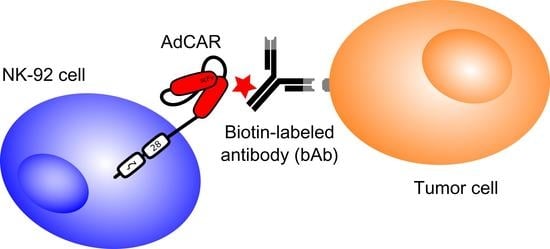
Adapter Chimeric Antigen Receptor (AdCAR)-Engineered NK-92 Cells for the Multiplex Targeting of Bone Metastases
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
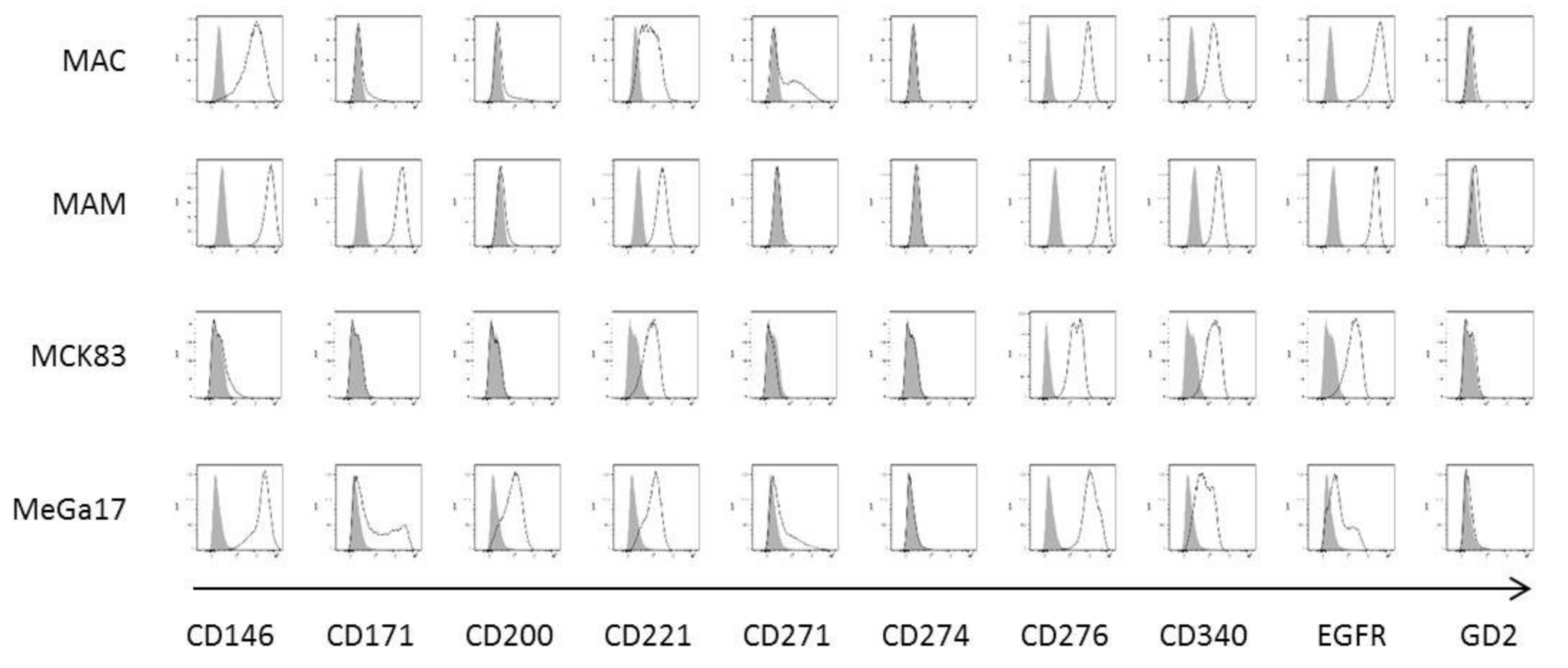
2.1. Establishment and Characterization of Newly Developed Bone Metastasis Cell Lines
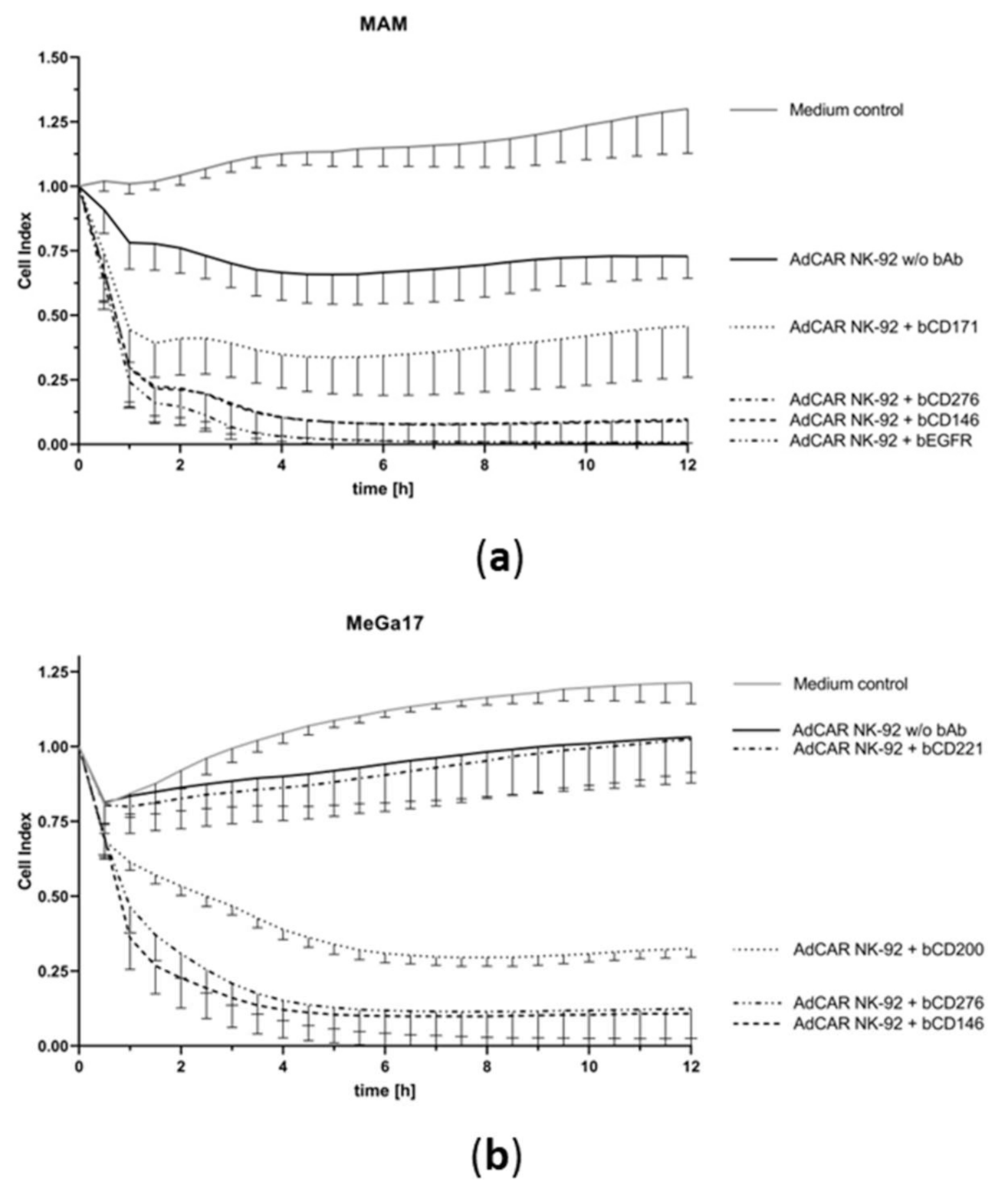
2.2. AdCAR NK-92 Cells Specifically Lyse Bone Metastasis Cell Lines In Vitro
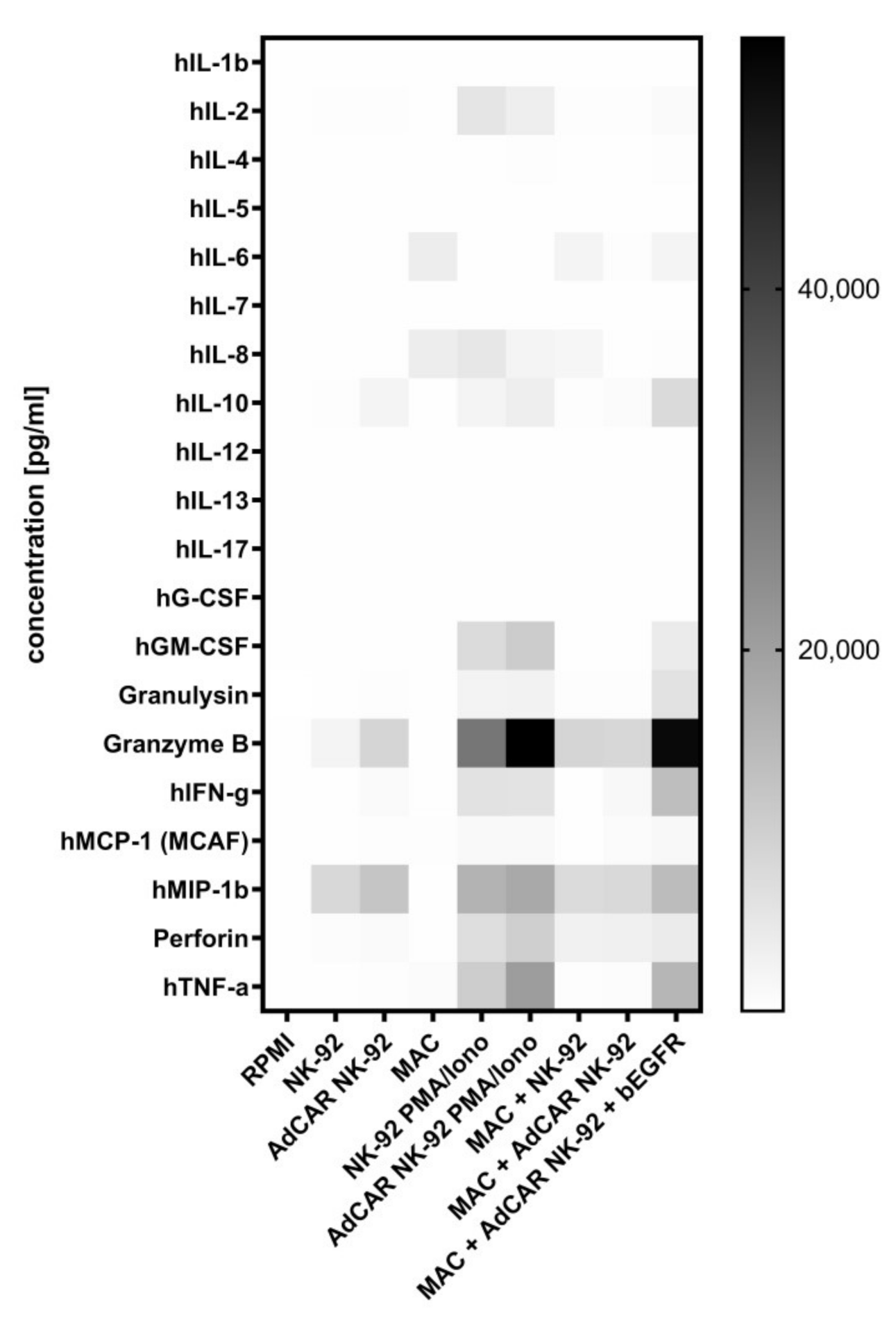
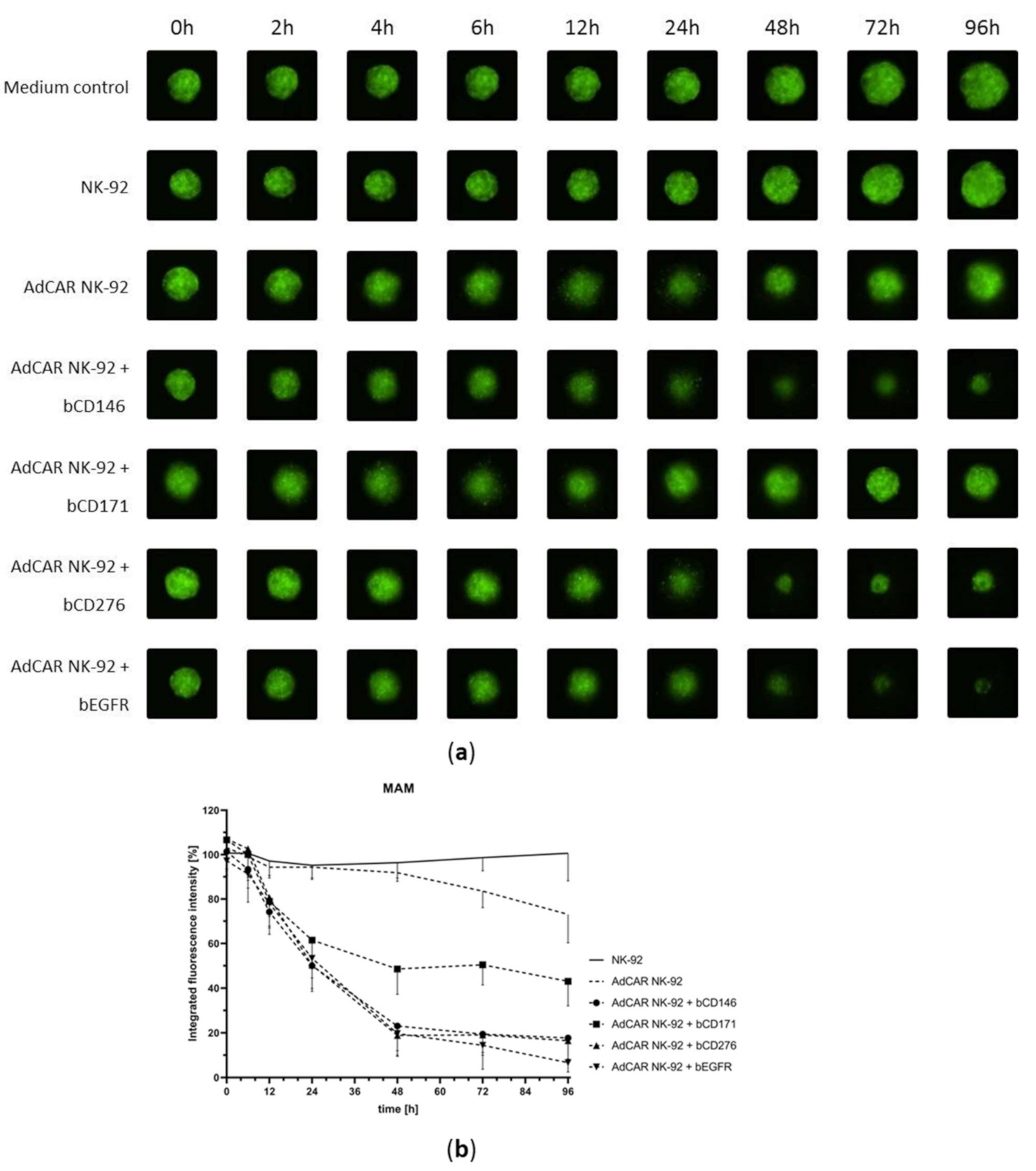
2.3. NK-92 Cells Exhibit Successful AdCAR-Mediated Cytotoxicity in a Three-Dimensional Tumor Cell Model
3. Discussion
4. Materials and Methods
4.1. Cell lines and Culturing Conditions
4.2. Design of the AdCAR System
4.3. Biotinylated Antibodies
| Antigen | Clone | Antibody | Order # | Lot | Supplier |
| CD146 | 541-10B2 | n/a | 130-092-852 | 5190627154 | Miltenyi Biotec, Bergisch Gladbach, Germany |
| CD171 | REA163 | n/a | 130-100-702 | 5190607129 | Miltenyi Biotec, Bergisch Gladbach, Germany |
| CD200 | OX-104 | n/a | 130-106-064 | 5191021606 | Miltenyi Biotec, Bergisch Gladbach, Germany |
| CD221 | REA271 | n/a | 130-103-973 | 5190627184 | Miltenyi Biotec, Bergisch Gladbach, Germany |
| CD271 | REA844 | n/a | 130-112-608 | 5190627191 | Miltenyi Biotec, Bergisch Gladbach, Germany |
| CD274 | n/a | Atezolizumab | n/a | n/a | Hoffmann-La Roche, Basel, Switzerland |
| CD276 | FM276 | n/a | 130-095-514 | 5190627174 | Miltenyi Biotec, Bergisch Gladbach, Germany |
| CD340 | n/a | Trastuzumab | n/a | n/a | Hoffmann-La Roche, Basel, Switzerland |
| EGFR | n/a | Cetuximab | n/a | n/a | Merck KgaA, Darmstadt, Germany |
| GD2 | n/a | Dinutuximab beta | n/a | n/a | Eusa Pharma, Hertfordshire, Great Britain |
4.4. Flow Cytometry
4.5. Calcein Release-Based Cytotoxicity Assay (CRA)
