Mouse Models of Peritoneal Carcinomatosis to Develop Clinical Applications
Abstract
Simple Summary
Abstract
1. Introduction
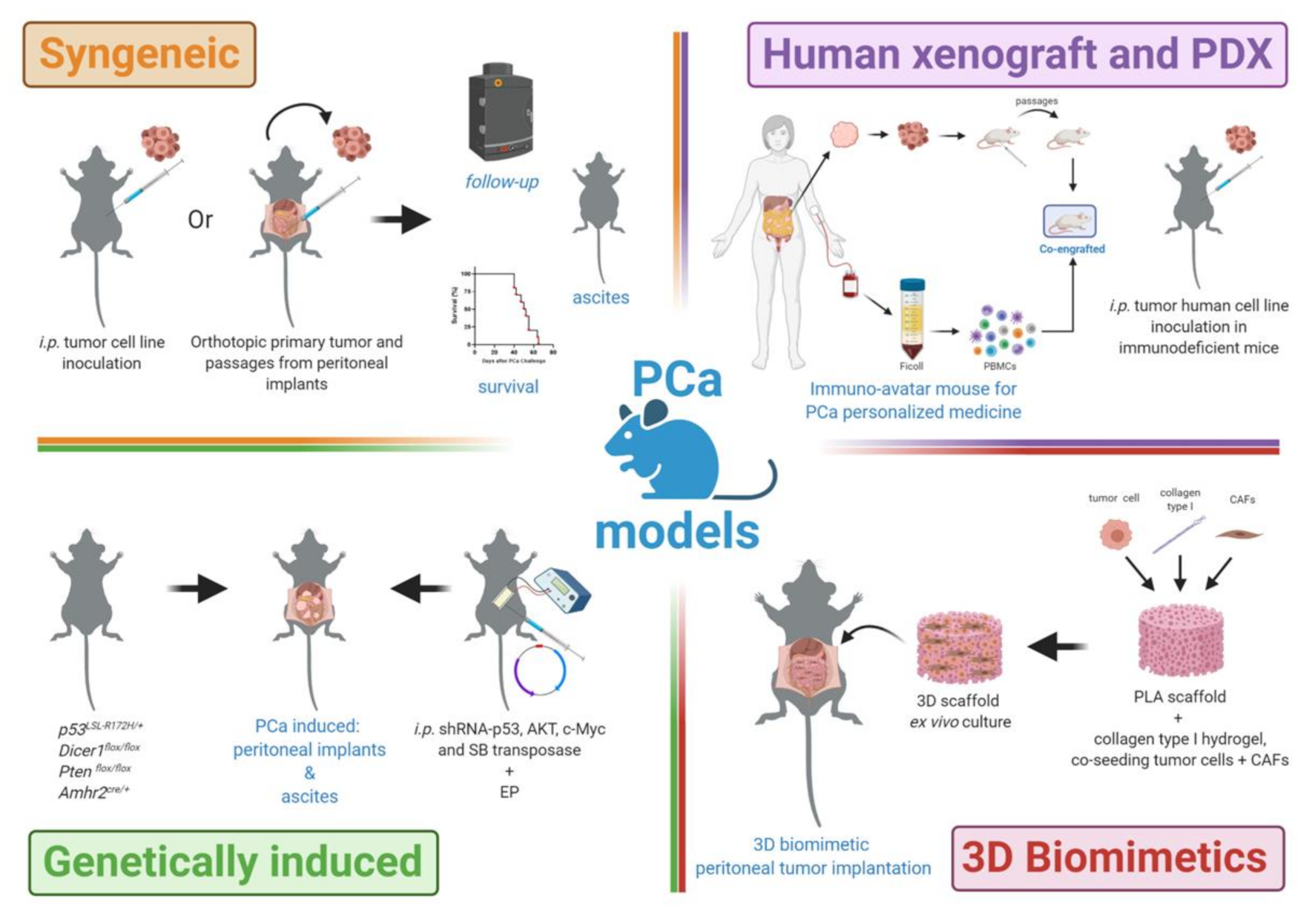
2. PCa Mouse Models
2.1. PCa Syngeneic Models
2.2. PCa Human Xenograft and PDX Models
2.3. PCa Genetically Induced Models
2.4. 3D-Biomimetics Peritoneal Implants
3. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coccolini, F.; Gheza, F.; Lotti, M.; Virzi, S.; Iusco, D.; Ghermandi, C.; Melotti, R.; Baiocchi, G.; Giulini, S.M.; Ansaloni, L.; et al. Peritoneal carcinomatosis. World J. Gastroenterol. 2013, 19, 6979–6994. [Google Scholar] [CrossRef] [PubMed]
- McMullen, J.R.W.; Selleck, M.; Wall, N.R.; Senthil, M. Peritoneal carcinomatosis: Limits of diagnosis and the case for liquid biopsy. Oncotarget 2017, 8, 43481–43490. [Google Scholar] [CrossRef]
- Gamboa, A.C.; Winer, J.H. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for gastric cancer. Cancers 2019, 11, 1662. [Google Scholar] [CrossRef]
- Chia, C.S.; You, B.; Decullier, E.; Vaudoyer, D.; Lorimier, G.; Abboud, K.; Bereder, J.M.; Arvieux, C.; Boschetti, G.; Glehen, O.; et al. Patients with peritoneal carcinomatosis from gastric cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: Is cure a possibility? Ann. Surg. Oncol. 2016, 23, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Teixeira Farinha, H.; Grass, F.; Labgaa, I.; Pache, B.; Demartines, N.; Hubner, M. Inflammatory response and toxicity after pressurized intraperitoneal aerosol chemotherapy. J. Cancer 2018, 9, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Malfroy, S.; Wallet, F.; Maucort-Boulch, D.; Chardonnal, L.; Sens, N.; Friggeri, A.; Passot, G.; Glehen, O.; Piriou, V. Complications after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis: Risk factors for ICU admission and morbidity prognostic score. Surg. Oncol. 2016, 25, 6–15. [Google Scholar] [CrossRef]
- Canda, A.E.; Sokmen, S.; Terzi, C.; Arslan, C.; Oztop, I.; Karabulut, B.; Ozzeybek, D.; Sarioglu, S.; Fuzun, M. Complications and toxicities after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2013, 20, 1082–1087. [Google Scholar] [CrossRef]
- Ireson, C.R.; Alavijeh, M.S.; Palmer, A.M.; Fowler, E.R.; Jones, H.J. The role of mouse tumour models in the discovery and development of anticancer drugs. Br. J. Cancer 2019, 121, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Buque, A.; Galluzzi, L. Modeling tumor immunology and immunotherapy in mice. Trends Cancer 2018, 4, 599–601. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, N.; Garcia, J.R.; Mohamed, A.; Benencia, F.; Rubin, S.C.; Allman, D.; Coukos, G. Generation of a syngeneic mouse model to study the effects of vascular endothelial growth factor in ovarian carcinoma. Am. J. Pathol. 2002, 161, 2295–2309. [Google Scholar] [CrossRef]
- Taylor, M.A.; Hughes, A.M.; Walton, J.; Coenen-Stass, A.M.L.; Magiera, L.; Mooney, L.; Bell, S.; Staniszewska, A.D.; Sandin, L.C.; Barry, S.T.; et al. Longitudinal immune characterization of syngeneic tumor models to enable model selection for immune oncology drug discovery. J. Immunother. Cancer 2019, 7, 328. [Google Scholar] [CrossRef] [PubMed]
