Evolution of the Role of Radiotherapy for Anal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction: Surgery Prior to the Introduction of Chemoradiotherapy
2. Chemoradiotherapy versus Radiotherapy Alone: ACT I and EORTC
3. Omission of Mitomycin C: RTOG 87-04/ECOG 1289
4. Cisplatin as a Possible Alternative to Mitomycin C: RTOG 98-11 and ACT II
5. Capecitabine as an Alternative to 5FU
6. Radiation Dose De-Escalation
7. Radiation Dose Escalation
8. Intensity-Modulated Radiation Therapy: RTOG 05-29
9. Improvements in Radiotherapy Simulation
10. Treatment Planning and Delivery: Institutional Practice and Recent Advances
11. Hematologic and Genitourinary Toxicity: Implications for Treatment Planning
12. Timing of Treatment Evaluation and Persistent/Recurrent Disease
13. Treatment of Persistent/Recurrent Disease
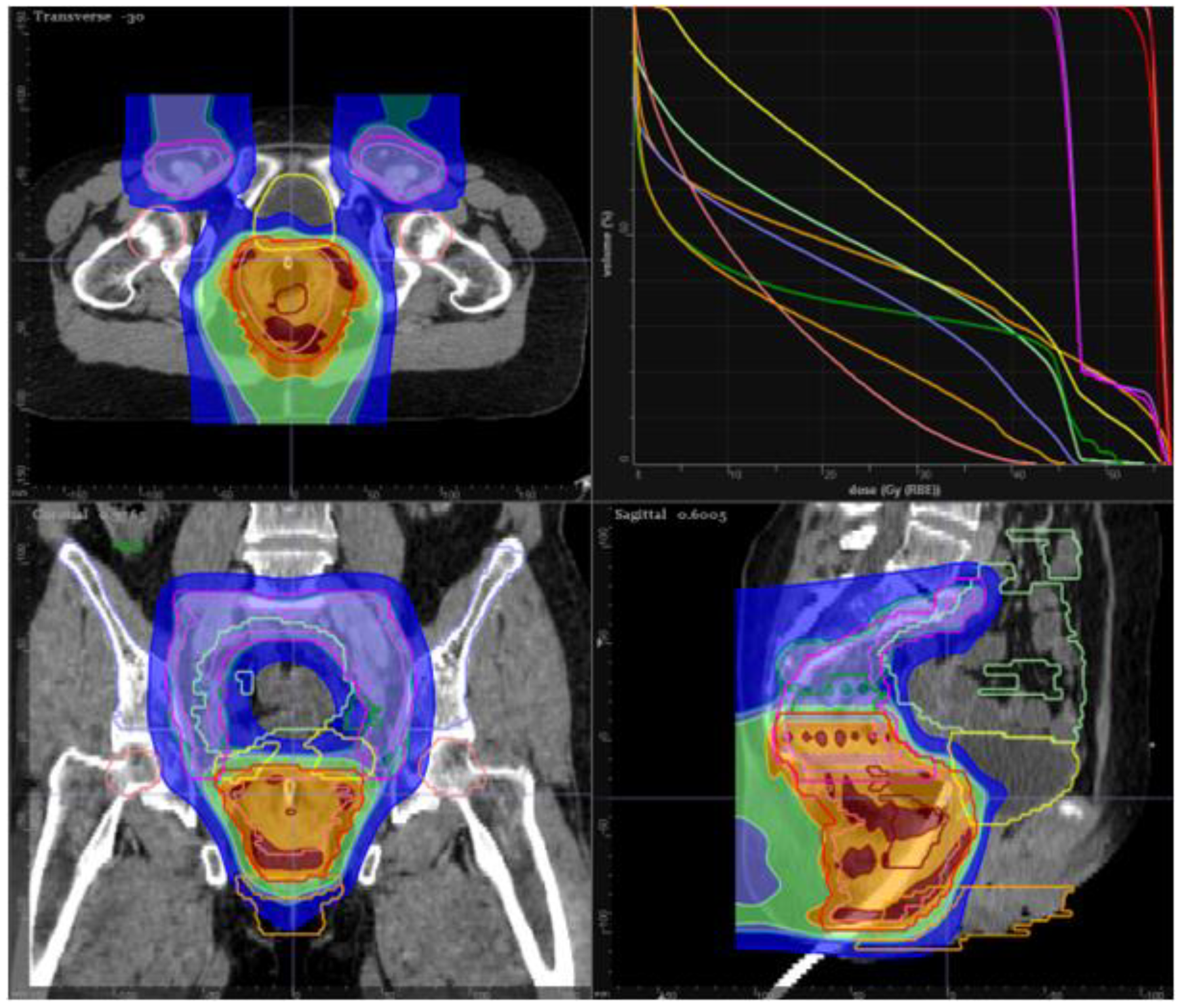
14. Proton Therapy in Anal Cancer
15. Ongoing Trials and Studies in Development: PLATO (ACT III, IV, V)
16. Immuno-oncology in the High-Risk and Metastatic Setting
17. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NIH National Cancer Institute. Cancer Facts & Figures 2020. CA. Cancer J. Clin. 2020, 15, 5323–5337. [Google Scholar]
- Wilkinson, J.R.; Morris, E.J.A.; Downing, A.; Finan, P.J.; Aravani, A.; Thomas, J.D.; Sebag-Montefiore, D. The rising incidence of anal cancer in England 1990–2010: A population-based study. Color. Dis. 2014, 16, O234–O239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palefsky, J.M.; Giuliano, A.R.; Goldstone, S.; Moreira, E.D.; Aranda, C.; Jessen, H.; Hillman, R.; Ferris, D.; Coutlee, F.; Stoler, M.H.; et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N. Engl. J. Med. 2011, 365, 1576–1585. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Madeleine, M.M.; Biggar, R.J.; Engels, E.A. Risk of human papillomavirus-associated cancers among persons with AIDS. J. Natl. Cancer Inst. 2009, 101, 1120–1130. [Google Scholar] [CrossRef] [Green Version]
- Goldie, S.J.; Kuntz, K.M.; Weinstein, M.C.; Freedberg, K.A.; Welton, M.L.; Palefsky, J.M. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men. J. Am. Med. Assoc. 1999, 281, 1822–1829. [Google Scholar] [CrossRef] [Green Version]
- Goldie, S.J.; Kuntz, K.M.; Weinstein, M.C.; Freedberg, K.A.; Palefsky, J.M. Cost-effectiveness of screening for anal squamous intraepithelial lesions and anal cancer in human immunodeficiency virus-negative homosexual and bisexual men. Am. J. Med. 2000, 108, 634–641. [Google Scholar] [CrossRef]
- Gunderson, L.L.; Winter, K.A.; Ajani, J.A.; Pedersen, J.E.; Moughan, J.; Benson, A.B.; Thomas, C.R.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; et al. Long-term update of US GI intergroup RTOG 98-11 Phase III trial for anal carcinoma: Survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J. Clin. Oncol. 2012, 30, 4344–4351. [Google Scholar] [CrossRef]
