Genetic Variation in the Vascular Endothelial Growth Factor (VEGFA) Gene at rs13207351 Is Associated with Overall Survival of Patients with Head and Neck Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Variant Distribution
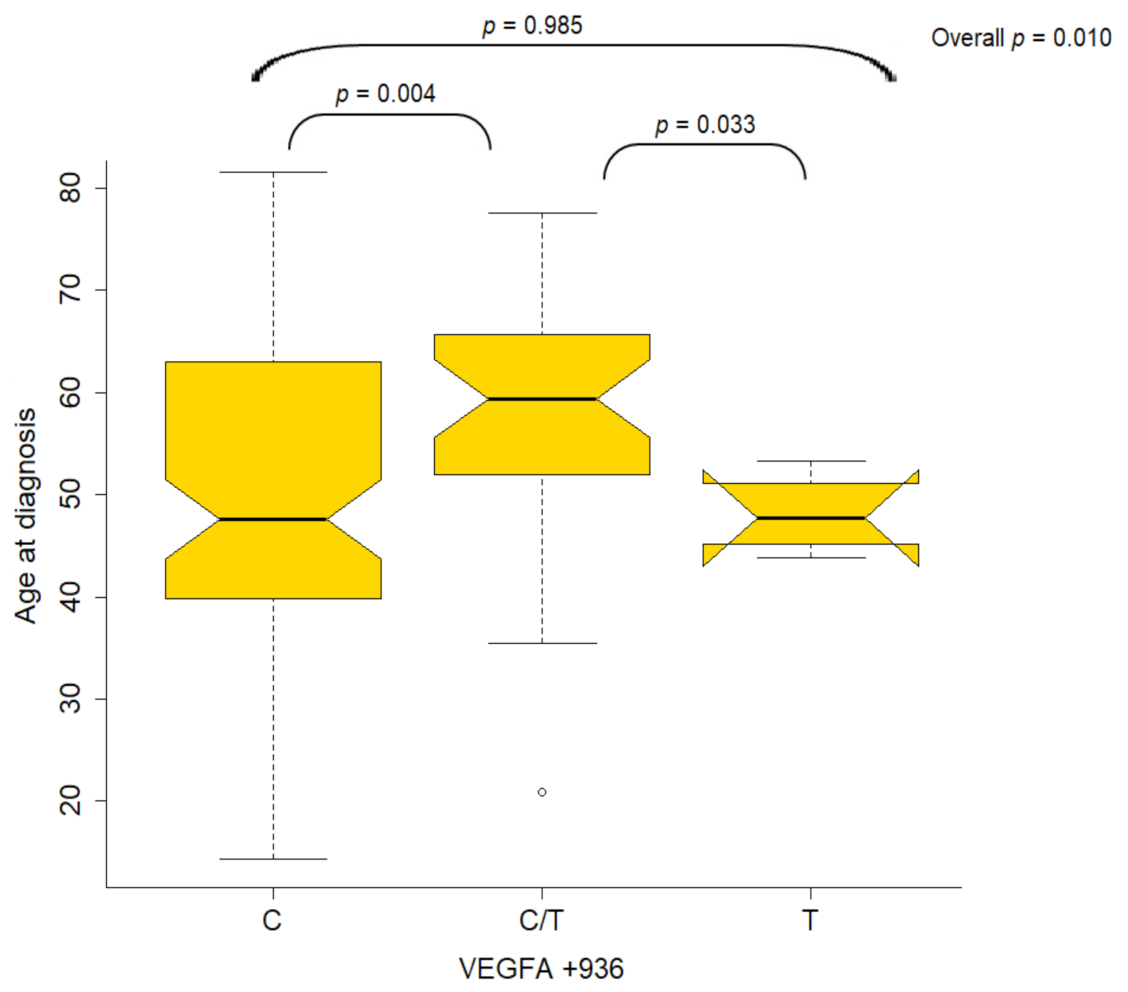
2.3. Association of Variants with Clinicopathological Characteristics
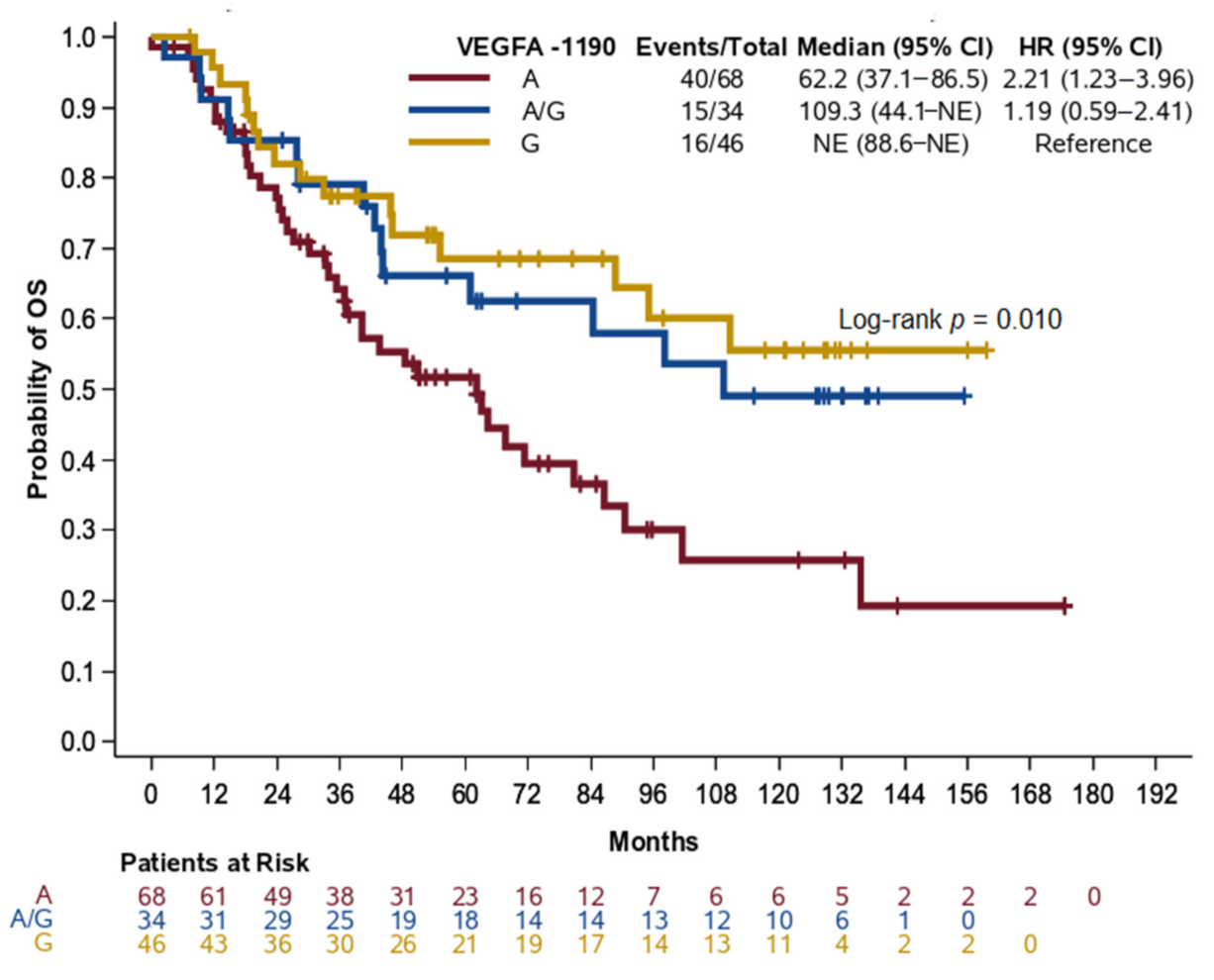
2.4. Improved Overall Survival for NPC Patients
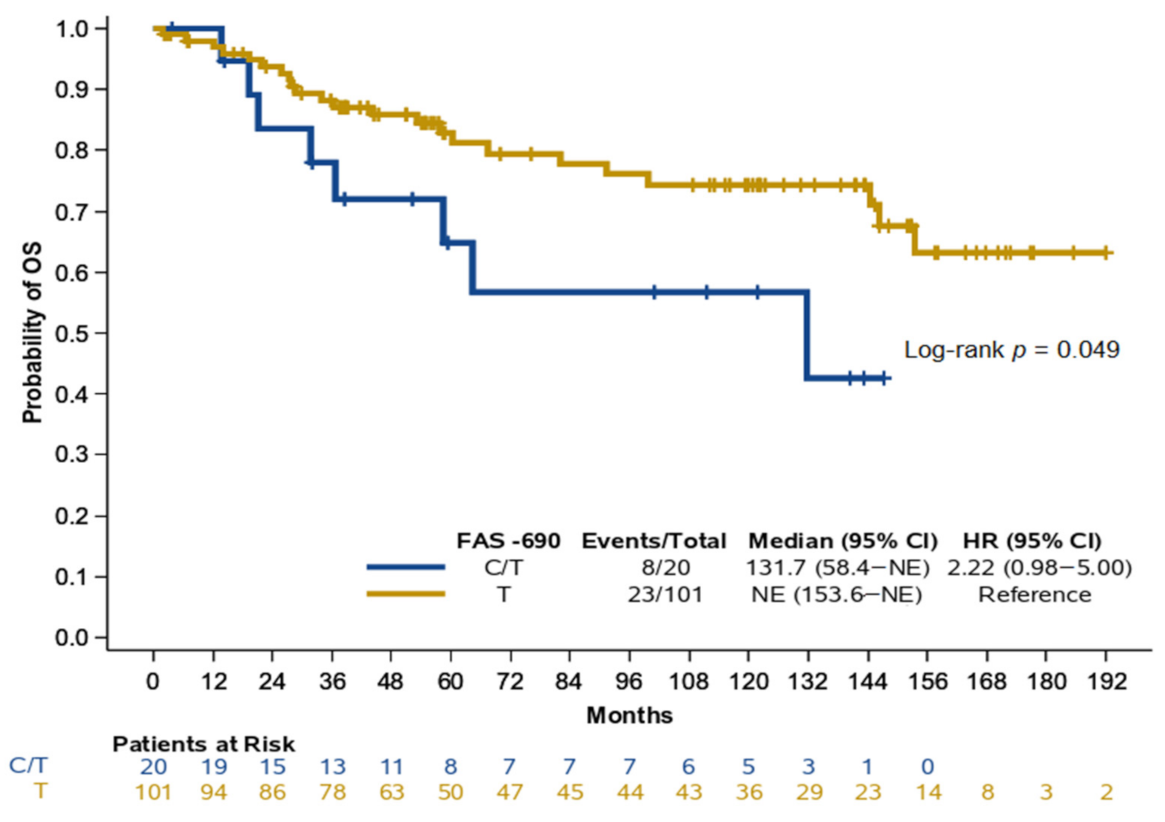
2.5. FAS −690 and Overall Survival in NPC Patients
2.6. LC Patients Carrying the A Allele of VEGFA rs13207351 Had a Higher Risk of Death and Poorer Outcomes
3. Discussion
4. Patients and Methods
4.1. Study Design, Population and Data Collection
4.2. Genetic Variant Selection
4.3. Variant Genotyping
4.4. Statistical Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2021. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Botta, L.; Sánchez, M.J.; Anderson, L.A.; Pierannunzio, D.; Licitra, L.; Hackl, M.; Zielonke, N.; Oberaigner, W.; Van Eycken, E.; et al. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur. J. Cancer 2015, 51, 2130–2143. [Google Scholar] [CrossRef]
- Lubin, J.H.; Purdue, M.; Kelsey, K.; Zhang, Z.-F.; Winn, D.; Wei, Q.; Talamini, R.; Szeszenia-Dabrowska, N.; Sturgis, E.M.; Smith, E.; et al. Total Exposure and Exposure Rate Effects for Alcohol and Smoking and Risk of Head and Neck Cancer: A Pooled Analysis of Case-Control Studies. Am. J. Epidemiol. 2009, 170, 937–947. [Google Scholar] [CrossRef]
- Mork, J.; Lie, A.K.; Glattre, E.; Clark, S.; Hallmans, G.; Jellum, E.; Koskela, P.; Møller, B.; Pukkala, E.; Schiller, J.T.; et al. Human Papillomavirus Infection as a Risk Factor for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2001, 344, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. EBV based cancer prevention and therapy in nasopharyngeal carcinoma. NPJ Precis. Oncol. 2017, 1, 10. [Google Scholar] [CrossRef] [Green Version]
- Pai, S.I.; Westra, W.H. Molecular pathology of head and neck cancer: Implications for diagnosis, prognosis, and treatment. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 49–70. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Tobias, P.V. The nasopharynx: Review of structure and development, with notes on speech, pharyngeal hypophysis, chordoma and the dens. J. Dent. Assoc. S. Afr. 1981, 36, 765–778. [Google Scholar]
- White, S.; Danowitz, M.; Solounias, N. Embryology and evolutionary history of the respiratory tract. Edorium J. Anat. Embryol. 2016, 3, 54–62. [Google Scholar]
- Chou, J.; Lin, Y.C.; Kim, J.; You, L.; Xu, Z.; He, B.; Jablons, D.M. Nasopharyngeal carcinoma—Review of the molecular mechanisms of tumorigenesis. Head Neck 2008, 30, 946–963. [Google Scholar] [CrossRef] [Green Version]
- Musil, M. Serological differences between some isolates of bean yellow mosaic virus. Acta Virol. 1975, 19, 473–480. [Google Scholar] [PubMed]
