Use of Measurable Residual Disease to Evolve Transplant Policy in Acute Myeloid Leukemia: A 20-Year Monocentric Observation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Transplantation Regimens
2.2. Immunophenotypic Studies and MRD Detection
2.3. Statistical Analysis and Outcome Definitions
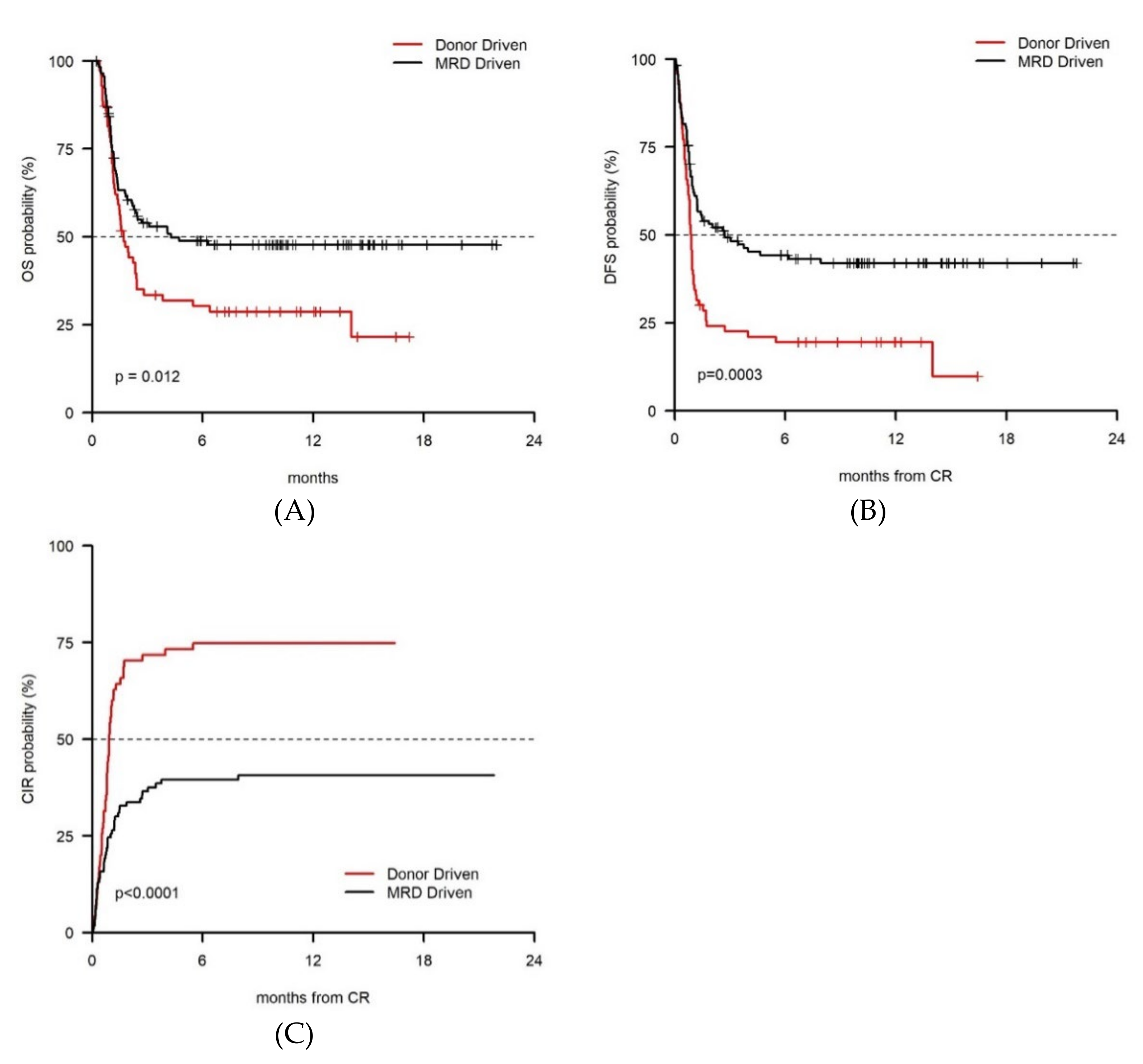
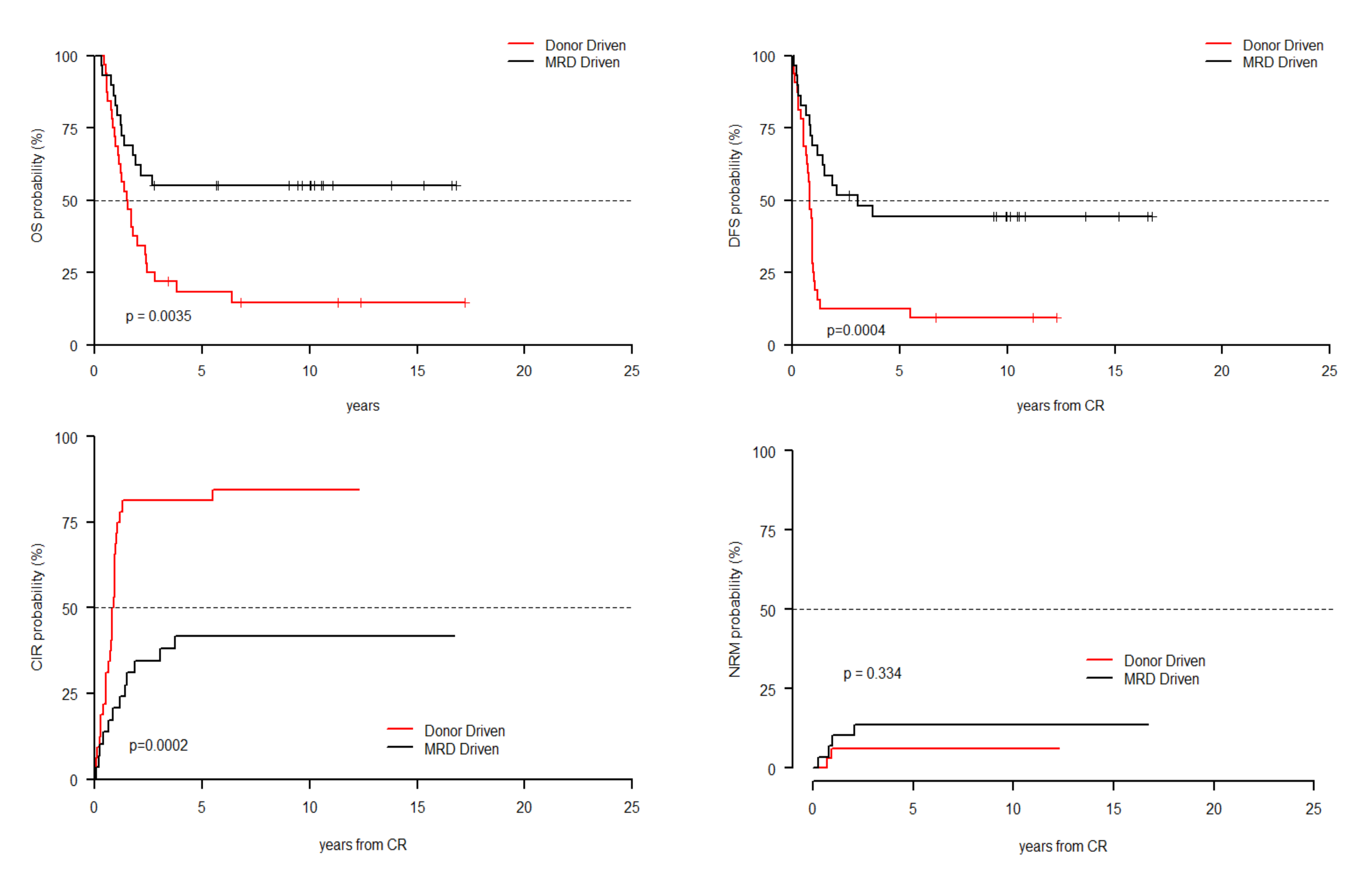
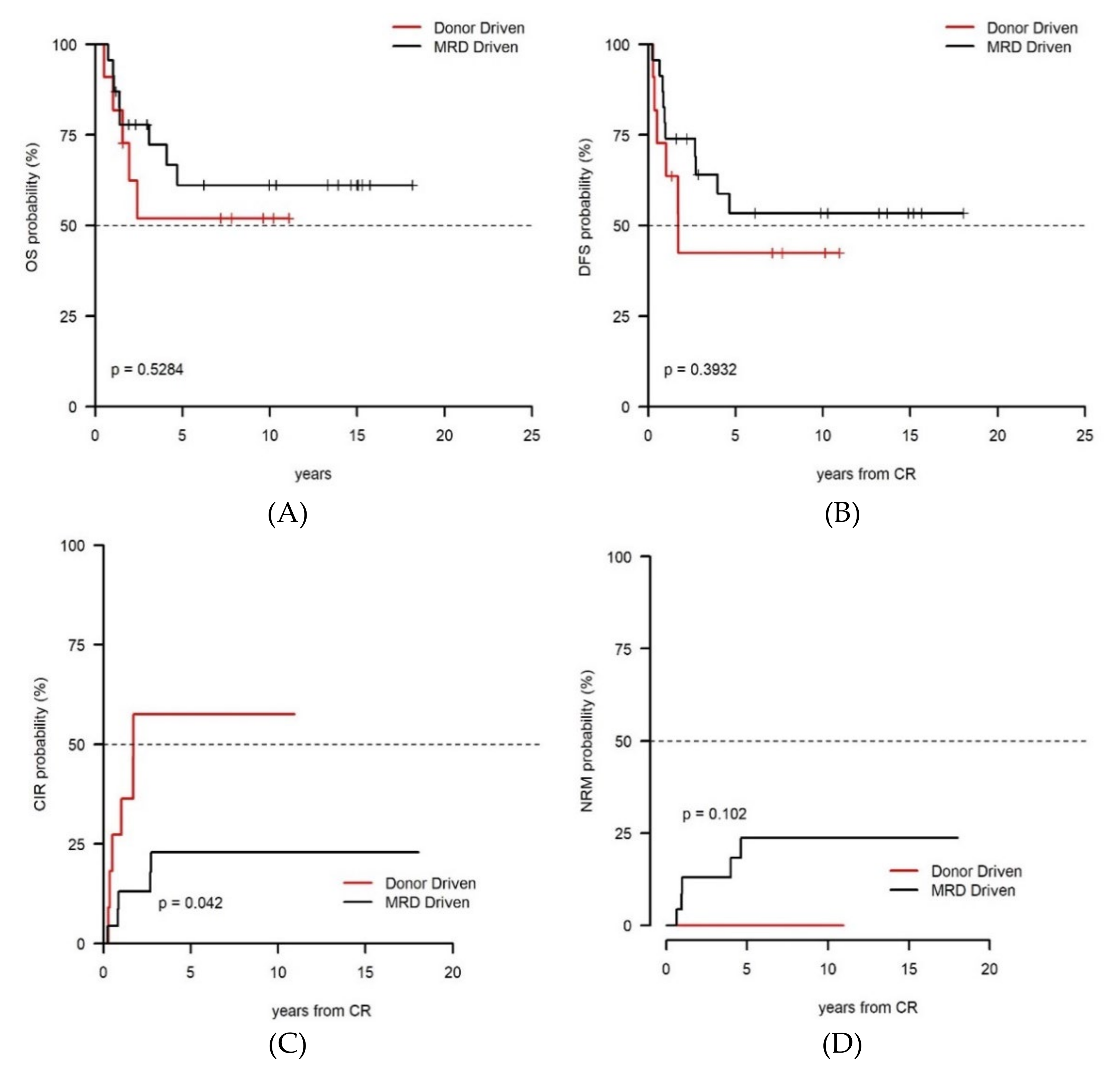
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ustun, C.; Le-Rademacher, J.; Wang, H.-L.; Othus, M.; Sun, Z.; Major, B.; Zhang, M.-J.; Storrick, E.; Lafky, J.M.; Chow, S.; et al. Allogeneic hematopoietic cell transplantation compared to chemotherapy consolidation in older acute myeloid leukemia (AML) patients 60-75 years in first complete remission (CR1): An alliance (A151509), SWOG, ECOG-ACRIN, and CIBMTR study. Leukemia 2019. [Google Scholar] [CrossRef]
- Estey, E.H. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am. J. Hematol. 2018, 93, 1267–1291. [Google Scholar] [CrossRef] [Green Version]
- Koreth, J.; Schlenk, R.; Kopecky, K.J.; Honda, S.; Sierra, J.; Djulbegovic, B.J.; Wadleigh, M.; DeAngelo, D.J.; Stone, R.M.; Sakamaki, H.; et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: Systematic review and meta-analysis of prospective clinical trials. JAMA 2009, 301, 2349–2361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornelissen, J.J.; Gratwohl, A.; Schlenk, R.F.; Sierra, J.; Bornhäuser, M.; Juliusson, G.; Råcil, Z.; Rowe, J.M.; Russell, N.; Mohty, M.; et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: An integrated-risk adapted approach. Nat. Rev. Clin. Oncol. 2012, 9, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, F.R. Hematopoietic cell transplantation for adults with acute myeloid leukemia with minimal residual disease. Best Pract. Res. Clin. Haematol. 2015, 28, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, F.R. Incorporating hematopoietic cell transplantation (HCT) into the management of adults aged under 60 years with acute myeloid leukemia (AML). Best Pract. Res. Clin. Haematol. 2008, 21, 85–92. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- Buccisano, F.; Maurillo, L.; del Principe, M.I.; di Veroli, A.; de Bellis, E.; Biagi, A.; Zizzari, A.; Rossi, V.; Rapisarda, V.; Amadori, S.; et al. Minimal residual disease as a biomarker for outcome prediction and therapy optimization in acute myeloid leukemia. Expert Rev. Hematol. 2018, 11. [Google Scholar] [CrossRef]
- Buccisano, F.; Maurillo, L.; del Principe, M.I.; del Poeta, G.; Sconocchia, G.; Lo-Coco, F.; Arcese, W.; Amadori, S.; Venditti, A. Prognostic and therapeutic implications of minimal residual disease detection in acute myeloid leukemia. Blood 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimwade, D.; Freeman, S.D. Defining minimal residual disease in acute myeloid leukemia: Which platforms are ready for “prime time”? Blood 2014, 124, 3345–3355. [Google Scholar] [CrossRef] [Green Version]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [Green Version]
- Buccisano, F.; Maurillo, L.; Spagnoli, A.; del Principe, M.I.; Fraboni, D.; Panetta, P.; Ottone, T.; Consalvo, M.I.; Lavorgna, S.; Bulian, P.; et al. Cytogenetic and molecular diagnostic characterization combined to postconsolidation minimal residual disease assessment by flow cytometry improves risk stratification in adult acute myeloid leukemia. Blood 2010, 116, 2295–2303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venditti, A.; Buccisano, F.; del Poeta, G.; Maurillo, L.; Tamburini, A.; Battaglia, A.; Catalano, G.; del Moro, B.; Cudillo, L.; Masi, M.; et al. Level of minimal residual disease after consolidation therapy predicts outcome in acute myeloid leukemia: Presented in part at the 41st Annual Level of minimal residual disease after consolidation therapy predicts outcome in acute myeloid leukemia. Blood 2000, 96, 3948–3952. [Google Scholar] [CrossRef]
- Buccisano, F.; Maurillo, L.; Gattei, V.; del Poeta, G.; del Principe, M.I.; Cox, M.C.; Panetta, P.; Consalvo, M.I.; Mazzone, C.; Neri, B.; et al. The kinetics of reduction of minimal residual disease impacts on duration of response and survival of patients with acute myeloid leukemia. Leukemia 2006, 20, 1783–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurillo, L.; Buccisano, F.; del Principe, M.I.; del Poeta, G.; Spagnoli, A.; Panetta, P.; Ammatuna, E.; Neri, B.; Ottaviani, L.; Sarlo, C.; et al. Toward optimization of postremission therapy for residual disease-positive patients with acute myeloid leukemia. J. Clin. Oncol. 