4.6. Real-Time Label-Free Live Cell Analysis
4.7. Quantification of Cytokine Release
4.8. 3D Spheroid Cytotoxicity Assay
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Hage, W.D.; Aboulafia, A.J.; Aboulafia, D.M. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop. Clin. North Am. 2000, 31, 515–528. [Google Scholar] [CrossRef]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone metastases: An overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Z.; Shen, J.-F.; Zhou, Y.; Chen, X.-Y.; Liu, J.-M.; Liu, Z.-L. Clinical characteristics and risk factors for developing bone metastases in patients with breast cancer. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [Green Version]
- Gross, G.; Waks, T.; Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity (chimeric genes/antibody variable region). Immunology 1989, 86, 10024–10028. [Google Scholar]
- Sadelain, M.; Brentjens, R.; Rivière, I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef]
- Gardner, R.A.; Finney, O.; Annesley, C.; Brakke, H.; Summers, C.; Leger, K.; Bleakley, M.; Brown, C.; Mgebroff, S.; Kelly-Spratt, K.S.; et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017, 129, 3322–3331. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Riddell, S.R. Chimeric antigen receptor T cell therapy: Challenges to bench-to-bedside efficacy. J. Immunol. 2018, 200, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Kerbauy, L.N.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Tonn, T.; Schwabe, D.; Klingemann, H.G.; Becker, S.; Esser, R.; Koehl, U.; Suttorp, M.; Seifried, E.; Ottmann, O.G.; Bug, G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 2013, 15, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Law, A.D.; Routy, B.; Denhollander, N.; Gupta, V.; Wang, X.