Pre-Operative Imaging and Pathological Diagnosis of Localized High-Grade Pancreatic Intra-Epithelial Neoplasia without Invasive Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Pathological and Genetic Characteristics of PanIN
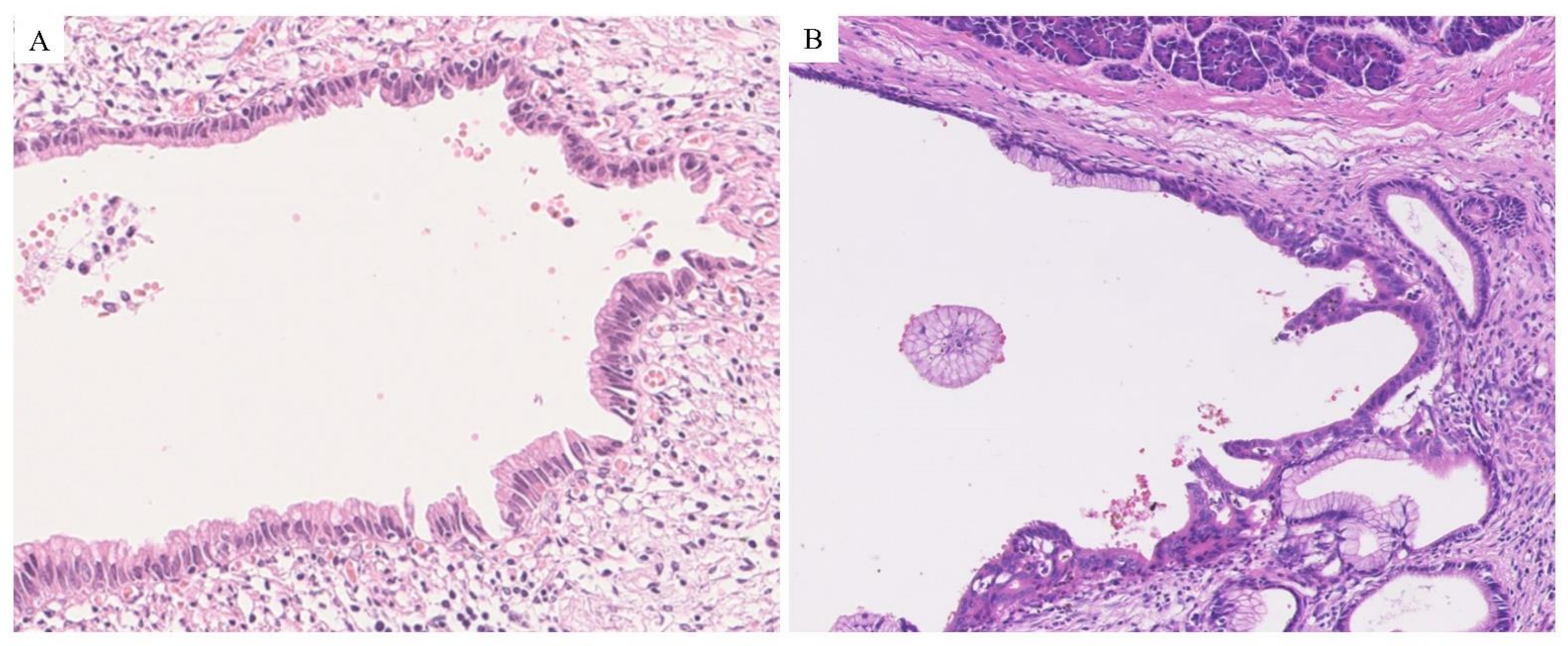
2.1. Pathological Features
2.2. Genetic Features
3. Pancreatic Diseases Associated with High-Grade PanIN
3.1. Pancreatic Ductal Adenocarcinoma
3.2. Chronic Pancreatitis
3.3. IPMN
3.3.1. IPMN with High-Grade Dysplasia
3.3.2. High-Grade PanIN Associated with IPMN
3.4. Other Pancreatic Diseases
3.5. Aging Pancreas
4. Imaging Characteristics of High-Grade PanIN
4.1. Relationship between Imaging Findings and Pathological Features Associated with of PanIN
4.2. Indirect Imaging Characteristics of High-Grade PanIN
4.2.1. Morphological Changes in the Main Pancreatic Duct (Stenosis and Dilation)
4.2.2. Retention Cysts (Dilation of BPDs)
4.2.3. Focal PPA
4.2.4. Hypoechoic Changes Around the MPD
4.3. Recommended Modalities for Detection of High-Grade PanIN
4.3.1. Recommended Modalities
4.3.2. Other Newer Imaging Modalities
4.4. Differential Diagnosis Between High-Grade PanIN and Benign Lesions (Combination of Indirect Imaging)
5. Pre-Operative Histopathological Diagnosis of High-Grade PanIN
6. Challenges to Be Solved in Diagnosis of High-Grade PanIN
6.1. Populations Requiring Imaging Analysis to Assess High-Grade PanIN
6.2. Challenges in Diagnosis and Follow-Up
6.3. Limitation of High-Grade PanIN Diagnosis
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanno, A.; Masamune, A.; Hanada, K.; Kikuyama, M.; Kitano, M. Advances in Early Detection of Pancreatic Cancer. Diagnostics 2019, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, A.Y.; Tan, M.L.; Wu, L.M.; Asrani, V.M.; Windsor, J.A.; Yadav, D.; Petrov, M.S. Global incidence and mortality of pancreatic diseases: A systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol. Hepatol. 2016, 1, 45–55. [Google Scholar] [CrossRef]
- Egawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, M. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas 2012, 41, 985–992. [Google Scholar] [CrossRef]
- Lennon, A.M.; Wolfgang, C.L.; Canto, M.I.; Klein, A.P.; Herman, J.M.; Goggins, M.; Fishman, E.K.; Kamel, I.; Weiss, M.J.; Diaz, L.A.; et al. The early detection of pancreatic cancer: What will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res. 2014, 74, 3381–3389. [Google Scholar] [CrossRef] [Green Version]
- Basturk, O.; Hong, S.M.; Wood, L.D.; Adsay, N.V.; Albores-Saavedra, J.; Biankin, A.V.; Brosens, L.A.; Fukushima, N.; Goggins, M.; Hruban, R.H.; et al. A Revised Classification System and Recommendations from the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am. J. Surg. Pathol. 2015, 39, 1730–1741. [Google Scholar] [CrossRef]
- Ren, B.; Liu, X.; Suriawinata, A.A. Pancreatic Ductal Adenocarcinoma and Its Precursor Lesions. Am. J. Pathol. 2019, 189, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Gill, A.J.; Klimstra, D.S.; Lam, A.K. Tumours of the pancreas. In WHO Classification of Tumours of the Digestive System, 5th ed.; World Health Organization: Geneva, Switzerland, 2019; pp. 295–340. [Google Scholar]
- Canto, M.I.; Almario, J.A.; Schulick, R.D.; Yeo, C.J.; Klein, A.; Blackford, A.; Shin, E.J.; Sanyal, A.; Yenokyan, G.; Lennon, A.M.; et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology 2018, 155, 740–751.e742. [Google Scholar] [CrossRef] [Green Version]
- Andea, A.; Sarkar, F.; Adsay, V.N. Clinicopathological correlates of pancreatic intraepithelial neoplasia: A comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod. Pathol. 2003, 16, 996–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konings, I.; Canto, M.I.; Almario, J.A.; Harinck, F.; Saxena, P.; Lucas, A.L.; Kastrinos, F.; Whitcomb, D.C.; Brand, R.E.; Lachter, J.; et al. Surveillance for pancreatic cancer in high-risk individuals. BJS Open 2019, 3, 656–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, R.; Kondo, F.; Yamaguchi, T.; Kato, K.; Sakai, Y.; Saisho, H.; Yamazaki, K. Pancreatic intraepithelial neoplasms in the normal appearing pancreas: On their precise relationship with age. Hepatogastroenterology 2008, 55, 1103–1106. [Google Scholar]
- Wilentz, R.E.; Geradts, J.; Maynard, R.; Offerhaus, G.J.; Kang, M.; Goggins, M.; Yeo, C.J.; Kern, S.E.; Hruban, R.H. Inactivation of the p16 (INK4A) tumor-suppressor gene in pancreatic duct lesions: Loss of intranuclear expression. Cancer Res. 1998, 58, 4740–4744. [Google Scholar]
- Wilentz, R.E.; Iacobuzio-Donahue, C.A.; Argani, P.; McCarthy, D.M.; Parsons, J.L.; Yeo, C.J.; Kern, S.E.; Hruban, R.H. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: Evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000, 60, 2002–2006. [Google Scholar] [PubMed]
- Maitra, A.; Adsay, N.V.; Argani, P.; Iacobuzio-Donahue, C.; De Marzo, A.; Cameron, J.L.; Yeo, C.J.; Hruban, R.H. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod. Pathol. 2003, 16, 902–912. [Google Scholar] [CrossRef] [Green Version]
- Löhr, M.; Klöppel, G.; Maisonneuve, P.; Lowenfels, A.B.; Lüttges, J. Frequency of K-ras mutations in pancreatic intraductal neoplasias associated with pancreatic ductal adenocarcinoma and chronic pancreatitis: A meta-analysis. Neoplasia 2005, 7, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, Y.; Furukawa, T.; Yachida, S.; Nishimura, M.; Seki, A.; Nonaka, K.; Aida, J.; Takubo, K.; Ishiwata, T.; Kimura, W.; et al. The Prevalence and Clinicopathological Characteristics of High-Grade Pancreatic Intraepithelial Neoplasia: Autopsy Study Evaluating the Entire Pancreatic Parenchyma. Pancreas 2017, 46, 658–664. [Google Scholar] [CrossRef]
- Corral, J.E.; Mareth, K.F.; Riegert-Johnson, D.L.; Das, A.; Wallace, M.B. Diagnostic Yield From Screening Asymptomatic Individuals at High Risk for Pancreatic Cancer: A Meta-analysis of Cohort Studies. Clin. Gastroenterol. Hepatol. 2019, 17, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Curry, S.J.; Doubeni, C.A.; Epling, J.W.; Kubik, M.; et al. Screening for Pancreatic Cancer. JAMA 2019, 322, 438. [Google Scholar]