- Urbute, A.; Rasmussen, C.L.; Belmonte, F.; Obermueller, T.; Prigge, E.S.; Arbyn, M.; Verdoodt, F.; Kjaer, S.K. Prognostic significance of HPV DNA and p16INK4a in anal cancer: A systematic review and meta-analysis. Cancer Epidemiol. Biomarkers Prev. 2020, 29, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Franco, P.; Montagnani, F.; Arcadipane, F.; Casadei, C.; Andrikou, K.; Martini, S.; Iorio, G.C.; Scartozzi, M.; Mistrangelo, M.; Fornaro, L.; et al. The prognostic role of hemoglobin levels in patients undergoing concurrent chemo-radiation for anal cancer. Radiat. Oncol. 2018, 13, 83. [Google Scholar] [CrossRef] [Green Version]
- Casadei-Gardini, A.; Montagnani, F.; Casadei, C.; Arcadipane, F.; Andrikou, K.; Aloi, D.; Prete, A.A.; Zampino, M.G.; Argentiero, A.; Pugliese, G.; et al. Immune inflammation indicators in anal cancer patients treated with concurrent chemoradiation: Training and validation cohort with online calculator (ARC: Anal Cancer Response Classifier). Cancer Manag. Res. 2019, 11, 3631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, D.T.; Thomas, C.R. Carcinoma of the anal canal. Oncol. Rev. 2009, 3, 27–40. [Google Scholar] [CrossRef]
- Beahrs, O.H.; Wilson, S.M. Carcinoma of the anus. Ann. Surg. 1976, 184, 422–428. [Google Scholar] [CrossRef]
- Pintor, M.P.; Northover, J.M.A.; Nicholls, R.J. Squamous cell carcinoma of the anus at one hospital from 1948 to 1984. Br. J. Surg. 1989, 76, 806–810. [Google Scholar] [CrossRef]
- Boman, B.M.; Moertel, C.G.; O’Connell, M.J.; Scott, M.; Weiland, L.H.; Beart, R.W.; Gunderson, L.L.; Spencer, R.J. Carcinoma of the anal canal, a clinical and pathologic study of 188 cases. Cancer 1984, 54, 114–125. [Google Scholar] [CrossRef]
- Singh, R.; Nime, F.; Mittelman, A. Malignant epithelial tumors of the anal canal. Cancer 1981, 48, 411–415. [Google Scholar] [CrossRef]
- Schraut, W.H.; Wang, C.-H.; Dawson, P.J.; Block, G.E. Depth of invasion, location, and size of cancer of the anus dictate operative treatment. Cancer 1983, 51, 1291–1296. [Google Scholar] [CrossRef]
- Frost, D.B.; Richards, P.C.; Montague, E.D.; Giacco, G.G.; Martin, R.G. Epidermoid cancer of the anorectum. Cancer 1984, 53, 1285–1293. [Google Scholar] [CrossRef]
- Grabenbauer, G.G.; Schneider, I.H.F.; Gall, F.P.; Sauer, R. Epidermoid carcinoma of the anal canal: Treatment by combined radiation and chemotherapy. Radiother. Oncol. 1993, 27, 59–62. [Google Scholar] [CrossRef]
- Nigro, N.D.; Vaitkevicius, V.K.; Considine, B. Combined therapy for cancer of the anal canal: A preliminary report. Dis. Colon Rectum 1974, 24, 73–75. [Google Scholar] [CrossRef]
- Nigro, N.D.; Seydel, H.G.; Considine, B.; Vaitkevicius, V.K.; Leichman, L.; Kinzie, J.J. Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer 1983, 51, 1826–1829. [Google Scholar] [CrossRef]
- Nigro, N.D. An evaluation of combined therapy for squamous cell cancer of the anal canal. Dis. Colon Rectum 1984, 27, 763–766. [Google Scholar] [CrossRef]