- Petersson, F. Nasopharyngeal carcinoma: A review. Semin Diagn. Pathol. 2015, 32, 54–73. [Google Scholar] [CrossRef] [PubMed]
- Rottey, S.; Madani, I.; Deron, P.; Van Belle, S. Modern treatment for nasopharyngeal carcinoma: Current status and prospects. Curr. Opin. Oncol. 2011, 23, 254–258. [Google Scholar] [CrossRef]
- Kim, T.J.; Lee, Y.S.; Kang, J.H.; Kim, Y.S.; Kang, C.S. Prognostic significance of expression of VEGF and Cox-2 in nasopharyngeal carcinoma and its association with expression of C-erbB2 and EGFR. J. Surg. Oncol. 2011, 103, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Saaristo, A.; Partanen, T.A.; Arola, J.; Jussila, L.; Hytönen, M.; Mäkitie, A.; Vento, S.; Kaipainen, A.; Malmberg, H.; Alitalo, K. Vascular Endothelial Growth Factor-C and Its Receptor VEGFR-3 in the Nasal Mucosa and in Nasopharyngeal Tumors. Am. J. Pathol. 2000, 157, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Sacks, D.; Baxter, B.; Campbell, B.C.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Psoma, E.; Koliou, G.A.; Dimitrakopoulos, F.I.; Papadopoulou, K.; Rontogianni, D.; Bobos, M.; Visvikis, A.; Kosmidis, P.A.; Fountzilas, G.; Constantinidis, J.; et al. Genetic Variations of VEGFA Gene Are Associated with Infiltration of Adjacent Tissues and the Clinical Outcome of Patients with Nasopharyngeal Carcinoma. Anticancer Res. 2020, 40, 677–688. [Google Scholar] [CrossRef]
- Koutras, A.K.; Antonacopoulou, A.G.; Eleftheraki, A.G.; Dimitrakopoulos, F.I.; Koumarianou, A.; Varthalitis, I.; Fostira, F.; Sgouros, J.; Briasoulis, E.; Bournakis, E.; et al. Vascular endothelial growth factor polymorphisms and clinical outcome in colorectal cancer patients treated with irinotecan-based chemotherapy and bevacizumab. Pharm. J. 2012, 12, 468–475. [Google Scholar] [CrossRef] [Green Version]
- Antonacopoulou, A.G.; Kottorou, A.E.; Dimitrakopoulos, F.-I.D.; Triantafyllia, V.; Marousi, S.; Koutras, A.; Kalofonos, H.P. VEGF polymorphisms may be associated with susceptibility to colorectal cancer: A case-control study. Cancer Biomark. 2011, 10, 213–217. [Google Scholar] [CrossRef]
- Jain, L.; Vargo, C.A.; Danesi, R.; Sissung, T.M.; Price, D.K.; Venzon, D.; Venitz, J.; Figg, W.D. The role of vascular endothelial growth factor SNPs as predictive and prognostic markers for major solid tumors. Mol. Cancer Ther. 2009, 8, 2496–2508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutras, A.; Kotoula, V.; Fountzilas, G. Prognostic and predictive role of vascular endothelial growth factor polymorphisms in breast cancer. Pharmacogenomics 2015, 16, 79–94. [Google Scholar] [CrossRef]
- Makni, L.; Stayoussef, M.; Ghazouani, E.; Mezlini, A.; Almawi, W.Y.; BesmaYacoubi, L. Distinct association of VEGF-A polymorphisms with laryngeal and nasopharyngeal cancer. Meta Gene 2016, 10, 90–94. [Google Scholar] [CrossRef]