-H.; Chaboureau, A.; Viswanathan, S.; Keating, A. A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget 2017, 8, 89256–89268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyiadzis, M.; Agha, M.; Redner, R.L.; Sehgal, A.; Im, A.; Hou, J.-Z.; Farah, R.; Dorritie, K.A.; Raptis, A.; Lim, S.H.; et al. Phase 1 clinical trial of adoptive immunotherapy using “off-the-shelf” activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy 2017, 19, 1225–1232. [Google Scholar] [CrossRef]
- Zhang, C.; Oberoi, P.; Oelsner, S.; Waldmann, A.; Lindner, A.; Tonn, T.; Wels, W.S. Chimeric antigen receptor-engineered NK-92 cells: An off-the-shelf cellular therapeutic for targeted elimination of cancer cells and induction of protective antitumor immunity. Front. Immunol. 2017, 8, 533. [Google Scholar] [CrossRef]
- Bin, H.; Tang, Y.; Li, W.; Zeng, Q.; Chang, D. Efficiency of CAR-T therapy for treatment of solid tumor in clinical trials: A meta-analysis. Dis. Markers 2019. [Google Scholar] [CrossRef] [Green Version]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017. [Google Scholar] [CrossRef] [Green Version]
- Darowski, D.; Kobold, S.; Jost, C.; Klein, C. Combining the best of two worlds: Highly flexible chimeric antigen receptor adaptor molecules (CAR-adaptors) for the recruitment of chimeric antigen receptor T cells. mAbs 2019, 11, 621–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grote, S.; Seitz, C.M.; Diepold, S.; Buchner, M.; Baden, C.; Malenke, E.; Dieckmann, S.M.; Schwaemmle, H.; Mittelstaet, J.; Kaiser, A.; et al. Adapter chimeric antigen receptor (aCAR)-engineered NK-92 cells: An off-the-shelf cellular therapeutic for universal tumor targeting. Blood 2018, 132, 3331. [Google Scholar] [CrossRef]
