The Frontal Aslant Tract and Supplementary Motor Area Syndrome: Moving towards a Connectomic Initiation Axis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Data Collection
2.2. Defining SMA Syndrome
2.3. Operative Technique
2.3.1. Traditional Surgical Approach
2.3.2. Modified Approach to Avoid the FAT
2.4. Outcome Assessment
2.5. Statistical Analysis of Clinical Outcomes
2.6. Construction of Network Maps and Anatomy of the “Command and Control” Axis
2.6.1. Literature Search Strategy
2.6.2. ALE Generation and Identification of Relevant Cortical Regions
2.6.3. Tractography
3. Results
3.1. Patient Population
3.2. Surgical Outcomes
4. Discussion
4.1. Challenges with Certainty
4.2. Onco-Functional Balance for Surgically Induced SMA Syndrome in Glioma Patients
4.3. Moving towards the Idea of Preserving the “Initiation” Axis
4.4. Techniques for Operating around the Initiation Axis
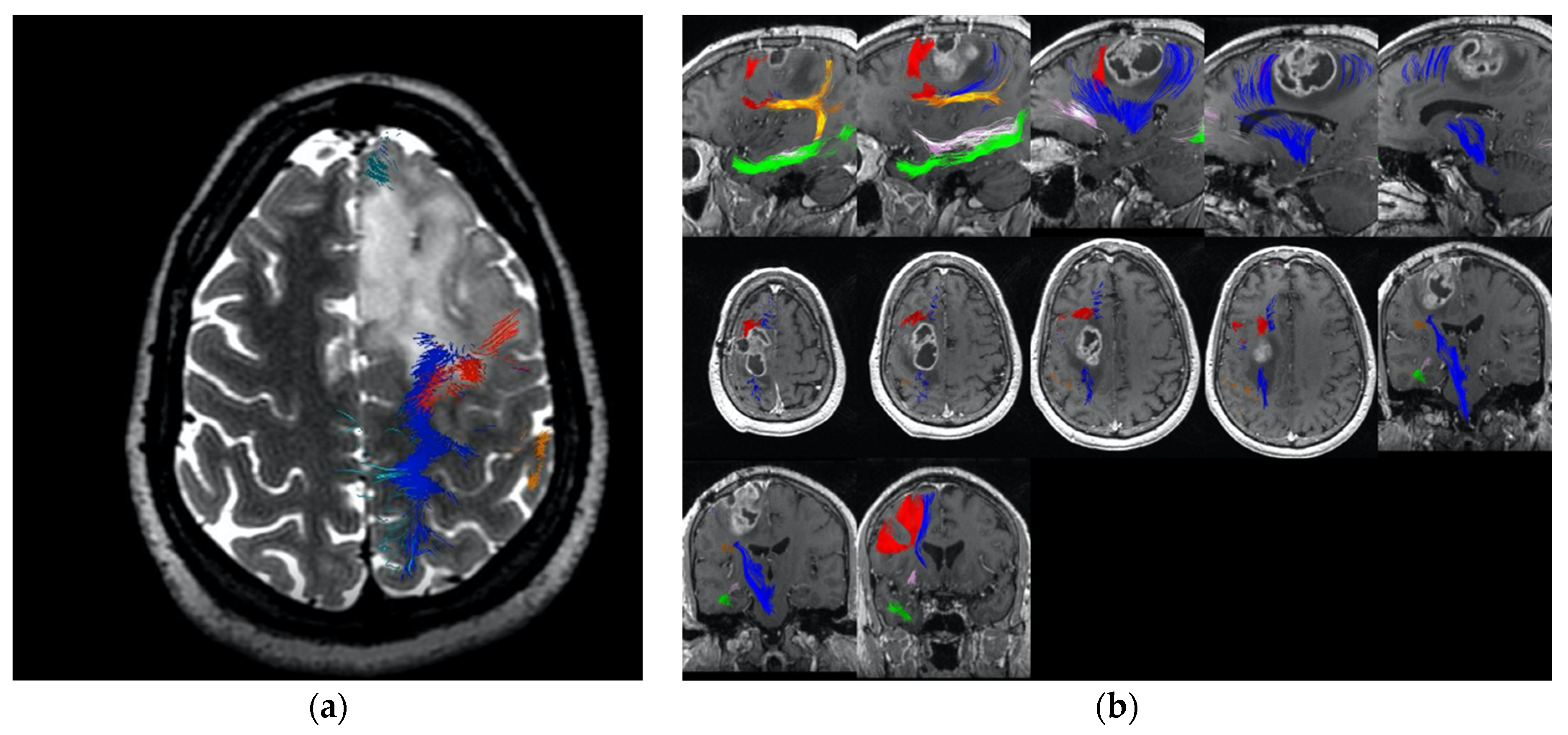
4.4.1. Coronal Cuts within the Motor System and along the Frontal Aslant Tract
4.4.2. Sagittal Cuts within the Cingulum
4.4.3. Tumours Which Split the FAT and Primary Motor Cortex
4.5. Future Work in Cognition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pallud, J.; Dezamis, E. Functional and Oncological Outcomes Following Awake Surgical Resection Using Intraoperative Cortico-Subcortical Functional Mapping for Supratentorial Gliomas Located in Eloquent Areas. Neurochirurgie 2017, 63, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Grafman, J. The structured event complex and the human prefrontal cortex. In Principles of Frontal Lobe Function; Oxford University Press: New York, NY, USA, 2002; pp. 292–310. [Google Scholar]
- Krainik, A.; Lehericy, S.; Duffau, H.; Vlaicu, M.; Poupon, F.; Capelle, L.; Cornu, P.; Clemenceau, S.; Sahel, M.; Valery, C.A.; et al. Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology 2001, 57, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Poologaindran, A.; Suckling, J.; Sughrue, M.E. Letter: Elucidating the Principles of Brain Network Organization Through Neurosurgery. Neurosurgery 2020, 87, E80–E81. [Google Scholar] [CrossRef] [PubMed]
- Avecillas-Chasin, J.M.; Hurwitz, T.A.; Bogod, N.M.; Honey, C.R. An Analysis of Clinical Outcome and Tractography following Bilateral Anterior Capsulotomy for Depression. Stereotact. Funct. Neurosurg. 2019, 97, 369–380. [Google Scholar] [CrossRef]
- Poologaindran, A.; Lowe, S.R.; Sughrue, M.E. The cortical organization of language: Distilling human connectome insights for supratentorial neurosurgery. J. Neurosurg. 2020, 1–8. [Google Scholar] [CrossRef]
- Abel, T.J.; Buckley, R.T.; Morton, R.P.; Gabikian, P.; Silbergeld, D.L. Recurrent Supplementary Motor Area Syndrome Following Repeat Brain Tumor Resection Involving Supplementary Motor Cortex. Neurosurgery 2015, 11 (Suppl. 3), 447–455, discussion 456. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.A.-O.X.; Bludau, S.; Palomero-Gallagher, N.; Caspers, S.; Mohlberg, H.; Eickhoff, S.B.; Seitz, R.J.; Amunts, K. Cytoarchitecture, probability maps, and functions of the human supplementary and pre-supplementary motor areas. Brain Struct. Funct. 2018, 223, 4169–4186. [Google Scholar] [CrossRef]