- Kogekar, N.; Diaz, K.E.; Weinberg, A.D.; Lucas, A.L. Surveillance of high-risk individuals for pancreatic cancer with EUS and MRI: A meta-analysis. Pancreatology 2020, 20, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Canto, M.I.; Hruban, R.H.; Fishman, E.K.; Kamel, I.R.; Schulick, R.; Zhang, Z.; Topazian, M.; Takahashi, N.; Fletcher, J.; Petersen, G.; et al. Frequent Detection of Pancreatic Lesions in Asymptomatic High-Risk Individuals. Gastroenterology 2012, 142, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, E.; Olson, S.H.; Bayuga, S.; Simon, J.; Schattner, M.A.; Gerdes, H.; Allen, P.J.; Jarnagin, W.R.; Kurtz, R.C. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am. J. Gastroenterol. 2011, 106, 946–954. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, D.K.; Slater, E.P.; Carrato, A.; Ibrahim, I.S.; Guillen-Ponce, C.; Vasen, H.F.; Matthäi, E.; Earl, J.; Jendryschek, F.S.; Figiel, J.; et al. Refinement of screening for familial pancreatic cancer. Gut 2016, 65, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Slater, E.P.; Sina, M.; Habbe, N.; Fendrich, V.; Matthäi, E.; Langer, P.; Bartsch, D.K. German national case collection for familial pancreatic cancer (FaPaCa): Ten years experience. FAM Cancer 2011, 10, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Potjer, T.P.; Schot, I.; Langer, P.; Heverhagen, J.T.; Wasser, M.N.; Slater, E.P.; Klöppel, G.; Morreau, H.M.; Bonsing, B.A.; de Vos Tot Nederveen Cappel, W.H.; et al. Variation in precursor lesions of pancreatic cancer among high-risk groups. Clin. Cancer Res. 2013, 19, 442–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasen, H.; Ibrahim, I.; Ponce, C.G.; Slater, E.P.; Matthäi, E.; Carrato, A.; Earl, J.; Robbers, K.; van Mil, A.M.; Potjer, T.; et al. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J. Clin. Oncol. 2016, 34, 2010–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology 2018, 18, 61–67. [Google Scholar] [CrossRef]
- Izumi, Y.; Hanada, K.; Okazaki, A.; Minami, T.; Hirano, N.; Ikemoto, J.; Kanemitsu, K.; Nakadoi, K.; Shishido, T.; Katamura, Y.; et al. Endoscopic ultrasound findings and pathological features of pancreatic carcinoma in situ. Endosc. Int. Open. 2019, 7, e585–e593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terada, S.; Kikuyama, M.; Kawaguchi, S.; Kanemoto, H.; Yokoi, Y.; Kamisawa, T.; Kuruma, S.; Chiba, K.; Honda, G.; Horiguchi, S.; et al. Proposal for Endoscopic Ultrasonography Classification for Small Pancreatic Cancer. Diagnostics 2019, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Yamao, K.; Takenaka, M.; Ishikawa, R.; Okamoto, A.; Yamazaki, T.; Nakai, A.; Omoto, S.; Kamata, K.; Minaga, K.; Matsumoto, I.; et al. Partial Pancreatic Parenchymal Atrophy Is a New Specific Finding to Diagnose Small Pancreatic Cancer (≤10 mm) Including Carcinoma in Situ: Comparison with Localized Benign Main Pancreatic Duct Stenosis Patients. Diagnostics 2020, 10, 445. [Google Scholar] [CrossRef] [PubMed]
- Nakahodo, J.; Kikuyama, M.; Nojiri, S.; Chiba, K.; Yoshimoto, K.; Kamisawa, T.; Horiguchi, S.I.; Honda, G. Focal parenchymal atrophy of pancreas: An important sign of underlying high-grade pancreatic intraepithelial neoplasia without invasive carcinoma, i.e., carcinoma in situ. Pancreatology 2020, 20, 1689–1697. [Google Scholar] [CrossRef]
- Hulst, S.P.L. Zur kenntnis der Genese des Adenokarzinoms und Karzinoms des Pankreas. Virchows Arch. 1905, 180, 288–316. [Google Scholar] [CrossRef]
- Klimstra, D.S.; Longnecker, D.S. K-ras mutations in pancreatic ductal proliferative lesions. Am. J. Pathol. 1994, 145, 1547–1550. [Google Scholar] [PubMed]
- Hruban, R.H.; Takaori, K.; Klimstra, D.S.; Adsay, N.V.; Albores-Saavedra, J.; Biankin, A.V.; Biankin, S.A.; Compton, C.; Fukushima, N.; Furukawa, T.; et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am. J. Surg. Pathol. 2004, 28, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Matthaei, H.; Wu, J.; Hong, S.M.; Yu, J.; Borges, M.; Hruban, R.H.; Maitra, A.; Kinzler, K.; Vogelstein, B.; et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012, 142, 730–733.e739. [Google Scholar] [CrossRef] [Green Version]
- Hingorani, S.R.; Petricoin, E.F.; Maitra, A.; Rajapakse, V.; King, C.; Jacobetz, M.A.; Ross, S.; Conrads, T.P.; Veenstra, T.D.; Hitt, B.A.; et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003, 4, 437–450. [Google Scholar] [CrossRef] [Green Version]
- Hruban, R.H.; Adsay, N.V.; Albores-Saavedra, J.; Anver, M.R.; Biankin, A.V.; Boivin, G.P.; Furth, E.E.; Furukawa, T.; Klein, A.; Klimstra, D.S.; et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: Consensus report and recommendations. Cancer Res. 2006, 66, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, C.G.; Wood, L.D. From somatic mutation to early detection: Insights from molecular characterization of pancreatic cancer precursor lesions. J. Pathol. 2018, 246, 395–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosty, C.; Geradts, J.; Sato, N.; Wilentz, R.E.; Roberts, H.; Sohn, T.; Cameron, J.L.; Yeo, C.J.; Hruban, R.H.; Goggins, M. p16 Inactivation in pancreatic intraepithelial neoplasias (PanINs) arising in patients with chronic pancreatitis. Am. J. Surg. Pathol. 2003, 27, 1495–1501. [Google Scholar] [CrossRef]
- Furukawa, T.; Fujisaki, R.; Yoshida, Y.; Kanai, N.; Sunamura, M.; Abe, T.; Takeda, K.; Matsuno, S.; Horii, A. Distinct progression pathways involving the dysfunction of DUSP6/MKP-3 in pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms of the pancreas. Mod. Pathol. 2005, 18, 1034–1042. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.J.; Hart, S.N.; Lima, J.F.; Kipp, B.R.; Klebig, M.; Winters, J.L.; Szabo, C.; Zhang, L.; Eckloff, B.W.; Petersen, G.M.; et al. Genetic alterations associated with progression from pancreatic intraepithelial neoplasia to invasive pancreatic tumor. Gastroenterology 2013, 145, 1098–1109.e1091. [Google Scholar] [CrossRef] [Green Version]
- Yokode, M.; Akita, M.; Fujikura, K.; Kim, M.J.; Morinaga, Y.; Yoshikawa, S.; Terada, T.; Matsukiyo, H.; Tajiri, T.; Abe-Suzuki, S.; et al. High-grade PanIN presenting with localised stricture of the main pancreatic duct: A clinicopathological and molecular study of 10 cases suggests a clue for the early detection of pancreatic cancer. Histopathology 2018, 73, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, W.; Chianchiano, P.; Griffin, J.F.; Pittman, M.E.; Brosens, L.A.; Noë, M.; Yu, J.; Shindo, K.; Suenaga, M.; Rezaee, N.; et al. Genetic analyses of isolated high-grade pancreatic intraepithelial neoplasia (HG-PanIN) reveal paucity of alterations in TP53 and SMAD4. J. Pathol. 2017, 242, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawa, Z.; Haque, I.; Ghosh, A.; Banerjee, S.; Harris, L.; Banerjee, S.K. The miRacle in Pancreatic Cancer by miRNAs: Tiny Angels or Devils in Disease Progression. Int. J. Mol. Sci. 2016, 17, 809. [Google Scholar] [CrossRef] [Green Version]
