The Chicken Chorioallantoic Membrane Tumor Assay as a Relevant In Vivo Model to Study the Impact of Hypoxia on Tumor Progression and Metastasis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. ccRCC Patient Tumors
2.4. Chorioallantoic Membrane Assays
2.5. RNA/DNA Isolation and qPCR Analysis
2.6. Immunohistochemistry
2.7. Image Acquisition and Quantification of Hypoxia and Vascularization
2.8. Statistical Analysis
3. Results
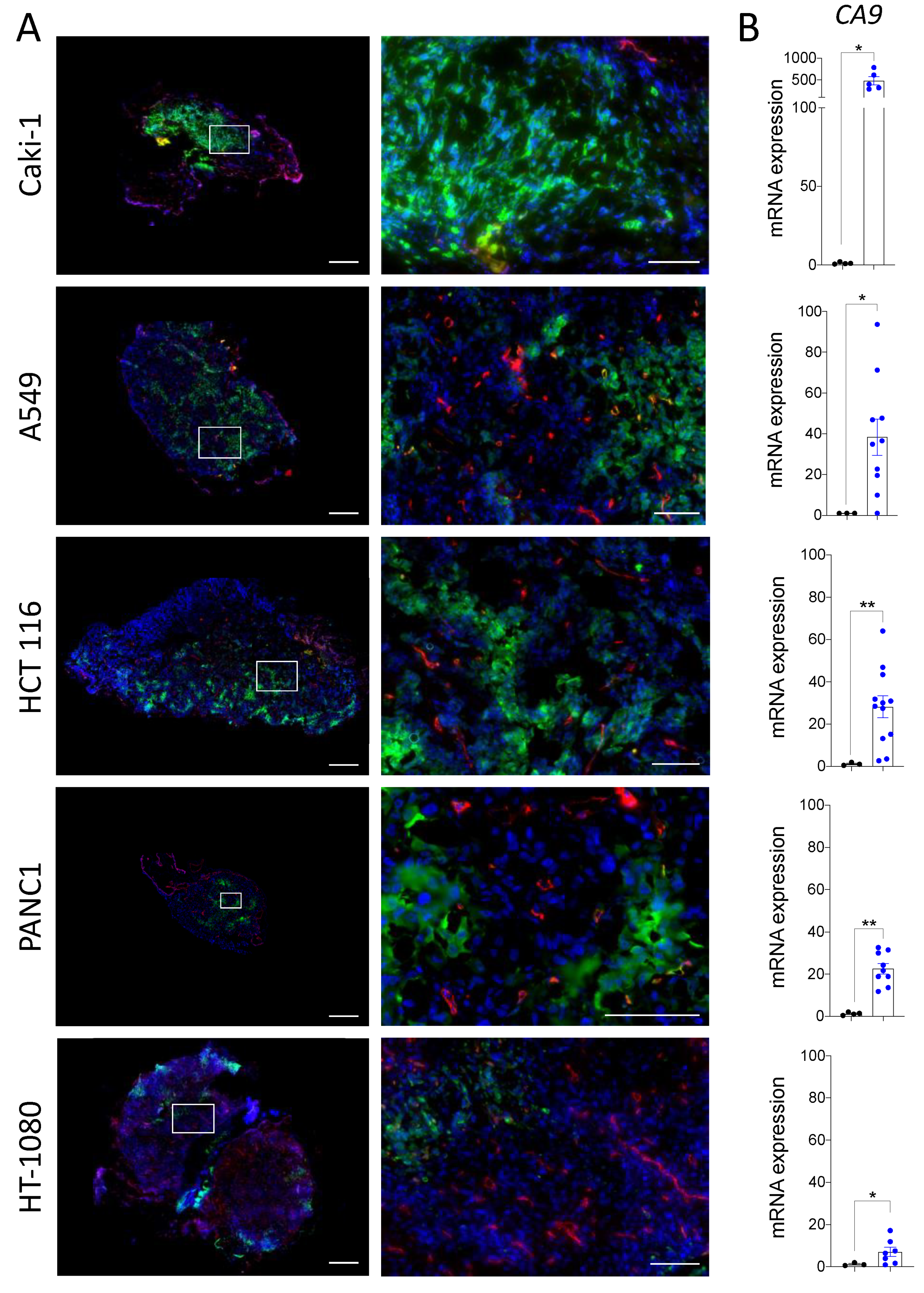
3.1. The Chicken Chorioallantoic Membrane Assay Supports the Development of Hypoxic Zones in Cancer Cell Line-Derived Xenografts
3.2. Hypoxia in Tumors Grown on the CAM Can Be Modulated by Pro- and Anti-Angiogenic Treatments
3.3. In Vivo Modulation of Hypoxia Affects Spontaneous Metastasis in the CAM Model
3.4. Modulation of Hypoxia in ccRCC Patient-Derived CAM Xenografts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stoyanova, R.; Huang, K.; Sandler, K.; Cho, H.; Carlin, S.; Zanzonico, P.B.; Koutcher, J.A.; Ackerstaff, E. Mapping Tumor Hypoxia in vivo Using Pattern Recognition of Dynamic Contrast-Enhanced MRI Data. Transl. Oncol. 2012, 5, 437–447. [Google Scholar] [CrossRef]
- Hockel, M.; Schlenger, K.; Aral, B.; Mitze, M.; Schaffer, U.; Vaupel, P. Association between Tumor Hypoxia and Malignant Progression in Advanced Cancer of the Uterine Cervix. Cancer Res. 1996, 56, 4509–4515. [Google Scholar]
- Luoto, K.R.; Kumareswaran, R.; Bristow, R.G. Tumor Hypoxia as a Driving Force in Genetic Instability. Genome Integr. 2013, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Wang, J.Z.; Yu, F.; Venkatachalam, M.A. Apoptosis-Resistance of Hypoxic Cells: Multiple Factors Involved and a Role for IAP-2. Am. J. Pathol. 2003, 163, 663–671. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-Inducible Factors: Mediators of Cancer Progression and Targets for Cancer Therapy. Trends Pharmacol. Sci. 2012, 33, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Krishnamachary, B.; Zagzag, D.; Nagasawa, H.; Rainey, K.; Okuyama, H.; Baek, J.H.; Semenza, G.L. Hypoxia-Inducible Factor-1-Dependent Repression of E-Cadherin in von Hippel-Lindau Tumor Suppressor-Null Renal Cell Carcinoma Mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006, 66, 2725–2731. [Google Scholar] [CrossRef] [Green Version]
- Staller, P.; Sulitkova, J.; Lisztwan, J.; Moch, H.; Oakeley, E.J.; Krek, W. Chemokine Receptor CXCR4 Downregulated by von Hippel-Lindau Tumour Suppressor PVHL. Nature 2003, 425, 307–311. [Google Scholar] [CrossRef]
- Pennacchietti, S.; Michieli, P.; Galluzzo, M.; Mazzone, M.; Giordano, S.; Comoglio, P.M. Hypoxia Promotes Invasive Growth by Transcriptional Activation of the Met Protooncogene. Cancer Cell 2003, 3, 347–361. [Google Scholar] [CrossRef] [Green Version]
- Cosse, J.-P.; Michiels, C. Tumour Hypoxia Affects the Responsiveness of Cancer Cells to Chemotherapy and Promotes Cancer Progression. Anticancer Agents. Med. Chem. 2008, 8, 790–797. [Google Scholar] [CrossRef]