- Gebreyohannes, Y.K.; Burton, E.A.; Wozniak, A.; Matusow, B.; Habets, G.; Wellens, J.; Cornillie, J.; Lin, J.; Nespi, M.; Wu, G.; et al. PLX9486 shows anti-tumor efficacy in patient-derived, tyrosine kinase inhibitor-resistant KIT-mutant xenograft models of gastrointestinal stromal tumors. Clin. Exp. Med. 2019, 19, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Chester, C.; Melero, I.; Kohrt, H. Defining the optimal murine models to investigate immune checkpoint blockers and their combination with other immunotherapies. Ann. Oncol. 2016, 27, 1190–1198. [Google Scholar] [CrossRef]
- Xu, C.; Li, X.; Liu, P.; Li, M.; Luo, F. Patient-derived xenograft mouse models: A high fidelity tool for individualized medicine. Oncol. Lett. 2019, 17, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Walrath, J.C.; Hawes, J.J.; Van Dyke, T.; Reilly, K.M. Genetically engineered mouse models in cancer research. Adv. Cancer Res. 2010, 106, 113–164. [Google Scholar] [PubMed]
- DuPage, M.; Jacks, T. Genetically engineered mouse models of cancer reveal new insights about the antitumor immune response. Curr. Opin. Immunol. 2013, 25, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.W.; Muller, W.J. Transgenic mouse models-a seminal breakthrough in oncogene research. Cold Spring Harb. Protoc. 2013, 2013, 1099–1108. [Google Scholar] [CrossRef]
- Hanahan, D.; Wagner, E.F.; Palmiter, R.D. The origins of oncomice: A history of the first transgenic mice genetically engineered to develop cancer. Genes Dev. 2007, 21, 2258–2270. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Im, S.K.; Fang, S. Mouse Cre-LoxP system: General principles to determine tissue-specific roles of target genes. Lab. Anim. Res. 2018, 34, 147–159. [Google Scholar] [CrossRef]
- Ng, S.R.; Rideout, W.M., 3rd; Akama-Garren, E.H.; Bhutkar, A.; Mercer, K.L.; Schenkel, J.M.; Bronson, R.T.; Jacks, T. CRISPR-mediated modeling and functional validation of candidate tumor suppressor genes in small cell lung cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Kemp, C.J. Animal models of chemical carcinogenesis: Driving breakthroughs in cancer research for 100 Years. Cold Spring Harb. Protoc. 2015, 2015, 865–874. [Google Scholar] [CrossRef]
- Kishimoto, H.; Zhao, M.; Hayashi, K.; Urata, Y.; Tanaka, N.; Fujiwara, T.; Penman, S.; Hoffman, R.M. In vivo internal tumor illumination by telomerase-dependent adenoviral GFP for precise surgical navigation. Proc. Natl. Acad. Sci. USA 2009, 106, 14514–14517. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.M.; Esakov, E.; Braley, C.; AlHilli, M.; Michener, C.; Reizes, O. Use of transabdominal ultrasound for the detection of intra-peritoneal tumor engraftment and growth in mouse xenografts of epithelial ovarian cancer. PLoS ONE 2020, 15, e0228511. [Google Scholar] [CrossRef] [PubMed]
- Chiriva-Internati, M.; Yu, Y.; Mirandola, L.; Jenkins, M.R.; Chapman, C.; Cannon, M.; Cobos, E.; Kast, W.M. Cancer testis antigen vaccination affords long-term protection in a murine model of ovarian cancer. PLoS ONE 2010, 5, e10471. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Lemke-Miltner, C.D.; Blackwell, S.; Tomanek-Chalkley, A.; Gibson-Corely, K.N.; Coleman, K.L.; Weiner, G.J.; Chan, C.H.F. Intraperitoneal CMP-001: A novel immunotherapy for treating peritoneal carcinomatosis of gastrointestinal and pancreaticobiliary cancer. Ann. Surg. Oncol. 2021, 28, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Saga, Y.; Koyanagi, T.; Takei, Y.; Machida, S.; Taneichi, A.; Mizukami, H.; Sato, Y.; Matsubara, S.; Fujiwara, H. The angiogenesis regulator vasohibin-1 inhibits ovarian cancer growth and peritoneal dissemination and prolongs host survival. Int. J. Oncol. 2015, 47, 2057–2063. [Google Scholar] [CrossRef]