- Abreu, F.B.; Liu, X.; Tsongalis, G.J. miRNA analysis in pancreatic cancer: The Dartmouth experience. Clin. Chem. Lab. Med. 2017, 55, 755–762. [Google Scholar] [CrossRef]
- Yu, J.; Li, A.; Hong, S.M.; Hruban, R.H.; Goggins, M. MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin. Cancer Res. 2012, 18, 981–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, X.; Zhang, J.; Wu, Q.; Wang, W.; Ye, A.Y.; Song, W.; Dai, H.; Wang, X.; Wu, F.; You, L.; et al. Challenges in detecting pre-malignant pancreatic lesions during acute pancreatitis using a serum microRNA assay: A study based on KrasG12D transgenic mice. Oncotarget 2016, 7, 22700–22710. [Google Scholar] [CrossRef] [Green Version]
- Slater, E.P.; Strauch, K.; Rospleszcz, S.; Ramaswamy, A.; Esposito, I.; Klöppel, G.; Matthäi, E.; Heeger, K.; Fendrich, V.; Langer, P.; et al. MicroRNA-196a and -196b as Potential Biomarkers for the Early Detection of Familial Pancreatic Cancer. Transl. Oncol. 2014, 7, 464–471. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Ohtsuka, T.; Kono, H.; Nagayoshi, Y.; Ideno, N.; Aso, T.; Kozono, S.; Ohuchida, K.; Takahata, S.; Nakamura, M.; et al. A minimally invasive and simple screening test for detection of pancreatic ductal adenocarcinoma using biomarkers in duodenal juice. Pancreas 2013, 42, 187–192. [Google Scholar] [CrossRef]
- Kozuka, S.; Sassa, R.; Taki, T.; Masamoto, K.; Nagasawa, S.; Saga, S.; Hasegawa, K.; Takeuchi, M. Relation of pancreatic duct hyperplasia to carcinoma. Cancer 1979, 43, 1418–1428. [Google Scholar] [CrossRef]
- Cubilla, A.L.; Fitzgerald, P.J. Morphological lesions associated with human primary invasive nonendocrine pancreas cancer. Cancer Res. 1976, 36, 2690–2698. [Google Scholar] [PubMed]
- Yu, D.-Y.; Yu, Y.-D.; Kim, W.-B.; Han, H.-J.; Choi, S.-B.; Kim, D.-S.; Choi, S.-Y.; Kim, J.-Y.; Chang, H.; Kim, B.-H. Clinical significance of pancreatic intraepithelial neoplasia in resectable pancreatic cancer on survivals. Ann. Surg. Treat. Res. 2018, 94, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recavarren, C.; Labow, D.M.; Liang, J.; Zhang, L.; Wong, M.; Zhu, H.; Wang, J.; Francis, F.; Xu, R. Histologic characteristics of pancreatic intraepithelial neoplasia associated with different pancreatic lesions. Hum. Pathol. 2011, 42, 18–24. [Google Scholar] [CrossRef]
- Oda, Y.; Aishima, S.; Morimatsu, K.; Shindo, K.; Fujino, M.; Mizuuchi, Y.; Hattori, M.; Miyazaki, T.; Tanaka, M.; Oda, Y. Pancreatic intraepithelial neoplasia in the background of invasive ductal carcinoma of the pancreas as a prognostic factor. Histopathology 2014, 65, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Hassid, B.G.; Lucas, A.L.; Salomao, M.; Weng, C.; Liu, F.; Khanna, L.G.; Kumar, S.; Hwang, C.; Chabot, J.A.; Frucht, H. Absence of pancreatic intraepithelial neoplasia predicts poor survival after resection of pancreatic cancer. Pancreas 2014, 43, 1073–1077. [Google Scholar] [CrossRef] [Green Version]
- Brune, K.; Abe, T.; Canto, M.; O’Malley, L.; Klein, A.P.; Maitra, A.; Volkan Adsay, N.; Fishman, E.K.; Cameron, J.L.; Yeo, C.J.; et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am. J. Surg. Pathol. 2006, 30, 1067–1076. [Google Scholar]
- Shi, C.; Klein, A.P.; Goggins, M.; Maitra, A.; Canto, M.; Ali, S.; Schulick, R.; Palmisano, E.; Hruban, R.H. Increased Prevalence of Precursor Lesions in Familial Pancreatic Cancer Patients. Clin. Cancer Res. 2009, 15, 7737–7743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthaei, H.; Hong, S.M.; Mayo, S.C.; dal Molin, M.; Olino, K.; Venkat, R.; Goggins, M.; Herman, J.M.; Edil, B.H.; Wolfgang, C.L.; et al. Presence of pancreatic intraepithelial neoplasia in the pancreatic transection margin does not influence outcome in patients with R0 resected pancreatic cancer. Ann. Surg. Oncol. 2011, 18, 3493–3499. [Google Scholar] [CrossRef]
- Duell, E.J.; Lucenteforte, E.; Olson, S.H.; Bracci, P.M.; Li, D.; Risch, H.A.; Silverman, D.T.; Ji, B.T.; Gallinger, S.; Holly, E.A.; et al. Pancreatitis and pancreatic cancer risk: A pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann. Oncol. 2012, 23, 2964–2970. [Google Scholar] [CrossRef]
- Raimondi, S.; Lowenfels, A.B.; Morselli-Labate, A.M.; Maisonneuve, P.; Pezzilli, R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 349–358. [Google Scholar] [CrossRef]
- Kirkegård, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef] [Green Version]
- Yadav, D.; Lowenfels, A.B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013, 144, 1252–1261. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, J.K.; Chen, J.H.; Al-Haddad, M.; Luz, L.; McHenry, L.; Sherman, S.; Juan, M.; Dewitt, J. Can endoscopic ultrasound predict pancreatic intraepithelial neoplasia lesions in chronic pancreatitis?: A retrospective study of pathologic correlation. Pancreas 2014, 43, 849–854. [Google Scholar] [CrossRef]
- Gupta, R.; Khosroshahi, A.; Shinagare, S.; Fernandez, C.; Ferrone, C.; Lauwers, G.Y.; Stone, J.H.; Deshpande, V. Does autoimmune pancreatitis increase the risk of pancreatic carcinoma?: A retrospective analysis of pancreatic resections. Pancreas 2013, 42, 506–510. [Google Scholar] [CrossRef]
- Hwang, I.K.; Kim, H.; Lee, Y.S.; Kim, J.; Cho, J.Y.; Yoon, Y.S.; Han, H.S.; Hwang, J.H. Presence of pancreatic intraepithelial neoplasia-3 in a background of chronic pancreatitis in pancreatic cancer patients. Cancer Sci. 2015, 106, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.; Saunders, B.; Wilkinson, K.; Rumbles, S.; Schofield, G.; Kamm, M.; Williams, C.; Price, A.; Talbot, I.; Forbes, A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 2004, 126, 451–459. [Google Scholar] [CrossRef]
- Tada, M.; Kawabe, T.; Arizumi, M.; Togawa, O.; Matsubara, S.; Yamamoto, N.; Nakai, Y.; Sasahira, N.; Hirano, K.; Tsujino, T.; et al. Pancreatic cancer in patients with pancreatic cystic lesions: A prospective study in 197 patients. Clin. Gastroenterol. Hepatol. 2006, 4, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, K.; Tada, M.; Isayama, H.; Sasahira, N.; Nakai, Y.; Yamamoto, K.; Kogure, H.; Sasaki, T.; Hirano, K.; Ijichi, H.; et al. Incidence of extrapancreatic malignancies in patients with intraductal papillary mucinous neoplasms of the pancreas. Gut 2011, 60, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef]
