.; Xu, G.; Guo, M.; Lian, X.; Zhang, H. Prognostic value of differentiation status in gastric cancer. BMC Cancer 2018, 18, 1–6. [Google Scholar] [CrossRef]
- Lu, M.; Yang, Z.; Feng, Q.; Yu, M.; Zhang, Y.; Mao, C.; Shen, L.; Tang, J. The characteristics and prognostic value of signet ring cell histology in gastric cancer: A retrospective cohort study of 2199 consecutive patients. Medicine 2016, 95, e4052. [Google Scholar] [CrossRef]
- Kim, S.M.; Min, B.H.; Lee, J.; An, J.Y.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; Kim, J.J.; Kang, W.K.; Kim, S.; et al. Protective Effects of Female Reproductive Factors on Lauren Intestinal-Type Gastric Adenocarcinoma. Yonsei Med. J. 2018, 59, 28–34. [Google Scholar] [CrossRef]
- Micheli, A.; Mariotto, A.; Rossi, A.G.; Gatta, G.; Muti, P. The prognostic role of gender in survival of adult cancer patients. Eur. J. Cancer 1998, 34, 2271–2278. [Google Scholar] [CrossRef]
- Athauda, A.; Nankivell, M.; Langley, R.E.; Alderson, D.; Allum, W.; Grabsch, H.I.; Starling, N.; Chau, I.; Cunningham, D. Impact of sex and age on chemotherapy efficacy, toxicity and survival in localised oesophagogastric cancer: A pooled analysis of 3265 individual patient data from four large randomised trials (OE02, OE05, MAGIC and ST03). Eur. J. Cancer 2020, 137, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Polom, K.; Marano, L.; Marrelli, D.; De Luca, R.; Roviello, G.; Savelli, V.; Tan, P. Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br. J. Surg. 2018, 105, 159–167. [Google Scholar] [CrossRef]
- Ratti, M.; Lampis, A.; Hahne, J.C.; Passalacqua, R.; Valeri, N. Microsatellite instability in gastric cancer: Molecular bases, clinical perspectives, and new treatment approaches. Cell. Mol. Life Sci. 2018, 75, 4151–4162. [Google Scholar] [CrossRef]
- Kohlruss, M.; Grosser, B.; Krenauer, M.; Slotta-Huspenina, J.; Jesinghaus, M.; Blank, S.; Novotny, A.; Reiche, M.; Schmidt, T.; Ismani, L.; et al. Prognostic implication of molecular subtypes and response to neoadjuvant chemotherapy in 760 gastric carcinomas: Role of Epstein—Barr virus infection and high- and low-microsatellite instability. J. Pathol. Clin. Res. 2019, 5, 227–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [Green Version]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.T.; Cristescu, R.; Bass, A.J.; Kim, K.-M.; Odegaard, J.I.; Kim, K.; Liu, X.Q.; Sher, X.; Jung, H.; Lee, M.; et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 2018, 24, 1449–1458. [Google Scholar] [CrossRef]
- Smyth, E.C.; Wotherspoon, A.; Peckitt, C.; Gonzalez, D.; Hulkki-Wilson, S.; Eltahir, Z.; Fassan, M.; Rugge, M.; Valeri, N.; Okines, A.; et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol. 2017, 3, 1197–1203. [Google Scholar] [CrossRef] [Green Version]
- Lordick, F. Chemotherapy for resectable microsatellite instability-high gastric cancer? Lancet Oncol. 2020, 21, 203. [Google Scholar] [CrossRef]
- Smyth, E.C. Chemotherapy for resectable microsatellite instability-high gastric cancer? Lancet Oncol. 2020, 21, 204. [Google Scholar] [CrossRef]
- Siewert, J.R.; Stein, H.J. Classification of adenocarcinoma of the oesophagogastric junction. Br. J. Surg. 1998, 85, 1457–1459. [Google Scholar]