- Mandonnet, E.; Sarubbo, S.; Duffau, H. Proposal of an optimized strategy for intraoperative testing of speech and language during awake mapping. Neurosurg. Rev. 2017, 40, 29–35. [Google Scholar] [CrossRef]
- Yu, Y.L.; Lee, M.S.; Juan, C.J.; Hueng, D.Y. Calculating the tumor volume of acoustic neuromas: Comparison of ABC/2 formula with planimetry method. Clin. Neurol. Neurosurg. 2013, 115, 1371–1374. [Google Scholar] [CrossRef]
- Laird, A.R.; Lancaster, J.L.; Fox, P.T. BrainMap: The social evolution of a human brain mapping database. Neuroinformatics 2005, 3, 65–78. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Laird, A.R.; Grefkes, C.; Wang, L.E.; Zilles, K.; Fox, P.T. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009, 30, 2907–2926. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Bzdok, D.; Laird, A.R.; Kurth, F.; Fox, P.T. Activation likelihood estimation meta-analysis revisited. Neuroimage 2012, 59, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Turkeltaub, P.E.; Eickhoff, S.B.; Laird, A.R.; Fox, M.; Wiener, M.; Fox, P. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum. Brain Mapp. 2012, 33, 1–13. [Google Scholar] [CrossRef]
- Baker, C.M.; Burks, J.D.; Briggs, R.G.; Sheets, J.R.; Conner, A.K.; Glenn, C.A.; Sali, G.; McCoy, T.M.; Battiste, J.D.; O’Donoghue, D.L.; et al. A Connectomic Atlas of the Human Cerebrum-Chapter 3: The Motor, Premotor, and Sensory Cortices. Oper. Neurosurg. 2018, 15, S75–S121. [Google Scholar] [CrossRef]
- Baker, C.M.; Burks, J.D.; Briggs, R.G.; Conner, A.K.; Glenn, C.A.; Morgan, J.P.; Stafford, J.; Sali, G.; McCoy, T.M.; Battiste, J.D.; et al. A Connectomic Atlas of the Human Cerebrum-Chapter 2: The Lateral Frontal Lobe. Oper. Neurosurg. 2018, 15, S10–S74. [Google Scholar] [CrossRef]
- Baker, C.M.; Burks, J.D.; Briggs, R.G.; Conner, A.K.; Glenn, C.A.; Sali, G.; McCoy, T.M.; Battiste, J.D.; O’Donoghue, D.L.; Sughrue, M.E. A Connectomic Atlas of the Human Cerebrum-Chapter 1: Introduction, Methods, and Significance. Oper. Neurosurg. 2018, 15, S1–S9. [Google Scholar] [CrossRef]
- Yeh, F.-C.; Wedeen, V.J.; Tseng, W.-Y.I. Generalized Q-Sampling Imaging. IEEE Trans. Med. Imaging 2010, 29, 1626–1635. [Google Scholar]
- Baker, C.M.; Burks, J.D.; Briggs, R.G.; Stafford, J.; Conner, A.K.; Glenn, C.A.; Sali, G.; McCoy, T.M.; Battiste, J.D.; O’Donoghue, D.L.; et al. A Connectomic Atlas of the Human Cerebrum-Chapter 4: The Medial Frontal Lobe, Anterior Cingulate Gyrus, and Orbitofrontal Cortex. Oper. Neurosurg. 2018, 15, S122–S174. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.G.; Conner, A.K.; Sali, G.; Rahimi, M.; Baker, C.M.; Burks, J.D.; Glenn, C.A.; Battiste, J.D.; Sughrue, M.E. A Connectomic Atlas of the Human Cerebrum-Chapter 17: Tractographic Description of the Cingulum. Oper. Neurosurg. 2018, 15, S462–S469. [Google Scholar] [CrossRef] [PubMed]