- Rockwell, S.; Dobrucki, I.T.; Kim, E.Y.; Marrison, S.T.; Vu, V.T. Hypoxia and Radiation Therapy: Past History, Ongoing Research, and Future Promise. Curr. Mol. Med. 2009, 9, 442–458. [Google Scholar] [CrossRef] [Green Version]
- Barsoum, I.B.; Koti, M.; Siemens, D.R.; Graham, C.H. Mechanisms of Hypoxia-Mediated Immune Escape in Cancer. Cancer Res. 2014, 74, 7185–7190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, J.C.; Lebedev, A.; Aten, E.; Madsen, K.; Marciano, L.; Kolb, H.C. The Clinical Importance of Assessing Tumor Hypoxia: Relationship of Tumor Hypoxia to Prognosis and Therapeutic Opportunities. Antioxid. Redox Signal. 2014, 21, 1516–1554. [Google Scholar] [CrossRef]
- Phillips, R.M. Targeting the Hypoxic Fraction of Tumours Using Hypoxia-Activated Prodrugs. Cancer Chemother Pharmacol. 2016, 77, 441–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campanile, C.; Arlt, M.J.E.; Krämer, S.D.; Honer, M.; Gvozdenovic, A.; Brennecke, P.; Fischer, C.R.; Sabile, A.A.; Müller, A.; Ametamey, S.M.; et al. Characterization of Different Osteosarcoma Phenotypes by PET Imaging in Preclinical Animal Models. J. Nucl. Med. 2013, 54, 1362–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahy, P.; De Bast, M.; Leveque, P.H.; Gillart, J.; Labar, D.; Marchand, J.; Gregoire, V. Preclinical Validation of the Hypoxia Tracer 2-(2-Nitroimidazol-1-Yl)- N-(3,3,3-[(18)F]Trifluoropropyl)Acetamide, [(18)F]EF3. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1263–1272. [Google Scholar] [CrossRef]
- Corroyer-Dulmont, A.; Pérès, E.A.; Petit, E.; Durand, L.; Marteau, L.; Toutain, J.; Divoux, D.; Roussel, S.; MacKenzie, E.T.; Barré, L.; et al. Noninvasive Assessment of Hypoxia with 3-[18F]-Fluoro-1-(2-Nitro-1-Imidazolyl)-2-Propanol ([18F]-FMISO): A PET Study in Two Experimental Models of Human Glioma. Biol. Chem. 2013, 394, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Wobb, J.; Krueger, S.A.; Kane, J.L.; Galoforo, S.; Grills, I.S.; Wilson, G.D.; Marples, B. The Effects of Pulsed Radiation Therapy on Tumor Oxygenation in 2 Murine Models of Head and Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 820–828. [Google Scholar] [CrossRef]
- Wyss, M.T.; Honer, M.; Schubiger, P.A.; Ametamey, S.M. NanoPET Imaging of [(18)F]Fluoromisonidazole Uptake in Experimental Mouse Tumours. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Gaustad, J.-V.; Simonsen, T.G.; Andersen, L.M.K.; Rofstad, E.K. Vascular Abnormalities and Development of Hypoxia in Microscopic Melanoma Xenografts. J. Transl. Med. 2017, 15, 241. [Google Scholar] [CrossRef] [Green Version]
- Mena-Romano, P.; Cheng, C.; Glowa, C.; Peschke, P.; Pan, L.; Haberkorn, U.; Dimitrakopoulou-Strauss, A.; Karger, C.P. Measurement of Hypoxia-Related Parameters in Three Sublines of a Rat Prostate Carcinoma Using Dynamic (18)F-FMISO-Pet-Ct and Quantitative Histology. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 348–362. [Google Scholar]