- Leichman, L.; Nigro, N.; Vaitkevicius, V.K.; Considine, B.; Buroker, T.; Bradley, G.; Seydel, H.G.; Olchowski, S.; Cummings, G.; Leichman, C.; et al. Cancer of the anal canal. Model for preoperative adjuvant combined modality therapy. Am. J. Med. 1985, 78, 211–215. [Google Scholar] [CrossRef]
- Papillon, J. Radiation therapy in the conservative management of cancers of the low rectum and anal canal. Int. J. Colorectal Dis. 1986, 1, 251–255. [Google Scholar] [CrossRef]
- Papillon, J.; Mayer, M.; Montbarbon, J.F.; Gerard, J.P.; Chassard, J.L.; Bailly, C. A new approach to the management of epidermoid carcinoma of the anal canal. Cancer 1983, 51, 1830–1837. [Google Scholar] [CrossRef]
- Papillon, J.; Montbarbon, J.F. Epidermoid carcinoma of the anal canal—A series of 276 cases. Dis. Colon Rectum 1987, 30, 324–333. [Google Scholar] [CrossRef]
- Enker, W.E.; Heilwell, M.; Janov, A.J.; Quan, S.H.; Magill, G.; Stearns, M.W.; Shank, B.; Learning, R.; Sternberg, S.S. Improved Survival in Epidermoid Carcinoma of the Anus in Association With Preoperative Multidisciplinary Therapy. Arch. Surg. 1986, 121, 1386–1390. [Google Scholar] [CrossRef]
- Michaelson, R.A.; Magill, G.B.; Quan, S.H.Q.; Leaming, R.H.; Nikrui, M.; Stearns, M.W. Preoperative chemotherapy and radiation therapy in the management of anal epidermoid carcinoma. Cancer 1983, 51, 390–395. [Google Scholar] [CrossRef]
- Nigro, N.D. Multidisciplinary management of cancer of the anus. World J. Surg. 1987, 11, 446–451. [Google Scholar] [CrossRef]
- Sischy, B. The use of radiation therapy combined with chemotherapy in the management of squamous cell carcinoma of the anus and marginally resectable adenocarcinoma of the rectum. Int. J. Radiat. Oncol. Biol. Phys. 1985, 1, 1587–1593. [Google Scholar] [CrossRef]
- John, M.J.; Flam, M.; Lovalvo, L.; Mowry, P.A. Feasibility of non-surgical definitive management of anal canal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 299–303. [Google Scholar] [CrossRef]
- Flam, M.S.; John, M.; Lovalvo, L.J.; Mills, R.J.; Ramalho, L.D.; Prather, C.; Mowry, P.A.; Morgan, D.R.; Lau, B.P. Definitive nonsurgical therapy of epithelial malignancies of the anal canal a report of 12 cases. Cancer 1983, 51, 1378–1387. [Google Scholar] [CrossRef]
- Sischy, B.; Doggett, R.L.S.; Krall, J.M.; Taylor, D.G.; Sause, W.T.; Lipsett, J.A.; Seydel, H.G. Definitive irradiation and chemotherapy for radiosensitization in management of anal carcinoma: Interim report on radiation therapy oncology group study no. 8314. J. Natl. Cancer Inst. 1989, 81, 850–857. [Google Scholar] [CrossRef]
- Hughes, L.L.; Rich, T.A.; Delclos, L.; Ajani, J.A.; Martin, R.G. Radiotherapy for anal cancer: Experience from 1979-1987. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 1153–1160. [Google Scholar] [CrossRef]