- Grote, S.; Mittelstaet, J.; Baden, C.; Chan, K.C.-H.; Seitz, C.; Schlegel, P.; Kaiser, A.; Handgretinger, R.; Schleicher, S. Adapter chimeric antigen receptor (AdCAR)-engineered NK-92 cells: An off-the-shelf cellular therapeutic for universal tumor targeting. Oncoimmunology 2020, 9, 1825177. [Google Scholar] [CrossRef]
- Seitz, C.M.; Schlegel, P.; Hau, J.; Krahl, A.-C.; Schroeder, S.; Bender, G.; Reiter, S.; Schleicher, S.; Schilbach, K.; Ebinger, M.; et al. Novel adapter chimeric antigen receptor (aCAR) T cells for temporally controllable targeting of single and multiple tumor antigens. Blood 2017, 130, 1912. [Google Scholar]
- Seitz, C.M.; Kieble, V.; Illi, C.; Reiter, S.; Grote, S.; Mittelstaet, J.; Lock, D.; Kaiser, A.; Schleicher, S.; Handgretinger, R.; et al. Combinatorial targeting of multiple shared antigens by adapter-CAR-T cells (aCAR-Ts) allows target cell discrimination and specific lysis based on differential expression profiles. Blood 2018, 132, 4543. [Google Scholar] [CrossRef]
- Kaiser, A.; Mittelstaet, J.; Huppert, V.; Miltenyi, S.; Schlegel, P.; Seitz, C.; Lang, P.; Handgretinger, R. Adapter chimeric antigen receptor expressing cells for targeting of multiple antigens. Europe Patent No. 3315511A1, 29 October 2016. [Google Scholar]
- Mitwasi, N.; Feldmann, A.; Arndt, C.; Koristka, S.; Berndt, N.; Jureczek, J.; Loureiro, L.R.; Bergmann, R.; Máthé, D.; Hegedüs, N.; et al. “UniCAR”-modified off-the-shelf NK-92 cells for targeting of GD2-expressing tumour cells. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Lohmueller, J.J.; Ham, J.D.; Kvorjak, M.; Finn, O.J. mSA2 affinity-enhanced biotin-binding CAR T cells for universal tumor targeting. OncoImmunology 2017, 7, e1368604. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, K.; Lanitis, E.; Poussin, M.; Lynn, R.C.; Gavin, B.P.; Kelderman, S.; Yu, J.; Scholler, N.; Powell, D.J. A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res. 2012, 72, 1844–1852. [Google Scholar] [CrossRef] [Green Version]
