Influence of Hepatocellular Carcinoma on Platelet Aggregation in Cirrhosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Demographics
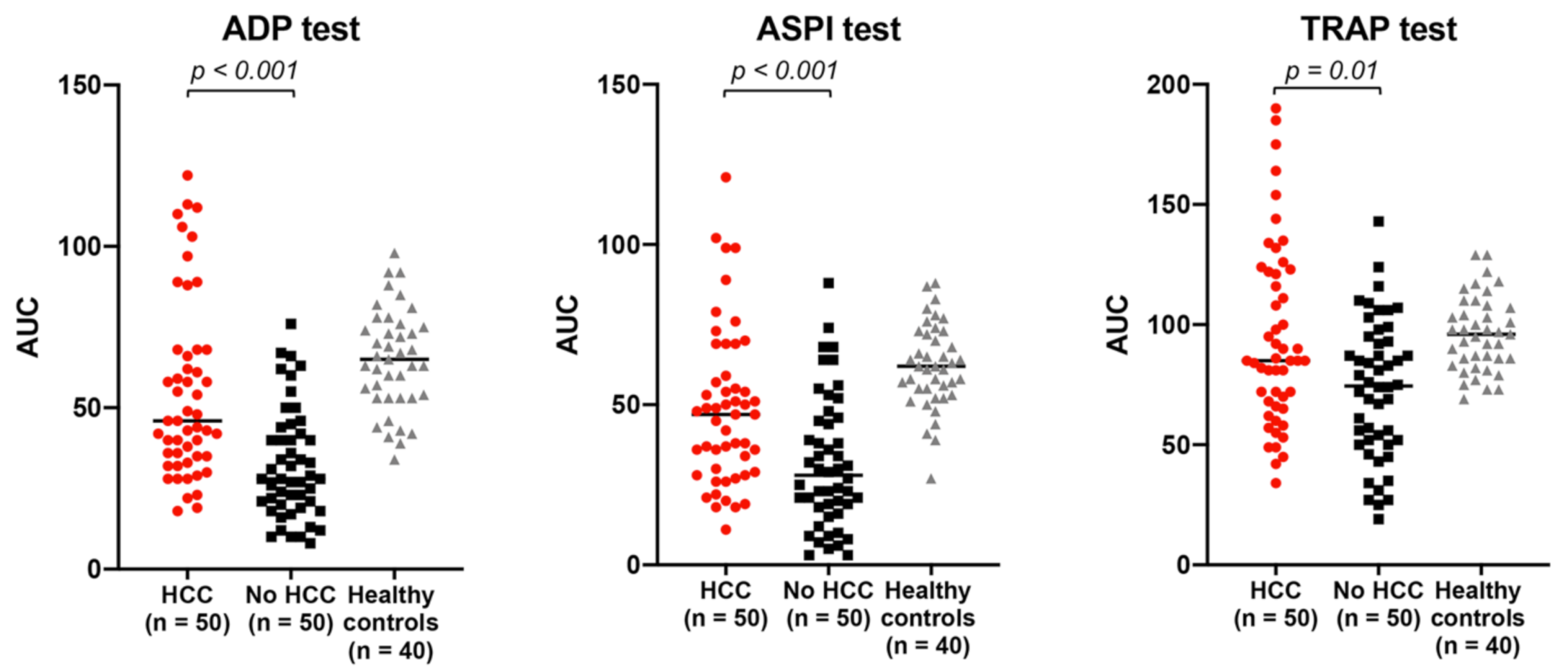
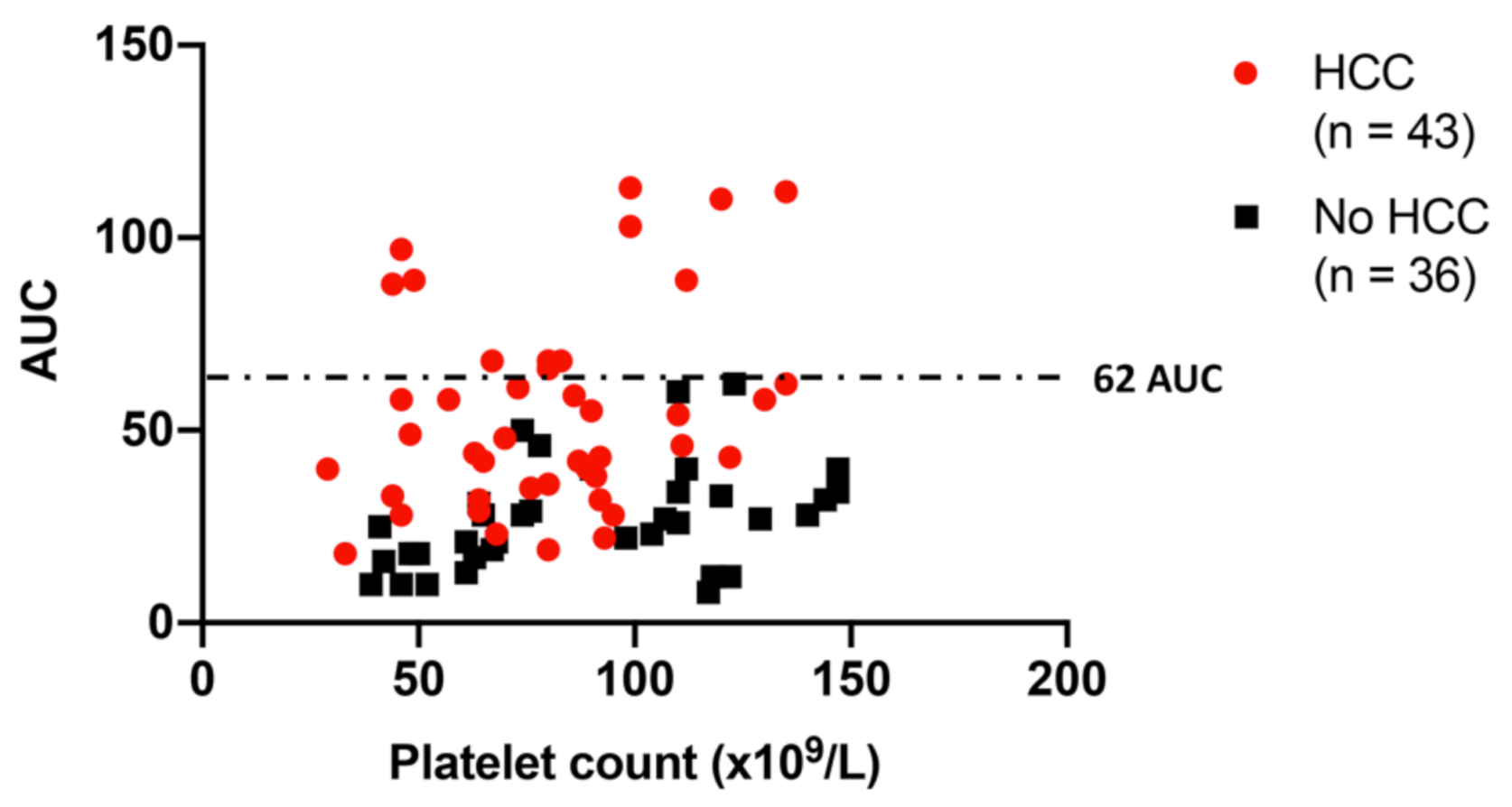
2.2. Platelet Function in Cirrhosis Patients with vs. without HCC: HCC Is Associated with Relatively Increased Platelet Aggregation, Independent of Platelet Count
2.3. Plasmatic Marker of Primary Hemostasis in Cirrhosis Patients with vs. without HCC: HCC Is Associated with Higher Levels of Platelet Adhesive Glycoprotein Von Willebrand Factor
3. Discussion
4. Materials and Methods
4.1. Patient Selection
4.2. Study Design
4.3. Sample Collection and Primary Hemostasis Assessment
4.3.1. Blood Sampling
4.3.2. Platelet Function Assessment
4.3.3. Von Willebrand Factor
4.4. Data Collection
4.5. Data Analysis
4.5.1. Sample Size Determination
4.5.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falanga, A.; Marchetti, M.; Vignoli, A. Coagulation and cancer: Biological and clinical aspects. J. Thromb. Haemost. 2013, 11, 223–233. [Google Scholar] [CrossRef]
- Franco, A.T.; Corken, A.; Ware, J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 2015, 126, 582–588. [Google Scholar] [CrossRef]
- Goubran, H.A.; Stakiw, J.; Radosevic, M.; Burnouf, T. Platelets effects on tumor growth. Semin. Oncol. 2014, 41, 359–369. [Google Scholar] [CrossRef]
- Cho, M.S.; Bottsford-Miller, J.; Vasquez, H.G.; Stone, R.; Zand, B.; Kroll, M.H.; Sood, A.K.; Afshar-Kharghan, V. Platelets increase the proliferation of ovarian cancer cells. Blood 2012, 120, 4869–4872. [Google Scholar] [CrossRef] [PubMed]
- Italiano, J.E., Jr.; Richardson, J.L.; Patel-Hett, S.; Battinelli, E.; Zaslavsky, A.; Short, S.; Ryeom, S.; Folkman, J.; Klement, G.L. Angiogenesis is regulated by a novel mechanism: Pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 2008, 111, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Mezouar, S.; Frere, C.; Darbousset, R.; Mege, D.; Crescence, L.; Dignat-George, F.; Panicot-Dubois, L.; Dubois, C. Role of platelets in cancer and cancer-associated thrombosis: Experimental and clinical evidences. Thromb. Res. 2016, 139, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, D.; Strilic, B.; Sivaraj, K.K.; Wettschureck, N.; Offermanns, S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 2013, 24, 130–137. [Google Scholar] [CrossRef]
- Hwang, S.J.; Luo, J.C.; Li, C.P.; Chu, C.W.; Wu, J.C.; Lai, C.R.; Chiang, J.H.; Chau, G.Y.; Lui, W.Y.; Lee, C.C.; et al. Thrombocytosis: A paraneoplastic syndrome in patients with hepatocellular carcinoma. World J. Gastroenterol. 2004, 10, 2472–2477. [Google Scholar] [CrossRef]
