Conversion Therapy of Intrahepatic Cholangiocarcinoma Is Associated with Improved Prognosis and Verified by a Case of Patient-Derived Organoid
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Data Collection
2.3. Follow-Up
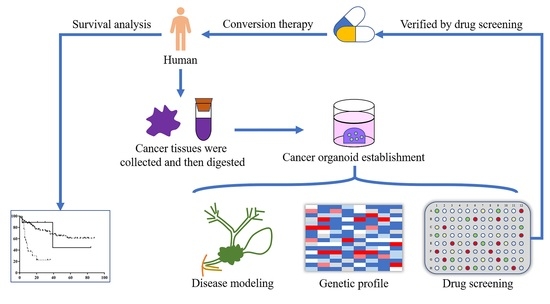
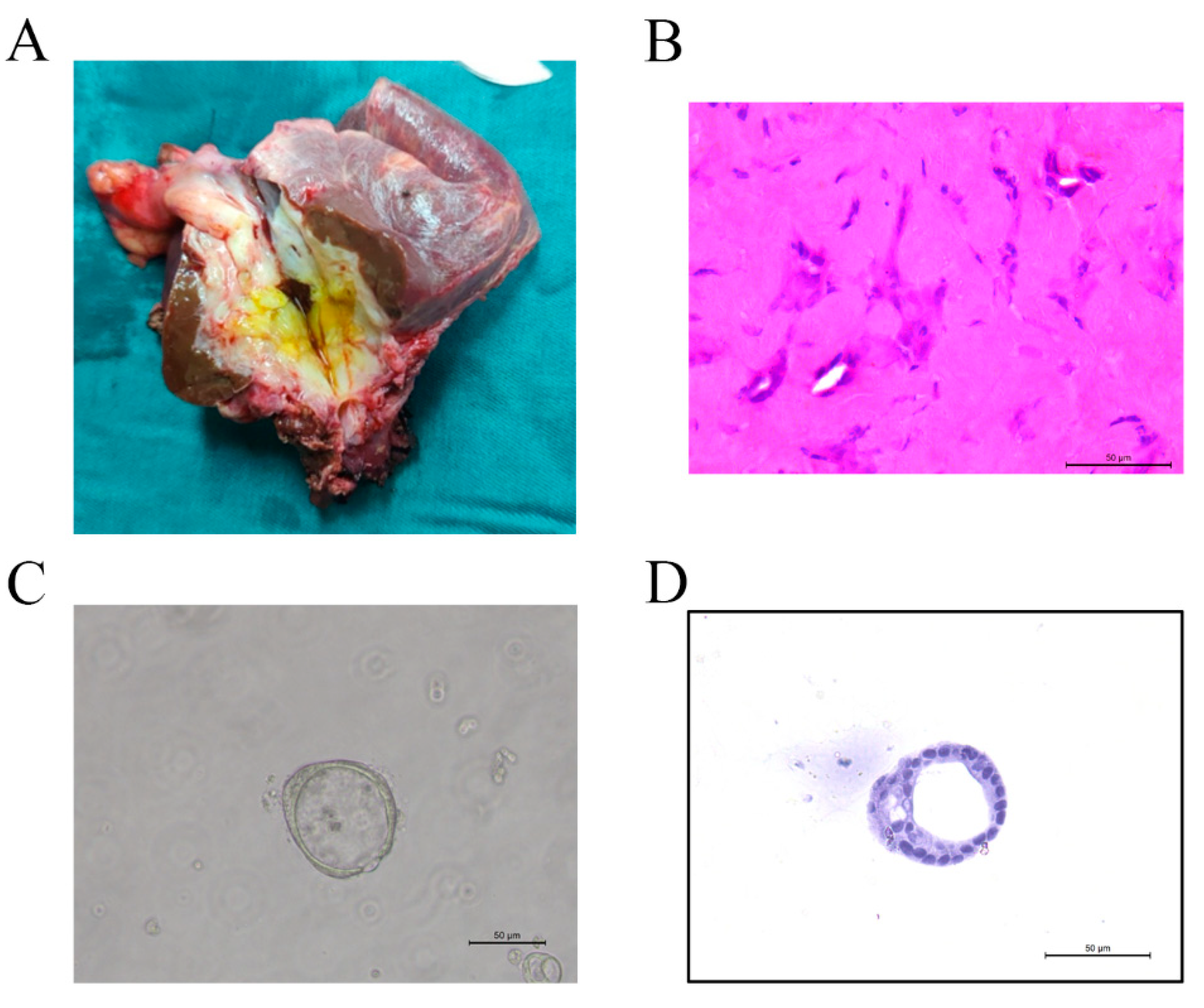
2.4. Organoid Establishment
2.5. Preparation of Histological Sections
2.6. Whole Exome Sequencing (WES)
2.7. Drug Treatment
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Survival Analysis
3.3. Organoid Establishment
3.4. Drug Screening
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.F.; Xue, F.; Dong, D.H.; Weiss, M.; Popescu, I.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; Pulitano, C.; Bauer, T.W.; et al. Number and station of lymph node metastasis after curative-intent resection of intrahepatic cholangiocarcinoma impact prognosis. Ann. Surg. 2020. [Google Scholar] [CrossRef]
- Sirica, A.E.; Gores, G.J.; Groopman, J.D.; Selaru, F.M.; Strazzabosco, M.; Wei Wang, X.; Zhu, A.X. Intrahepatic cholangiocarcinoma: Continuing challenges and translational advances. Hepatology 2019, 69, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Gorgen, A.; Roayaie, S.; Droz Dit Busset, M.; Sapisochin, G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 72, 364–377. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, F.; Yao, L.; Fang, T.; Liao, M.; Long, J.; Xiao, L.; Deng, G. Nomogram based on inflammatory indices for differentiating intrahepatic cholangiocarcinoma from hepatocellular carcinoma. Cancer Med. 2020, 9, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.P.; Drake, J.; Wach, M.M.; Ruff, S.M.; Diggs, L.P.; Wan, J.Y.; Good, M.L.; Dominguez, D.A.; Ayabe, R.I.; Glazer, E.S.; et al. Resection and chemotherapy is the optimal treatment approach for patients with clinically node positive intrahepatic cholangiocarcinoma. HPB 2020, 22, 129–135. [Google Scholar] [CrossRef]
- Zhou, R.; Lu, D.; Li, W.; Tan, W.; Zhu, S.; Chen, X.; Min, J.; Shang, C.; Chen, Y. Is lymph node dissection necessary for resectable intrahepatic cholangiocarcinoma? A systematic review and meta-analysis. HPB 2019, 21, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Bismuth, H.; Adam, R.; Levi, F.; Farabos, C.; Waechter, F.; Castaing, D.; Majno, P.; Engerran, L. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann. Surg. 1996, 224, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, Y.; Yang, M.; Jiang, D.; Chen, Y.; Jiang, J.; Chen, Z.; Yang, L.; Huang, D. Conversion therapy for advanced pancreatic cancer: The case series and literature review. Front Pharmacol. 2020, 11, 579239. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamaguchi, K.; Okumura, N.; Tanahashi, T.; Kodera, Y. Is conversion therapy possible in stage iv gastric cancer: The proposal of new biological categories of classification. Gastric Cancer 2016, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Shi, S.; Hua, J.; Xu, J.; Yu, X.; Chinese Study Group for Pancreatic Cancer. Simultaneous resection of the primary tumour and liver metastases after conversion chemotherapy versus standard therapy in pancreatic cancer with liver oligometastasis: Protocol of a multicentre, prospective, randomised phase iii control trial (cspac-1). BMJ Open 2019, 9, e033452. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Lorgis, V.; Vincent, J.; Ladoire, S.; Guiu, B. Hepatic arterial infusion of gemcitabine plus oxaliplatin as second-line treatment for locally advanced intrahepatic cholangiocarcinoma: Preliminary experience. Chemotherapy 2013, 59, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Shimizu, H.; Ohtsuka, M.; Yoshidome, H.; Yoshitomi, H.; Furukawa, K.; Takeuchi, D.; Takayashiki, T.; Kimura, F.; Miyazaki, M. Surgical resection after downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer: A retrospective single-center study. Ann. Surg. Oncol. 2013, 20, 318–324. [Google Scholar] [CrossRef]
- Mouli, S.; Memon, K.; Baker, T.; Benson, A.B., 3rd; Mulcahy, M.F.; Gupta, R.; Ryu, R.K.; Salem, R.; Lewandowski, R.J. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: Safety, response, and survival analysis. J. Vasc. Interv. Radiol. 2013, 24, 1227–1234. [Google Scholar] [CrossRef]
- Rayar, M.; Sulpice, L.; Edeline, J.; Garin, E.; Levi Sandri, G.B.; Meunier, B.; Boucher, E.; Boudjema, K. Intra-arterial yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann. Surg. Oncol. 2015, 22, 3102–3108. [Google Scholar] [CrossRef] [PubMed]
- Servajean, C.; Gilabert, M.; Piana, G.; Monges, G.; Delpero, J.R.; Brenot, I.; Raoul, J.L. One case of intrahepatic cholangiocarcinoma amenable to resection after radioembolization. World J. Gastroenterol. 2014, 20, 5131–5134. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.B.; Bal, C.K.; Schaberg, K.; Longacre, T.A.; Chatrath, B.S.; Poultsides, G.A. Locally advanced intrahepatic cholangiocarcinoma: Complete pathologic response to neoadjuvant chemotherapy followed by left hepatic trisectionectomy and caudate lobectomy. Dig. Dis. Sci. 2015, 60, 3226–3229. [Google Scholar] [CrossRef] [PubMed]
- Uji, M.; Mizuno, T.; Ebata, T.; Sugawara, G.; Igami, T.; Uehara, K.; Nagino, M. A case of advanced intrahepatic cholangiocarcinoma accidentally, but successfully, treated with capecitabine plus oxaliplatin (capox) therapy combined with bevacizumab: A case report. Surg. Case Rep. 2016, 2, 63. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Touchefeu, Y.; Guiu, B.; Farge, O.; Tougeron, D.; Baumgaertner, I.; Ayav, A.; Campillo-Gimenez, B.; Beuzit, L.; Pracht, M.; et al. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma: A phase 2 clinical trial. JAMA Oncol. 