- Topp, M.S.; Gökbuget, N.; Zugmaier, G.; Degenhard, E.; Goebeler, M.-E.; Klinger, M.; Neumann, S.A.; Horst, H.A.; Raff, T.; Viardot, A.; et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood 2012, 120, 5185–5187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topp, M.S.; Gökbuget, N.; Zugmaier, G.; Klappers, P.; Stelljes, M.; Neumann, S.; Viardot, A.; Marks, R.; Diedrich, H.; Faul, C.; et al. Phase II trial of the anti-CD19 bispecific T cell–engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J. Clin. Oncol. 2014, 32, 4134–4140. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Han, W. Chimeric antigen receptor-modified T cells for solid tumors: Challenges and prospects. J. Immunol. Res. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, J.H.; Maki, G.; Klingemann, H.G. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 1994, 4, 652–658. [Google Scholar]
- Ward, J.P.; Bonaparte, M.I.; Barker, E. HLA-C and HLA-E reduce antibody-dependent natural killer cell-mediated cytotoxicity of HIV-infected primary T cell blasts. AIDS 2004, 18, 1769–1779. [Google Scholar] [CrossRef]
- Sarkar, S.; Van Gelder, M.; Noort, W.; Xu, Y.; Rouschop, K.M.A.; Groen, R.; Schouten, H.C.; Tilanus, M.G.J.; Germeraad, W.T.V.; Martens, A.C.M.; et al. Optimal selection of natural killer cells to kill myeloma: The role of HLA-E and NKG2A. Cancer Immunol. Immunother. 2015, 64, 951–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Yang, L.; Li, Z.; Nalin, A.P.; Dai, H.; Xu, T.; Yin, J.; You, F.; Zhu, M.; Shen, W.; et al. First-in-man clinical trial of CAR NK-92 cells: Safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am. J. Cancer Res. 2018, 8, 1899. [Google Scholar] [PubMed]