- Jin, H.; Pinheiro, P.S.; Callahan, K.E.; Altekruse, S.F. Examining the gastric cancer survival gap between Asians and whites in the United States. Gastric Cancer 2017, 20, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Cummings, B.J.; Keane, T.J.; O’Sullivan, B.; Wong, C.S.; Catton, C.N. Epidermoid anal cancer: Treatment by radiation alone or by radiation and 5-fluorouracil with and without mitomycin C. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 1115–1125. [Google Scholar] [CrossRef]
- John, M.; Pajak, T.; Flam, M.; Hoffman, J.; Markoe, A.; Wolkov, H.; Paris, K. Dose escalation in chemoradiation for anal cancer: Preliminary results of RTOG 92-08. Cancer J. Sci. Am. 1996, 24, 205–211. [Google Scholar]
- Doci, R.; Zucali, R.; Bombelli, L.; Montalto, F.; Lamonica, G. Combined chemoradiation therapy for anal cancer: A report of 56 cases. Ann. Surg. 1992, 215, 150–156. [Google Scholar] [CrossRef]
- Martenson, J.A.; Lipsitz, S.R.; Lefkopoulou, M.; Engstrom, P.F.; Dayal, Y.Y.; Cobau, C.D.; Oken, M.M.; Hatter, D.G. Results of combined modality therapy for patients with anal cancer (E7283). An eastern cooperative oncology group study. Cancer 1995, 76, 1731–1736. [Google Scholar] [CrossRef]
- Martenson, J.A.; Lipsitz, S.R.; Wagner, H.; Kaplan, E.H.; Otteman, L.A.; Schuchter, L.M.; Mansour, E.G.; Talamonti, M.S.; Benson, A.B. Initial results of a phase II trial of high dose radiation therapy, 5-fluorouracil, and cisplatin for patients with anal cancer (E4292): An Eastern Cooperative Oncology Group study. Int. J. Radiat. Oncol. Biol. Phys. 1996, 35, 745–749. [Google Scholar] [CrossRef]
- Green, J.P.; Schaupp, W.C.; Cantril, S.T.; Schall, G. Anal carcinoma: Current therapeutic concepts. Am. J. Surg. 1980, 140, 151–155. [Google Scholar] [CrossRef]
- James, R.D.; Pointon, R.S.; Martin, S. Local radiotherapy in the management of squamous carcinoma of the anus. Br. J. Surg. 1985, 72, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F. Combined modality treatment with radiotherapy and chemotherapy. Radiother. Oncol. 1989, 16, 83–101. [Google Scholar] [CrossRef]
- Cummings, B.; Keane, T.; Thomas, G.; Harwood, A.; Rider, W. Results and toxicity of the treatment of anal canal carcinoma by radiation therapy or radiation therapy and chemotherapy. Cancer 1984, 54, 2062–2068. [Google Scholar] [CrossRef]
- Northover, J.M.A.; Arnott, S.J.; Cunningham, D.; Gallagher, J.; Gray, R.; Hardcastle, J.; Houghton, J.; James, R.D.; Lennon, T.A.; Meadows, H.M.; et al. Epidermoid anal cancer: Results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. Lancet 1996, 348, 1049–1054. [Google Scholar]
- Northover, J.; Glynne-Jones, R.; Sebag-Montefiore, D.; James, R.; Meadows, H.; Wan, S.; Jitlal, M.; Ledermann, J. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br. J. Cancer 2010, 102, 1123–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartelink, H.; Roelofsen, F.; Eschwege, F.; Rougier, P.; Bosset, J.F.; Gonzalez, D.G.; Peiffert, D.; van Glabbeke, M.; Pierart, M. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: Results of a phase III randomized trial of the European organization for research and treatment of cancer radiotherapy and gastro. J. Clin. Oncol. 1997, 15, 2040–2049. [Google Scholar] [CrossRef]