- Irani, S.; Salajegheh, A.; Smith, R.A.; Lam, A.K.-Y. A review of the profile of endothelin axis in cancer and its management. Crit. Rev. Oncol. 2014, 89, 314–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, H.-Q.; Zeng, Z.-Y.; Zhang, H.-Z.; Hou, J.-H.; Mo, H.-Y.; Guo, X.; Min, H.-Q.; Hong, M.-H. [Correlation of endothelin A receptor expression to prognosis of nasopharyngeal carcinoma]. Ai Zheng 2005, 24, 611–615. [Google Scholar]
- Wen, Y.-F.; Qi, B.; Liu, H.; Mo, H.-Y.; Chen, Q.-Y.; Li, J.; Huang, P.-Y.; Ye, Y.-F.; Zhang, Y.; Deng, M.-Q.; et al. Polymorphisms in the Endothelin-1 and Endothelin A Receptor Genes and Survival in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma. Clin. Cancer Res. 2011, 17, 2451–2458. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.-L.; Liu, R.; Huang, L.-H.; Zou, C.; Huang, J.; Wang, J.; Chen, S.-J.; Meng, X.-G.; Yang, J.-K.; Li, H.; et al. Impact of polymorphisms in angiogenesis-related genes on clinical outcomes of radiotherapy in patients with nasopharyngeal carcinoma. Clin. Exp. Pharmacol. Physiol. 2017, 44, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.E.; Hadji, A.; Murmann, A.E.; Brockway, S.; Putzbach, W.; Pattanayak, A.; Ceppi, P. The role of CD95 and CD95 ligand in cancer. Cell Death Differ. 2015, 22, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Villa-Morales, M.; Fernández-Piqueras, J. Targeting the Fas/FasL signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 85–101. [Google Scholar] [CrossRef]
- Xu, Y.; He, B.; Li, R.; Pan, Y.; Gao, T.; Deng, Q.; Sun, H.; Song, G.; Wang, S. Association of the polymorphisms in the Fas/FasL promoter regions with cancer susceptibility: A systematic review and meta-analysis of 52 studies. PLoS ONE 2014, 9, e90090. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zuo, L.; Li, L.; Yin, L.; Liang, K.; Yu, H.; Ren, H.; Zhou, W.; Jing, H.; Liu, Y.; et al. Significant association among the Fas -670 A/G (rs1800682) polymorphism and esophageal cancer, hepatocellular carcinoma, and prostate cancer susceptibility: A meta-analysis. Tumor Biol. 2014, 35, 10911–10918. [Google Scholar] [CrossRef]
- Eun, Y.G.; Lee, Y.C.; Kim, S.K.; Chung, J.-H.; Kwon, K.H.; Park, I.S. Single nucleotide polymorphisms of the Fas gene are associated with papillary thyroid cancer. Auris Nasus Larynx 2015, 42, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tong, S.; Guan, L.; Na, F.; Zhao, W.; Wei, L. CD95 rs1800682 polymorphism and cervical cancer risk: Evidence from a meta-analysis. Tumor Biol. 2014, 35, 1785–1790. [Google Scholar] [CrossRef]
- Friedlander, P.L. Genomic instability in head and neck cancer patients. Head Neck 2001, 23, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Gollin, S.M. Mechanisms leading to chromosomal instability. Semin. Cancer Biol. 2005, 15, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Berardinelli, F.; Di Masi, A.; Antoccia, A. NBN Gene Polymorphisms and Cancer Susceptibility: A Systemic Review. Curr. Genom. 2013, 14, 425–440. [Google Scholar] [CrossRef] [Green Version]