- Becker, K.; Langer, R.; Reim, D.; Novotny, A.; zum Buschenfelde, C.M.; Engel, J.; Friess, H.; Hofler, H. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: A summary of 480 cases. Ann. Surg. 2011, 253, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Sicic, L.; Blank, S.; Becker, K.; Weichert, W.; Bruckner, T.; Parakonthun, T.; Langer, R.; Büchler, M.W.; Siewert, J.-R.; et al. Prognostic value of histopathological regression in 850 neoadjuvantly treated oesophagogastric adenocarcinomas. Br. J. Cancer 2014, 110, 1712–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, L.; Hapfelmeier, A.; Blank, S.; Reiche, M.; Slotta-Huspenina, J.; Jesinghaus, M.; Novotny, A.; Schmidt, T.; Grosser, B.; Kohlruss, M.; et al. A novel pretherapeutic gene expression-based risk score for treatment guidance in gastric cancer. Ann. Oncol. 2018, 29, 127–132. [Google Scholar] [CrossRef]
- Boland, C.R.; Thibodeau, S.N.; Hamilton, S.R.; Sidransky, D.; Eshleman, J.R.; Burt, R.W.; Meltzer, S.J.; A Rodriguez-Bigas, M.; Fodde, R.; Ranzani, G.N.; et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998, 58, 5248–5257. [Google Scholar] [PubMed]
- Bacher, J.W.; Flanagan, L.A.; Smalley, R.L.; Nassif, N.A.; Burgart, L.J.; Halberg, R.B.; Megid, W.M.A.; Thibodeau, S.N. Development of a Fluorescent Multiplex Assay for Detection of MSI-High Tumors. Dis. Markers 2004, 20, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Nardon, E.; Glavač, D.; Benhattar, J.; Groenen, P.J.; Höfler, G.; Höfler, H.; Jung, A.; Keller, G.; Kirchner, T.; Lessi, F.; et al. A Multicenter Study to Validate the Reproducibility of MSI Testing with a Panel of 5 Quasimonomorphic Mononucleotide Repeats. Diagn. Mol. Pathol. 2010, 19, 236–242. [Google Scholar] [CrossRef]
- Geller, S.E.; Koch, A.; Pellettieri, B.; Carnes, M. Inclusion, Analysis, and Reporting of Sex and Race/Ethnicity in Clinical Trials: Have We Made Progress? J. Women Health 2011, 20, 315–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheatley-Price, P.; Blackhall, F.; Lee, S.-M.; Ma, C.; Ashcroft, L.; Jitlal, M.; Qian, W.; Hackshaw, A.; Rudd, R.; Booton, R.; et al. The influence of sex and histology on outcomes in non-small-cell lung cancer: A pooled analysis of five randomized trials. Ann. Oncol. 2010, 21, 2023–2028. [Google Scholar] [CrossRef]
- Elsaleh, H.; Joseph, D.; Grieu, F.; Zeps, N.; Spry, N.; Iacopetta, B. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet 2000, 355, 1745–1750. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Merz, N.B.; Barnes, P.J.; Brinton, R.D.; Carrero, J.-J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Rottenberg, S.; Disler, C.; Perego, P. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 2021, 21, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Rivera Vargas, T.; Apetoh, L. Danger signals: Chemotherapy enhancers? Immunol. Rev. 2017, 280, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Mlecnik, B.; Bifulco, C.; Bindea, G.; Marliot, F.; Lugli, A.; Lee, J.J.; Zlobec, I.; Rau, T.T.; Berger, M.D.; Nagtegaal, I.D.; et al. Multicenter International Society for Immunotherapy of Cancer Study of the Consensus Immunoscore for the Prediction of Survival and Response to Chemotherapy in Stage III Colon Cancer. J. Clin. Oncol. 2020, 38, 3638–3651. [Google Scholar] [CrossRef] [PubMed]