2008, 26, 4944–4951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandelli, F.; Vignetti, M.; Suciu, S.; Stasi, R.; Petti, M.-C.; Meloni, G.; Muus, P.; Marmont, F.; Marie, J.-P.; Labar, B.; et al. Daunorubicin versus mitoxantrone versus idarubicin as induction and consolidation chemotherapy for adults with acute myeloid leukemia: The EORTC and GIMEMA Groups Study AML-10. J. Clin. Oncol. 2009, 27, 5397–5403. [Google Scholar] [CrossRef] [Green Version]
- Willemze, R.; Suciu, S.; Meloni, G.; Labar, B.; Marie, J.-P.; Halkes, C.J.M.; Muus, P.; Mistrik, M.; Amadori, S.; Specchia, G.; et al. High-Dose Cytarabine in Induction Treatment Improves the Outcome of Adult Patients Younger Than Age 46 Years With Acute Myeloid Leukemia: Results of the EORTC-GIMEMA AML-12 Trial. J. Clin. Oncol. 2014, 32, 219–228. [Google Scholar] [CrossRef]
- Venditti, A.; Piciocchi, A.; Candoni, A.; Melillo, L.; Calafiore, V.; Cairoli, R.; de Fabritiis, P.; Storti, G.; Salutari, P.; Lanza, F.; et al. GIMEMA AML1310 trial of risk-adapted, MRD-directed therapy for young adults with newly diagnosed acute myeloid leukemia. Blood 2019, 134, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Zeijlemaker, W.; Gratama, J.W.; Schuurhuis, G.J. Tumor heterogeneity makes AML a “moving target” for detection of residual disease. Cytom. Part B Clin. Cytom. 2014, 86, 3–14. [Google Scholar] [CrossRef]
- Grimwade, D.; Hills, R.K.; Moorman, A.V.; Walker, H.; Chatters, S.; Goldstone, A.H.; Wheatley, K.; Harrison, C.J.; Burnett, A.K. National Cancer Research Institute Adult Leukaemia Working Group Refinement of cytogenetic classification in acute myeloid leukemia: Determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010, 116, 354–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acute Myeloid Leukemia, Version 1.2009, NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf (accessed on 6 May 2018).