- Haynes, J.; McKee, T.D.; Haller, A.; Wang, Y.; Leung, C.; Gendoo, D.M.A.; Lima-Fernandes, E.; Kreso, A.; Wolman, R.; Szentgyorgyi, E.; et al. Administration of Hypoxia-Activated Prodrug Evofosfamide after Conventional Adjuvant Therapy Enhances Therapeutic Outcome and Targets Cancer-Initiating Cells in Preclinical Models of Colorectal Cancer. Clin. Cancer Res. 2018, 24, 2116–2127. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.-H.; Tilan, J.U.; Galli, S.; Acree, R.; Connors, K.; Mahajan, A.; Wietlisbach, L.; Polk, T.; Izycka-Swieszewska, E.; Lee, Y.-C.; et al. In vivo Model for Testing Effect of Hypoxia on Tumor Metastasis. J. Vis. Exp. 2016. [Google Scholar] [CrossRef] [Green Version]
- Rockwell, S.; Moulder, J.E.; Martin, D.F. Effectiveness and Biological Effects of Techniques Used to Induce Hypoxia in Solid Tumors. Radiother Oncol. 1986, 5, 311–319. [Google Scholar] [CrossRef]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in Translation: Animal Models and Clinical Trials in Cancer Treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar] [PubMed]
- Beekhuijzen, M. The Era of 3Rs Implementation in Developmental and Reproductive Toxicity (DART) Testing: Current Overview and Future Perspectives. Reprod. Toxicol. 2017, 72, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane (CAM). A Multifaceted Experimental Model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef]
- Durupt, F.; Koppers-Lalic, D.; Balme, B.; Budel, L.; Terrier, O.; Lina, B.; Thomas, L.; Hoeben, R.C.; Rosa-Calatrava, M. The Chicken Chorioallantoic Membrane Tumor Assay as Model for Qualitative Testing of Oncolytic Adenoviruses. Cancer Gene Ther. 2012, 19, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D.; Vacca, A.; Roncali, L.; Dammacco, F. The Chick Embryo Chorioallantoic Membrane as a Model for in vivo Research on Angiogenesis. Int. J. Dev. Biol. 1996, 40, 1189–1197. [Google Scholar] [CrossRef]
- Norrby, K. In vivo Models of Angiogenesis. J. Cell Mol. Med. 2006, 10, 588–612. [Google Scholar] [CrossRef]
- Busch, C.; Krochmann, J.; Drews, U. The Chick Embryo as an Experimental System for Melanoma Cell Invasion. PLoS ONE 2013, 8, e53970. [Google Scholar] [CrossRef] [Green Version]
- Kunzi-Rapp, K.; Genze, F.; Küfer, R.; Reich, E.; Hautmann, R.E.; Gschwend, J.E. Chorioallantoic Membrane Assay: Vascularized 3-Dimensional Cell Culture System for Human Prostate Cancer Cells as an Animal Substitute Model. J. Urol. 2001, 166, 1502–1507. [Google Scholar] [CrossRef]