- Ludmir, E.B.; Kachnic, L.A.; Czito, B.G. Evolution and Management of Treatment-Related Toxicity in Anal Cancer. Surg. Oncol. Clin. N. Am. 2017, 26, 91–113. [Google Scholar] [CrossRef]
- Flam, M.; John, M.; Pajak, T.F.; Petrelli, N.; Myerson, R.; Doggett, S.; Quivey, J.; Rotman, M.; Kerman, H.; Coia, L.; et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: Results of a phase III randomized intergroup study. J. Clin. Oncol. 1996, 14, 2527–2539. [Google Scholar] [CrossRef]
- Ajani, J.A.; Winter, K.A.; Gunderson, L.L.; Pedersen, J.; Benson, A.B.; Thomas, C.R.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; Willett, C. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: A randomized controlled trial. JAMA J. Am. Med. Assoc. 2008, 299, 1914–1921. [Google Scholar] [CrossRef]
- James, R.D.; Glynne-Jones, R.; Meadows, H.M.; Cunningham, D.; Myint, A.S.; Saunders, M.P.; Maughan, T.; McDonald, A.; Essapen, S.; Leslie, M.; et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): A randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol. 2013, 14, 516–524. [Google Scholar] [CrossRef]
- Konski, A.; Garcia, M.; John, M.; Krieg, R.; Pinover, W.; Myerson, R.; Willett, C. Evaluation of Planned Treatment Breaks During Radiation Therapy for Anal Cancer: Update of RTOG 92-08. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 114–118. [Google Scholar] [CrossRef] [Green Version]
- Peiffert, D.; Tournier-Rangeard, L.; Gérard, J.P.; Lemanski, C.; François, E.; Giovannini, M.; Cvitkovic, F.; Mirabel, X.; Bouché, O.; Luporsi, E.; et al. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: Final analysis of the randomized UNICANCER ACCORD 03 trial. J. Clin. Oncol. 2012, 30, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Kachnic, L.A.; Winter, K.; Myerson, R.J.; Goodyear, M.D.; Willins, J.; Esthappan, J.; Haddock, M.G.; Rotman, M.; Parikh, P.J.; Safran, H.; et al. RTOG 0529: A phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 27–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wo, J.Y.; Plastaras, J.P.; Metz, J.M.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Adams, J.; Baglini, C.; Ryan, D.P.; Murphy, J.E.; et al. Pencil Beam Scanning Proton Beam Chemoradiation Therapy With 5-Fluorouracil and Mitomycin-C for Definitive Treatment of Carcinoma of the Anal Canal: A Multi-institutional Pilot Feasibility Study. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 90–95. [Google Scholar] [CrossRef]
- Morris, V.K.; Salem, M.E.; Nimeiri, H.; Iqbal, S.; Singh, P.; Ciombor, K.; Polite, B.; Deming, D.; Chan, E.; Wade, J.L.; et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Ho, G.Y.; Woodward, N.; Coward, J.I.G. Cisplatin versus carboplatin: Comparative review of therapeutic management in solid malignancies. Crit. Rev. Oncol. Hematol. 2016, 102, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Rich, T.A.; Ajani, J.A.; Morrison, W.H.; Ota, D.; Levin, B. Chemoradiation therapy for anal cancer: Radiation plus continuous infusion of 5-fluorouracil with or without cisplatin. Radiother. Oncol. 1993, 27, 209–215. [Google Scholar] [CrossRef]