2019, 6, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Roodsant, T.; Navis, M.; Aknouch, I.; Renes, I.B.; van Elburg, R.M.; Pajkrt, D.; Wolthers, K.C.; Schultsz, C.; van der Ark, K.C.H.; Sridhar, A.; et al. A human 2d primary organoid-derived epithelial monolayer model to study host-pathogen interaction in the small intestine. Front. Cell. Infect. Microbiol. 2020, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Tuveson, D.; Clevers, H. Cancer modeling meets human organoid technology. Science 2019, 364, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Wu, C.; O’Rourke, K.P.; Szeglin, B.C.; Zheng, Y.; Sauve, C.G.; Adileh, M.; Wasserman, I.; Marco, M.R.; Kim, A.S.; et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat. Med. 2019, 25, 1607–1614. [Google Scholar] [CrossRef]
- Frappart, P.O.; Walter, K.; Gout, J.; Beutel, A.K.; Morawe, M.; Arnold, F.; Breunig, M.; Barth, T.F.; Marienfeld, R.; Schulte, L.; et al. Pancreatic cancer-derived organoids-a disease modeling tool to predict drug response. United Eur. Gastroenterol. J. 2020, 8, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.; Francies, H.E.; Gavarro, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Driehuis, E.; van Hoeck, A.; Moore, K.; Kolders, S.; Francies, H.E.; Gulersonmez, M.C.; Stigter, E.C.A.; Burgering, B.; Geurts, V.; Gracanin, A.; et al. Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proc. Natl. Acad. Sci. USA 2019, 116, 26580–26590. [Google Scholar] [CrossRef] [PubMed]
- Fruscione, M.; Pickens, R.C.; Baker, E.H.; Martinie, J.B.; Iannitti, D.A.; Hwang, J.J.; Vrochides, D. Conversion therapy for intrahepatic cholangiocarcinoma and tumor downsizing to increase resection rates: A systematic review. Curr. Probl. Cancer 2020, 45, 100614. [Google Scholar] [CrossRef] [PubMed]
- Riby, D.; Mazzotta, A.D.; Bergeat, D.; Verdure, L.; Sulpice, L.; Bourien, H.; Lievre, A.; Rolland, Y.; Garin, E.; Boudjema, K.; et al. Downstaging with radioembolization or chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2020, 27, 3729–3737. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, B.; Gelli, M.; Pittau, G.; Allard, M.A.; Pereira, B.; Serji, B.; Vibert, E.; Castaing, D.; Adam, R.; Cherqui, D.; et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br. J. Surg. 2018, 105, 839–847. [Google Scholar] [CrossRef]
- Bruun, J.; Kryeziu, K.; Eide, P.W.; Moosavi, S.H.; Eilertsen, I.A.; Langerud, J.; Rosok, B.I.; Totland, M.Z.; Brunsell, T.H.; Pellinen, T.; et al. Patient-derived organoids from multiple colorectal cancer liver metastases reveal moderate intra-patient pharmacotranscriptomic heterogeneity. Clin. Cancer Res. 2020, 26, 4107–4119. [Google Scholar] [CrossRef]
- Li, X.; Pan, B.; Song, X.; Li, N.; Zhao, D.; Li, M.; Zhao, Z. Breast cancer organoids from a patient with giant papillary carcinoma as a high-fidelity model. Cancer Cell Int. 2020, 20, 86. [Google Scholar] [CrossRef]
