Baseline Hedgehog Pathway Activation and Increase of Plasma Wnt1 Protein Are Associated with Resistance to Immune Checkpoint Inhibitors in Advanced Non-Small-Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
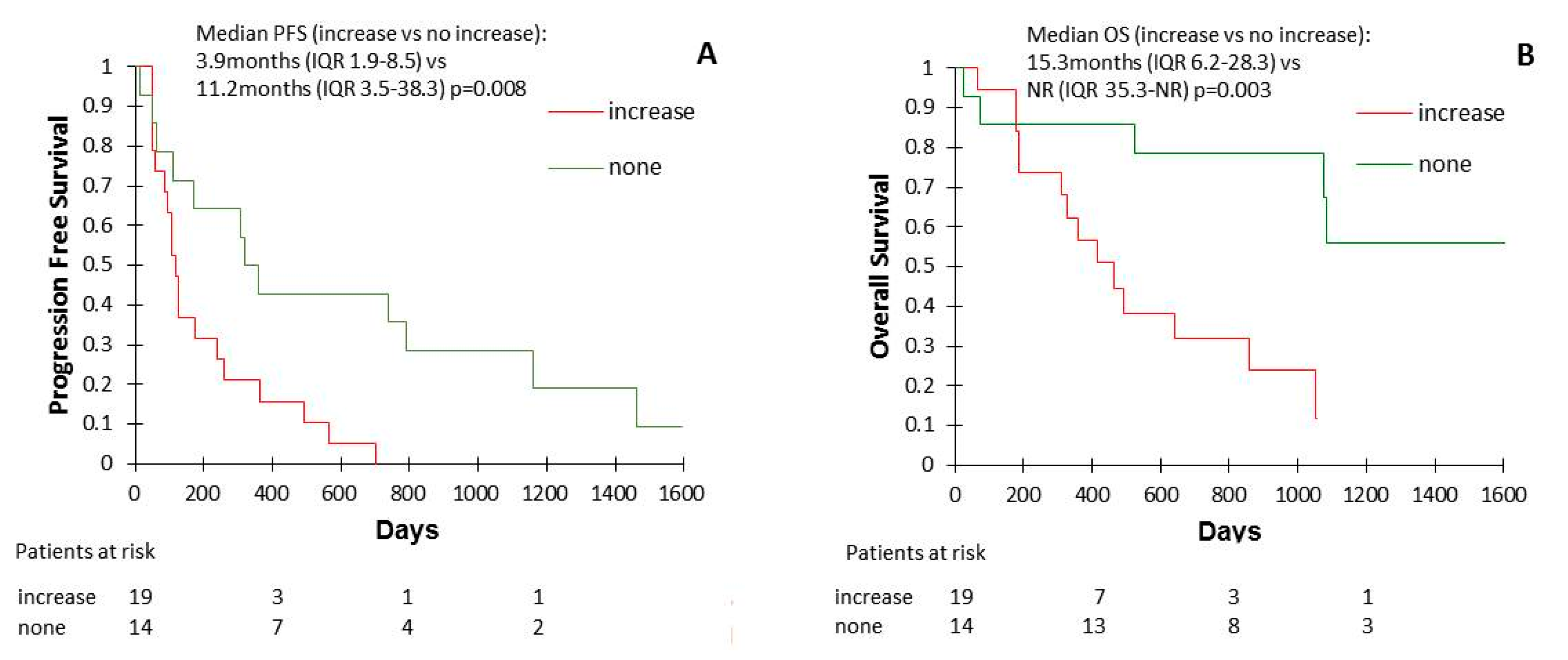
2.1. Clinical Characteristics
2.2. Hh Pathway Expression and Activation
2.2.1. Gli1 Expression
2.2.2. Plasma Shh Concentrations
2.3. Wnt-Beta Catenin Pathway Expression
2.3.1. Beta-Catenin Expression
2.3.2. Wnt1 Plasma Concentrations
2.3.3. Wnt2 Plasma Concentrations
2.3.4. Wnt3 Plasma Concentrations
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Plasma and Tumor Tissues
4.3. ELISA
4.4. Immunohistochemistry (IHC)
4.5. Reverse Transcription and Quantitative Polymerase Chain Reaction
4.6. Ethical Considerations
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| CSCs | Cancer stem cells |
| ECOG | Eastern Cooperative Oncology Group |
| ICIs | Immune checkpoint inhibitors |
| IHC | Immunohistochemistry |
| IQR | Interquartile range |
| NR | Not reached |
| NSCLC | Non-small-cell lung cancer |
| ORR | Objective response rate |
| OS | Overall survival |
| PCR | Polimerase chain reaction |
| PFS | Progression free survival |
| PD-1 | Programmed death-1 |
| PD-L1 | Programmed death-ligand 1 |
| Shh | Sonic Hedgehog |
References
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Hellmann, M.D.; Rizvi, A.N.; Goldman, J.W.; Gettinger, S.N.; Borghaei, H.; Brahmer, J.R.; Ready, E.N.; Gerber, E.D.; Chow, L.Q.; Juergens, A.R.; et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): Results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017, 18, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.M.; Marabelle, A.; Soria, J.-C.; et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin. Cancer Res. 2017, 23, 1920–1928. [Google Scholar] [CrossRef] [Green Version]
- Ready, N.; Hellmann, M.D.; Awad, M.M.; Otterson, G.A.; Gutierrez, M.; Gainor, J.F.; Borghaei, H.; Jolivet, J.; Horn, L.; Mates, M.; et al. First-Line Nivolumab Plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J. Clin. Oncol. 