- Belluco, C.; Forlin, M.; Delrio, P.; Rega, D.; Degiuli, M.; Sofia, S.; Olivieri, M.; Pucciarelli, S.; Zuin, M.; De Manzoni, G.; et al. Elevated platelet count is a negative predictive and prognostic marker in locally advanced rectal cancer undergoing neoadjuvant chemoradiation: A retrospective multi-institutional study on 965 patients. BMC Cancer 2018, 18, 1094. [Google Scholar] [CrossRef] [PubMed]
- Simanek, R.; Vormittag, R.; Ay, C.; Alguel, G.; Dunkler, D.; Schwarzinger, I.; Steger, G.; Jaeger, U.; Zielinski, C.; Pabinger, I. High platelet count associated with venous thromboembolism in cancer patients: Results from the Vienna Cancer and Thrombosis Study (CATS). J. Thromb. Haemost. 2010, 8, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Jiang, H.; Huang, S.; Hong, H.; Huang, X.; Wang, X.; Liao, W.; Wang, X.; Chen, X.; Jiang, L. The prognostic role of pretreatment thrombocytosis in gastric cancer: A systematic review and meta-analysis. Medicine 2018, 97, e11763. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Burra, P.; Zanetto, A.; Germani, G. Liver Transplantation for Alcoholic Liver Disease and Hepatocellular Carcinoma. Cancers 2018, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Basili, S.; Carnevale, R.; Nocella, C.; Bartimoccia, S.; Raparelli, V.; Talerico, G.; Stefanini, L.; Romiti, G.F.; Perticone, F.; Corazza, G.R.; et al. Serum Albumin Is Inversely Associated With Portal Vein Thrombosis in Cirrhosis. Hepatol. Commun. 2019, 3, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Raparelli, V.; Basili, S.; Carnevale, R.; Napoleone, L.; Del Ben, M.; Nocella, C.; Bartimoccia, S.; Lucidi, C.; Talerico, G.; Riggio, O.; et al. Low-grade endotoxemia and platelet activation in cirrhosis. Hepatology 2017, 65, 571–581. [Google Scholar] [CrossRef]
- Russo, F.P.; Zanetto, A.; Campello, E.; Bulato, C.; Shalaby, S.; Spiezia, L.; Gavasso, S.; Franceschet, E.; Radu, C.; Senzolo, M.; et al. Reversal of hypercoagulability in patients with HCV-related cirrhosis after treatment with direct-acting antivirals. Liver Int. 2018, 38, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Basili, S.; Raparelli, V.; Chowdary, P.; Gatt, A.; Burroughs, A.K. Patients with liver cirrhosis suffer from primary haemostatic defects? Fact or fiction? J. Hepatol. 2011, 55, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Zanetto, A.; Rinder, H.M.; Campello, E.; Saggiorato, G.; Deng, Y.; Ciarleglio, M.; Wilson, F.P.; Senzolo, M.; Gavasso, S.; Bulato, C.; et al. Acute kidney injury in decompensated cirrhosis is associated with both hypo- and hyper-coagulable features. Hepatology 2020, 72, 1327–1340. [Google Scholar] [CrossRef]