- Bruni, D.; Angell, H.K.; Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Miceli, R.; Raimondi, A.; Kim, Y.W.; Kang, W.K.; Langley, R.E.; Choi, Y.Y.; Kim, K.-M.; Nankivell, M.G.; Morano, F.; et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability as a Biomarker in Gastric Cancer. J. Clin. Oncol. 2019, 37, 3392–3400. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.; Hubner, R.; Houlston, R. Systematic Review of Microsatellite Instability and Colorectal Cancer Prognosis. J. Clin. Oncol. 2005, 23, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Morihiro, T.; Kuroda, S.; Kanaya, N.; Kakiuchi, Y.; Kubota, T.; Aoyama, K.; Tanaka, T.; Kikuchi, S.; Nagasaka, T.; Nishizaki, M.; et al. PD-L1 expression combined with microsatellite instability/CD8+ tumor infiltrating lymphocytes as a useful prognostic biomarker in gastric cancer. Sci. Rep. 2019, 9, 4633. [Google Scholar] [CrossRef] [Green Version]
- Supek, F.; Lehner, B. Differential DNA mismatch repair underlies mutation rate variation across the human genome. Nat. Cell Biol. 2015, 521, 81–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoos, A.; Britten, C.M. The immuno-oncology framework: Enabling a new era of cancer therapy. Oncoimmunology 2012, 1, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Liu, L.; Chen, H.; Wang, Y.; Xu, Y.; Mao, H.; Li, J.; Mills, G.B.; Shu, Y.; Li, L.; et al. Comprehensive Characterization of Molecular Differences in Cancer between Male and Female Patients. Cancer Cell 2016, 29, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Grosser, B.; Kohlruss, M.; Slotta-Huspenina, J.; Jesinghaus, M.; Pfarr, N.; Steiger, K.; Novotny, A.; Gaida, M.M.; Schmidt, T.; Hapfelmeier, A.; et al. Impact of Tumor Localization and Molecular Subtypes on the Prognostic and Predictive Significance of p53 Expression in Gastric Cancer. Cancers 2020, 12, 1689. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; de Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Derakhshan, M.H.; Liptrot, S.; Paul, J.; Brown, I.L.; Morrison, D.; McColl, K.E. Oesophageal and gastric intestinal-type adenocarcinomas show the same male predominance due to a 17 year delayed development in females. Gut 2009, 58, 16–23. [Google Scholar] [CrossRef]
- Ławniczak, M.; Gawin, A.; Jaroszewicz-Heigelmann, H.; Rogoza-Mateja, W.; Białek, A.; Kulig, J.; Kaczmarczyk, M.; Starzyńska, T. Analysis of clinicopathologic characteristics of gastric cancer in patients ≤40 and ≥40 years of age. Scand. J. Gastroenterol. 2020, 55, 62–66. [Google Scholar] [CrossRef] [PubMed]




| Category | Value | Female n (%) | Male n (%) | p-Value 1 | <55 Years n (%) | ≥55
Years n (%) | p-Value 1 |
|---|---|---|---|---|---|---|---|
| Cases | Total | 188 (100) | 529 (100) | 174 (100) | 543 (100) | ||
| Tumor localization | Proximal | 75 (39.9) | 298 (56.3) | 0.001 | 94 (54.0) | 279 (51.4) | 0.581 |
| Middle | 52 (27.7) | 116 (21.9) | 39 (22.4) | 129 (23.7) | |||
| Distal | 47 (25.0) | 92 (17.4) | 36 (20.7) | 103 (19.0) | |||
| Total/linitis | 14 (7.4) | 16 (3.6) | 5 (2.9) | 28 (5.2) | |||
| n/a | - | 4 (<1) | - | 4 (<1) | |||