- Nowakowska, P.; Romanski, A.; Miller, N.; Odendahl, M.; Bonig, H.; Zhang, C.; Seifried, E.; Wels, W.S.; Tonn, T. Clinical grade manufacturing of genetically modified, CAR-expressing NK-92 cells for the treatment of ErbB2-positive malignancies. Cancer Immunol. Immunother. 2017, 67, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Schürch, C.M. CARving up colorectal cancer organoids in vitro. Genes Immun. 2019, 21, 1–3. [Google Scholar] [CrossRef]
- Wallstabe, L.; Göttlich, C.; Nelke, L.C.; Kühnemundt, J.; Schwarz, T.; Nerreter, T.; Einsele, H.; Walles, H.; Dandekar, G.; Nietzer, S.L.; et al. ROR1-CAR T cells are effective against lung and breast cancer in advanced microphysiologic 3D tumor models. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- Hart, A.L.; Ng, S.C.; Mann, E.; Al-Hassi, H.O.; Bernardo, D.; Knight, S.C. Homing of immune cells: Role in homeostasis and intestinal inflammation. Inflamm. Bowel Dis. 2010, 16, 1969–1977. [Google Scholar] [CrossRef]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Burger, M.C.; Jennewein, L.; Genβler, S.; Schönfeld, K.; Zeiner, P.; Hattingen, E.; Harter, P.N.; Mittelbronn, M.; Tonn, T.; et al. ErbB2/HER2-specific NK cells for targeted therapy of glioblastoma. J. Natl. Cancer Inst. 2015, 108. [Google Scholar] [CrossRef]
- Jiao, S.; Subudhi, S.K.; Aparicio, A.; Ge, Z.; Guan, B.; Miura, Y.; Sharma, P. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell 2019, 179, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Grote, S.; Chan, K.C.; Baden, C.; Bösmüller, H.; Sulyok, M.; Frauenfeld, L.; Ebinger, M.; Handgretinger, R.; Schleicher, S. CD276 as a novel CAR NK-92 therapeutic target for neuroblastoma. Adv. Cell Gene Ther. 2020, 4. [Google Scholar] [CrossRef]