- Vege, S.S.; Ziring, B.; Jain, R.; Moayyedi, P. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148, 819–822. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Fernández-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.; Donahue, T.R.; Reber, H.A.; Hines, O.J. Pancreatic Cyst Disease: A Review. JAMA 2016, 315, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Kawazoe, A.; Mishima, S.; Wada, T.; Shimbo, T.; Sekine, K.; Watanabe, K.; Imbe, K.; Kojima, Y.; Kumazawa, K.; et al. Development of Pancreatic Cancer, Disease-specific Mortality, and All-Cause Mortality in Patients with Nonresected IPMNs: A Long-term Cohort Study. Radiology 2016, 278, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Pergolini, I.; Sahora, K.; Ferrone, C.R.; Morales-Oyarvide, V.; Wolpin, B.M.; Mucci, L.A.; Brugge, W.R.; Mino-Kenudson, M.; Patino, M.; Sahani, D.V.; et al. Long-term Risk of Pancreatic Malignancy in Patients With Branch Duct Intraductal Papillary Mucinous Neoplasm in a Referral Center. Gastroenterology 2017, 153, 1284–1294.e1281. [Google Scholar] [CrossRef] [PubMed]
- Petrone, M.C.; Magnoni, P.; Pergolini, I.; Capurso, G.; Traini, M.; Doglioni, C.; Mariani, A.; Crippa, S.; Arcidiacono, P.G. Long-term follow-up of low-risk branch-duct IPMNs of the pancreas: Is main pancreatic duct dilatation the most worrisome feature? Clin. Transl. Gastroenterol. 2018, 9, 158. [Google Scholar] [CrossRef]
- Kamata, K.; Kitano, M.; Kudo, M.; Sakamoto, H.; Kadosaka, K.; Miyata, T.; Imai, H.; Maekawa, K.; Chikugo, T.; Kumano, M.; et al. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy 2014, 46, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Nakaizumi, A.; Ishikawa, O.; Iishi, H.; Tatsumi, K.; Takakura, R.; Ishida, T.; Takano, Y.; Tanaka, S.; Takenaka, A. Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut 2008, 57, 1561–1565. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Kanemitsu, S.; Hatori, T.; Maguchi, H.; Shimizu, Y.; Tada, M.; Nakagohri, T.; Hanada, K.; Osanai, M.; Noda, Y.; et al. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas 2011, 40, 571–580. [Google Scholar] [CrossRef]
- Patra, K.C.; Bardeesy, N.; Mizukami, Y. Diversity of Precursor Lesions for Pancreatic Cancer: The Genetics and Biology of Intraductal Papillary Mucinous Neoplasm. Clin. Transl. Gastroenterol. 2017, 8, e86. [Google Scholar] [CrossRef]
- Oyama, H.; Tada, M.; Takagi, K.; Tateishi, K.; Hamada, T.; Nakai, Y.; Hakuta, R.; Ijichi, H.; Ishigaki, K.; Kanai, S.; et al. Long-term Risk of Malignancy in Branch-Duct Intraductal Papillary Mucinous Neoplasms. Gastroenterology 2020, 158, 226–237.e225. [Google Scholar] [CrossRef] [Green Version]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchegiani, G.; Andrianello, S.; Borin, A.; Dal Borgo, C.; Perri, G.; Pollini, T.; Romanò, G.; D’Onofrio, M.; Gabbrielli, A.; Scarpa, A.; et al. Systematic review, meta-analysis, and a high-volume center experience supporting the new role of mural nodules proposed by the updated 2017 international guidelines on IPMN of the pancreas. Surgery 2018, 163, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Tanno, S.; Nakano, Y.; Nishikawa, T.; Nakamura, K.; Sasajima, J.; Minoguchi, M.; Mizukami, Y.; Yanagawa, N.; Fujii, T.; Obara, T.; et al. Natural history of branch duct intraductal papillary-mucinous neoplasms of the pancreas without mural nodules: Long-term follow-up results. Gut 2008, 57, 339–343. [Google Scholar] [CrossRef]
- Kawamoto, S.; Horton, K.M.; Lawler, L.P.; Hruban, R.H.; Fishman, E.K. Intraductal papillary mucinous neoplasm of the pancreas: Can benign lesions be differentiated from malignant lesions with multidetector CT? Radiographics 2005, 25, 1451–1468; discussion 1468–1470. [Google Scholar] [CrossRef] [PubMed]
- Kulzer, M.; Singhi, A.D.; Furlan, A.; Heller, M.T.; Katabathina, V.S.; McGrath, K.M.; Zeh, H.J.; Zureikat, A.; Dasyam, A.K. Current concepts in molecular genetics and management guidelines for pancreatic cystic neoplasms: An essential update for radiologists. Abdom. Radiol. (N. Y.) 2018, 43, 2351–2368. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.L.; Lee, L.S. Clinical approach to incidental pancreatic cysts. World J. Gastroenterol. 2016, 22, 1236–1245. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, M.J.; Lee, J.Y.; Lim, J.S.; Chung, J.J.; Kim, K.W.; Yoo, H.S. Typical and atypical manifestations of serous cystadenoma of the pancreas: Imaging findings with pathologic correlation. AJR Am. J. Roentgenol. 2009, 193, 136–142. [Google Scholar] [CrossRef]
- Ono, J.; Yaeger, K.A.; Genevay, M.; Mino-Kenudson, M.; Brugge, W.R.; Pitman, M.B. Cytological analysis of small branch-duct intraductal papillary mucinous neoplasms provides a more accurate risk assessment of malignancy than symptoms. Cytojournal 2011, 8, 21. [Google Scholar] [CrossRef]
- Kobayashi, N.; Sugimori, K.; Shimamura, T.; Hosono, K.; Watanabe, S.; Kato, S.; Ueda, M.; Endo, I.; Inayama, Y.; Maeda, S.; et al. Endoscopic ultrasonographic findings predict the risk of carcinoma in branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatology 2012, 12, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Yamaue, H.; Maguchi, H.; Yamao, K.; Hirono, S.; Osanai, M.; Hijioka, S.; Hosoda, W.; Nakamura, Y.; Shinohara, T.; et al. Predictors of malignancy in intraductal papillary mucinous neoplasm of the pancreas: Analysis of 310 pancreatic resection patients at multiple high-volume centers. Pancreas 2013, 42, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, G.; Fujita, N.; Maguchi, H.; Tanno, S.; Mizuno, N.; Hanada, K.; Hatori, T.; Sadakari, Y.; Yamaguchi, T.; Tobita, K.; et al. Natural history of branch duct intraductal papillary mucinous neoplasm with mural nodules: A Japan Pancreas Society multicenter study. Pancreas 2014, 43, 532–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, Y.; Nakazato, T.; Yokoyama, M.; Kogure, M.; Matsuki, R.; Abe, N.; Mori, T.; Ohkura, Y.; Sugiyama, M. Development and Potential Utility of a New Scoring Formula for Prediction of Malignant Intraductal Papillary Mucinous Neoplasm of the Pancreas. Pancreas 2016, 45, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Tawada, K.; Ishihara, T.; Yamaguchi, T.; Tsuyuguchi, T.; Hara, T.; Tada, M.; Mikata, R.; Sakai, Y.; Sugiyama, H.; Saito, M.; et al. Comparison of branch duct and main pancreatic duct mural nodules in intraductal papillary mucinous neoplasm. Pancreas 2013, 42, 1193–1195. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Song, T.J.; Hwang, J.H.; Yoo, K.S.; Lee, W.J.; Lee, K.H.; Dong, S.H.; Park, C.H.; Park, E.T.; Moon, J.H.; et al. Predictors of malignancy in pure branch duct type intraductal papillary mucinous neoplasm of the pancreas: A nationwide multicenter study. Pancreatology 2015, 15, 405–410. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Repici, A.; Koçollari, A.; Auriemma, F.; Bianchetti, M.; Mangiavillano, B. Pancreatoscopy: An update. World J. Gastrointest Endosc. 2019, 11, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Yamaguchi, T.; Ishihara, T.; Tsuyuguchi, T.; Kondo, F.; Kato, K.; Asano, T.; Saisho, H. Diagnosis and patient management of intraductal papillary-mucinous tumor of the pancreas by using peroral pancreatoscopy and intraductal ultrasonography. Gastroenterology 2002, 122, 34–43. [Google Scholar] [CrossRef]