- Abou-Elkacem, L.; Arns, S.; Brix, G.; Gremse, F.; Zopf, D.; Kiessling, F.; Lederle, W. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol. Cancer Ther. 2013, 12, 1322–1331. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, W.S.; Kim, C.W.; Lee, S.J.; Yang, H.; Kong, S.J.; Ning, J.; Yang, K.M.; Kang, B.; Kim, W.R.; et al. Oncolytic vaccinia virus reinvigorates peritoneal immunity and cooperates with immune checkpoint inhibitor to suppress peritoneal carcinomatosis in colon cancer. J. Immunother. Cancer 2020, 8, e000857. [Google Scholar] [CrossRef] [PubMed]
- Sedlacek, A.L.; Gerber, S.A.; Randall, T.D.; Van Rooijen, N.; Frelinger, J.G.; Lord, E.M. Generation of a dual-functioning antitumor immune response in the peritoneal cavity. Am. J. Pathol. 2013, 183, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Taibi, A.; Albouys, J.; Jacques, J.; Perrin, M.L.; Yardin, C.; Durand Fontanier, S.; Bardet, S.M. Comparison of implantation sites for the development of peritoneal metastasis in a colorectal cancer mouse model using non-invasive bioluminescence imaging. PLoS ONE 2019, 14, e0220360. [Google Scholar] [CrossRef]
- Chen, C.H.; Kuo, C.Y.; Chen, S.H.; Mao, S.H.; Chang, C.Y.; Shalumon, K.T.; Chen, J.P. Thermosensitive injectable hydrogel for simultaneous intraperitoneal delivery of doxorubicin and prevention of peritoneal adhesion. Int. J. Mol. Sci. 2018, 19, 1373. [Google Scholar] [CrossRef]
- Yoshimura, M.; Fujiwara, H.; Kubota, T.; Amaike, H.; Takashima, K.; Inada, S.; Atsuji, K.; Araki, Y.; Matsumoto, K.; Nakamura, T.; et al. Possible inhibition of cancer cell adhesion to the extracellular matrix in NK4-induced suppression of peritoneal implantation. Anticancer Res. 2005, 25, 3847–3854. [Google Scholar] [PubMed]
- Cai, Q.; Yan, L.; Xu, Y. Anoikis resistance is a critical feature of highly aggressive ovarian cancer cells. Oncogene 2015, 34, 3315–3324. [Google Scholar] [CrossRef]
- Flies, D.B.; Higuchi, T.; Harris, J.C.; Jha, V.; Gimotty, P.A.; Adams, S.F. Immune checkpoint blockade reveals the stimulatory capacity of tumor-associated CD103(+) dendritic cells in late-stage ovarian cancer. Oncoimmunology 2016, 5, e1185583. [Google Scholar] [CrossRef] [PubMed]
- Janat-Amsbury, M.M.; Yockman, J.W.; Anderson, M.L.; Kieback, D.G.; Kim, S.W. Comparison of ID8 MOSE and VEGF-modified ID8 cell lines in an immunocompetent animal model for human ovarian cancer. Anticancer Res. 2006, 26, 2785–2789. [Google Scholar]
- Wilkinson-Ryan, I.; Pham, M.M.; Sergent, P.; Tafe, L.J.; Berwin, B.L. A syngeneic mouse model of epithelial ovarian cancer Port site metastases. Transl. Oncol. 2019, 12, 62–68. [Google Scholar] [CrossRef]