- Goulden, N.; Khusnulina, A.; Davis, N.J.; Bracewell, R.M.; Bokde, A.L.; McNulty, J.P.; Mullins, P.G. The salience network is responsible for switching between the default mode network and the central executive network: Replication from DCM. NeuroImage 2014, 99, 180–190. [Google Scholar] [CrossRef]
- Nimsky, C.; Bauer, M.; Carl, B. Merits and Limits of Tractography Techniques for the Uninitiated. Adv. Tech. Stand. Neurosurg. 2016, 37–60. [Google Scholar] [CrossRef]
- Gong, S.; Zhang, F.; Norton, I.; Essayed, W.I.; Unadkat, P.; Rigolo, L.; Pasternak, O.; Rathi, Y.; Hou, L.; Golby, A.J.; et al. Free water modeling of peritumoral edema using multi-fiber tractography: Application to tracking the arcuate fasciculus for neurosurgical planning. PLoS ONE 2018, 13, e0197056. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, C.H.; Kim, J.S.; Lee, S.K.; Han, J.H.; Kim, C.Y.; Chung, C.K. Risk factor analysis of the development of new neurological deficits following supplementary motor area resection. J. Neurosurg. 2013, 119, 7–14. [Google Scholar] [CrossRef]
- Zentner, J.; Hufnagel, A.; Pechstein, U.; Wolf, H.K.; Schramm, J. Functional results after resective procedures involving the supplementary motor area. J. Neurosurg. 1996, 85, 542–549. [Google Scholar] [CrossRef]
- Kasasbeh, A.S.; Yarbrough, C.K.; Limbrick, D.D.; Steger-May, K.; Leach, J.L.; Mangano, F.T.; Smyth, M.D. Characterization of the supplementary motor area syndrome and seizure outcome after medial frontal lobe resections in pediatric epilepsy surgery. Neurosurgery 2012, 70, 1152–1168; discussion 1168. [Google Scholar] [CrossRef]
- Sun, M.Z.; Oh, T.; Ivan, M.E.; Clark, A.J.; Safaee, M.; Sayegh, E.T.; GKaur, u.; Parsa, A.T.; Bloch, O. Survival impact of time to initiation of chemoradiotherapy after resection of newly diagnosed glioblastoma. J. Neurosurg. 2015, 122, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Gabarrós, A.; Martino, J.; Juncadella, M.; Plans, G.; Pujol, R.; Deus, J.; Godino, O.; Torres, A.; Aparicio, A.; Conesa, G.; et al. Intraoperative identification of the supplementary motor area in neurooncological surgery. Neurocirugía 2011, 22, 123–132. [Google Scholar] [CrossRef]
- Kushner, D.S.; Amidei, C. Rehabilitation of motor dysfunction in primary brain tumor patients. Neuro-Oncol. Pract. 2015, 2, 185–191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chaichana, K.L.; Pendleton, C.; Jackson, C.; Martinez-Gutierrez, J.C.; Diaz-Stransky, A.; Aguayo, J.; Olivi, A.; Weingart, J.; Gallia, G.; Lim, M.; et al. Deep venous thrombosis and pulmonary embolisms in adult patients undergoing craniotomy for brain tumors. Neurol. Res. 2013, 35, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Burks, J.D.; Bonney, P.A.; Conner, A.K.; Glenn, C.A.; Briggs, R.G.; Battiste, J.D.; McCoy, T.; O’Donoghue, D.L.; Wu, D.H.; Sughrue, M.E. A method for safely resecting anterior butterfly gliomas: The surgical anatomy of the default mode network and the relevance of its preservation. J. Neurosurg. 2017, 126, 1795–1811. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G.; Trillo, G.; Picotti, V.; Raco, A. Functional Magnetic Resonance Imaging (fMRI), Pre-intraoperative Tractography in Neurosurgery: The Experience of Sant’ Andrea Rome University Hospital. In Trends in Reconstructive Neurosurgery; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Maesawa, S.; Fujii, M.; Nakahara, N.; Watanabe, T.; Wakabayashi, T.; Yoshida, J. Intraoperative tractography and motor evoked potential (MEP) monitoring in surgery for gliomas around the corticospinal tract. World Neurosurg. 2010, 74, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Vassal, F.; Schneider, F.; Sontheimer, A.; Lemaire, J.J.; Nuti, C. Intraoperative visualisation of language fascicles by diffusion tensor imaging-based tractography in glioma surgery. Acta Neurochir. 2013, 155, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Bubb, E.J.; Metzler-Baddeley, C.; Aggleton, J.P. The cingulum bundle: Anatomy, function, and dysfunction. Neurosci. Biobehav. Rev. 2018, 92, 104–127. [Google Scholar] [CrossRef]
- Conner, A.K.; Briggs, R.G.; Rahimi, M.; Sali, G.; Baker, C.M.; Burks, J.D.; Glenn, C.A.; Battiste, J.D.; Sughrue, M.E. A Connectomic Atlas of the Human Cerebrum-Chapter 12: Tractographic Description of the Middle Longitudinal Fasciculus. Oper. Neurosurg. 2018, 15, S429–S435. [Google Scholar] [CrossRef]
- Le Gars, D.; Lejeune, J.P.; Peltier, J. Surgical anatomy and surgical approaches to the lateral ventricles. Adv. Tech. Stand. Neurosurg. 2009, 34, 147–187. [Google Scholar] [CrossRef] [PubMed]
- Noll, K.R.; Bradshaw, M.E.; Rexer, J.; Wefel, J.S. Neuropsychological Practice in the Oncology Setting. Arch. Clin. Neuropsychol. 2018, 33, 344–353. [Google Scholar] [CrossRef]
- Canas, A.; Juncadella, M.; Lau, R.; Gabarros, A.; Hernandez, M. Working Memory Deficits After Lesions Involving the Supplementary Motor Area. Front. Psychol. 2018, 9, 765. [Google Scholar] [CrossRef]
- Dick, A.S.; Garic, D.; Graziano, P.; Tremblay, P. The frontal aslant tract (FAT) and its role in speech, language and executive function. Cortex 2019, 111, 148–163. [Google Scholar] [CrossRef]
- Varriano, F.; Pascual-Diaz, S.; Prats-Galino, A. When the FAT goes wide: Right extended Frontal Aslant Tract volume predicts performance on working memory tasks in healthy humans. PLoS ONE 2018, 13, e0200786. [Google Scholar] [CrossRef]
- Løvstad, M.; Funderud, I.; Meling, T.; Krämer, U.M.; Voytek, B.; Due-Tønnessen, P.; Endestad, T.; Lindgren, M.; Knight, R.T.; Solbakk, A.K. Anterior cingulate cortex and cognitive control: Neuropsychological and electrophysiological findings in two patients with lesions to dorsomedial prefrontal cortex. Brain Cogn. 2012, 80, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Romero-Garcia, R.; Erez, Y.; Oliver, G.; Owen, M.; Merali, S.; Poologaindran, A.; Morris, R.C.; Price, S.J.; Santarius, T.; Suckling, J.; et al. Practical Application of Networks in Neurosurgery: Combined 3-Dimensional Printing, Neuronavigation, and Preoperative Surgical Planning. World Neurosurg. 2020, 137, e126–e137. [Google Scholar] [CrossRef] [PubMed]