- Cheson, B.D.; Bennett, J.M.; Kopecky, K.J.; Büchner, T.; Willman, C.L.; Estey, E.H.; Schiffer, C.A.; Doehner, H.; Tallman, M.S.; Lister, T.A.; et al. Revised Recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J. Clin. Oncol. 2003, 21, 4642–4649. [Google Scholar] [CrossRef] [PubMed]
- Prentice, R.L.; Kalbfleisch, J.D.; Peterson, A.V.; Flournoy, N.; Farewell, V.T.; Breslow, N.E. The analysis of failure times in the presence of competing risks. Biometrics 1978, 34, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Buccisano, F.; Walter, R.B. Should patients with acute myeloid leukemia and measurable residual disease be transplanted in first complete remission? Curr. Opin. Hematol. 2017, 24, 132–138. [Google Scholar] [CrossRef]
- Buckley, S.A.; Wood, B.L.; Othus, M.; Hourigan, C.S.; Ustun, C.; Linden, M.A.; Defor, T.E.; Malagola, M.; Anthias, C.; Valkova, V.; et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: A meta-analysis. Haematologica 2017, 102, 865–873. [Google Scholar] [CrossRef] [Green Version]
- Hourigan, C.S.; Goswami, M.; Battiwalla, M.; Barrett, A.J.; Sheela, S.; Karp, J.E.; Lai, C. When the Minimal Becomes Measurable. J. Clin. Oncol. 2016, 34, 2557–2558. [Google Scholar] [CrossRef]
- Freeman, S.D.; Hills, R.K.; Virgo, P.; Khan, N.; Couzens, S.; Dillon, R.; Gilkes, A.; Upton, L.; Nielsen, O.J.; Cavenagh, J.D.; et al. Measurable Residual Disease at Induction Redefines Partial Response in Acute Myeloid Leukemia and Stratifies Outcomes in Patients at Standard Risk Without NPM1 Mutations. J. Clin. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buccisano, F.; Maurillo, L.; Piciocchi, A.; del Principe, M.I.; Picardi, A.; Cerretti, R.; Cudillo, L.; de Angelis, G.; Sarlo, C.; Cefalo, M.; et al. Pre-transplant persistence of minimal residual disease does not contraindicate allogeneic stem cell transplantation for adult patients with acute myeloid leukemia. Bone Marrow Transplant. 2017, 52, 473–475. [Google Scholar] [CrossRef]