- Fujimori, D.; Kinoshita, J.; Yamaguchi, T.; Nakamura, Y.; Gunjigake, K.; Ohama, T.; Sato, K.; Yamamoto, M.; Tsukamoto, T.; Nomura, S.; et al. Established fibrous peritoneal metastasis in an immunocompetent mouse model similar to clinical immune microenvironment of gastric cancer. BMC Cancer 2020, 20, 1014. [Google Scholar] [CrossRef] [PubMed]
- Banan, B.; Beckstead, J.A.; Dunavant, L.E.; Sohn, Y.; Adcock, J.M.; Nomura, S.; Abumrad, N.; Goldenring, J.R.; Fingleton, B. Development of a novel murine model of lymphatic metastasis. Clin. Exp. Metastasis. 2020, 37, 247–255. [Google Scholar] [CrossRef]
- Greco, S.H.; Tomkotter, L.; Vahle, A.K.; Rokosh, R.; Avanzi, A.; Mahmood, S.K.; Deutsch, M.; Alothman, S.; Alqunaibit, D.; Ochi, A.; et al. TGF-beta blockade reduces mortality and metabolic changes in a validated murine model of pancreatic cancer cachexia. PLoS ONE 2015, 10, e0132786. [Google Scholar] [CrossRef]
- Akimoto, M.; Iizuka, M.; Kanematsu, R.; Yoshida, M.; Takenaga, K. Anticancer effect of ginger extract against pancreatic cancer cells mainly through reactive oxygen species-mediated autotic cell death. PLoS ONE 2015, 10, e0126605. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, S.; Ren, L.; Wang, L.; Chen, Z.; Hoffman, R.M.; Zhou, J. Pancreatic cancer-derived exosomes promote tumor metastasis and liver pre-metastatic niche formation. Oncotarget 2017, 8, 63461–63483. [Google Scholar] [CrossRef] [PubMed]
- Nowacki, M.; Wisniewski, M.; Werengowska-Ciecwierz, K.; Roszek, K.; Czarnecka, J.; Lakomska, I.; Kloskowski, T.; Tyloch, D.; Debski, R.; Pietkun, K.; et al. Nanovehicles as a novel target strategy for hyperthermic intraperitoneal chemotherapy: A multidisciplinary study of peritoneal carcinomatosis. Oncotarget 2015, 6, 22776–22798. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Takeoka, M.; Ehara, T.; Hashimoto, S.; Shibuki, H.; Yoshimura, N.; Shigematsu, H.; Takahashi, K.; Katsuki, M. Structural fragility of blood vessels and peritoneum in calponin h1-deficient mice, resulting in an increase in hematogenous metastasis and peritoneal dissemination of malignant tumor cells. Cancer Res. 2001, 61, 7627–7634. [Google Scholar] [PubMed]
- Weiss, J.M.; Davies, L.C.; Karwan, M.; Ileva, L.; Ozaki, M.K.; Cheng, R.Y.; Ridnour, L.A.; Annunziata, C.M.; Wink, D.A.; McVicar, D.W. Itaconic acid mediates crosstalk between macrophage metabolism and peritoneal tumors. J. Clin. Investig. 2018, 128, 3794–3805. [Google Scholar] [CrossRef] [PubMed]
- Conejo-Garcia, J.R.; Benencia, F.; Courreges, M.C.; Kang, E.; Mohamed-Hadley, A.; Buckanovich, R.J.; Holtz, D.O.; Jenkins, A.; Na, H.; Zhang, L.; et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004, 10, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Wang, C.; Beiss, V.; Steinmetz, N.F. Antibody response against cowpea mosaic viral nanoparticles improves in situ vaccine efficacy in ovarian cancer. ACS Nano 2020, 14, 2994–3003. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Ruiz, J.R.; Engle, X.; Scarlett, U.K.; Martinez, D.; Barber, A.; Elgueta, R.; Wang, L.; Nesbeth, Y.; Durant, Y.; Gewirtz, A.T.; et al. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J. Clin. Investig. 2009, 119, 2231–2244. [Google Scholar] [CrossRef] [PubMed]