- Zermatten, M.G.; Fraga, M.; Moradpour, D.; Bertaggia Calderara, D.; Aliotta, A.; Stirnimann, G.; De Gottardi, A.; Alberio, L. Hemostatic Alterations in Patients With Cirrhosis: From Primary Hemostasis to Fibrinolysis. Hepatology 2020, 71, 2135–2148. [Google Scholar] [CrossRef]
- Zanetto, A.; Rinder, H.M.; Senzolo, M.; Simioni, P.; Garcia-Tsao, G. Reduced Clot Stability by Thromboelastography as a Potential Indicator of Procedure-Related Bleeding in Decompensated Cirrhosis. Hepatol. Commun. 2021, 5, 272–282. [Google Scholar] [CrossRef]
- Caldwell, S.; Lisman, T. The cirrhotic platelet: Shedding light on an enigma. Hepatology 2017, 65, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhu, J.; Ma, X.; Wang, H.; Qiu, S.; Pan, B.; Zhou, J.; Fan, J.; Yang, X.; Guo, W.; et al. Platelet activation status in the diagnosis and postoperative prognosis of hepatocellular carcinoma. Clin. Chim. Acta 2019, 495, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Guo, H.; Xu, J.; Li, B.; Liu, Y.J.; Cheng, C.; Zhou, C.; Zhao, Y.; Liu, Y. Activated platelets inhibit hepatocellular carcinoma cell differentiation and promote tumor progression via platelet-tumor cell binding. Oncotarget 2016, 7, 60609–60622. [Google Scholar] [CrossRef]
- Mitrugno, A.; Tassi Yunga, S.; Sylman, J.L.; Zilberman-Rudenko, J.; Shirai, T.; Hebert, J.F.; Kayton, R.; Zhang, Y.; Nan, X.; Shatzel, J.J.; et al. The role of coagulation and platelets in colon cancer-associated thrombosis. Am. J. Physiol. Cell Physiol. 2019, 316, C264–C273. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupairmoole, R.; Armaiz-Pena, G.N.; Pecot, C.V.; Coward, J.; et al. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Padickakudy, R.; Pereyra, D.; Offensperger, F.; Jonas, P.; Oehlberger, L.; Schwarz, C.; Haegele, S.; Assinger, A.; Brostjan, C.; Gruenberger, T.; et al. Bivalent role of intra-platelet serotonin in liver regeneration and tumor recurrence in humans. J. Hepatol. 2017, 67, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Hsu, Y.C.; Tseng, H.C.; Lin, J.T.; Wu, M.S.; Wu, C.Y. Association of Daily Aspirin Therapy with Hepatocellular Carcinoma Risk in Patients with Chronic Hepatitis C Virus Infection. Clin. Gastroenterol. Hepatol. 2020, 18, 2784–2792.e7. [Google Scholar] [CrossRef]
- Simon, T.G.; Duberg, A.S.; Aleman, S.; Chung, R.T.; Chan, A.T.; Ludvigsson, J.F. Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality. N. Engl. J. Med. 2020, 382, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Chung, G.E.; Lee, J.H.; Oh, S.; Nam, J.Y.; Chang, Y.; Cho, H.; Ahn, H.; Cho, Y.Y.; Yoo, J.J.; et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology 2017, 66, 1556–1569. [Google Scholar] [CrossRef]