- Maker, A.V.; Lee, L.S.; Raut, C.P.; Clancy, T.E.; Swanson, R.S. Cytology from pancreatic cysts has marginal utility in surgical decision-making. Ann. Surg. Oncol. 2008, 15, 3187–3192. [Google Scholar] [CrossRef]
- Majumder, S.; Raimondo, M.; Taylor, W.R.; Yab, T.C.; Berger, C.K.; Dukek, B.A.; Cao, X.; Foote, P.H.; Wu, C.W.; Devens, M.E.; et al. Methylated DNA in Pancreatic Juice Distinguishes Patients With Pancreatic Cancer From Controls. Clin. Gastroenterol. Hepatol. 2020, 18, 676–683.e673. [Google Scholar] [CrossRef]
- Singhi, A.D.; McGrath, K.; Brand, R.E.; Khalid, A.; Zeh, H.J.; Chennat, J.S.; Fasanella, K.E.; Papachristou, G.I.; Slivka, A.; Bartlett, D.L.; et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 2018, 67, 2131–2141. [Google Scholar] [CrossRef] [Green Version]
- Krishna, S.G.; Hart, P.A.; DeWitt, J.M.; DiMaio, C.J.; Kongkam, P.; Napoleon, B.; Othman, M.O.; Yew Tan, D.M.; Strobel, S.G.; Stanich, P.P.; et al. EUS-guided confocal laser endomicroscopy: Prediction of dysplasia in intraductal papillary mucinous neoplasms (with video). Gastrointest. Endosc. 2020, 91, 551–563.e555. [Google Scholar] [CrossRef]
- Machicado, J.D.; Chao, W.L.; Carlyn, D.E.; Pan, T.Y.; Poland, S.; Alexander, V.L.; Maloof, T.G.; Dubay, K.; Ueltschi, O.; Middendorf, D.M.; et al. High performance in risk stratification of intraductal papillary mucinous neoplasms by confocal laser endomicroscopy image analysis with convolutional neural networks (with video). Gastrointest. Endosc. 2021. [Google Scholar] [CrossRef]
- Biankin, A.V.; Kench, J.G.; Biankin, S.A.; Lee, C.S.; Morey, A.L.; Dijkman, F.P.; Coleman, M.J.; Sutherland, R.L.; Henshall, S.M. Pancreatic intraepithelial neoplasia in association with intraductal papillary mucinous neoplasms of the pancreas: Implications for disease progression and recurrence. Am. J. Surg. Pathol. 2004, 28, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Nehra, D.; Oyarvide, V.M.; Mino-Kenudson, M.; Thayer, S.P.; Ferrone, C.R.; Wargo, J.A.; Muzikansky, A.; Finkelstein, D.; Warshaw, A.L.; Castillo, C.F. Intraductal papillary mucinous neoplasms: Does a family history of pancreatic cancer matter? Pancreatology 2012, 12, 358–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maire, F.; Couvelard, A.; Palazzo, L.; Aubert, A.; Vullierme, M.P.; Rebours, V.; Hammel, P.; Sauvanet, A.; Levy, P.; Ruszniewski, P. Pancreatic intraepithelial neoplasia in patients with intraductal papillary mucinous neoplasms: The interest of endoscopic ultrasonography. Pancreas 2013, 42, 1262–1266. [Google Scholar] [CrossRef]
- Bartsch, D.K.; Dietzel, K.; Bargello, M.; Matthaei, E.; Kloeppel, G.; Esposito, I.; Heverhagen, J.T.; Gress, T.M.; Slater, E.P.; Langer, P. Multiple small “imaging” branch-duct type intraductal papillary mucinous neoplasms (IPMNs) in familial pancreatic cancer: Indicator for concomitant high grade pancreatic intraepithelial neoplasia? FAM Cancer 2013, 12, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, I.T.; Vinuela, E.F.; Tang, L.H.; Klimstra, D.S.; D’Angelica, M.I.; Dematteo, R.P.; Kingham, T.P.; Fong, Y.; Jarnagin, W.R.; Allen, P.J. Incidentally discovered pancreatic intraepithelial neoplasia: What is its clinical significance? Ann. Surg. Oncol. 2013, 20, 3643–3647. [Google Scholar] [CrossRef]
- Matsuda, Y. Age-related morphological changes in the pancreas and their association with pancreatic carcinogenesis. Pathol. Int. 2019, 69, 450–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meckler, K.A.; Brentnall, T.A.; Haggitt, R.C.; Crispin, D.; Byrd, D.R.; Kimmey, M.B.; Bronner, M.P. Familial fibrocystic pancreatic atrophy with endocrine cell hyperplasia and pancreatic carcinoma. Am. J. Surg. Pathol. 2001, 25, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Azuma, K.; Nonaka, Y.; Kamada, Y.; Tomoeda, M.; Kishida, M.; Tanemura, M.; Miyoshi, E. Pancreatic fatty degeneration and fibrosis as predisposing factors for the development of pancreatic ductal adenocarcinoma. Pancreas 2014, 43, 1032–1041. [Google Scholar] [CrossRef]
- Detlefsen, S.; Sipos, B.; Feyerabend, B.; Klöppel, G. Pancreatic fibrosis associated with age and ductal papillary hyperplasia. Virchows. Arch. 2005, 447, 800–805. [Google Scholar] [CrossRef]
- Detlefsen, S.; Sipos, B.; Feyerabend, B.; Klöppel, G. Fibrogenesis in alcoholic chronic pancreatitis: The role of tissue necrosis, macrophages, myofibroblasts and cytokines. Mod. Pathol. 2006, 19, 1019–1026. [Google Scholar] [CrossRef]
- Petrone, M.C.; Arcidiacono, P.G. New strategies for the early detection of pancreatic cancer. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 157–159. [Google Scholar] [CrossRef] [Green Version]
- Ito, H.; Kawaguchi, Y.; Kawashima, Y.; Maruno, A.; Ogawa, M.; Hirabayashi, K.; Mine, T. A case of pancreatic intraepithelial neoplasia that was difficult to diagnose preoperatively. Case Rep. Oncol. 2015, 8, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Nakaizumi, A.; Ioka, T.; Oshikawa, O.; Uehara, H.; Nakao, M.; Yamamoto, K.; Ishikawa, O.; Ohigashi, H.; Kitamra, T. Main pancreatic duct dilatation: A sign of high risk for pancreatic cancer. Jpn. J. Clin. Oncol. 2002, 32, 407–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, S.; Nakao, M.; Ioka, T.; Takakura, R.; Takano, Y.; Tsukuma, H.; Uehara, H.; Suzuki, R.; Fukuda, J. Slight dilatation of the main pancreatic duct and presence of pancreatic cysts as predictive signs of pancreatic cancer: A prospective study. Radiology 2010, 254, 965–972. [Google Scholar] [CrossRef]
- Hanada, K.; Okazaki, A.; Hirano, N.; Izumi, Y.; Teraoka, Y.; Ikemoto, J.; Kanemitsu, K.; Hino, F.; Fukuda, T.; Yonehara, S. Diagnostic strategies for early pancreatic cancer. J. Gastroenterol. 2015, 50, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Gangi, S.; Fletcher, J.G.; Nathan, M.A.; Christensen, J.A.; Harmsen, W.S.; Crownhart, B.S.; Chari, S.T. Time interval between abnormalities seen on CT and the clinical diagnosis of pancreatic cancer: Retrospective review of CT scans obtained before diagnosis. AJR Am. J. Roentgenol. 2004, 182, 897–903. [Google Scholar] [CrossRef]