- Liu, M.; Scanlon, C.S.; Banerjee, R.; Russo, N.; Inglehart, R.C.; Willis, A.L.; Weiss, S.J.; D’Silva, N.J. The Histone Methyltransferase EZH2 Mediates Tumor Progression on the Chick Chorioallantoic Membrane Assay, a Novel Model of Head and Neck Squamous Cell Carcinoma. Transl. Oncol. 2013, 6, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Lokman, N.A.; Elder, A.S.F.; Ricciardelli, C.; Oehler, M.K. Chick Chorioallantoic Membrane (CAM) Assay as an in vivo Model to Study the Effect of Newly Identified Molecules on Ovarian Cancer Invasion and Metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quigley, J.P.; Armstrong, P.B. Tumor Cell Intravasation Alu-Cidated: The Chick Embryo Opens the Window. Cell 1998, 94, 281–284. [Google Scholar] [CrossRef] [Green Version]
- Taizi, M.; Deutsch, V.R.; Leitner, A.; Ohana, A.; Goldstein, R.S. A Novel and Rapid in vivo System for Testing Therapeutics on Human Leukemias. Exp. Hematol. 2006, 34, 1698–1708. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Koshida, K.; Endo, Y.; Imao, T.; Uchibayashi, T.; Sasaki, T.; Namiki, M. A Chick Embryo Model for Metastatic Human Prostate Cancer. Eur. Urol. 1998, 34, 154–160. [Google Scholar] [CrossRef]
- Lucien, F.; Pelletier, P.-P.; Lavoie, R.R.; Lacroix, J.-M.; Roy, S.; Parent, J.-L.; Arsenault, D.; Harper, K.; Dubois, C.M. Hypoxia-Induced Mobilization of NHE6 to the Plasma Membrane Triggers Endosome Hyperacidification and Chemoresistance. Nat. Commun. 2017, 8, 15884. [Google Scholar] [CrossRef] [Green Version]
- Harper, K.; Lavoie, R.R.; Charbonneau, M.; Brochu-Gaudreau, K.; Dubois, C.M. The Hypoxic Tumor Microenvironment Promotes Invadopodia Formation and Metastasis through LPA1 Receptor and EGFR Cooperation. Mol. Cancer Res. 2018, 16, 1601–1613. [Google Scholar] [CrossRef] [Green Version]
- Zijlstra, A.; Mellor, R.; Panzarella, G.; Aimes, R.T.; Hooper, J.D.; Marchenko, N.D.; Quigley, J.P. A Quantitative Analysis of Rate-Limiting Steps in the Metastatic Cascade Using Human-Specific Real-Time Polymerase Chain Reaction. Cancer Res. 2002, 62, 7083–7092. [Google Scholar] [PubMed]
- Jilani, S.M.; Murphy, T.J.; Thai, S.N.M.; Eichmann, A.; Alva, J.A.; Iruela-Arispe, M.L. Selective Binding of Lectins to Embryonic Chicken Vasculature. J. Histochem. Cytochem. 2003, 51, 597–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deryugina, E.I.; Quigley, J.P. Chapter 2. Chick Embryo Chorioallantoic Membrane Models to Quantify Angiogenesis Induced by Inflammatory and Tumor Cells or Purified Effector Molecules. Methods Enzymol. 2008, 444, 21–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subauste, M.C.; Kupriyanova, T.A.; Conn, E.M.; Ardi, V.C.; Quigley, J.P.; Deryugina, E.I. Evaluation of Metastatic and Angiogenic Potentials of Human Colon Carcinoma Cells in Chick Embryo Model Systems. Clin. Exp. Metastasis 2009, 26, 1033–1047. [Google Scholar] [CrossRef] [Green Version]
- Mangir, N.; Raza, A.; Haycock, J.W.; Chapple, C.; Macneil, S. An Improved in vivo Methodology to Visualise Tumour Induced Changes in Vasculature Using the Chick Chorionic Allantoic Membrane Assay. In Vivo 2018, 32, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Mangir, N.; Dikici, S.; Claeyssens, F.; MacNeil, S. Using Ex Ovo Chick Chorioallantoic Membrane (CAM) Assay To Evaluate the Biocompatibility and Angiogenic Response to Biomaterials. ACS Biomater. Sci. Eng. 2019, 5, 3190–3200. [Google Scholar] [CrossRef]