- Abiko, K.; Mandai, M.; Hamanishi, J.; Yoshioka, Y.; Matsumura, N.; Baba, T.; Yamaguchi, K.; Murakami, R.; Yamamoto, A.; Kharma, B.; et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin. Cancer Res. 2013, 19, 1363–1374. [Google Scholar] [CrossRef]
- Ward, K.K.; Tancioni, I.; Lawson, C.; Miller, N.L.; Jean, C.; Chen, X.L.; Uryu, S.; Kim, J.; Tarin, D.; Stupack, D.G.; et al. Inhibition of focal adhesion kinase (FAK) activity prevents anchorage-independent ovarian carcinoma cell growth and tumor progression. Clin. Exp. Metastasis. 2013, 30, 579–594. [Google Scholar] [CrossRef]
- Yang, T.; Wall, E.M.; Milne, K.; Theiss, P.; Watson, P.; Nelson, B.H. CD8+ T cells induce complete regression of advanced ovarian cancers by an interleukin (IL)-2/IL-15 dependent mechanism. Clin. Cancer Res. 2007, 13, 7172–7180. [Google Scholar] [CrossRef]
- Martin, S.D.; Brown, S.D.; Wick, D.A.; Nielsen, J.S.; Kroeger, D.R.; Twumasi-Boateng, K.; Holt, R.A.; Nelson, B.H. Low mutation burden in ovarian cancer may limit the utility of neoantigen-targeted vaccines. PLoS ONE 2016, 11, e0155189. [Google Scholar] [CrossRef]
- Sekiya, A.; Suzuki, S.; Tanaka, A.; Hattori, S.; Shimizu, Y.; Yoshikawa, N.; Koya, Y.; Kajiyama, H.; Kikkawa, F. Interleukin33 expression in ovarian cancer and its possible suppression of peritoneal carcinomatosis. Int. J. Oncol. 2019, 55, 755–765. [Google Scholar]
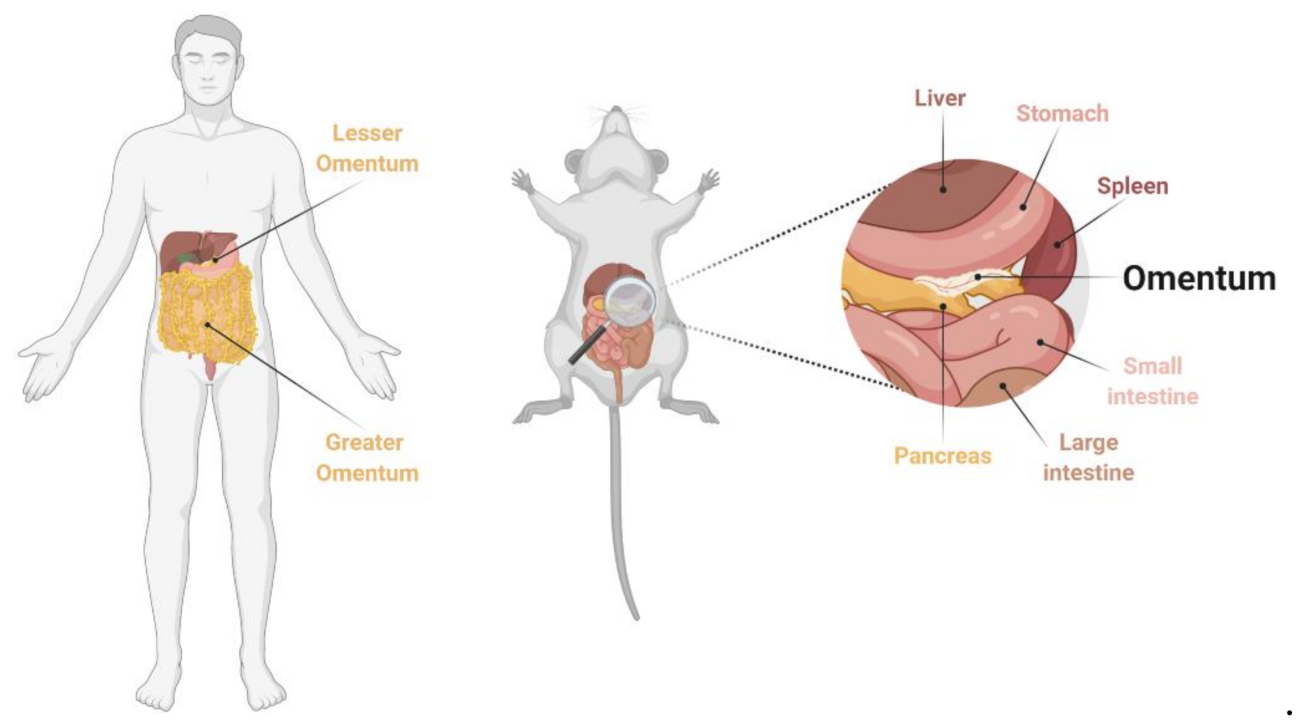
- Wilkosz, S.; Ireland, G.; Khwaja, N.; Walker, M.; Butt, R.; De Giorgio-Miller, A.; Herrick, S.E. A comparative study of the structure of human and murine greater omentum. Anat. Embryol. (Berl.) 2005, 209, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Etzerodt, A.; Moulin, M.; Doktor, T.K.; Delfini, M.; Mossadegh-Keller, N.; Bajenoff, M.; Sieweke, M.H.; Moestrup, S.K.; Auphan-Anezin, N.; Lawrence, T. Tissue-resident macrophages in omentum promote metastatic spread of ovarian cancer. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Krishnan, V.; Tallapragada, S.; Schaar, B.; Kamat, K.; Chanana, A.M.; Zhang, Y.; Patel, S.; Parkash, V.; Rinker-Schaeffer, C.; Folkins, A.K.; et al. Omental macrophages secrete chemokine ligands that promote ovarian cancer colonization of the omentum via CCR1. Commun. Biol. 2020, 3, 524. [Google Scholar] [CrossRef] [PubMed]
- Van der Bij, G.J.; Bogels, M.; Oosterling, S.J.; Kroon, J.; Schuckmann, D.T.; De Vries, H.E.; Meijer, S.; Beelen, R.H.; Van Egmond, M. Tumor infiltrating macrophages reduce development of peritoneal colorectal carcinoma metastases. Cancer Lett. 2008, 262, 77–86. [Google Scholar] [CrossRef]
- Miyoshi, J.; Toden, S.; Yoshida, K.; Toiyama, Y.; Alberts, S.R.; Kusunoki, M.; Sinicrope, F.A.; Goel, A. MiR-139-5p as a novel serum biomarker for recurrence and metastasis in colorectal cancer. Sci. Rep. 2017, 7, 43393. [Google Scholar] [CrossRef]
- Bastiaenen, V.P.; Klaver, C.E.L.; Van Der Heijden, M.C.S.; Nijman, L.E.; Lecca, M.C.; Tanis, P.J.; Lenos, P.J.; Vermeulen, L. A mouse model for peritoneal metastases of colorectal origin recapitulates patient heterogeneity. Lab. Investig. 2020, 100, 1465–1474. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Su, X.; Dai, L.; Yu, L.; Deng, H.; Gou, L.; Yang, J. Establishment and characterization of intraperitoneal xenograft models by co-injection of human tumor cells and extracellular matrix gel. Oncol. Lett. 2015, 10, 3450–3456. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Li, Y.; Liang, X.; Qi, Y.; Chen, Y.; Meng, X.; Zheng, H.; Xu, Y.; Cai, S.; Cai, G.; et al. A study of peritoneal metastatic xenograft model of colorectal cancer in the treatment of hyperthermic intraperitoneal chemotherapy with Raltitrexed. Biomed. Pharm. 2017, 92, 149–156. [Google Scholar] [CrossRef]