- Balsat, M.; Renneville, A.; Thomas, X.; de Botton, S.; Caillot, D.; Marceau, A.; Lemasle, E.; Marolleau, J.P.; Nibourel, O.; Berthon, C.; et al. Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: A study by the acute leukemia French association group. J. Clin. Oncol. 2017, 35, 185–193. [Google Scholar] [CrossRef]
- Zhu, H.-H.; Zhang, X.-H.; Qin, Y.-Z.; Liu, D.-H.; Jiang, H.; Chen, H.; Jiang, Q.; Xu, L.-P.; Lu, J.; Han, W.; et al. MRD-directed risk-stratification treatment may improve outcome of t (8;21) AML in the first complete remission: Results from AML05 Multicenter Trial. Blood 2013, 121, 4056–4063. [Google Scholar] [CrossRef]
- Venditti, A.; Maurillo, L.; Buccisano, F.; Del Poeta, G.; Mazzone, C.; Tamburini, A.; del Principe, M.I.; Consalvo, M.I.; de Fabritiis, P.; Cudillo, L.; et al. Pretransplant minimal residual disease level predicts clinical outcome in patients with acute myeloid leukemia receiving high-dose chemotherapy and autologous stem cell transplantation. Leukemia 2003, 17, 2178–2182. [Google Scholar] [CrossRef]
- Leung, W.; Pui, C.-H.; Coustan-Smith, E.; Yang, J.; Pei, D.; Gan, K.; Srinivasan, A.; Hartford, C.; Triplett, B.M.; Dallas, M.; et al. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood 2012, 120, 468–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platzbecker, U.; Middeke, J.M.; Sockel, K.; Herbst, R.; Wolf, D.; Baldus, C.D.; Oelschlägel, U.; Mütherig, A.; Fransecky, L.; Noppeney, R.; et al. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): An open-label, multicentre, phase 2 trial. Lancet Oncol. 2018, 19, 1668–1679. [Google Scholar] [CrossRef]
- Morsink, L.M.; Othus, M.; Bezerra, E.D.; Wood, B.L.; Fang, M.; Sandmaier, B.M.; Mielcarek, M.; Schoch, G.; Storb, R.; Deeg, H.J.; et al. Impact of pretransplant measurable residual disease on the outcome of allogeneic hematopoietic cell transplantation in adult monosomal karyotype AML. Leukemia 2020. [Google Scholar] [CrossRef] [PubMed]
- Suciu, S.; Mandelli, F.; de Witte, T.; Zittoun, R.; Gallo, E.; Labar, B.; De Rosa, G.; Belhabri, A.; Giustolisi, R.; Delarue, R.; et al. Allogeneic compared with autologous stem cell transplantation in the treatment of patients younger than 46 years with acute myeloid leukemia (AML) in first complete remission (CR1): An intention-to-treat analysis of the EORTC/GIMEMAAML-10 trial. Blood 2003, 102, 1232–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameter | Level | Donor-Driven Cohort (n = 70, 37.8%) | MRD-Driven Cohort (n = 115, 62.2%) | p-Value |