- Løkkegaard, A.; Nyengaard, J.R.; West, M.J. Stereological Estimates of Number and Length of Capillaries in Subdivisions of the Human Hippocampal Region. Hippocampus 2001, 11, 726–740. [Google Scholar] [CrossRef]
- Ivanov, S.; Liao, S.Y.; Ivanova, A.; Danilkovitch-Miagkova, A.; Tarasova, N.; Weirich, G.; Merrill, M.J.; Proescholdt, M.A.; Oldfield, E.H.; Lee, J.; et al. Expression of Hypoxia-Inducible Cell-Surface Transmembrane Carbonic Anhydrases in Human Cancer. Am. J. Pathol. 2001, 158, 905–919. [Google Scholar] [CrossRef] [Green Version]
- Shin, K.H.; Diaz-Gonzalez, J.A.; Russell, J.; Chen, Q.; Burgman, P.; Li, X.-F.; Ling, C.C. Detecting Changes in Tumor Hypoxia with Carbonic Anhydrase IX and Pimonidazole. Cancer Biol. Ther. 2007, 6, 70–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eales, K.L.; Hollinshead, K.E.R.; Tennant, D.A. Hypoxia and Metabolic Adaptation of Cancer Cells. Oncogenesis 2016, 5, e190. [Google Scholar] [CrossRef] [Green Version]
- Secomb, T.W. Mechanics of Blood Flow in the Microcirculation. Symp. Soc. Exp. Biol. 1995, 49, 305–321. [Google Scholar] [PubMed]
- Rankin, E.B.; Giaccia, A.J. Hypoxic Control of Metastasis. Science 2016, 352, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Nobre, A.R.; Entenberg, D.; Wang, Y.; Condeelis, J.; Aguirre-Ghiso, J.A. The Different Routes to Metastasis via Hypoxia-Regulated Programs. Trends Cell Biol. 2018, 28, 941–956. [Google Scholar] [CrossRef]
- Hutson, T.E.; Lesovoy, V.; Al-Shukri, S.; Stus, V.P.; Lipatov, O.N.; Bair, A.H.; Rosbrook, B.; Chen, C.; Kim, S.; Vogelzang, N.J. Axitinib versus Sorafenib as First-Line Therapy in Patients with Metastatic Renal-Cell Carcinoma: A Randomised Open-Label Phase 3 Trial. Lancet Oncol. 2013, 14, 1287–1294. [Google Scholar] [CrossRef]
- Wang, H.; Man, L.; Li, G.; Huang, G.; Wang, J. Comparative Efficacy and Safety of Axitinib versus Sorafenib in Metastatic Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Onco. Targets. Ther. 2016, 9, 3423–3432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, B.; Yang, Y.; Guo, S.; Duoerkun, S.; Deng, X.; Chen, D.; Yu, S.; Qian, W.; Li, Q.; Li, Q.; et al. Intra-Tumour Molecular Heterogeneity of Clear Cell Renal Cell Carcinoma Reveals the Diversity of the Response to Targeted Therapies Using Patient-Derived Xenograft Models. Oncotarget 2017, 8, 49839–49850. [Google Scholar] [CrossRef]
- Martinez, P.; Birkbak, N.J.; Gerlinger, M.; McGranahan, N.; Burrell, R.A.; Rowan, A.J.; Joshi, T.; Fisher, R.; Larkin, J.; Szallasi, Z.; et al. Parallel Evolution of Tumour Subclones Mimics Diversity between Tumours. J. Pathol. 2013, 230, 356–364. [Google Scholar] [CrossRef] [Green Version]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The Role of Hypoxia in Cancer Progression, Angiogenesis, Metastasis, and Resistance to Therapy. Hypoxia (Auckl) 2015, 3, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaupel, P.; Harrison, L. Tumor Hypoxia: Causative Factors, Compensatory Mechanisms, and Cellular Response. Oncologist 2004, 9 (Suppl. 5), 4–9. [Google Scholar] [CrossRef] [Green Version]