- Mikula-Pietrasik, J.; Sosinska, P.; Maksin, K.; Kucinska, M.G.; Piotrowska, H.; Murias, M.; Wozniak, A.; Szpurek, D.; Ksiazek, K. Colorectal cancer-promoting activity of the senescent peritoneal mesothelium. Oncotarget 2015, 6, 29178–29195. [Google Scholar] [CrossRef] [PubMed]
- Menen, R.S.; Hassanein, M.K.; Momiyama, M.; Suetsugu, A.; Moossa, A.R.; Hoffman, R.M.; Bouvet, M. Tumor-educated macrophages promote tumor growth and peritoneal metastasis in an orthotopic nude mouse model of human pancreatic cancer. Vivo 2012, 26, 565–569. [Google Scholar]
- Saimura, M.; Nagai, E.; Mizumoto, K.; Maehara, N.; Okino, H.; Katano, M.; Matsumoto, K.; Nakamura, T.; Narumi, K.; Nukiwa, T.; et al. Intraperitoneal injection of adenovirus-mediated NK4 gene suppresses peritoneal dissemination of pancreatic cancer cell line AsPC-1 in nude mice. Cancer Gene Ther. 2002, 9, 799–806. [Google Scholar] [CrossRef]
- Yanagihara, K.; Kubo, T.; Mihara, K.; Kuwata, T.; Ochiai, A.; Seyama, T.; Yokozaki, H. Development and biological analysis of a novel orthotopic peritoneal dissemination mouse model generated using a pancreatic ductal adenocarcinoma cell line. Pancreas 2019, 48, 315–322. [Google Scholar] [CrossRef]
- Okazaki, M.; Fushida, S.; Harada, S.; Tsukada, T.; Kinoshita, J.; Oyama, K.; Miyashita, T.; Ninomiya, I.; Ohta, T. Establishing a xenograft mouse model of peritoneal dissemination of gastric cancer with organ invasion and fibrosis. BMC Cancer 2017, 17, 23. [Google Scholar] [CrossRef]
- Yanagihara, K.; Takigahira, M.; Tanaka, H.; Komatsu, T.; Fukumoto, H.; Koizumi, F.; Nishio, K.; Ochiya, T.; Ino, Y.; Hirohashi, S. Development and biological analysis of peritoneal metastasis mouse models for human scirrhous stomach cancer. Cancer Sci. 2005, 96, 323–332. [Google Scholar] [CrossRef]
- Fujita, T.; Yanagihara, K.; Takeshita, F.; Aoyagi, K.; Nishimura, T.; Takigahira, M.; Chiwaki, F.; Fukagawa, T.; Katai, H.; Ochiya, T.; et al. Intraperitoneal delivery of a small interfering RNA targeting NEDD1 prolongs the survival of scirrhous gastric cancer model mice. Cancer Sci. 2013, 104, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, K.; Koizumi, K.; Kawashima, A.; Saitoh, Y.; Arita, Y.; Shinohara, K.; Minami, T.; Nakayama, T.; Sakurai, H.; Takahashi, Y.; et al. Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of gastric cancer. Cancer Res. 2006, 66, 2181–2187. [Google Scholar] [CrossRef]
- Mori, T.; Fujiwara, Y.; Yano, M.; Yasuda, T.; Takiguchi, S.; Miyata, H.; Viliotou, V.; Monden, M. A mouse model of early-stage peritoneal metastasis: Optimal RT-PCR-based method for detection of peritoneal micrometastases. Oncol. Rep. 2005, 13, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Rezniczek, G.A.; Buggisch, J.; Sobilo, J.; Launay, A.; Lerondel, S.; Le Pape, A.; Ouaissi, M.; Gohler, D.; Senkal, M.; Giger-Pabst, U.; et al. Establishment of a mouse ovarian cancer and peritoneal metastasis model to study intraperitoneal chemotherapy. Cancers 2020, 12, 3818. [Google Scholar] [CrossRef]