- Malehmir, M.; Pfister, D.; Gallage, S.; Szydlowska, M.; Inverso, D.; Kotsiliti, E.; Leone, V.; Peiseler, M.; Surewaard, B.G.J.; Rath, D.; et al. Platelet GPIbalpha is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat. Med. 2019, 25, 641–655. [Google Scholar] [CrossRef]
- Starlinger, P.; Pereyra, D.; Hackl, H.; Ortmayr, G.; Braunwarth, E.; Santol, J.; Najarnia, S.; Driedger, M.R.; Gregory, L.; Alva-Ruiz, R.; et al. Consequences of Perioperative Serotonin Reuptake Inhibitor Treatment during Hepatic Surgery. Hepatology 2020. [Google Scholar] [CrossRef]
- Sitia, G.; Aiolfi, R.; Di Lucia, P.; Mainetti, M.; Fiocchi, A.; Mingozzi, F.; Esposito, A.; Ruggeri, Z.M.; Chisari, F.V.; Iannacone, M.; et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc. Natl. Acad. Sci. USA 2012, 109, E2165–E2172. [Google Scholar] [CrossRef]
- Shin, S.; Lee, S.H.; Lee, M.; Kim, J.H.; Lee, W.; Lee, H.W.; Park, M.S.; Park, S.; Kim, T.S.; Choi, D.H. Aspirin and the risk of hepatocellular carcinoma development in patients with alcoholic cirrhosis. Medicine 2020, 99, e19008. [Google Scholar] [CrossRef]
- Lai, Q.; Vitale, A.; Manzia, T.M.; Foschi, F.G.; Levi Sandri, G.B.; Gambato, M.; Melandro, F.; Russo, F.P.; Miele, L.; Vigano, L.; et al. Platelets and Hepatocellular Cancer: Bridging the Bench to the Clinics. Cancers 2019, 11, 1568. [Google Scholar] [CrossRef]
- Liu, P.H.; Hsu, C.Y.; Su, C.W.; Huang, Y.H.; Hou, M.C.; Rich, N.E.; Fujiwara, N.; Hoshida, Y.; Singal, A.G.; Huo, T.I. Thrombocytosis is associated with worse survival in patients with hepatocellular carcinoma. Liver Int. 2020, 40, 2522–2534. [Google Scholar] [CrossRef]
- Pavlovic, N.; Rani, B.; Gerwins, P.; Heindryckx, F. Platelets as Key Factors in Hepatocellular Carcinoma. Cancers 2019, 11, 1022. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.A. Von Willebrand factor, Jedi knight of the bloodstream. Blood 2014, 124, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.J.; Sussman, I.I.; Hoyer, L.W. Stabilization of factor VIII in plasma by the von Willebrand factor. Studies on posttransfusion and dissociated factor VIII and in patients with von Willebrand’s disease. J. Clin. Investig. 1977, 60, 390–404. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.M.; Preston, R.J.S.; Robson, T.; O’Donnell, J.S. Emerging Roles for von Willebrand Factor in Cancer Cell Biology. Semin. Thromb. Hemost. 2018, 44, 159–166. [Google Scholar] [CrossRef]