2019, 37, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Arbour, K.C.; Hellmann, M.D.; Patil, P.D.; Marmarelis, M.E.; Awad, M.M.; Murray, J.C.; Hellyer, J.; Gainor, J.F.; Dimou, A.; et al. Association of STK11/LKB1 genomic alterations with lack of benefit from the addition of pembrolizumab to platinum doublet chemotherapy in non-squamous non-small cell lung cancer. J. Clin. Oncol. 2019, 37, 102. [Google Scholar] [CrossRef]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.; Choi, J.; Hastings, K.; Truini, A.; Datar, I.; Sowell, R.; Wurtz, A.; Dong, W.; Cai, G.; Melnick, M.A.; et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov. 2017, 7, 1420–1435. [Google Scholar] [CrossRef] [Green Version]
- Anagnostou, V.; Smith, K.N.; Forde, P.M.; Niknafs, N.; Bhattacharya, R.; White, J.; Zhang, T.; Adleff, V.; Phallen, J.; Wali, N.; et al. Evolution of Neoantigen Landscape during Immune Checkpoint Blockade in Non–Small Cell Lung Cancer. Cancer Discov. 2017, 7, 264–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Lewis, M.T.; Huang, J.; Gutierrez, C.; Osborne, C.K.; Wu, M.-F.; Hilsenbeck, S.G.; Pavlick, A.; Zhang, X.; Chamness, G.C.; et al. Intrinsic Resistance of Tumorigenic Breast Cancer Cells to Chemotherapy. J. Natl. Cancer Inst. 2008, 100, 672–679. [Google Scholar] [CrossRef]
- Leung, E.L.-H.; Fiscus, R.R.; Tung, J.W.; Tin, V.P.-C.; Cheng, L.C.; Sihoe, A.D.-L.; Fink, L.M.; Ma, Y.; Wong, M.P. Non-Small Cell Lung Cancer Cells Expressing CD44 Are Enriched for Stem Cell-Like Properties. PLoS ONE 2010, 5, e14062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salnikov, A.V.; Gladkich, J.; Moldenhauer, G.; Volm, M.; Herr, I. CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients. Int. J. Cancer 2009, 126, 950–958. [Google Scholar] [CrossRef]
- Maccalli, C.; Parmiani, G.; Ferrone, S. Immunomodulating and Immunoresistance Properties of Cancer-Initiating Cells: Implications for the Clinical Success of Immunotherapy. Immunol. Investig. 2017, 46, 221–238. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, C.; Zheng, X.; Nie, X.; Wang, Z.; Liu, H.; Zhao, Y. LIN28/let-7/PD-L1 Pathway as a Target for Cancer Immunotherapy. Cancer Immunol. Res. 2019, 7, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Lemjabbar-Alaoui, H.; Dasari, V.; Sidhu, S.S.; Mengistab, A.; Finkbeiner, W.; Gallup, M.; Basbaum, C. Wnt and Hedgehog Are Critical Mediators of Cigarette Smoke-Induced Lung Cancer. PLoS ONE 2006, 1, e93. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Y.; Wang, X.; Wang, Y.; Ma, D. Wnt/β-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem. Biophys. Res. Commun. 2010, 392, 373–379. [Google Scholar] [CrossRef]