- Mitra, A.K.; Davis, D.A.; Tomar, S.; Roy, L.; Gurler, H.; Xie, J.; Lantvit, D.D.; Cardenas, H.; Fang, F.; Liu, Y.; et al. In vivo tumor growth of high-grade serous ovarian cancer cell lines. Gynecol. Oncol. 2015, 138, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Tanizaki, Y.; Kobayashi, A.; Toujima, S.; Shiro, M.; Mizoguchi, M.; Mabuchi, Y.; Yagi, S.; Minami, S.; Takikawa, O.; Ino, K. Indoleamine 2,3-dioxygenase promotes peritoneal metastasis of ovarian cancer by inducing an immunosuppressive environment. Cancer Sci. 2014, 105, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Bhimani, J.; Ball, K.; Stebbing, J. Patient-derived xenograft models-the future of personalised cancer treatment. Br. J. Cancer 2020, 122, 601–602. [Google Scholar] [CrossRef] [PubMed]
- De Thaye, E.; Van De Vijver, K.; Van Der Meulen, J.; Taminau, J.; Wagemans, G.; Denys, H.; Van Dorpe, J.; Berx, G.; Ceelen, W.; Van Bocxlaer, J.; et al. Establishment and characterization of a cell line and patient-derived xenograft (PDX) from peritoneal metastasis of low-grade serous ovarian carcinoma. Sci. Rep. 2020, 10, 6688. [Google Scholar] [CrossRef]
- Shultz, L.D.; Brehm, M.A.; Garcia-Martinez, J.V.; Greiner, D.L. Humanized mice for immune system investigation: Progress, promise and challenges. Nat. Rev. Immunol. 2012, 12, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Huang, G.; Cheng, L.; Li, Z.; Xiao, Y.; Deng, Q.; Jiang, Y.; Li, B.; Lin, S.; Wang, S.; et al. Establishment of peripheral blood mononuclear cell-derived humanized lung cancer mouse models for studying efficacy of PD-L1/PD-1 targeted immunotherapy. MAbs 2018, 10, 1301–1311. [Google Scholar] [CrossRef]
- Gopinathan, A.; Tuveson, D.A. The use of GEM models for experimental cancer therapeutics. Dis Model Mech. 2008, 1, 83–86. [Google Scholar] [CrossRef]
- Kersten, K.; De Visser, K.E.; Van Miltenburg, M.H.; Jonkers, J. Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol. Med. 2017, 9, 137–153. [Google Scholar] [CrossRef]
- Abdul-Wahid, A.; Huang, E.H.; Lu, H.; Flanagan, J.; Mallick, A.I.; Gariepy, J. A focused immune response targeting the homotypic binding domain of the carcinoembryonic antigen blocks the establishment of tumor foci in vivo. Int. J. Cancer 2012, 131, 2839–2851. [Google Scholar] [CrossRef]
- Kim, J.; Coffey, D.M.; Creighton, C.J.; Yu, Z.; Hawkins, S.M.; Matzuk, M.M. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc. Natl. Acad. Sci. USA 2012, 109, 3921–3926. [Google Scholar] [CrossRef]
- Kim, O.; Park, E.Y.; Klinkebiel, D.L.; Pack, S.D.; Shin, Y.H.; Abdullaev, Z.; Emerson, R.E.; Coffey, D.M.; Kwon, S.Y.; Creighton, C.J.; et al. In vivo modeling of metastatic human high-grade serous ovarian cancer in mice. PLoS Genet. 2020, 16, e1008808. [Google Scholar] [CrossRef] [PubMed]