- Yang, A.J.; Wang, M.; Wang, Y.; Cai, W.; Li, Q.; Zhao, T.T.; Zhang, L.H.; Houck, K.; Chen, X.; Jin, Y.L.; et al. Cancer cell-derived von Willebrand factor enhanced metastasis of gastric adenocarcinoma. Oncogenesis 2018, 7, 12. [Google Scholar] [CrossRef]
- Mojiri, A.; Stoletov, K.; Carrillo, M.A.; Willetts, L.; Jain, S.; Godbout, R.; Jurasz, P.; Sergi, C.M.; Eisenstat, D.D.; Lewis, J.D.; et al. Functional assessment of von Willebrand factor expression by cancer cells of non-endothelial origin. Oncotarget 2017, 8, 13015–13029. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, W.; Fan, J.; Guo, J.; Shen, F.; Ma, Z.; Ruan, C.; Guo, L.; Jiang, M.; Zhao, Y. Von Willebrand factor promotes platelet-induced metastasis of osteosarcoma through activation of the VWF-GPIb axis. J. Bone Oncol. 2020, 25, 100325. [Google Scholar] [CrossRef] [PubMed]
- Schellerer, V.S.; Mueller-Bergh, L.; Merkel, S.; Zimmermann, R.; Weiss, D.; Schlabrakowski, A.; Naschberger, E.; Sturzl, M.; Hohenberger, W.; Croner, R.S. The clinical value of von Willebrand factor in colorectal carcinomas. Am. J. Transl. Res. 2011, 3, 445–453. [Google Scholar]
- Gadducci, A.; Baicchi, U.; Marrai, R.; Del Bravo, B.; Fosella, P.V.; Facchini, V. Pretreatment plasma levels of fibrinopeptide-A (FPA), D-dimer (DD), and von Willebrand factor (vWF) in patients with ovarian carcinoma. Gynecol. Oncol. 1994, 53, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Zietek, Z.; Iwan-Zietek, I.; Paczulski, R.; Kotschy, M.; Wolski, Z. Von Willebrand factor antigen in blood plasma of patients with urinary bladder carcinoma. Thromb. Res. 1996, 83, 399–402. [Google Scholar] [CrossRef]
- Rhone, P.; Zarychta, E.; Bielawski, K.; Ruszkowska-Ciastek, B. Pre-surgical level of von Willebrand factor as an evident indicator of breast cancer recurrence. Cancer Biomark. 2020, 29, 359–372. [Google Scholar] [CrossRef]
- Aryal, B.; Yamakuchi, M.; Shimizu, T.; Kadono, J.; Furoi, A.; Gejima, K.; Takenouchi, K.; Komokata, T.; Hashiguchi, T.; Imoto, Y. Bivalent property of intra-platelet VWF in liver regeneration and HCC recurrence: A prospective multicenter study. Cancer Biomark. 2019, 26, 51–61. [Google Scholar] [CrossRef]
- Patmore, S.; Dhami, S.P.S.; O’Sullivan, J.M. Von Willebrand factor and cancer; metastasis and coagulopathies. J. Thromb. Haemost. 2020, 18, 2444–2456. [Google Scholar] [CrossRef] [PubMed]
- Borchiellini, A.; Fijnvandraat, K.; ten Cate, J.W.; Pajkrt, D.; van Deventer, S.J.; Pasterkamp, G.; Meijer-Huizinga, F.; Zwart-Huinink, L.; Voorberg, J.; van Mourik, J.A. Quantitative analysis of von Willebrand factor propeptide release in vivo: Effect of experimental endotoxemia and administration of 1-deamino-8-D-arginine vasopressin in humans. Blood 1996, 88, 2951–2958. [Google Scholar] [CrossRef] [PubMed]