- Burger, M.C.; Zhang, C.; Harter, P.N.; Romanski, A.; Strassheimer, F.; Senft, C.; Tonn, T.; Steinbach, J.P.; Wels, W.S. CAR-Engineered NK cells for the treatment of glioblastoma: Turning innate effectors into precision tools for cancer immunotherapy. Front. Immunol. 2019, 10, 2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Patient Age [years] | Patient Sex | Metastatic Site | Tumor Entity | Designation | |
|---|---|---|---|---|---|
| Patient 1 | 63 | f | Scapula | Mammary carcinoma | MAC |
| Patient 2 | 17 | m | Spine L3/L4 | Renal cell carcinoma | MAM |
| Patient 3 | 54 | m | Spine C7 | Colorectal carcinoma | MCK83 |
| Patient 4 | 47 | m | Acetabulum | Melanoma | MeGa17 |
| Cell Line | Percentage of Stained Cells | |||||||||
| CD146 | CD171 | CD200 | CD221 | CD271 | CD274 | CD276 | CD340 | EGFR | GD2 | |
| MAC | 97.70% | 11.80% | 13.90% | 68.50% | 44.60% | 0.40% | 100.00% | 97.90% | 99.90% | 1.78% |
| MAM | 99.80% | 99.90% | 5.29% | 99.40% | 2.06% | 0.65% | 99.90% | 99.70% | 100.00% | 4.04% |
| MCK83 | 5.62% | 0.45% | 0.31% | 59.60% | 0.13% | 0.46% | 99.50% | 81.20% | 85.80% | 0.44% |
| MeGa17 | 99.20% | 42.90% | 62.10% | 70.40% | 22.80% | 1.23% | 99.00% | 44.10% | 27.20% | 1.91% |
| Median Fluorescence Index (MFI) | ||||||||||
| CD146 | CD171 | CD200 | CD221 | CD271 | CD274 | CD276 | CD340 | EGFR | GD2 | |
| MAC | 45.48 | 1.15 | 1.23 | 4.10 | 1.91 | 1.03 | 106.65 | 9.39 | 183.42 | 1.21 |
| MAM | 194.20 | 79.54 | 1.16 | 11.27 | 0.99 | 1.03 | 113.92 | 11.59 | 92.86 | 1.35 |
| MCK83 | 1.22 | 1.00 | 0.96 | 6.26 | 0.77 | 0.97 | 31.39 | 10.08 | 12.41 | 1.14 |
| MeGa17 | 172.52 | 3.49 | 7.71 | 9.06 | 1.96 | 0.96 | 67.70 | 5.00 | 2.78 | 1.18 |
| Cell Line | CD48 | CD50 | CD54 | CD58 | CD95 | CD102 | CD112 | CD155 | CD261 | CD262 |
| MAC | 1.47 | 1.69 | 5.46 | 73.22 | 23.35 | 1.27 | 34.41 | 128.96 | 3.01 | 16.87 |