- Tamada, T.; Ito, K.; Kanomata, N.; Sone, T.; Kanki, A.; Higaki, A.; Hayashida, M.; Yamamoto, A. Pancreatic adenocarcinomas without secondary signs on multiphasic multidetector CT: Association with clinical and histopathologic features. Eur. Radiol. 2016, 26, 646–655. [Google Scholar] [CrossRef]
- Gonoi, W.; Hayashi, T.Y.; Okuma, H.; Akahane, M.; Nakai, Y.; Mizuno, S.; Tateishi, R.; Isayama, H.; Koike, K.; Ohtomo, K. Development of pancreatic cancer is predictable well in advance using contrast-enhanced CT: A case-cohort study. Eur. Radiol. 2017, 27, 4941–4950. [Google Scholar] [CrossRef] [PubMed]
- Higashi, M.; Tanabe, M.; Onoda, H.; Nakao, S.; Miyoshi, K.; Iida, E.; Okada, M.; Furukawa, M.; Ito, K. Incidentally detected pancreatic adenocarcinomas on computed tomography obtained during the follow-up for other diseases. Abdom. Radiol. (N. Y.) 2020, 45, 774–781. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, S.H.; Lee, D.H.; Lee, S.M.; Kim, Y.S.; Jang, J.Y.; Han, J.K. Isolated Main Pancreatic Duct Dilatation: CT Differentiation Between Benign and Malignant Causes. AJR Am. J. Roentgenol. 2017, 209, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Okusaka, T.; Shimizu, K.; Furuse, J.; Ito, Y.; Hanada, K.; Shimosegawa, T.; Okazaki, K. Clinical Practice Guidelines for Pancreatic Cancer 2016 From the Japan Pancreas Society. Pancreas 2017, 46, 595–604. [Google Scholar] [CrossRef]
- Zhang, X.M.; Mitchell, D.G.; Dohke, M.; Holland, G.A.; Parker, L. Pancreatic cysts: Depiction on single-shot fast spin-echo MR images. Radiology 2002, 223, 547–553. [Google Scholar] [CrossRef]
- Kimura, W.; Nagai, H.; Kuroda, A.; Muto, T.; Esaki, Y. Analysis of small cystic lesions of the pancreas. Int. J. Pancreatol. 1995, 18, 197–206. [Google Scholar] [PubMed]
- Kim, J.H.; Park, S.H.; Yu, E.S.; Kim, M.H.; Kim, J.; Byun, J.H.; Lee, S.S.; Hwang, H.J.; Hwang, J.Y.; Lee, S.S.; et al. Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: Frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology 2010, 257, 87–96. [Google Scholar] [CrossRef]
- Yoon, S.H.; Lee, J.M.; Cho, J.Y.; Lee, K.B.; Kim, J.E.; Moon, S.K.; Kim, S.J.; Baek, J.H.; Kim, S.H.; Kim, S.H.; et al. Small (≤20 mm) pancreatic adenocarcinomas: Analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology 2011, 259, 442–452. [Google Scholar] [CrossRef] [Green Version]
- Zaheer, A.; Singh, V.K.; Akshintala, V.S.; Kawamoto, S.; Tsai, S.D.; Gage, K.L.; Fishman, E.K. Differentiating autoimmune pancreatitis from pancreatic adenocarcinoma using dual-phase computed tomography. J. Comput. Assist. Tomogr. 2014, 38, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Klöppel, G.; Detlefsen, S.; Feyerabend, B. Fibrosis of the pancreas: The initial tissue damage and the resulting pattern. Virchows Arch. 2004, 445, 1–8. [Google Scholar]
- Kato, S.; Zakimi, M.; Yamada, K.; Chinen, K.; Kubota, T.; Arashiro, M.; Kikuchi, K.; Murakami, T.; Kunishima, F. Efficacy of repeated cytology of pancreatic juice obtained by endoscopic nasopancreatic drainage tube for early diagnosis of pancreatic cancer: A case series including a case of carcinoma in situ. Clin. J. Gastroenterol. 2015, 8, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Yoshida, T.; Itonaga, M.; Tamura, T.; Hatamaru, K.; Yamashita, Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J. Gastroenterol. 2019, 54, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, H.; Kitano, M.; Suetomi, Y.; Maekawa, K.; Takeyama, Y.; Kudo, M. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med. Biol. 2008, 34, 525–532. [Google Scholar] [CrossRef]
- Krishna, S.G.; Rao, B.B.; Ugbarugba, E.; Shah, Z.K.; Blaszczak, A.; Hinton, A.; Conwell, D.L.; Hart, P.A. Diagnostic performance of endoscopic ultrasound for detection of pancreatic malignancy following an indeterminate multidetector CT scan: A systemic review and meta-analysis. Surg. Endosc. 2017, 31, 4558–4567. [Google Scholar] [CrossRef]
- Harinck, F.; Konings, I.C.; Kluijt, I.; Poley, J.W.; van Hooft, J.E.; van Dullemen, H.M.; Nio, C.Y.; Krak, N.C.; Hermans, J.J.; Aalfs, C.M.; et al. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut 2016, 65, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.T.; Hu, D.M.; Zhu, Q. Contrast-enhanced EUS for differential diagnosis of pancreatic mass lesions: A meta-analysis. Gastrointest. Endosc. 2012, 76, 301–309. [Google Scholar] [CrossRef]
- He, X.K.; Ding, Y.; Sun, L.M. Contrast-enhanced endoscopic ultrasound for differential diagnosis of pancreatic cancer: An updated meta-analysis. Oncotarget 2017, 8, 66392–66401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand, B.; Pfaff, T.; Binmoeller, K.F.; Sriram, P.V.; Fritscher-Ravens, A.; Knöfel, W.T.; Jäckle, S.; Soehendra, N. Endoscopic ultrasound for differential diagnosis of focal pancreatic lesions, confirmed by surgery. Scand. J. Gastroenterol. 2000, 35, 1221–1228. [Google Scholar] [PubMed]
- Pei, Q.; Zou, X.; Zhang, X.; Chen, M.; Guo, Y.; Luo, H. Diagnostic value of EUS elastography in differentiation of benign and malignant solid pancreatic masses: A meta-analysis. Pancreatology 2012, 12, 402–408. [Google Scholar] [CrossRef]
- Mei, M.; Ni, J.; Liu, D.; Jin, P.; Sun, L. EUS elastography for diagnosis of solid pancreatic masses: A meta-analysis. Gastrointest. Endosc. 2013, 77, 578–589. [Google Scholar] [CrossRef]
- Ying, L.; Lin, X.; Xie, Z.L.; Hu, Y.P.; Tang, K.F.; Shi, K.Q. Clinical utility of endoscopic ultrasound elastography for identification of malignant pancreatic masses: A meta-analysis. J. Gastroenterol. Hepatol. 2013, 28, 1434–1443. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Shi, J.; Lin, Y.; Zeng, X. Endoscopic ultrasound elastography for differentiating between pancreatic adenocarcinoma and inflammatory masses: A meta-analysis. World J. Gastroenterol. 2013, 19, 6284–6291. [Google Scholar] [CrossRef]
- Hu, D.M.; Gong, T.T.; Zhu, Q. Endoscopic ultrasound elastography for differential diagnosis of pancreatic masses: A meta-analysis. Dig. Dis. Sci. 2013, 58, 1125–1131. [Google Scholar] [CrossRef]
- Xu, W.; Shi, J.; Li, X.; Zeng, X.; Lin, Y. Endoscopic ultrasound elastography for differentiation of benign and malignant pancreatic masses: A systemic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2013, 25, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, L.; Li, C.; Chen, H.; Chen, J. Diagnostic utility of endoscopic ultrasonography-elastography in the evaluation of solid pancreatic masses: A meta-analysis and systematic review. Med. Ultrason. 2017, 19, 150–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, T.; Yamaguchi, A.; Kuwai, T.; Kouno, H.; Matsuura, N.; Toyota, N.; Nakahira, S.; Kuraoka, K.; Kohno, H. Carcinoma in situ of the pancreas with fibrosis area around the carcinoma: A case report. Medicine (Baltimore) 2020, 99, e22645. [Google Scholar] [CrossRef]