- Xu, X.; Sun, P.-L.; Li, J.-Z.; Jheon, S.; Lee, C.-T.; Chung, J.-H. Aberrant Wnt1/β-Catenin Expression is an Independent Poor Prognostic Marker of Non-small Cell Lung Cancer After Surgery. J. Thorac. Oncol. 2011, 6, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, N.; Liu, D.; Huang, C.-L.; Ueno, M.; Zhang, X.; Yokomise, H. Wnt3 gene expression promotes tumor progression in non-small cell lung cancer. Lung Cancer 2012, 76, 228–234. [Google Scholar] [CrossRef]
- Calderaro, J.; Couchy, G.; Imbeaud, S.; Amaddeo, G.; Letouzé, E.; Blanc, J.-F.; Laurent, C.; Hajji, Y.; Azoulay, D.; Bioulac-Sage, P.; et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J. Hepatol. 2017, 67, 727–738. [Google Scholar] [CrossRef]
- Pai, S.G.; Carneiro, B.A.; Mota, J.M.; Costa, R.; Leite, C.A.; Barroso-Sousa, R.; Kaplan, J.B.; Chae, Y.K.; Giles, F.J. Wnt/beta-catenin pathway: Modulating anticancer immune response. J. Hematol. Oncol. 2017, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nat. Cell Biol. 2015, 523, 231–235. [Google Scholar] [CrossRef]
- Giroux Leprieur, E.; Hélias-Rodzewicz, Z.; Kamga, P.T.; Costantini, A.; Julie, C.; Corjon, A.; Dumenil, C.; Dumoulin, J.; Giraud, V.; Labrune, S.; et al. Sequential ctDNA whole-exome sequencing in advanced lung adenocarcinoma with initial durable tumor response on immune checkpoint inhibitor and late progression. J. Immunother. Cancer 2020, 8, e000527. [Google Scholar] [CrossRef]
- Giroux Leprieur, E.; Jablons, D.M.; He, B. Old Sonic Hedgehog, new tricks: A new paradigm in thoracic malignancies. Oncotarget 2018, 9, 14680–14691. [Google Scholar] [CrossRef] [Green Version]
- Giroux-Leprieur, E.; Costantini, A.; Ding, V.W.; He, B. Hedgehog Signaling in Lung Cancer: From Oncogenesis to Cancer Treatment Resistance. Int. J. Mol. Sci. 2018, 19, 2835. [Google Scholar] [CrossRef] [Green Version]
- Giroux Leprieur, E.; Tolani, B.; Li, H.; Leguay, F.; Hoang, N.T.; Acevedo, L.A.; Jin, J.Q.; Tseng, H.-H.; Yue, D.; Kim, I.-J.; et al. Membrane-bound full-length Sonic Hedgehog identifies cancer stem cells in human non-small cell lung cancer. Oncotarget 2017, 8, 103744–103757. [Google Scholar] [CrossRef] [Green Version]