- Brachova, P.; Thiel, K.W.; Leslie, K.K. The consequence of oncomorphic TP53 mutations in ovarian cancer. Int. J. Mol. Sci. 2013, 14, 19257–19275. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.H.; Park, S.T.; Lam, B.; Tsai, Y.C.; Cheng, M.A.; Farmer, E.; Xing, D.; Hung, C.F. Novel, genetically induced mouse model that recapitulates the histological morphology and immunosuppressive tumor microenvironment of metastatic peritoneal carcinomatosis. J. Immunother. Cancer 2020, 8, e000480. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Zhang, S.; Yucel, S.; Horn, H.; Smith, S.G.; Reinhardt, F.; Hoefsmit, E.; Assatova, B.; Casado, J.; Meinsohn, M.C.; et al. Genetically defined syngeneic mouse models of ovarian cancer as tools for the discovery of combination immunotherapy. Cancer Discov. 2020, 11, 384–407. [Google Scholar] [CrossRef]
- Turetta, M.; Ben, F.D.; Brisotto, G.; Biscontin, E.; Bulfoni, M.; Cesselli, D.; Colombatti, A.; Scoles, G.; Gigli, G.; Del Mercato, L.L. Emerging technologies for cancer research: Towards personalized medicine with microfluidic platforms and 3D tumor models. Curr. Med. Chem. 2018, 25, 4616–4637. [Google Scholar] [CrossRef] [PubMed]
- De Jaeghere, E.; De Vlieghere, E.; Van Hoorick, J.; Van Vlierberghe, S.; Wagemans, G.; Pieters, L.; Melsens, E.; Praet, M.; Van Dorpe, J.; Boone, M.N.; et al. Heterocellular 3D scaffolds as biomimetic to recapitulate the tumor microenvironment of peritoneal metastases in vitro and in vivo. Biomaterials 2018, 158, 95–105. [Google Scholar] [CrossRef]
- Loessner, D.; Rockstroh, A.; Shokoohmand, A.; Holzapfel, B.M.; Wagner, F.; Baldwin, J.; Boxberg, M.; Schmalfeldt, B.; Lengyel, E.; Clements, J.A.; et al. A 3D tumor microenvironment regulates cell proliferation, peritoneal growth and expression patterns. Biomaterials 2019, 190–191, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Loessner, D.; Rizzi, S.C.; Stok, K.S.; Fuehrmann, T.; Hollier, B.; Magdolen, V.; Hutmacher, D.W.; Clements, J.A. A bioengineered 3D ovarian cancer model for the assessment of peptidase-mediated enhancement of spheroid growth and intraperitoneal spread. Biomaterials 2013, 34, 7389–7400. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bella, Á.; Di Trani, C.A.; Fernández-Sendin, M.; Arrizabalaga, L.; Cirella, A.; Teijeira, Á.; Medina-Echeverz, J.; Melero, I.; Berraondo, P.; Aranda, F. Mouse Models of Peritoneal Carcinomatosis to Develop Clinical Applications. Cancers 2021, 13, 963. https://doi.org/10.3390/cancers13050963
Bella Á, Di Trani CA, Fernández-Sendin M, Arrizabalaga L, Cirella A, Teijeira Á, Medina-Echeverz J, Melero I, Berraondo P, Aranda F. Mouse Models of Peritoneal Carcinomatosis to Develop Clinical Applications. Cancers. 2021; 13(5):963. https://doi.org/10.3390/cancers13050963
Chicago/Turabian StyleBella, Ángela, Claudia Augusta Di Trani, Myriam Fernández-Sendin, Leire Arrizabalaga, Assunta Cirella, Álvaro Teijeira, José Medina-Echeverz, Ignacio Melero, Pedro Berraondo, and Fernando Aranda. 2021. "Mouse Models of Peritoneal Carcinomatosis to Develop Clinical Applications" Cancers 13, no. 5: 963. https://doi.org/10.3390/cancers13050963
APA StyleBella, Á., Di Trani, C. A., Fernández-Sendin, M., Arrizabalaga, L., Cirella, A., Teijeira, Á., Medina-Echeverz, J., Melero, I., Berraondo, P., & Aranda, F. (2021). Mouse Models of Peritoneal Carcinomatosis to Develop Clinical Applications. Cancers, 13(5), 963. https://doi.org/10.3390/cancers13050963