- Lowerison, M.R.; Huang, C.; Lucien, F.; Chen, S.; Song, P. Ultrasound Localization Microscopy of Renal Tumor Xenografts in Chicken Embryo Is Correlated to Hypoxia. Sci. Rep. 2020, 10, 2478. [Google Scholar] [CrossRef] [Green Version]
- Ostergaard, L.; Tietze, A.; Nielsen, T.; Drasbek, K.R.; Mouridsen, K.; Jespersen, S.N.; Horsman, M.R. The Relationship between Tumor Blood Flow, Angiogenesis, Tumor Hypoxia, and Aerobic Glycolysis. Cancer Res. 2013, 73, 5618–5624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood Flow, Oxygen and Nutrient Supply, and Metabolic Microenvironment of Human Tumors: A Review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar]
- Vaupel, P.; Thews, O.; Hoeckel, M. Treatment Resistance of Solid Tumors: Role of Hypoxia and Anemia. Med. Oncol. 2001, 18, 243–259. [Google Scholar] [CrossRef]
- Siemeister, G.; Weindel, K.; Mohrs, K.; Barleon, B.; Martiny-Baron, G.; Marmé, D. Reversion of Deregulated Expression of Vascular Endothelial Growth Factor in Human Renal Carcinoma Cells by von Hippel-Lindau Tumor Suppressor Protein. Cancer Res. 1996, 56, 2299–2301. [Google Scholar] [PubMed]
- Lin, Y.-T.; Wu, K.-J. Epigenetic Regulation of Epithelial-Mesenchymal Transition: Focusing on Hypoxia and TGF-β Signaling. J. Biomed. Sci. 2020, 27, 39. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ohh, M. Oxygen-Mediated Endocytosis in Cancer. J. Cell Mol. Med. 2010, 14, 496–503. [Google Scholar] [CrossRef] [Green Version]
- Cassavaugh, J.; Lounsbury, K.M. Hypoxia-Mediated Biological Control. J. Cell Biochem. 2011, 112, 735–744. [Google Scholar] [CrossRef]
- Lucien, F.; Brochu-Gaudreau, K.; Arsenault, D.; Harper, K.; Dubois, C.M. Hypoxia-Induced Invadopodia Formation Involves Activation of NHE-1 by the P90 Ribosomal S6 Kinase (P90RSK). PLoS ONE 2011, 6, e28851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtnay, R.; Ngo, D.C.; Malik, N.; Ververis, K.; Tortorella, S.M.; Karagiannis, T.C. Cancer Metabolism and the Warburg Effect: The Role of HIF-1 and PI3K. Mol. Biol. Rep. 2015, 42, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Lo, L.W.; Cheng, J.J.; Chiu, J.J.; Wung, B.S.; Liu, Y.C.; Wang, D.L. Endothelial Exposure to Hypoxia Induces Egr-1 Expression Involving PKCalpha-Mediated Ras/Raf-1/ERK1/2 Pathway. J. Cell. Physiol. 2001, 188, 304–312. [Google Scholar] [CrossRef]
- Qiu, G.-Z.; Jin, M.-Z.; Dai, J.-X.; Sun, W.; Feng, J.-H.; Jin, W.-L. Reprogramming of the Tumor in the Hypoxic Niche: The Emerging Concept and Associated Therapeutic Strategies. Trends Pharmacol. Sci. 2017, 38, 669–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardman, P. Nitroimidazoles as Hypoxic Cell Radiosensitizers and Hypoxia Probes: Misonidazole, Myths and Mistakes. Br. J. Radiol. 2019, 92, 20170915. [Google Scholar] [CrossRef]