- Gerard, J.P.; Ayzac, L.; Hun, D.; Romestaing, P.; Coquard, R.; Ardiet, J.M.; Mornex, F. Treatment of anal canal carcinoma with high dose radiation therapy and concomitant fluorouracil-cisplatinum. Long-term results in 95 patients. Radiother. Oncol. 1998, 46, 249–256. [Google Scholar] [CrossRef]
- Gunderson, L.L.; Winter, K.A.; Ajani, J.A.; Pedersen, J.E.; Benson, A.B.; Thomas, C.R.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; Willett, C.G. Long-term update of U.S. GI Intergroup RTOG 98-11 phase III trial for anal carcinoma: Comparison of concurrent chemoradiation with 5FU-mitomycin versus 5FU-cisplatin for disease-free and overall survival. J. Clin. Oncol. 2011, 29, 367. [Google Scholar] [CrossRef]
- Ben-Josef, E.; Moughan, J.; Ajani, J.A.; Flam, M.; Gunderson, L.; Pollock, J.D.; Myerson, R.; Anne, R.; Rosenthal, S.A.; Willett, C. Impact of overall treatment time on survival and local control in patients with anal cancer: A pooled data analysis of radiation therapy oncology group trials 87-04 and 98-11. J. Clin. Oncol. 2010, 28, 5061–5066. [Google Scholar] [CrossRef]
- White, E.C.; Goldman, K.; Aleshin, A.; Lien, W.W.; Rao, A.R. Chemoradiotherapy for squamous cell carcinoma of the anal canal: Comparison of one versus two cycles mitomycin-C. Radiother. Oncol. 2015, 117, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Swellengrebel, H.A.M.; Marijnen, C.A.M.; Verwaal, V.J.; Vincent, A.; Heuff, G.; Gerhards, M.F.; Van Geloven, A.A.W.; Van Tets, W.F.; Verheij, M.; Cats, A. Toxicity and complications of preoperative chemoradiotherapy for locally advanced rectal cancer. Br. J. Surg. 2011, 98, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.C.R.; Moniz, C.M.V.; Riechelmann, R.; Alex, A.K.; Braghirolli, M.I.; Bariani, G.; Nahas, C.; Hoff, P.M.G. Phase II Study of Capecitabine in Substitution of 5-FU in the Chemoradiotherapy Regimen for Patients with Localized Squamous Cell Carcinoma of the Anal Canal. J. Gastrointest. Cancer 2016, 47, 75–81. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Meadows, H.; Wan, S.; Gollins, S.; Leslie, M.; Levine, E.; McDonald, A.C.; Myint, S.; Samuel, L.; Sebag-Montefiore, D. EXTRA-A Multicenter Phase II Study of Chemoradiation Using a 5 Day per Week Oral Regimen of Capecitabine and Intravenous Mitomycin C in Anal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 119–126. [Google Scholar] [CrossRef]