- Giroux Leprieur, E.; Vieira, T.; Antoine, M.; Rozensztajn, N.; Rabbe, N.; Ruppert, A.-M.; Lavole, A.; Cadranel, J.; Wislez, M. Sonic Hedgehog Pathway Activation Is Associated With Resistance to Platinum-Based Chemotherapy in Advanced Non–Small-Cell Lung Carcinoma. Clin. Lung Cancer 2016, 17, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Della Corte, C.M.; Malapelle, U.; Vigliar, E.; Pepe, F.; Troncone, G.; Ciaramella, V.; Troiani, T.; Martinelli, E.; Belli, V.; Ciardiello, F.; et al. Efficacy of continuous EGFR-inhibition and role of Hedgehog in EGFR acquired resistance in human lung cancer cells with activating mutation of EGFR. Oncotarget 2017, 8, 23020–23032. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Tian, L.; Cheng, J.; Chen, Z.; Xu, B.; Wang, L.; Li, C.; Huang, Q. Sonic Hedgehog Signaling Pathway Supports Cancer Cell Growth during Cancer Radiotherapy. PLoS ONE 2013, 8, e65032. [Google Scholar] [CrossRef] [Green Version]
- Grund-Gröschke, S.; Ortner, D.; Szenes-Nagy, A.B.; Zaborsky, N.; Weiss, R.; Neureiter, D.; Wipplinger, M.; Risch, A.; Hammerl, P.; Greil, R.; et al. Epidermal activation of Hedgehog signaling establishes an immunosuppressive microenvironment in basal cell carcinoma by modulating skin immunity. Mol. Oncol. 2020, 14, 1930–1946. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Genomic testing, tumor microenvironment and targeted therapy of Hedgehog-related human cancers. Clin. Sci. 2019, 133, 953–970. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.; Metge, B.J.; Bailey, S.K.; Chen, D.; Chandrashekar, D.S.; Varambally, S.; Samant, R.S.; Shevde, L.A. Inhibition of Hedgehog signaling reprograms the dysfunctional immune microenvironment in breast cancer. OncoImmunology 2019, 8, 1548241. [Google Scholar] [CrossRef]