- Chrzanowska, K.H.; Gregorek, H.; Dembowska-Bagińska, B.; Kalina, M.A.; Digweed, M. Nijmegen breakage syndrome (NBS). Orphanet J. Rare Dis. 2012, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Kong, S.Y.; Park, J.W.; Lee, J.A.; Park, J.E.; Park, K.W.; Hong, E.K.; Kim, C.M. Association between vascular endothelial growth factor gene polymorphisms and survival in hepatocellular carcinoma patients. Hepatology 2007, 46, 446–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drobkova, H.; Jurečeková, J.; Sivoňová, M.K.; Mazuchová, J.; Škorvanová, M.; Šarlinová, M.; Halašová, E.; Kliment, J. Associations Between Gene Polymorphisms of Vascular Endothelial Growth Factor and Prostate Cancer. Anticancer Res. 2019, 39, 2903–2909. [Google Scholar] [CrossRef]
- Gingerich, M.A.; Smith, J.D.; Michmerhuizen, N.L.; Ludwig, M.; Devenport, S.; Matovina, C.; Brenner, C.; Chinn, S.B. Comprehensive review of genetic factors contributing to head and neck squamous cell carcinoma development in low-risk, nontraditional patients. Head Neck 2018, 40, 943–954. [Google Scholar] [CrossRef]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, A.D. The Human Genome Browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Consortium, E.P. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef]
- Yang, X.; Deng, Y.; Gu, H.; Lim, A.; Altankhuyag, A.; Jia, W.; Ma, K.; Xu, J.; Zou, Y.; Snellingen, T.; et al. Polymorphisms in the vascular endothelial growth factor gene and the risk of diabetic retinopathy in Chinese patients with type 2 diabetes. Mol. Vis. 2011, 17, 3088–3096. [Google Scholar] [PubMed]
- Li, X.; Lu, Y.; Wei, P. Association between VEGF genetic variants and diabetic foot ulcer in Chinese Han population: A case-control study. Medicine 2018, 97, e10672. [Google Scholar] [CrossRef] [PubMed]
- Koutras, A.K.; Kotoula, V.; Papadimitriou, C.; Dionysopoulos, D.; Zagouri, F.; Kalofonos, H.P.; Kourea, H.P.; Skarlos, D.V.; Samantas, E.; Papadopoulou, K.; et al. Vascular endothelial growth factor polymorphisms and clinical outcome in patients with metastatic breast cancer treated with weekly docetaxel. Pharm. J. 2014, 14, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-Y.; Guo, H.-R.; Chen, H.H.W.; Hsiao, J.-R.; Jin, Y.-T.; Tsai, S.-T. Prognostic implications of Fas-ligand expression in nasopharyngeal carcinoma. Head Neck 2004, 26, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Jrad, B.B.; Mahfouth, W.; Bouaouina, N.; Gabbouj, S.; Ahmed, S.B.; Ltaïef, M.; Jalbout, M.; Chouchane, L. A polymorphism in FAS gene promoter associated with increased risk of nasopharyngeal carcinoma and correlated with anti-nuclear autoantibodies induction. Cancer Lett. 2006, 233, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wang, T.; Ren, J.; Hu, K.; Liu, W.; Wu, G. FAS-670A/G polymorphism: A biomarker for the metastasis of nasopharyngeal carcinoma in a Chinese population. Clin. Chim. Acta 2010, 411, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Miao, X.-P.; Huang, M.-Y.; Deng, L.; Lin, D.-X.; Zeng, Y.-X.; Shao, J.-Y. Polymorphisms of death pathway genes FAS and FASL and risk of nasopharyngeal carcinoma. Mol. Carcinog. 2010, 49, 944–950. [Google Scholar] [CrossRef]