| MAM | 1.36 | 1.78 | 7.31 | 30.55 | 9.54 | 1.13 | 69.73 | 192.65 | 6.06 | 59.84 |
| MCK83 | 1.84 | 1.75 | 1.29 | 5.54 | 4.26 | 1.27 | 15.32 | 89.62 | 4.15 | 14.91 |
| MeGa17 | 1.25 | 1.58 | 9.66 | 31.32 | 2.18 | 1.13 | 16.27 | 50.20 | 1.22 | 15.03 |
| HLA-ABC | HLA-DR | HLA-E | HLA-G | MICA/B | ULBP1 | ULBP2/5/6 | ULBP3 | ULBP4 | ||
| MAC | 52.41 | 0.90 | 9.18 | 0.98 | 6.53 | 3.45 | 1.26 | 0.68 | 0.75 | |
| MAM | 19.07 | 0.87 | 8.07 | 0.81 | 21.25 | 3.15 | 1.00 | 1.67 | 1.53 | |
| MCK83 | 0.47 | 0.92 | 10.01 | 0.82 | 9.07 | 6.09 | 1.09 | 1.25 | 1.41 | |
| MeGa17 | 23.33 | 12.10 | 7.55 | 1.34 | 7.27 | 2.45 | 1.37 | 0.92 | 0.77 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grote, S.; Traub, F.; Mittelstaet, J.; Seitz, C.; Kaiser, A.; Handgretinger, R.; Schleicher, S. Adapter Chimeric Antigen Receptor (AdCAR)-Engineered NK-92 Cells for the Multiplex Targeting of Bone Metastases. Cancers 2021, 13, 1124. https://doi.org/10.3390/cancers13051124
Grote S, Traub F, Mittelstaet J, Seitz C, Kaiser A, Handgretinger R, Schleicher S. Adapter Chimeric Antigen Receptor (AdCAR)-Engineered NK-92 Cells for the Multiplex Targeting of Bone Metastases. Cancers. 2021; 13(5):1124. https://doi.org/10.3390/cancers13051124
Chicago/Turabian StyleGrote, Stefan, Frank Traub, Joerg Mittelstaet, Christian Seitz, Andrew Kaiser, Rupert Handgretinger, and Sabine Schleicher. 2021. "Adapter Chimeric Antigen Receptor (AdCAR)-Engineered NK-92 Cells for the Multiplex Targeting of Bone Metastases" Cancers 13, no. 5: 1124. https://doi.org/10.3390/cancers13051124
APA StyleGrote, S., Traub, F., Mittelstaet, J., Seitz, C., Kaiser, A., Handgretinger, R., & Schleicher, S. (2021). Adapter Chimeric Antigen Receptor (AdCAR)-Engineered NK-92 Cells for the Multiplex Targeting of Bone Metastases. Cancers, 13(5), 1124. https://doi.org/10.3390/cancers13051124