- Hyseni, A.; Kemperman, H.; de Lange, D.W.; Kesecioglu, J.; de Groot, P.G.; Roest, M. Active von Willebrand factor predicts 28-day mortality in patients with systemic inflammatory response syndrome. Blood 2014, 123, 2153–2156. [Google Scholar] [CrossRef] [PubMed]
- Van Mourik, J.A.; Boertjes, R.; Huisveld, I.A.; Fijnvandraat, K.; Pajkrt, D.; van Genderen, P.J.; Fijnheer, R. Von Willebrand factor propeptide in vascular disorders: A tool to distinguish between acute and chronic endothelial cell perturbation. Blood 1999, 94, 179–185. [Google Scholar] [CrossRef]
- Toya, T.; Sara, J.D.; Corban, M.T.; Taher, R.; Godo, S.; Herrmann, J.; Lerman, L.O.; Lerman, A. Assessment of peripheral endothelial function predicts future risk of solid-tumor cancer. Eur. J. Prev. Cardiol. 2020, 27, 608–618. [Google Scholar] [CrossRef]
- Solimando, A.G.; Summa, S.; Vacca, A.; Ribatti, D. Cancer-Associated Angiogenesis: The Endothelial Cell as a Checkpoint for Immunological Patrolling. Cancers 2020, 12, 3380. [Google Scholar] [CrossRef]
- Campello, E.; Zanetto, A.; Radu, C.M.; Bulato, C.; Truma, A.; Spiezia, L.; Senzolo, M.; Garcia-Tsao, G.; Simioni, P. Acute kidney injury is associated with increased levels of circulating microvesicles in patients with decompensated cirrhosis. Dig. Liver Dis. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, S.; Simioni, P.; Campello, E.; Spiezia, L.; Gavasso, S.; Bizzaro, D.; Cardin, R.; D’Amico, F.; Gringeri, E.; Cillo, U.; et al. Endothelial Damage of the Portal Vein Is Associated with Heparin-Like Effect in Advanced Stages of Cirrhosis. Thromb. Haemost. 2020, 120, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Campello, E.; Zanetto, A.; Spiezia, L.; Radu, C.M.; Gavasso, S.; Ferrarese, A.; Farinati, F.; Senzolo, M.; Simioni, P. Hypercoagulability detected by circulating microparticles in patients with hepatocellular carcinoma and cirrhosis. Thromb. Res. 2016, 143, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Alkozai, E.M.; Porte, R.J.; Adelmeijer, J.; Zanetto, A.; Simioni, P.; Senzolo, M.; Lisman, T. No evidence for increased platelet activation in patients with hepatitis B- or C-related cirrhosis and hepatocellular carcinoma. Thromb. Res. 2015, 135, 292–297. [Google Scholar] [CrossRef]
- Zanetto, A.; Senzolo, M.; Vitale, A.; Cillo, U.; Radu, C.; Sartorello, F.; Spiezia, L.; Campello, E.; Rodriguez-Castro, K.; Ferrarese, A.; et al. Thromboelastometry hypercoagulable profiles and portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma. Dig. Liver Dis. 2017, 49, 440–445. [Google Scholar] [CrossRef]