- Miyata, T.; Takenaka, M.; Omoto, S.; Kamata, K.; Minaga, K.; Yamao, K.; Imai, H.; Kudo, M. A Case of Pancreatic Carcinoma in situ Diagnosed by Repeated Pancreatic Juice Cytology. Oncology 2017, 93 (Suppl. 1), 98–101. [Google Scholar] [CrossRef] [PubMed]
- El, H., II; Brauer, B.C.; Wani, S.; Fukami, N.; Attwell, A.R.; Shah, R.J. Role of per-oral pancreatoscopy in the evaluation of suspected pancreatic duct neoplasia: A 13-year U.S. single-center experience. Gastrointest. Endosc. 2017, 85, 737–745. [Google Scholar]
- Chandrasekhara, V.; Chathadi, K.V.; Acosta, R.D.; Decker, G.A.; Early, D.S.; Eloubeidi, M.A.; Evans, J.A.; Faulx, A.L.; Fanelli, R.D.; Fisher, D.A.; et al. The role of endoscopy in benign pancreatic disease. Gastrointest. Endosc. 2015, 82, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Schabath, M.B. Radiomics Improves Cancer Screening and Early Detection. Cancer Epidemiol. Biomarkers. Prev. 2020, 29, 2556–2567. [Google Scholar] [CrossRef] [PubMed]
- Polk, S.L.; Choi, J.W.; McGettigan, M.J.; Rose, T.; Ahmed, A.; Kim, J.; Jiang, K.; Balagurunathan, Y.; Qi, J.; Farah, P.T.; et al. Multiphase computed tomography radiomics of pancreatic intraductal papillary mucinous neoplasms to predict malignancy. World J. Gastroenterol. 2020, 26, 3458–3471. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Koshita, S.; Ogawa, T.; Kusunose, H.; Masu, K.; Sakai, T.; Yonamine, K.; Kawakami, Y.; Fujii, Y.; Miyamoto, K.; et al. Predictive Value of Localized Stenosis of the Main Pancreatic Duct for Early Detection of Pancreatic Cancer. Clin. Endoscopy 2019, 52, 588–597. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.X.; Ben, Q.W.; Jin, Z.D.; Du, Y.Q.; Zou, D.W.; Liao, Z.; Li, Z.S. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest. Endosc. 2011, 73, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, R.; Lu, Y.; Xia, Y.; Zhou, H. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: A systematic review. J. Cancer Res. Clin. Oncol. 2012, 138, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, S.; Zhao, Y.; Dai, M.; Zhang, T. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer: A meta-analysis. Pancreatology 2013, 13, 298–304. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Brown, L.J.; Hong, S.K.; Draganova-Tacheva, R.A.; Korenblit, J.; Loren, D.E.; Kowalski, T.E.; Solomides, C. Relationship of pancreatic mass size and diagnostic yield of endoscopic ultrasound-guided fine needle aspiration. Dig. Dis. Sci. 2011, 56, 3370–3375. [Google Scholar] [CrossRef]
- Uehara, H.; Ikezawa, K.; Kawada, N.; Fukutake, N.; Katayama, K.; Takakura, R.; Takano, Y.; Ishikawa, O.; Takenaka, A. Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic malignancy in relation to the size of lesions. J. Gastroenterol. Hepatol. 2011, 26, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Nakaizumi, A.; Tatsuta, M.; Uehara, H.; Yamamoto, R.; Takenaka, A.; Kishigami, Y.; Takemura, K.; Kitamura, T.; Okuda, S. Cytologic examination of pure pancreatic juice in the diagnosis of pancreatic carcinoma. The endoscopic retrograde intraductal catheter aspiration cytologic technique. Cancer 1992, 70, 2610–2614. [Google Scholar] [CrossRef]
- Kimura, H.; Ohtsuka, T.; Matsunaga, T.; Watanabe, Y.; Tamura, K.; Ideno, N.; Aso, T.; Miyazaki, T.; Osoegawa, T.; Aishima, S.; et al. Predictors and Diagnostic Strategies for Early-Stage Pancreatic Ductal Adenocarcinoma: A Retrospective Study. Pancreas 2015, 44, 1148–1154. [Google Scholar] [CrossRef]
- Wakatsuki, T.; Irisawa, A.; Bhutani, M.S.; Hikichi, T.; Shibukawa, G.; Takagi, T.; Yamamoto, G.; Takahashi, Y.; Yamada, Y.; Watanabe, K.; et al. Comparative study of diagnostic value of cytologic sampling by endoscopic ultrasonography-guided fine-needle aspiration and that by endoscopic retrograde pancreatography for the management of pancreatic mass without biliary stricture. J. Gastroenterol. Hepatol. 2005, 20, 1707–1711. [Google Scholar] [CrossRef]
- Uchida, N.; Kamada, H.; Tsutsui, K.; Ono, M.; Aritomo, Y.; Masaki, T.; Kushida, Y.; Haba, R.; Nakatsu, T.; Kuriyama, S. Utility of pancreatic duct brushing for diagnosis of pancreatic carcinoma. J. Gastroenterol. 2007, 42, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Kikuyama, M.; Kamisawa, T.; Kuruma, S.; Chiba, K.; Kawaguchi, S.; Terada, S.; Satoh, T. Early Diagnosis to Improve the Poor Prognosis of Pancreatic Cancer. Cancers 2018, 10, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athanassiadou, P.; Grapsa, D. Value of endoscopic retrograde cholangiopancreatography-guided brushings in preoperative assessment of pancreaticobiliary strictures: What’s new? Acta Cytol. 2008, 52, 24–34. [Google Scholar] [CrossRef]
- Iiboshi, T.; Hanada, K.; Fukuda, T.; Yonehara, S.; Sasaki, T.; Chayama, K. Value of cytodiagnosis using endoscopic nasopancreatic drainage for early diagnosis of pancreatic cancer: Establishing a new method for the early detection of pancreatic carcinoma in situ. Pancreas 2012, 41, 523–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikata, R.; Ishihara, T.; Tada, M.; Tawada, K.; Saito, M.; Kurosawa, J.; Sugiyama, H.; Sakai, Y.; Tsuyuguchi, T.; Miyazaki, M.; et al. Clinical usefulness of repeated pancreatic juice cytology via endoscopic naso-pancreatic drainage tube in patients with pancreatic cancer. J. Gastroenterol. 2013, 48, 866–873. [Google Scholar] [CrossRef]
- Iwata, T.; Kitamura, K.; Yamamiya, A.; Ishii, Y.; Sato, Y.; Nomoto, T.; Ikegami, A.; Yoshida, H. Evaluation of diagnostic cytology via endoscopic naso-pancreatic drainage for pancreatic tumor. World J. Gastrointest. Endosc. 2014, 6, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Wilentz, R.E.; Chung, C.H.; Sturm, P.D.; Musler, A.; Sohn, T.A.; Offerhaus, G.J.; Yeo, C.J.; Hruban, R.H.; Slebos, R.J. K-ras mutations in the duodenal fluid of patients with pancreatic carcinoma. Cancer 1998, 82, 96–103. [Google Scholar] [CrossRef]