- Patterson, A.V.; Ferry, D.M.; Edmunds, S.J.; Gu, Y.; Singleton, R.S.; Patel, K.; Pullen, S.M.; Hicks, K.O.; Syddall, S.P.; Atwell, G.J.; et al. Mechanism of Action and Preclinical Antitumor Activity of the Novel Hypoxia-Activated DNA Cross-Linking Agent PR-104. Clin. Cancer Res. 2007, 13, 3922–3932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, S.; Calar, K.; de la Puente, P. Mimicking Tumor Hypoxia and Tumor-Immune Interactions Employing Three-Dimensional in vitro Models. J. Exp. Clin. Cancer Res. 2020, 39, 75. [Google Scholar] [CrossRef]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane (CAM) Assay. Reprod. Toxicol. 2017, 70, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska, P.; Tayoun, T.; Oulhen, M.; Faugeroux, V.; Rouffiac, V.; Aberlenc, A.; Pommier, A.L.; Honore, A.; Marty, V.; Bawa, O.; et al. Exploitation of the Chick Embryo Chorioallantoic Membrane (CAM) as a Platform for Anti-Metastatic Drug Testing. Sci. Rep. 2020, 10, 16876. [Google Scholar] [CrossRef]
- Jung, J.; Seol, H.S.; Chang, S. The Generation and Application of Patient-Derived Xenograft Model for Cancer Research. Cancer Res. Treat. 2018, 50, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komatsu, A.; Matsumoto, K.; Saito, T.; Muto, M.; Tamanoi, F. Patient Derived Chicken Egg Tumor Model (PDcE Model): Current Status and Critical Issues. Cells 2019, 8, 440. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harper, K.; Yatsyna, A.; Charbonneau, M.; Brochu-Gaudreau, K.; Perreault, A.; Jeldres, C.; McDonald, P.P.; Dubois, C.M. The Chicken Chorioallantoic Membrane Tumor Assay as a Relevant In Vivo Model to Study the Impact of Hypoxia on Tumor Progression and Metastasis. Cancers 2021, 13, 1093. https://doi.org/10.3390/cancers13051093
Harper K, Yatsyna A, Charbonneau M, Brochu-Gaudreau K, Perreault A, Jeldres C, McDonald PP, Dubois CM. The Chicken Chorioallantoic Membrane Tumor Assay as a Relevant In Vivo Model to Study the Impact of Hypoxia on Tumor Progression and Metastasis. Cancers. 2021; 13(5):1093. https://doi.org/10.3390/cancers13051093
Chicago/Turabian StyleHarper, Kelly, Anna Yatsyna, Martine Charbonneau, Karine Brochu-Gaudreau, Alexis Perreault, Claudio Jeldres, Patrick P. McDonald, and Claire M. Dubois. 2021. "The Chicken Chorioallantoic Membrane Tumor Assay as a Relevant In Vivo Model to Study the Impact of Hypoxia on Tumor Progression and Metastasis" Cancers 13, no. 5: 1093. https://doi.org/10.3390/cancers13051093
APA StyleHarper, K., Yatsyna, A., Charbonneau, M., Brochu-Gaudreau, K., Perreault, A., Jeldres, C., McDonald, P. P., & Dubois, C. M. (2021). The Chicken Chorioallantoic Membrane Tumor Assay as a Relevant In Vivo Model to Study the Impact of Hypoxia on Tumor Progression and Metastasis. Cancers, 13(5), 1093. https://doi.org/10.3390/cancers13051093