- Garcia-Tsao, G.; Abraldes, J.G.; Berzigotti, A.; Bosch, J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 Practice guidance by the American Association for the study of liver diseases. Hepatology 2017, 65, 310–335. [Google Scholar] [CrossRef]
- Zanetto, A.; Garcia-Tsao, G. Management of acute variceal hemorrhage. F1000Research 2019, 8, 966. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; the Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Boscolo, A.; Spiezia, L.; Campello, E.; Bertini, D.; Lucchetta, V.; Piasentini, E.; De Cassai, A.; Simioni, P. Whole-blood hypocoagulable profile correlates with a greater risk of death within 28 days in patients with severe sepsis. Korean J. Anesthesiol. 2020, 73, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Campello, E.; Spiezia, L.; Zabeo, E.; Maggiolo, S.; Vettor, R.; Simioni, P. Hypercoagulability detected by whole blood thromboelastometry (ROTEM(R)) and impedance aggregometry (MULTIPLATE(R)) in obese patients. Thromb. Res. 2015, 135, 548–553. [Google Scholar] [CrossRef]
- Spiezia, L.; Cuzzolin, M.; Elssy, H.; Di Gregorio, G.; Campello, E.; Rea, F.; Zuin, A.; Simioni, P. Post-operative hypercoagulable whole blood profiles in patients undergoing open thoracotomy vs video-assisted thoracoscopic surgery. Blood Transfus. 2021, 19, 144–151. [Google Scholar] [CrossRef] [PubMed]
- La Mura, V.; Reverter, J.C.; Flores-Arroyo, A.; Raffa, S.; Reverter, E.; Seijo, S.; Abraldes, J.G.; Bosch, J.; Garcia-Pagan, J.C. Von Willebrand factor levels predict clinical outcome in patients with cirrhosis and portal hypertension. Gut 2011, 60, 1133–1138. [Google Scholar] [CrossRef]
- Semmler, G.; Binter, T.; Kozbial, K.; Schwabl, P.; Hametner-Schreil, S.; Zanetto, A.; Gavasso, S.; Chromy, D.; Bauer, D.J.M.; Simbrunner, B.; et al. Non-invasive risk stratification after HCV-eradication in patients with advanced chronic liver disease. Hepatology 2020. [Google Scholar] [CrossRef]
- Lisman, T.; Bongers, T.N.; Adelmeijer, J.; Janssen, H.L.; de Maat, M.P.; de Groot, P.G.; Leebeek, F.W. Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology 2006, 44, 53–61. [Google Scholar] [CrossRef]
- Peck-Radosavljevic, M. Thrombocytopenia in chronic liver disease. Liver Int. 2017, 37, 778–793. [Google Scholar] [CrossRef]
- Toso, C.; Trotter, J.; Wei, A.; Bigam, D.L.; Shah, S.; Lancaster, J.; Grant, D.R.; Greig, P.D.; Shapiro, A.M.; Kneteman, N.M. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2008, 14, 1107–1115. [Google Scholar] [CrossRef] [PubMed]