| No FAT Preservation | FAT Preservation | Chi-Squared Test | |
|---|---|---|---|
| Number of Patients (N) | 23 | 22 | |
| Gender (M/F) | 13/10 | 11/11 | p = 0.76 |
| Age | 44 ± 0.2 | 49 ± 2.6 | p = 0.06 |
| Tumour Laterality (L/R) | 13/10 | 16/6 | p = 0.35 |
| Tumour Size (cc) | 48 ± 1.4 | 37 ± 8.6 | p = 0.22 |
| Extent of Resection | p = 0.23 | ||
| <80% | 2/23 (9%) | 3/22 (14%) | |
| 80–90% | 1/23 (4%) | 5/22 (23%) | |
| 91–99% | 5/23 (22%) | 5/22 (23%) | |
| 100% | 15/23 (65%) | 9/22 (40%) | |
| Tumour Grade | p = 0.29 | ||
| Grade 2 | 9/23 (39%) | 4/22 (18%) | |
| Grade 3 | 4/23 (17%) | 6/22 (27%) | |
| Grade 4 | 10/23 (43%) | 12/22 (55%) | |
| Location | p = 1.000 | ||
| SMA Proper | 15/23 (65%) | 14/22 (64%) | |
| Near the FAT | 8/23 (35%) | 8/22 (36%) |
| No FAT Preservation | FAT Preservation | Chi-Squared Test | |
|---|---|---|---|
| Number of Patients (N) | 23 | 22 | |
| Temporary SMA Syndrome | 11/23 (47%) | 2/22 (8.3%) | p = 0.003 |
| Permanent SMA Syndrome | 3/23 (13%) | 0/22 (0%) | p = 0.232 |
| In SMA Proper | FAT Involved Only | Chi-Squared Test | |
|---|---|---|---|
| Number of Patients (N) | 29 | 16 | |
| Temporary SMA Syndrome | 8/29 (28%) | 5/16 (31%) | p = 1.000 |
| Permanent SMA Syndrome | 1/29 (3%) | 2/16 (13%) | p = 0.285 |
| In SMA Proper | FAT Involved Only | |||||
|---|---|---|---|---|---|---|
| No FAT Preservation | FAT Preservation | Chi-Squared Test | No FAT Preservation | FAT Preservation | Chi-Squared Test | |
| Number of Patients (N) | 14 | 15 | 8 | 8 | ||
| Temporary SMA Syndrome | 6/14 (43%) | 2/15 (13%) | p = 0.11 | 5/8 (63%) | 0/8 (0%) | p = 0.03 |
| Permanent SMA Syndrome | 1/14 (7%) | 0/15 (0%) | p = 0.48 | 2/8 (13%) | 0/8 (0%) | p = 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briggs, R.G.; Allan, P.G.; Poologaindran, A.; Dadario, N.B.; Young, I.M.; Ahsan, S.A.; Teo, C.; Sughrue, M.E. The Frontal Aslant Tract and Supplementary Motor Area Syndrome: Moving towards a Connectomic Initiation Axis. Cancers 2021, 13, 1116. https://doi.org/10.3390/cancers13051116
Briggs RG, Allan PG, Poologaindran A, Dadario NB, Young IM, Ahsan SA, Teo C, Sughrue ME. The Frontal Aslant Tract and Supplementary Motor Area Syndrome: Moving towards a Connectomic Initiation Axis. Cancers. 2021; 13(5):1116. https://doi.org/10.3390/cancers13051116
Chicago/Turabian StyleBriggs, Robert G., Parker G. Allan, Anujan Poologaindran, Nicholas B. Dadario, Isabella M. Young, Syed A. Ahsan, Charles Teo, and Michael E. Sughrue. 2021. "The Frontal Aslant Tract and Supplementary Motor Area Syndrome: Moving towards a Connectomic Initiation Axis" Cancers 13, no. 5: 1116. https://doi.org/10.3390/cancers13051116
APA StyleBriggs, R. G., Allan, P. G., Poologaindran, A., Dadario, N. B., Young, I. M., Ahsan, S. A., Teo, C., & Sughrue, M. E. (2021). The Frontal Aslant Tract and Supplementary Motor Area Syndrome: Moving towards a Connectomic Initiation Axis. Cancers, 13(5), 1116. https://doi.org/10.3390/cancers13051116