| Proximal versus non-proximal | Proximal | 75 (39.9) | 298 (56.3) | <0.001 | 94 (54.0) | 279 (51.4) | 0.604 |
| Non-proximal | 113. (60.1) | 227 (42.9) | 80 (46.0) | 260 (47.9) | |||
| n/a | - | 4 (<1) | - | 4 (<1) | |||
| Laurén classification | Intestinal | 82 (43.6) | 315 (59.5) | <0.001 | 81 (46.6) | 316 (58.2) | 0.007 |
| Non-intestinal | 106 (56.4) | 214 (40.5) | 93 (53.4) | 227 (41.8) | |||
| Tumor grade | G1/2 | 36 (19.1) | 111 (21.0) | 0.485 | 27 (15.5) | 120 (22.1) | 0.061 |
| G3/4 | 131 (69.7) | 347 (65.6) | 124 (71.3) | 354 (65.2) | |||
| n/a | 21 (11.2) | 71 (13.4) | 23 (13.2) | 69 (12.7) | |||
| Clinical tumor stage | cT2 | 46 (24.5) | 103 (19.5) | 0.123 | 29 (16.7) | 120 (22.1) | 0.135 |
| cT3/cT4 | 138 (73.4) | 422 (79.8) | 142 (81.6) | 418 (77.0) | |||
| n/a | 4 (2.1) | 4 (<1) | 3 (1.7) | 5 (<1) | |||
| (y)pT 2 | (y)pT0 | 5 (2.7) | 4 (0.8) | 0.030 | 4 (2.3) | 5 (0.9) | 0.293 |
| (y)pT1 | 19 (10.1) | 48 (9.1) | 14 (8.1) | 53 (9.8) | |||
| (y)pT2 | 24 (12.8) | 70 (13.2) | 17 (9.8) | 77 (14.2) | |||
| (y)pT3 | 85 (45.2) | 297 (56.1) | 99 (56.9) | 283 (52.1) | |||
| (y)pT4 | 53 (28.2) | 110 (20.8) | 39 (22.4) | 124 (22.8) | |||
| n/a | 2 (1.1) | - | 1 (<1) | 1 (<1) | |||
| (y)pN 2 | Negative | 60 (31.9) | 179 (33.8) | 0.695 | 48 (27.6) | 191 (35.2) | 0.069 |
| Positive | 126 (67.0) | 350 (66.2) | 125 (71.8) | 351 (64.6) | |||
| n/a | 2 (1.1) | - | 1 (<1) | 1 (<1) | |||
| Metastasis status | No | 156 (83.0) | 442 (83.6) | 0.920 | 136 (78.2) | 462 (85.1) | 0.040 |
| Yes | 30 (15.9) | 87 (16.4) | 37 (21.3) | 80 (14.7) | |||
| n/a | 2 (1.1) | - | 1 (<1) | 1 (<1) | |||
| Resection category | R0 | 148 (78.7) | 402 (76.0) | 0.319 | 130 (74.7) | 420 (77.3) | 0.524 |
| R1 | 38 (20.2) | 127 (24.0) | 43 (27.7) | 122 (22.5) | |||
| n/a | 2 (1.1) | - | 1 (<1) | 1 (<1) | |||
| Neoadjuvant CTx | No | 98 (52.1) | 193 (36.5) | <0.001 | 50 (28.7) | 241 (44.4) | <0.001 |
| Yes | 90 (47.9) | 336 (63.5) | 124 (71.3) | 302 (55.6) | |||
| Response 3 | Responder (TRG1) | 12 (13.3) | 33 (9.8) | 0.336 | 16 (12.9) | 29 (9.6) | 0.314 |
| Non-responder (TRG2/3) 4 | 78 (86.7) | 303 (90.2) | 108 (87.1) | 273 (90.4) | |||
| Total 3 | 90 (100) | 336 (100) | 124 (100) | 302 (100) | |||
| MSI status | MSS | 159 (84.6) | 454 (85.8) | 0.278 | 162 (93.1) | 451 (83.1) | 0.004 |
| MSI-L | 7 (3.7) | 30 (5.7) | 5 (2.9) | 32 (5.9) | |||
| MSI-H | 22 (11.7) | 45 (8.5) | 7 (4.0) | 60 (11.0) | |||
| EBV status | EBV negative | 186 (98.9) | 502 (94.9) | 0.016 | 163 (93.7) | 525 (96.7) | 0.080 |
| EBV positive | 2 (1.1) | 27 (5.1) | 11 (6.3) | 18 (3.3) |
| Patient Cohort and Subgroups | Sex | No. | Events | Survival Probability (%) | Median Survival (Months) | HR | p-Value 1 | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 Year | 3 Years | 5 Years | (95% CI) | (95% CI) | |||||
| All tumor specimens | Female | 188 | 78 | 79.7 | 61.4 | 55.2 | 75.8 (42.6–109.0) | 0.77 (0.60–0.99) | 0.035 |
| Male | 529 | 266 | 78.4 | 51.4 | 43.8 | 41.7 (29.8–53.6) | 1 ref. | - | |
| Total | 717 | 344 | 78.8 | 54.0 | 46.8 | 46.3 (33.1–59.5) | - | - | |
| Tumors with neoadjuvant CTx | Female | 90 | 41 | 79.8 | 61.1 | 53.4 | 71.6 (42.4–100.9) | 0.72 (0.51–1.01) | 0.054 |