- Huang, Q.R.; Morris, D.; Manolios, N. Identification and characterisation of polymorphisms in the promoter region of the human Apo-1/Fas (CD95) gene. Mol. Immunol. 1997, 34, 577–582. [Google Scholar] [CrossRef]
- Sibley, K.; Rollinson, S.; Allan, J.M.; Smith, A.G.; Law, G.R.; Roddam, P.L.; Skibola, C.F.; Smith, M.T.; Morgan, G.J. Functional FAS promoter polymorphisms are associated with increased risk of acute myeloid leukemia. Cancer Res. 2003, 63, 4327–4330. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Ricceri, F.; Matullo, G.; Vineis, P. Is there evidence of involvement of DNA repair polymorphisms in human cancer? Mutat. Res. Mol. Mech. Mutagen. 2012, 736, 117–121. [Google Scholar] [CrossRef]
- Adjadj, E.; Schlumberger, M.; de Vathaire, F. Germ-line DNA polymorphisms and susceptibility to differentiated thyroid cancer. Lancet Oncol. 2009, 10, 181–190. [Google Scholar] [CrossRef]
- Tan, J.; Jiang, L.; Cheng, X.; Wang, C.; Chen, J.; Huang, X.; Xie, P.; Xia, D.; Wang, R.; Zhang, Y. Association between VEGF-460T/C gene polymorphism and clinical outcomes of nasopharyngeal carcinoma treated with intensity-modulated radiation therapy. Onco Targets Ther. 2017, 10, 909–918. [Google Scholar] [CrossRef] [Green Version]
- Ungerbäck, J.; Elander, N.; Dimberg, J.; Söderkvist, P. Analysis of VEGF polymorphisms, tumor expression of VEGF mRNA and colorectal cancer susceptibility in a Swedish population. Mol. Med. Rep. 2009, 2, 435–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, T.; Li, G.; Zhao, C.; Zheng, R.; Wei, Q.; Sturgis, E.M. Fas single nucleotide polymorphisms and risk of thyroid and salivary gland carcinomas: A case-control analysis. Head Neck 2008, 30, 297–305. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Total (N = 333) | Non-NPC (N = 189) | NPC (N = 144) |
|---|---|---|---|
| Age (N = 314) | |||
| Median (min, max) | 60.2 (14.3, 88.3) | 64.9 (37.1, 88.3) | 51.6 (14.3, 81.5) |
| N (%) | N (%) | N (%) | |
| Sex (N = 333) | |||
| Female | 52 (15.6) | 16 (8.5) | 36 (24.8) |
| Male | 282 (84.4) | 173 (91.5) | 109 (75.2) |
| Alcohol Abuse (N = 292) | |||
| No | 145 (49.7) | 47 (26.6) | 98 (85.2) |
| Yes | 147 (50.3) | 130 (73.4) | 17 (14.8) |
| Smoking (N = 293) | |||
| No | 60 (20.5) | 19 (10.8) | 41 (35.0) |
| Yes | 233 (79.5) | 157 (89.2) | 76 (65.0) |
| Histological Type (N = 307) | |||
| Non-Keratinizing Carcinoma | 37 (12.1) | 0 (0.0) | 37 (28.7) |
| Squamous Cell Carcinoma | 189 (61.6) | 172 (96.6) | 17 (13.2) |