|---|---|---|---|---|
| Sex (%) | Female | 29 (41.4) | 47 (40.9) | 1.000 |
| Male | 41 (58.6) | 68 (59.1) | ||
| Age (median [range]) | 49.50 [17.00, 72.00] | 44.00 [18.00, 64.00] | 0.046 | |
| WBC count (%) | <50.0 × 109/L | 45 (64.3) | 88 (76.5) | 0.104 |
| >50.0 × 109/L | 25 (35.7) | 27 (23.5) | ||
| FLT3-ITD (%) | FLT3 negative | 53 (80.3) | 75 (72.8) | 0.356 |
| FLT3 positive | 13 (19.7) | 28 (27.2) | ||
| NPM1-mutated (%) | NPM negative | 36 (55.4) | 76 (74.5) | 0.017 |
| NPM positive | 29 (44.6) | 26 (25.5) | ||
| Karyotype (%) | Favorable risk * | 11 (16.4) | 23 (21.1) | 0.514 |
| Intermediate risk * | 53 (79.1) | 78 (71.6) | ||
| Adverse risk * | 3 (4.5) | 8 (7.3) | ||
| Post-remission therapy | alloHCT | 4 (5.7) | 49 (43.0) | <0.001 |
| (%) | AuSCT | 35 (50.0) | 37 (32.5) | |
| No SCT | 31 (44.3) | 28 (24.6) |
| Parameter | Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | Lower 95% CI | Higher 95% CI | p | HR | Lower 95% CI | Higher 95% CI | p | |
| Treatment strategy (MRD-oriented vs. donor-oriented) | 0.62 | 0.42 | 0.9 | 0.0129 | 0.587 | 0.396 | 0.872 | 0.008 |
| Sex (male vs. female) | 1.08 | 0.73 | 1.59 | 0.7129 | ||||
| Age (>60 vs. <60 years) | 1.02 | 1 | 1.03 | 0.067 | ||||
| WBC count (× 109/L) (>50.0 vs. <50.0) | 1.589 | 1.0618 | 2.3771 | 0.0243 | ||||
| FLT3-ITD (positive vs. negative) | 1.232 | 0.771 | 1.968 | 0.3839 | ||||
| NPM1-mutated (positive vs. negative) | 0.94 | 0.61 | 1.46 | 0.7907 | ||||
| Karyotype risk group (Intermediate vs. Favorable) | 2.05 | 1.14 | 3.68 | 0.0166 | 1.973 | 1.097 | 3.549 | 0.023 |
| Karyotype risk group (Adverse vs. Favorable) | 2.22 | 0.89 | 5.57 | 0.0891 | 2.301 | 0.917 | 5.776 | 0.076 |
| Post-remission therapy (AuSCT vs. alloHCT) | 1.4 | 0.84 | 2.32 | 0.1926 | ||||
| Post-remission therapy (no SCT vs. alloHCT) | 2.16 | 1.28 | 3.62 | 0.0037 | ||||