| Male | 336 | 181 | 77.7 | 46.4 | 38.9 | 31.4 (22.1–40.7) | 1 ref. | - | |
| Total | 426 | 222 | 78.1 | 49.7 | 42.0 | 35.9 (27.2–44.6) | - | - | |
| Tumors without neoadjuvant CTx | Female | 98 | 37 | 79.8 | 61.9 | 57.2 | nr | 0.90 (0.61–1.32) | 0.587 |
| Male | 193 | 85 | 79.8 | 59.9 | 52.4 | 77.3 (36.7–117.9) | 1 ref. | - | |
| Total | 291 | 122 | 79.7 | 60.5 | 54.0 | 85.0 (51.7–118.3) | - | - | |
| Patient Cohort and Subgroups | Sex and MSI Status | No. | Events | Survival Probability (%) | Median Survival (Months) | HR | p-Value 1 | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 Year | 3 Years | 5 Years | (95% CI) | (95% CI) | |||||
| All tumor specimens | 0.031 | ||||||||
| Female, MSI-H | 22 | 6 | 81.8 | 77.0 | 71.1 | nr | 0.39 (0.18–0.89) | 0.024 | |
| Female, MSS/EBV(−) | 158 | 69 | 79.7 | 59.1 | 52.6 | 66.9 (33.9–99.9) | 0.79 (0.61–1.04) | 0.095 | |
| Male, MSI-H | 45 | 18 | 78.5 | 62.6 | 59.3 | 87.5 (-) | 0.69 (0.43–1.11) | 0.126 | |
| Male, MSS/EBV(−) | 428 | 222 | 78.5 | 49.3 | 41.4 | 33.8 (24.1–43.5) | 1 ref. | - | |
| Total 2 | 653 | 315 | 78.9 | 53.6 | 46.5 | 45.3 (31.5–59.1) | - | - | |
| Tumors with neoadjuvant CTx | 0.056 | ||||||||
| Female, MSI-H | 8 | 1 | 100 | 100 | 83.3 | 0.13 (0.02–0.94) | 0.043 | ||
| Female, MSS/EBV(−) | 78 | 39 | 78.1 | 56.7 | 49.7 | 44.9 (14.2–75.6) | 0.75 (0.53–1.07) | 0.114 | |
| Male, MSI-H | 24 | 10 | 82.4 | 57.7 | 51.3 | nr | 0.66 (0.35–1.25) | 0.198 | |
| Male, MSS/EBV(−) | 274 | 156 | 76.3 | 43.4 | 36.1 | 29.1 (25.2–33.0) | 1 ref. | - | |
| Total 2 | 384 | 206 | 77.8 | 48.5 | 40.9 | 33.8 (25.5–42.1) | - | - | |
| Tumors without neoadjuvant CTx | 0.898 | ||||||||
| Female, MSI-H | 14 | 5 | 71.4 | 63.5 | 63.5 | nr | 0.76 (0.30–1.88) | 0.548 | |
| Female, MSS/EBV(−) | 80 | 30 | 81.7 | 62.1 | 56.1 | 89.5 (-) | 0.94 (0.61–1.45) | 0.772 | |
| Male, MSI-H | 21 | 8 | 73.9 | 68.2 | 68.2 | 87.5 (-) | 0.82 (0.39–1.72) | 0.604 | |
| Male, MSS/EBV(−) | 154 | 66 | 81.7 | 59.9 | 51.2 | 61.1 (25.5–96.7) | 1 ref. | - | |
| Total 2 | 269 | 109 | 80.5 | 61.5 | 54.9 | 87.5 (-) | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohlruss, M.; Ott, K.; Grosser, B.; Jesinghaus, M.; Slotta-Huspenina, J.; Novotny, A.; Hapfelmeier, A.; Schmidt, T.; Gaida, M.M.; Weichert, W.; et al. Sexual Difference Matters: Females with High Microsatellite Instability Show Increased Survival after Neoadjuvant Chemotherapy in Gastric Cancer. Cancers 2021, 13, 1048. https://doi.org/10.3390/cancers13051048
Kohlruss M, Ott K, Grosser B, Jesinghaus M, Slotta-Huspenina J, Novotny A, Hapfelmeier A, Schmidt T, Gaida MM, Weichert W, et al. Sexual Difference Matters: Females with High Microsatellite Instability Show Increased Survival after Neoadjuvant Chemotherapy in Gastric Cancer. Cancers. 2021; 13(5):1048. https://doi.org/10.3390/cancers13051048
Chicago/Turabian StyleKohlruss, Meike, Katja Ott, Bianca Grosser, Moritz Jesinghaus, Julia Slotta-Huspenina, Alexander Novotny, Alexander Hapfelmeier, Thomas Schmidt, Matthias M. Gaida, Wilko Weichert, and et al. 2021. "Sexual Difference Matters: Females with High Microsatellite Instability Show Increased Survival after Neoadjuvant Chemotherapy in Gastric Cancer" Cancers 13, no. 5: 1048. https://doi.org/10.3390/cancers13051048