| Variables | HCC (n = 50) | No HCC (n = 50) |
|---|---|---|
| Age, years | 65 (58–69) | 61 (55–71) |
| Male gender, % | 80 | 66 |
| Etiology of cirrhosis, % | ||
| Alcohol | 46 | 36 |
| HCV | 26 | 42 |
| NASH | 10 | 8 |
| HBV ± HDV | 16 | 12 |
| Other | 2 | 2 |
| Child class A/B/C, % | 46/36/18 | 62/22/16 |
| MELD score | 11 (8–16) | 10 (8–14) |
| History of decompensation, % | ||
| Ascites | 46 | 28 |
| Variceal hemorrhage | 22 | 22 |
| Hepatic encephalopathy | 6 | 20 |
| Diabetes, % | 28 | 32 |
| Hemoglobin, g/dL | 12 (11–14) | 12 (10–14) |
| Platelet count, 109/L | 95 (64–114) | 108 (67–140) |
| Thrombocytopenia, (%) | ||
| Present | 86 | 72 |
| Mild 100–150 × 109/L | 19 | 33 |
| Moderate 50–100 × 109/L | 60 | 50 |
| Severe <50 × 109/L | 21 | 17 |
| Total bilirubin, mg/dL | 1.2 (0.9–2.7) | 1.2 (0.8–3.5) |
| INR | 1.3 (1.2–1.5) | 1.2 (1.1–1.6) |
| Creatinine, mg/dL | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) |
| Albumin, g/dL | 31 (29–36) | 34 (29–38) |
| AFP, ng/mL | 9 (4–47) | 3 (2–4) |
| Multinodular, % | 68 | - |
| Number of nodules | 3 (2–7) | - |
| TTV, cm3 | 9 (5–16) | - |
| TTV > 10 cm3, % | 45 | - |
| History of previous treatment, % | - | |
| Yes/No | 45 | |
| TACE § | 39 in 19 | |
| RF § | 31 in 20 | |
| Resection § | 5 in 5 | |
| PEI | 2 in 2 | |
| Capecitabin | 1 | |
| BCLC staging 0/A/B/C/D, % | 10/19/57/8/6 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanetto, A.; Senzolo, M.; Campello, E.; Bulato, C.; Gavasso, S.; Shalaby, S.; Gambato, M.; Vitale, A.; Cillo, U.; Farinati, F.; et al. Influence of Hepatocellular Carcinoma on Platelet Aggregation in Cirrhosis. Cancers 2021, 13, 1150. https://doi.org/10.3390/cancers13051150
Zanetto A, Senzolo M, Campello E, Bulato C, Gavasso S, Shalaby S, Gambato M, Vitale A, Cillo U, Farinati F, et al. Influence of Hepatocellular Carcinoma on Platelet Aggregation in Cirrhosis. Cancers. 2021; 13(5):1150. https://doi.org/10.3390/cancers13051150
Chicago/Turabian StyleZanetto, Alberto, Marco Senzolo, Elena Campello, Cristiana Bulato, Sabrina Gavasso, Sarah Shalaby, Martina Gambato, Alessandro Vitale, Umberto Cillo, Fabio Farinati, and et al. 2021. "Influence of Hepatocellular Carcinoma on Platelet Aggregation in Cirrhosis" Cancers 13, no. 5: 1150. https://doi.org/10.3390/cancers13051150
APA StyleZanetto, A., Senzolo, M., Campello, E., Bulato, C., Gavasso, S., Shalaby, S., Gambato, M., Vitale, A., Cillo, U., Farinati, F., Russo, F. P., Simioni, P., & Burra, P. (2021). Influence of Hepatocellular Carcinoma on Platelet Aggregation in Cirrhosis. Cancers, 13(5), 1150. https://doi.org/10.3390/cancers13051150