- Shi, C.; Fukushima, N.; Abe, T.; Bian, Y.; Hua, L.; Wendelburg, B.J.; Yeo, C.J.; Hruban, R.H.; Goggins, M.G.; Eshleman, J.R. Sensitive and quantitative detection of KRAS2 gene mutations in pancreatic duct juice differentiates patients with pancreatic cancer from chronic pancreatitis, potential for early detection. Cancer Biol. Ther. 2008, 7, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Eshleman, J.R.; Norris, A.L.; Sadakari, Y.; Debeljak, M.; Borges, M.; Harrington, C.; Lin, E.; Brant, A.; Barkley, T.; Almario, J.A.; et al. KRAS and guanine nucleotide-binding protein mutations in pancreatic juice collected from the duodenum of patients at high risk for neoplasia undergoing endoscopic ultrasound. Clin. Gastroenterol. Hepatol. 2015, 13, 963–969.e964. [Google Scholar] [CrossRef] [Green Version]
- Suenaga, M.; Yu, J.; Shindo, K.; Tamura, K.; Almario, J.A.; Zaykoski, C.; Witmer, P.D.; Fesharakizadeh, S.; Borges, M.; Lennon, A.-M.; et al. Pancreatic Juice Mutation Concentrations Can Help Predict the Grade of Dysplasia in Patients Undergoing Pancreatic Surveillance. Clin. Cancer Res. 2018, 24, 2963–2974. [Google Scholar] [CrossRef] [Green Version]
- Suenaga, M.; Dudley, B.; Karloski, E.; Borges, M.; Irene Canto, M.; Brand, R.E.; Goggins, M. The Effect of Pancreatic Juice Collection Time on the Detection of KRAS Mutations. Pancreas 2018, 47, 35–39. [Google Scholar] [CrossRef]
- Matsumoto, K.; Takeda, Y.; Onoyama, T.; Kawata, S.; Kurumi, H.; Ueki, M.; Miura, N.; Isomoto, H. Role of the preoperative usefulness of the pathological diagnosis of pancreatic diseases. World J. Gastrointest. Oncol. 2016, 8, 656–662. [Google Scholar] [CrossRef]
- Matsumoto, K.; Takeda, Y.; Harada, K.; Horie, Y.; Yashima, K.; Murawaki, Y. Effect of pancreatic juice cytology and/or endoscopic ultrasound-guided fine-needle aspiration biopsy for pancreatic tumor. J. Gastroenterol. Hepatol. 2014, 29, 223–227. [Google Scholar] [CrossRef]
- Henrikson, N.B.; Aiello Bowles, E.J.; Blasi, P.R.; Morrison, C.C.; Nguyen, M.; Pillarisetty, V.G.; Lin, J.S. Screening for Pancreatic Cancer. JAMA 2019, 322, 445. [Google Scholar] [CrossRef] [Green Version]
- Makohon-Moore, A.P.; Matsukuma, K.; Zhang, M.; Reiter, J.G.; Gerold, J.M.; Jiao, Y.; Sikkema, L.; Attiyeh, M.A.; Yachida, S.; Sandone, C.; et al. Precancerous neoplastic cells can move through the pancreatic ductal system. Nature 2018, 561, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, Y.; Ohtsuka, T.; Matsuda, R.; Mori, Y.; Nakata, K.; Ohuchida, K.; Nakamura, M. High-risk lesions in the remnant pancreas: Fate of the remnant pancreas after pancreatic resection for pancreatic cancer and intraductal papillary mucinous neoplasms. Surg. Today 2020, 50, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Suda, K.; Nobukawa, B.; Sonoue, H. Intraductal spread of pancreatic cancer. Clinicopathologic study of 54 pancreatectomized patients. Pancreatology 2002, 2, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Yanagisawa, A.; Seki, M.; Sasaki, K.; Takano, K.; Kato, Y. The early state of invasive pancreatic ductal adenocarcinomas: Characteristics of the low papillary type and flat type intraductal carcinoma. Pancreas 2006, 33, 135–141. [Google Scholar] [CrossRef]
- Yachida, S.; Jones, S.; Bozic, I.; Antal, T.; Leary, R.; Fu, B.; Kamiyama, M.; Hruban, R.H.; Eshleman, J.R.; Nowak, M.A.; et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010, 467, 1114–1117. [Google Scholar] [CrossRef] [Green Version]
- Yachida, S.; Iacobuzio-Donahue, C.A. Evolution and dynamics of pancreatic cancer progression. Oncogene 2013, 32, 5253–5260. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Study | Total Cases | Imaging Modality | Location Head/Body-tail, n (%) | MPD Dilation, n (%) | MPD Stenosis, n (%) | Retention Cyst, n (%) | Focal PPA, n (%) | Hypoechoic Changes around MPD, n (%) |
|---|---|---|---|---|---|---|---|---|
| Yokode [44] | 10 | CT/MRI/EUS/ERCP | 4 (40)/6 (60) | CT 7/10 (70) MRI 7/10 (70) ERCP 4/7 (57) | CT 7/10 (70) MRI 9/10 (90) ERCP 7/7 (100) | NA | NA | 3/8 (38) |
| Kanno [29] | 51 | US/CT/MRI/ EUS/ERCP | 17 (33)/34 (67) | US 26/34 (77) CT 36/50 (72) MRI 34/46 (74) EUS 35/41 (85) ERCP 39/47 (83) | US 2/34 (6) EUS 28/41 (68) ERCP 39/47 (83) | NA | CT 21/50 (42) | NA |
| Izumi [30] | 16 | EUS | 4 (25)/12 (75) | 15/16 (94) | 15/16 (94) | 5/16 (31) | NA | 9/16 (56) |
| Terada [31] | 6 | EUS | NA | 6/6 (100) | 3/6 (50) | NA | NA | 2/6 (33) |
| Yamao [32] | 11 | CT | NA | NA | NA | NA | 7/11 (64) | NA |
| Nakahodo [33] | 27 | CT/MRI/EUS/ERCP | 5 (19)/22 (82) | CT/MRI 14/27 (52) ERCP 12/27 (44) | EUS 20/27 (74) ERCP 12/27 (44) | CT/MRI 20/27 (74) | CT/MRI 15/27 (56) | 20/27 (74) |
| Study | Imaging Finding | Accuracy (%) | Sensitivity (%) | Specifisity (%) | False Positive (%) | High-Grade PanIN vs. Non-Malignant Lesion p Value |
|---|---|---|---|---|---|---|
| Yamao [32] | Focal PPA (CT) | 71 | 46 | 93 | 7 | 0.004 |
| Nakahodo [33] | MPD dilation (CT, MRI) | 57 | 52 | 63 | 37 | 0.314 |
| Focal PPA (CT, MRI) | 67 | 56 | 84 | 16 | 0.013 | |
| Retention cyst (CT, MRI) | 52 | 74 | 21 | 79 | 1.000 | |
| Stenosis and hypoecho (EUS) | 76 | 74 | 79 | 21 | 0.001 |
| Characteristic of Modalities | MPD Dilation | MPD Stricture | Retention Cyst | Focal PPA | Hypoechoic Changes around MPD |
|---|---|---|---|---|---|
| Most sensitive imaging modality | EUS > ERCP > US > MRI > CT | ERCP > EUS / MRI > CT | EUS > MRI > CT > ERCP | CT > MRI | EUS |
| Invasion of modality | ERCP > EUS > CT > MRI > US | ERCP > EUS > CT > MRI | ERCP > EUS > CT > MRI | CT > MRI | EUS |
| Appropriate imaging modality | MRI/EUS/US | EUS/MRI | EUS/MRI | CT | EUS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagami, R.; Yamao, K.; Nakahodo, J.; Minami, R.; Tsurusaki, M.; Murakami, K.; Amano, Y. Pre-Operative Imaging and Pathological Diagnosis of Localized High-Grade Pancreatic Intra-Epithelial Neoplasia without Invasive Carcinoma. Cancers 2021, 13, 945. https://doi.org/10.3390/cancers13050945
Sagami R, Yamao K, Nakahodo J, Minami R, Tsurusaki M, Murakami K, Amano Y. Pre-Operative Imaging and Pathological Diagnosis of Localized High-Grade Pancreatic Intra-Epithelial Neoplasia without Invasive Carcinoma. Cancers. 2021; 13(5):945. https://doi.org/10.3390/cancers13050945
Chicago/Turabian StyleSagami, Ryota, Kentaro Yamao, Jun Nakahodo, Ryuki Minami, Masakatsu Tsurusaki, Kazunari Murakami, and Yuji Amano. 2021. "Pre-Operative Imaging and Pathological Diagnosis of Localized High-Grade Pancreatic Intra-Epithelial Neoplasia without Invasive Carcinoma" Cancers 13, no. 5: 945. https://doi.org/10.3390/cancers13050945
APA StyleSagami, R., Yamao, K., Nakahodo, J., Minami, R., Tsurusaki, M., Murakami, K., & Amano, Y. (2021). Pre-Operative Imaging and Pathological Diagnosis of Localized High-Grade Pancreatic Intra-Epithelial Neoplasia without Invasive Carcinoma. Cancers, 13(5), 945. https://doi.org/10.3390/cancers13050945