- Chakrabarti, J.; Holokai, L.; Syu, L.; Steele, N.G.; Chang, J.; Wang, J.; Ahmed, S.; Dlugosz, A.; Zavros, Y. Hedgehog signaling induces PD-L1 expression and tumor cell proliferation in gastric cancer. Oncotarget 2018, 9, 37439–37457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Chen, X.; Hou, J.; Wang, C.; Xiang, Z.; Shen, Y.; Han, X. The Shh/Gli signaling cascade regulates myofibroblastic activation of lung-resident mesenchymal stem cells via the modulation of Wnt10a expression during pulmonary fibrogenesis. Lab. Investig. 2019, 100, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tian, T.; Kalland, K.-H.; Ke, X.; Qu, Y. Targeting Wnt/β-Catenin Signaling for Cancer Immunotherapy. Trends Pharmacol. Sci. 2018, 39, 648–658. [Google Scholar] [CrossRef]
- Pathi, S.; Pagan-Westphal, S.; Baker, D.P.; Garber, A.E.; Rayhorn, P.; Bumcrot, D.; Tabin, C.J.; Pepinsky, R.B.; Williams, K.P. Comparative biological responses to human Sonic, Indian, and Desert hedgehog. Mech. Dev. 2001, 106, 107–117. [Google Scholar] [CrossRef]
- Kakanj, P.; Reuter, K.; Séquaris, G.; Wodtke, C.; Schettina, P.; Frances, D.; Zouboulis, C.C.; Lanske, B.; Niemann, C. Indian Hedgehog Controls Proliferation and Differentiation in Skin Tumorigenesis and Protects against Malignant Progression. Cell Rep. 2013, 4, 340–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aberger, F.; Hutterer, E.; Sternberg, C.; Del Burgo, P.J.; Hartmann, T.N. Acute myeloid leukemia-strategies and challenges for targeting oncogenic Hedgehog/GLI signaling. Cell Commun. Signal. 2017, 15, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, C.D.; Flores, C.; Yang, C.; Pinheiro, E.M.; Yearley, J.H.; Sayour, E.J.; Pei, Y.; E Moore, C.; McLendon, R.E.; Huang, J.; et al. Differential Immune Microenvironments and Response to Immune Checkpoint Blockade among Molecular Subtypes of Murine Medulloblastoma. Clin. Cancer Res. 2016, 22, 582–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briscoe, J.; Thérond, P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013, 14, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, D.; Zhou, H.; Oliphant, M.U.J.; Zhang, X.; Simon, L.M.; Henke, D.M.; Shaw, C.A.; Wu, M.-F.; Hilsenbeck, S.G.; White, L.D.; et al. EMT cells increase breast cancer metastasis via paracrine GLI activation in neighbouring tumour cells. Nat. Commun. 2017, 8, 15773. [Google Scholar] [CrossRef]