| Undifferentiated Carcinoma | 77 (25.1) | 2 (1.1) | 75 (58.1) |
| Other | 4 (1.3) | 4 (2.2) | 0 (0.0) |
| Primary Tumor Location (N = 318) | |||
| Hypopharynx | 1 (0.3) | 1 (0.57) | 0 (0.0) |
| Larynx | 149 (46.9) | 149 (85.6) | 0 (0.0) |
| Major Salivary Glands | 2 (0.6) | 2 (1.1) | 0 (0.0) |
| Nasopharynx | 144 (45.3) | 0 (0.0) | 144 (100.0) |
| Oral Cavity | 11 (3.5) | 11 (6.3) | 0 (0.0) |
| Oropharynx | 7 (2.2) | 7 (4.0) | 0 (0.0) |
| Paranasal Sinuses | 4 (1.3) | 4 (2.3) | 0 (0.0) |
| T (N = 272) | |||
| T1 | 45 (16.5) | 19 (11.3) | 26 (25.0) |
| T2 | 79 (29.0) | 34 (20.2) | 45 (43.3) |
| T3 | 94 (34.6) | 77 (45.8) | 17 (16.3) |
| T4 | 54 (19.9) | 38 (22.6) | 16 (15.4) |
| N (N = 275) | |||
| N0 | 126 (45.8) | 114 (67.1) | 12 (11.4) |
| N1 | 42 (15.3) | 14 (8.2) | 28 (26.7) |
| N2 | 80 (29.1) | 33 (19.4) | 47 (44.8) |
| N3 | 23 (8.4) | 5 (2.9) | 18 (17.1) |
| Nx | 4 (1.5) | 4 (2.4) | 0 (0.0) |
| Stage (N = 281) | |||
| I | 19 (6.8) | 15 (9.0) | 4 (3.5) |
| II | 52 (18.5) | 23 (13.8) | 29 (25.4) |
| III | 105 (37.4) | 61 (36.5) | 44 (38.6) |
| IV | 105 (37.4) | 68 (40.7) | 37 (32.5) |
| Surgery (N = 176) | |||
| No | 33 (18.8) | 33 (18.8) | - |
| Yes | 143 (81.3) | 143 (81.3) | - |
| Type of Surgery (N = 142) | |||
| Chordectomy | 8 (5.6) | 8 (5.6) | - |
| Hemiglossectomy | 5 (3.5) | 5 (3.5) | - |
| Hemiglossectomy and Laryngectomy | 2 (1.4) | 2 (1.4) | - |
| Laryngectomy | 113 (79.6) | 113 (79.6) | - |
| Other | 14 (9.9) | 14 (9.9) | - |
| Treatment Received (N = 311) | |||
| No | 44 (14.1) | 43 (23.9) | 1 (0.8) |
| Yes | 267 (85.9) | 137 (76.1) | 130 (99.2) |
| Type of First Treatment Received (N = 267) | |||
| Induction Chemotherapy | 10 (3.8) | 0 (0.0) | 10 (7.7) |
| Primary concomitant CT and RT | 179 (67.0) | 65 (47.4) | 114 (87.7) |
| Primary RT | 34 (12.7) | 33 (24.1) | 1 (0.8) |
| RT after First Progression | 1 (0.4) | 1 (0.7) | 0 (0.0) |
| Adjuvant | 32 (12.0) | 29 (21.2) | 3 (2.3) |
| First-Line | 11 (4.1) | 9 (6.6) | 2 (1.5) |
| Parameter | Total (N = 293) | Non-NPC (N = 149) | NPC (N = 144) |
|---|---|---|---|
| VEGFA rs699947 (−2578) (N = 286) | |||
| A | 40 (14.0) | 20 (13.4) | 20 (14.6) |
| AC | 136 (47.6) | 73 (49.0) | 63 (46.0) |
| C | 110 (38.5) | 56 (37.6) | 54 (39.4) |
| VEGFA rs12664104 (N = 285) | |||
| G | 285 (100.0) | 149 (100.0) | 136 (100.0) |
| VEGFA rs34376996 (N = 258) | |||
| T | 258 (100.0) | 149 (100.0) | 109 (100.0) |
| VEGFA rs144854329 (N = 289) | |||
| het del18bp | 148 (51.2) | 73 (49.0) | 75 (53.6) |
| hom del18bp | 101 (34.9) | 56 (37.6) | 45 (32.1) |
| reference | 40 (13.8) | 20 (13.4) | 20 (14.3) |
| VEGFA rs35864111 (N = 289) | |||
| het ins1bp | 148 (51.2) | 73 (49.0) | 75 (53.6) |
| hom ins1bp | 101 (34.9) | 56 (37.6) | 45 (32.1) |
| reference | 40 (13.8) | 20 (13.4) | 20 (14.3) |
| VEGFA rs833061 (-1498) (N = 272) | |||
| C | 55 (20.2) | 31 (20.8) | 24 (19.5) |
| CT | 135 (49.6) | 75 (50.3) | 60 (48.8) |