| Parameter | Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | Lower 95% CI | Higher 95% CI | p | HR | Lower 95% CI | Higher 95% CI | p | |
| Treatment strategy (MRD-oriented vs. donor-oriented) | 0.52 | 0.36 | 0.74 | 0.0004 | ||||
| Sex (male vs. female) | 1.15 | 0.79 | 1.66 | 0.4654 | ||||
| Age (>60 vs. <60 years) | 1.02 | 1 | 1.03 | 0.0615 | ||||
| WBC count (× 109/L) (>50.0 vs. <50.0) | 1.596 | 1.0918 | 2.3339 | 0.0158 | ||||
| FLT3-ITD (positive vs. negative) | 1.253 | 0.812 | 1.933 | 0.3081 | ||||
| NPM1-mutated (positive vs. negative) | 0.88 | 0.58 | 1.33 | 0.5552 | ||||
| Karyotype risk group (Intermediate vs. Favorable) | 1.96 | 1.15 | 3.35 | 0.013 | 2.543 | 1.483 | 4.36 | 0.001 |
| Karyotype risk group (Adverse vs. Favorable) | 2.31 | 0.99 | 5.41 | 0.0535 | 3.853 | 1.596 | 9.302 | 0.003 |
| Post-remission therapy (AuSCT vs. alloHCT) | 1.67 | 1.03 | 2.73 | 0.0389 | 2.168 | 1.28 | 3.671 | 0.004 |
| Post-remission therapy (no SCT vs. alloHCT) | 3.2 | 1.95 | 5.24 | <0.001 | 4.191 | 2.482 | 7.077 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buccisano, F.; Palmieri, R.; Piciocchi, A.; Maurillo, L.; Del Principe, M.I.; Paterno, G.; Soddu, S.; Cerretti, R.; De Angelis, G.; Mariotti, B.; et al. Use of Measurable Residual Disease to Evolve Transplant Policy in Acute Myeloid Leukemia: A 20-Year Monocentric Observation. Cancers 2021, 13, 1083. https://doi.org/10.3390/cancers13051083
Buccisano F, Palmieri R, Piciocchi A, Maurillo L, Del Principe MI, Paterno G, Soddu S, Cerretti R, De Angelis G, Mariotti B, et al. Use of Measurable Residual Disease to Evolve Transplant Policy in Acute Myeloid Leukemia: A 20-Year Monocentric Observation. Cancers. 2021; 13(5):1083. https://doi.org/10.3390/cancers13051083
Chicago/Turabian StyleBuccisano, Francesco, Raffaele Palmieri, Alfonso Piciocchi, Luca Maurillo, Maria Ilaria Del Principe, Giovangiacinto Paterno, Stefano Soddu, Raffaella Cerretti, Gottardo De Angelis, Benedetta Mariotti, and et al. 2021. "Use of Measurable Residual Disease to Evolve Transplant Policy in Acute Myeloid Leukemia: A 20-Year Monocentric Observation" Cancers 13, no. 5: 1083. https://doi.org/10.3390/cancers13051083