- Mallery, S.R.; Wang, D.; Santiago, B.; Pei, P.; Bissonnette, C.; Jayawardena, J.A.; Schwendeman, S.P.; Spinney, R.; Lang, J. Fenretinide, Tocilizumab, and Reparixin Provide Multifaceted Disruption of Oral Squamous Cell Carcinoma Stem Cell Properties: Implications for Tertiary Chemoprevention. Mol. Cancer Ther. 2019, 18, 2308–2320. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-F.; Chang, C.-J.; Lin, C.-P.; Chang, S.-Y.; Chu, P.-Y.; Tai, S.-K.; Li, W.-Y.; Chao, K.C.; Chen, Y.-J. Expression of hedgehog signaling molecules as a prognostic indicator of oral squamous cell carcinoma. Head Neck 2012, 34, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Tanese, K.; Emoto, K.; Kubota, N.; Fukuma, M.; Sakamoto, M. Immunohistochemical visualization of the signature of activated Hedgehog signaling pathway in cutaneous epithelial tumors. J. Dermatol. 2018, 45, 1181–1186. [Google Scholar] [CrossRef]
- Carlson, J.W.; Fletcher, C.D.M. Immunohistochemistry for β?-catenin in the differential diagnosis of spindle cell lesions: Analysis of a series and review of the literature. Histopathology 2007, 51, 509–514. [Google Scholar] [CrossRef] [PubMed]
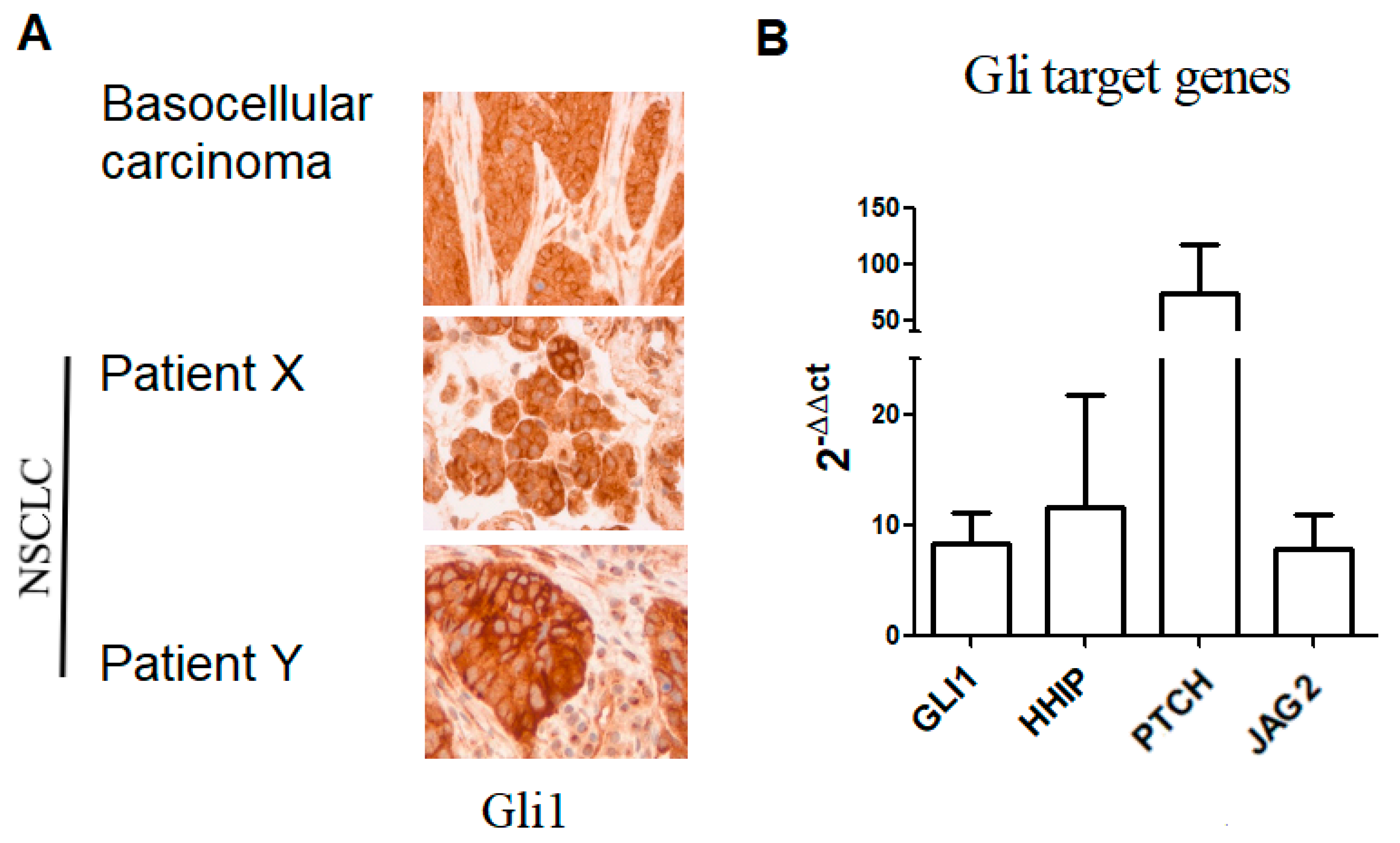


| Characteristics | Overall Population n = 63 |
|---|---|
| Age at the introduction of ICI (year-old) | |
| Median | 68 |
| Range | 62.0–73.5 |
| Gender | |
| Female | 28 (44.5%) |
| Male | 35 (55.5%) |
| Smoking status | |
| Current | 18 (28.6 %) |
| Former | 39 (61.9 %) |
| Never | 6 (9.5 %) |
| Histology | |
| Non squamous | 43 (68.2 %) |
| Squamous | 12 (19.0 %) |
| Other | 8 (12.7 %) |
| Molecular alteration at the diagnostic | |
| KRAS mutation | 22 (34.9 %) |
| BRAF mutation | 4 (6.3 %) |
| EGFR mutation | 2 (3.2 %) |
| MET amplification | 1 (1.6 %) |
| No alteration | 34 (54.0 %) |
| ECOG performance-status at the introduction of ICI | |
| 0–1 | 44 (69.8 %) |
| 2 | 19 (30.2 %) |
| Type of ICI and number of lines before ICI | |
| Pembrolizumab | 20 (31.7 %) |
| First line | 18 (28.6 %) |
| Second line and more | 2 (3.2 %) |
| Nivolumab | 43 (68.3 %) |
| First line | 0 (0 %) |
| Second line and more | 43 (68.3 %) |
| Tumor Response | Primary Resistance | ||||||
|---|---|---|---|---|---|---|---|
| Responders (pg/mL) | Non-Responders (pg/mL) | p | Yes (pg/mL) | No (pg/mL) | p | ||
| Shh | Introduction | 365.5 (IQR 282.3–507.6) | 286.4 (IQR 142.2–434.6) | 0.11 | 274.8 (IQR 139.6–372.1) | 386.0 (IQR 212.9–532.5) | 0.03 |