- Nuciforo, S.; Fofana, I.; Matter, M.S.; Blumer, T.; Calabrese, D.; Boldanova, T.; Piscuoglio, S.; Wieland, S.; Ringnalda, F.; Schwank, G.; et al. Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep. 2018, 24, 1363–1376. [Google Scholar] [CrossRef]
- Lamberti, D.; Cristinziano, G.; Porru, M.; Leonetti, C.; Egan, J.B.; Shi, C.X.; Buglioni, S.; Amoreo, C.A.; Castellani, L.; Borad, M.J.; et al. Hsp90 inhibition drives degradation of fgfr2 fusion proteins: Implications for treatment of cholangiocarcinoma. Hepatology 2019, 69, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, C.; Lu, G.; Hu, Z.; Chen, Q.; Du, X. Fgf/fgfr signaling pathway involved resistance in various cancer types. J. Cancer 2020, 11, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.; Lee, L.Y.; Goh, K.Y.; Ong, R.; Hao, H.X.; Huang, A.; Wang, Y.; Graus Porta, D.; Chow, P.; Chung, A. Infigratinib mediates vascular normalization, impairs metastasis, and improves chemotherapy in hepatocellular carcinoma. Hepatology 2019, 69, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Boscoe, A.N.; Rolland, C.; Kelley, R.K. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: A systematic literature review. J. Gastrointest. Oncol. 2019, 10, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Lowery, M.A.; Burris, H.A., 3rd; Janku, F.; Shroff, R.T.; Cleary, J.M.; Azad, N.S.; Goyal, L.; Maher, E.A.; Gore, L.; Hollebecque, A.; et al. Safety and activity of ivosidenib in patients with idh1-mutant advanced cholangiocarcinoma: A phase 1 study. Lancet Gastroenterol. Hepatol. 2019, 4, 711–720. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in idh1-mutant, chemotherapy-refractory cholangiocarcinoma (claridhy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Driehuis, E.; Kretzschmar, K.; Clevers, H. Establishment of patient-derived cancer organoids for drug-screening applications. Nat. Protoc. 2020, 15, 3380–3409. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid modeling of the tumor immune microenvironment. Cell 2018, 175, 1972–1988 e1916. [Google Scholar] [CrossRef]





| Variables | Successfully Downstaged Patients | Unsuccessfully Downstaged Patients | p Value |
|---|---|---|---|
| Number | 9 | 33 | |
| Age, years | 64 (61–71) | 62 (52.5–68.5) | 0.450 |
| Gender | 0.784 | ||
| Male | 5 (55.6%) | 20 (60.6%) | |
| Female | 4 (44.4%) | 13 (39.4%) | |
| Liver cirrhosis | 1 (11.1%) | 5 (15.2%) | 0.759 |
| Multiple lesions (≥2) | 2 (22.2%) | 20 (60.6%) | 0.062 |
| Tumor size | 0.655 | ||
| <5cm | 1 (11.1%) | 8 (24.2%) | |
| ≥5cm | 8 (88.9%) | 25 (75.8%) | |
| Vascular invasion | 3 (33.3%) | 20 (60.6%) | 0.257 |
| Perineural invasion | 1 (11.1%) | 0 (0%) | 0.214 |
| Histological grade | 0.004 | ||
| Well | 0 (0%) | 1 (3.0%) | |
| Moderate | 1 (11.1%) | 0 (0%) | |