| T | 82 (30.1) | 43 (28.9) | 39 (31.7) |
| VEGFA rs149983590 (N = 288) | |||
| C | 287 (99.7) | 149 (100.0) | 138 (99.3) |
| CA | 1 (0.35) | 0 (0.0) | 1 (0.72) |
| VEGFA rs833062 (N = 276) | |||
| CT | 8 (2.9) | 7 (4.7) | 1 (0.79) |
| T | 268 (97.1) | 142 (95.3) | 126 (99.2) |
| VEGFA rs1570360 (−1154) (N = 289) | |||
| A | 91 (31.5) | 60 (40.3) | 31 (22.1) |
| G | 97 (33.6) | 36 (24.2) | 61 (43.6) |
| GA | 101 (34.9) | 53 (35.6) | 48 (34.3) |
| VEGFA rs28357093 (N = 289) | |||
| A | 289 (100.0) | 149 (100.0) | 140 (100.0) |
| VEGFA rs13207351 (−1190) (N = 289) | |||
| A | 136 (47.1) | 69 (46.3) | 67 (47.9) |
| AG | 64 (22.1) | 34 (22.8) | 30 (21.4) |
| G | 89 (30.8) | 46 (30.9) | 43 (30.7) |
| VEGFA rs79469752 (N = 289) | |||
| C | 289 (100.0) | 149 (100.0) | 140 (100.0) |
| VEGFA rs59260042 (N = 289) | |||
| C | 289 (100.0) | 149 (100.0) | 140 (100.0) |
| VEGFA rs3025039 (+936) (N = 285) | |||
| C | 200 (70.2) | 104 (70.3) | 96 (70.1) |
| CT | 71 (24.9) | 34 (23.0) | 37 (27.0) |
| T | 14 (4.9) | 10 (6.8) | 4 (2.9) |
| VEGFA rs149179279 (N = 285) | |||
| C | 285 (100.0) | 148 (100.0) | 137 (100.0) |
| VEGFA rs112256643 (N = 285) | |||
| C | 285 (100.0) | 148 (100.0) | 137 (100.0) |
| VEGFA rs112005313 (N = 285) | |||
| G | 285 (100.0) | 148 (100.0) | 137 (100.0) |
| VEGFA rs187429037 (N = 283) | |||
| A | 283 (100.0) | 148 (100.0) | 135 (100.0) |
| VEGFA rs111933757 (N = 280) | |||
| T | 280 (100.0) | 148 (100.0) | 132 (100.0) |
| EDNRA rs5333 (p.H323H) (N = 289) | |||
| C | 13 (4.5) | 6 (4.1) | 7 (5.0) |
| CT | 114 (39.4) | 58 (39.2) | 56 (39.7) |
| T | 162 (56.1) | 84 (56.8) | 78 (55.3) |
| EDNRA rs5334 (p.E335E) (N = 287) | |||
| A | 12 (4.2) | 6 (4.1) | 6 (4.3) |
| AG | 113 (39.4) | 58 (39.2) | 55 (39.6) |
| G | 162 (56.4) | 84 (56.8) | 78 (56.1) |
| EDNRA rs10305924 (N = 285) | |||
| AG | 1 (0.35) | 0 (0.0) | 1 (0.73) |
| G | 284 (99.6) | 148 (100.0) | 136 (99.3) |
| EDNRA rs17856670 (p.L322V) (N = 289) | |||
| C | 289 (100.0) | 148 (100.0) | 141 (100.0) |
| EDNRA rs112710542 (N = 288) | |||
| AG | 6 (2.1) | 0 (0.0) | 6 (4.3) |
| G | 282 (97.9) | 147 (100.0) | 135 (95.7) |
| FAS rs1800682 (-670) (N = 290) | |||
| A | 74 (25.5) | 40 (26.8) | 34 (24.1) |
| AG | 165 (56.9) | 75 (50.3) | 90 (63.8) |
| G | 51 (17.6) | 34 (22.8) | 17 (12.1) |
| FAS rs34995925 (N = 290) | |||
| T | 290 (100.0) | 149 (100.0) | 141 (100.0) |
| FAS rs2234768 (−690) (N = 290) | |||
| C | 1 (0.34) | 1 (0.67) | 0 (0.0) |
| CT | 52 (17.9) | 27 (18.1) | 25 (17.7) |
| T | 237 (81.7) | 121 (81.2) | 116 (82.3) |
| FAS rs150130637 (N = 286) | |||
| A | 286 (100.0) | 149 (100.0) | 137 (100.0) |
| NBS1 rs1805794 (p.E185Q) (N = 291) | |||
| C | 37 (12.7) | 21 (14.1) | 16 (11.3) |
| G | 109 (37.5) | 61 (40.9) | 48 (33.8) |
| GC | 145 (49.8) | 67 (45.0) | 78 (54.9) |
| NBS1 rs192240705 (N = 291) | |||
| T | 291 (100.0) | 149 (100.0) | 142 (100.0) |
| NBS1 rs780661058 (p.A183A) (N = 291) | |||