| First evaluation | 467.4 (IQR 282.6–693.2) | 268.2 (IQR 142.3–461.3) | 0.01 | 242.2 (IQR 147.4–304.5) | 454.7 (IQR 236.1–640.2) | 0.009 | |
| Wnt1 | Introduction | 444.5 (IQR 290.6–727.4) | 396.1 (IQR 282.0–668.4) | 0.62 | 557.1 (IQR 296.3–707.1) | 397,3 (IQR 260.4–560.3) | 0.23 |
| First evaluation | 503.3 (IQR 367.8–688.6) | 346.6 (IQR 261.9–567.5) | 0.07 | 371.4 (IQR 266.3–540.8) | 424.4 (IQR 330.0–681.3) | 0.30 | |
| Wnt2 | Introduction | 689.2 (IQR 446.0–1201.5) | 918.6 (IQR 508.0–2138.4) | 0.09 | 1551.4 (IQR 513.8–2381.2) | 778.1 (IQR 502.2–1410.9) | 0.10 |
| First evaluation | 937.8 (IQR 469.4–1098.7) | 835.2 (IQR 563.3–1530.5) | 0.71 | 831.2 (IQR 534.1–1773.3) | 928.6 (IQR 482.4–1131.8) | 0.90 | |
| Wnt3 | Introduction | 270.4 (IQR 95.7–382.8) | 331.6 (IQR 244.6–409.7) | 0.20 | 320.6 (IQR 234.7–487.1) | 318.7 (IQR155.4–386.5) | 0.67 |
| First evaluation | 358.8 (IQR 189.5–398.0) | 319.2 (IQR 59.5–474.0) | 0.78 | 335.8 (IQR 64.5–441.7) | 354.6 (IQR 140.9–449.4) | 0.63 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehlman, C.; Takam Kamga, P.; Costantini, A.; Julié, C.; Dumenil, C.; Dumoulin, J.; Ouaknine, J.; Giraud, V.; Chinet, T.; Emile, J.-F.; et al. Baseline Hedgehog Pathway Activation and Increase of Plasma Wnt1 Protein Are Associated with Resistance to Immune Checkpoint Inhibitors in Advanced Non-Small-Cell Lung Cancer. Cancers 2021, 13, 1107. https://doi.org/10.3390/cancers13051107
Mehlman C, Takam Kamga P, Costantini A, Julié C, Dumenil C, Dumoulin J, Ouaknine J, Giraud V, Chinet T, Emile J-F, et al. Baseline Hedgehog Pathway Activation and Increase of Plasma Wnt1 Protein Are Associated with Resistance to Immune Checkpoint Inhibitors in Advanced Non-Small-Cell Lung Cancer. Cancers. 2021; 13(5):1107. https://doi.org/10.3390/cancers13051107
Chicago/Turabian StyleMehlman, Camille, Paul Takam Kamga, Adrien Costantini, Catherine Julié, Coraline Dumenil, Jennifer Dumoulin, Julia Ouaknine, Violaine Giraud, Thierry Chinet, Jean-François Emile, and et al. 2021. "Baseline Hedgehog Pathway Activation and Increase of Plasma Wnt1 Protein Are Associated with Resistance to Immune Checkpoint Inhibitors in Advanced Non-Small-Cell Lung Cancer" Cancers 13, no. 5: 1107. https://doi.org/10.3390/cancers13051107