| Poor | 8 (88.9%) | 12 (36.4%) | |
| Unknown | 0 (0%) | 20 (60.6%) | |
| AJCC T stage | 0.008 | ||
| T1a | 1 (11.1%) | 2 (6.1%) | |
| T1b | 3 (33.3%) | 0 (0%) | |
| T2 | 2 (22.2%) | 23 (69.7%) | |
| T3 | 1 (11.1%) | 5 (15.2%) | |
| T4 | 2 (22.2%) | 5 (15.2%) | |
| AJCC N stage | 0.593 | ||
| N0 | 3 (33.3%) | 4 (12.1%) | |
| N1 | 6 (66.7%) | 29 (87.9%) | |
| AJCC M stage | 1.000 | ||
| M0 | 9 (100%) | 33 (100%) | |
| M1 | 0 (0%) | 0 (0%) |
| Patient No. | Age | Gender | Tumor Size (cm) | Histological Grade | Tnm Stage | Treatment Pattern | Radial Margin Status | Recurrence Time (Months) | Status | Survival Time (Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 73 | Female | 5.8 | Poor | T1bN0M0 | TACE | R0 | No | Alive | 84 |
| Patient 2 | 44 | Male | 3 | Poor | T1aN1M0 | Gemcitabine | R0 | No | Alive | 8 |
| Patient 3 | 72 | Male | 8.9 | Poor | T3N1M0 | Gemcitabine+Oxaliplatin | R0 | 29 | Dead | 39 |
| Patient 4 | 62 | Female | 6 | Moderate | T4N0M0 | Radiotherapy | R0 | 21 | Alive | 21 |
| Patient 5 | 64 | Female | 6.6 | Poor | T1bN1M0 | TACE | R0 | No | Alive | 30 |
| Patient 6 | 70 | Female | 5 | Poor | T1bN1M0 | Gemcitabine+Oxaliplatin+Toripalimab | R0 | No | Alive | 8 |
| Patient 7 | 67 | Male | 5.5 | Poor | T2N0M0 | Gemcitabine+Cisplatin | R0 | 6 | Alive | 11 |
| Patient 8 | 62 | Male | 8.6 | Poor | T2N1M0 | Gemcitabine+albumin-bound paclitaxel | R0 | No | Alive | 5 |
| Patient 9 | 60 | Male | 9.2 | Poor | T4N1M0 | Gemcitabine+albumin-bound paclitaxel | R0 | No | Dead | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Jin, Y.; Guo, Y.; Tan, Z.; Zhang, X.; Ye, D.; Yu, Y.; Peng, S.; Zheng, L.; Li, J. Conversion Therapy of Intrahepatic Cholangiocarcinoma Is Associated with Improved Prognosis and Verified by a Case of Patient-Derived Organoid. Cancers 2021, 13, 1179. https://doi.org/10.3390/cancers13051179
Wang Z, Jin Y, Guo Y, Tan Z, Zhang X, Ye D, Yu Y, Peng S, Zheng L, Li J. Conversion Therapy of Intrahepatic Cholangiocarcinoma Is Associated with Improved Prognosis and Verified by a Case of Patient-Derived Organoid. Cancers. 2021; 13(5):1179. https://doi.org/10.3390/cancers13051179
Chicago/Turabian StyleWang, Zhiwei, Yun Jin, Yinghao Guo, Zhenhua Tan, Xiaoxiao Zhang, Dan Ye, Yuanquan Yu, Shuyou Peng, Lei Zheng, and Jiangtao Li. 2021. "Conversion Therapy of Intrahepatic Cholangiocarcinoma Is Associated with Improved Prognosis and Verified by a Case of Patient-Derived Organoid" Cancers 13, no. 5: 1179. https://doi.org/10.3390/cancers13051179
APA StyleWang, Z., Jin, Y., Guo, Y., Tan, Z., Zhang, X., Ye, D., Yu, Y., Peng, S., Zheng, L., & Li, J. (2021). Conversion Therapy of Intrahepatic Cholangiocarcinoma Is Associated with Improved Prognosis and Verified by a Case of Patient-Derived Organoid. Cancers, 13(5), 1179. https://doi.org/10.3390/cancers13051179