| A | 291 (100.0) | 149 (100.0) | 142 (100.0) |
| NBS1 rs151070415 (p.A183T) (N = 291) | |||
| G | 291 (100.0) | 149 (100.0) | 142 (100.0) |
| NBS1 rs61754966 (p.I171V) (N = 291) | |||
| A | 289 (99.3) | 148 (99.3) | 141 (99.3) |
| AG | 2 (0.7) | 1 (0.7) | 1 (0.7) |
| NBS1 rs182756889 (p.R169C) (N = 291) | |||
| C | 291 (100.0) | 149 (100.0) | 142 (100.0) |
| Parameter | Univariate | p-Value | Multivariate * | p-Value | ||
|---|---|---|---|---|---|---|
| Event/Total | HR (95% CI) | Event/Total | HR (95% CI) | |||
| VEGFA rs13207351 (−1190) | ||||||
| Additive model | 0.012 | 0.031 | ||||
| A | 40/68 | 2.21 (1.23–3.96) | 0.008 | 38/64 | 2.06 (1.14–3.72) | 0.017 |
| AG | 15/34 | 1.19 (0.59–2.41) | 0.624 | 14/33 | 1.19 (0.57–2.45) | 0.645 |
| G | 16/46 | Reference | 1 | 16/46 | Reference | - |
| Dominant model | ||||||
| A+AG | 55/102 | 1.79 (1.02–3.12) | 0.042 | 52/97 | 1.72 (0.98–3.03) | 0.059 |
| G | 16/46 | Reference | - | 16/46 | Reference | - |
| Recessive model | ||||||
| A | 40/68 | 2.04 (1.27–3.28) | 0.003 | 38/64 | 1.91 (1.17–3.10) | 0.009 |
| AG+G | 31/80 | Reference | - | 30/79 | Reference | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrakopoulos, F.-I.; Koliou, G.-A.; Kotoula, V.; Papadopoulou, K.; Markou, K.; Vlachtsis, K.; Angouridakis, N.; Karasmanis, I.; Nikolaou, A.; Psyrri, A.; et al. Genetic Variation in the Vascular Endothelial Growth Factor (VEGFA) Gene at rs13207351 Is Associated with Overall Survival of Patients with Head and Neck Cancer. Cancers 2021, 13, 1163. https://doi.org/10.3390/cancers13051163
Dimitrakopoulos F-I, Koliou G-A, Kotoula V, Papadopoulou K, Markou K, Vlachtsis K, Angouridakis N, Karasmanis I, Nikolaou A, Psyrri A, et al. Genetic Variation in the Vascular Endothelial Growth Factor (VEGFA) Gene at rs13207351 Is Associated with Overall Survival of Patients with Head and Neck Cancer. Cancers. 2021; 13(5):1163. https://doi.org/10.3390/cancers13051163
Chicago/Turabian StyleDimitrakopoulos, Foteinos-Ioannis, Georgia-Angeliki Koliou, Vassiliki Kotoula, Kyriaki Papadopoulou, Konstantinos Markou, Konstantinos Vlachtsis, Nikolaos Angouridakis, Ilias Karasmanis, Angelos Nikolaou, Amanda Psyrri, and et al. 2021. "Genetic Variation in the Vascular Endothelial Growth Factor (VEGFA) Gene at rs13207351 Is Associated with Overall Survival of Patients with Head and Neck Cancer" Cancers 13, no. 5: 1163. https://doi.org/10.3390/cancers13051163
APA StyleDimitrakopoulos, F.-I., Koliou, G.-A., Kotoula, V., Papadopoulou, K., Markou, K., Vlachtsis, K., Angouridakis, N., Karasmanis, I., Nikolaou, A., Psyrri, A., Visvikis, A., Kosmidis, P., Fountzilas, G., & Koutras, A. (2021). Genetic Variation in the Vascular Endothelial Growth Factor (VEGFA) Gene at rs13207351 Is Associated with Overall Survival of Patients with Head and Neck Cancer. Cancers, 13(5), 1163. https://doi.org/10.3390/cancers13051163