- Allegra, C.J.; Yothers, G.; O’Connell, M.J.; Beart, R.W.; Wozniak, T.F.; Pitot, H.C.; Shields, A.F.; Landry, J.C.; Ryan, D.P.; Arora, A.; et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: A phase III randomized clinical trial. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, L.C.; Healey, T.; Michele, T.; Price, T.J. Capecitabine in locally advanced anal cancer, do we need randomised evidence? Expert Rev. Anticancer Ther. 2017, 17, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Adams, R.; Downing, A.; Glynne-Jones, R.; Harrison, M.; Hawkins, M.; Sebag-Montefiore, D.; Gilbert, D.C.; Muirhead, R. Toxicity, Tolerability, and Compliance of Concurrent Capecitabine or 5-Fluorouracil in Radical Management of Anal Cancer With Single-dose Mitomycin-C and Intensity Modulated Radiation Therapy: Evaluation of a National Cohort. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1202–1211. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Tan, D.; Hughes, R.; Hoskin, P. Squamous-cell carcinoma of the anus: Progress in radiotherapy treatment. Nat. Rev. Clin. Oncol. 2016, 13, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Saunders, M.P.; Schofield, P.F.; O’Dwyer, S.T. Patterns of local disease failure and outcome after salvage surgery in patients with anal cancer. Br. J. Surg. 2005, 92, 605–614. [Google Scholar] [CrossRef]
- Ferrigno, R.; Nakamura, R.A.; Dos Santos Novaes, P.E.R.; Assis Pellizzon, A.C.; Conte Maia, M.A.; Fogarolli, R.C.; Salvajoli, J.V.; Filho, W.J.D.; Lopes, A. Radiochemotherapy in the conservative treatment of anal canal carcinoma: Retrospective analysis of results and radiation dose effectiveness. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1136–1142. [Google Scholar] [CrossRef]
- Huang, K.; Haas-Kogan, D.; Weinberg, V.; Krieg, R. Higher radiation dose with a shorter treatment duration improves outcome for locally carcinoma of anal canal. World J. Gastroenterol. 2007, 13, 895. [Google Scholar] [CrossRef] [Green Version]
- Ajani, J.A.; Winter, K.A.; Gunderson, L.L.; Pedersen, J.; Benson, A.B.; Thomas, C.R.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; Willett, C.G. Prognostic factors derived from a prospective database dictate clinical biology of anal cancer: The intergroup trial (RTOG 98-11). Cancer 2010, 116, 4007–4013. [Google Scholar] [CrossRef] [Green Version]
- Gunderson, L.L.; Moughan, J.; Ajani, J.A.; Pedersen, J.E.; Winter, K.A.; Benson, A.B.; Thomas, C.R.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; et al. Anal carcinoma: Impact of TN category of disease on survival, disease relapse, and colostomy failure in US gastrointestinal intergroup RTOG 98-11 phase 3 trial. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Bentzen, A.G.; Guren, M.G.; Vonen, B.; Wanderås, E.H.; Frykholm, G.; Wilsgaard, T.; Dahl, O.; Balteskard, L. Faecal incontinence after chemoradiotherapy in anal cancer survivors: Long-term results of a national cohort. Radiother. Oncol. 2013, 108, 55–60. [Google Scholar] [CrossRef]
- Lower-Dose Chemoradiation in Treating Patients With Early-Stage Anal Cancer, the DECREASE Study. Case Med. Res. 2019, 105, 591–605.
- Smith, C.A.; Kachnic, L.A. Randomized Clinical Trials in Localized Anal Cancer. Surg. Oncol. Clin. N. Am. 2017, 26, 705–718. [Google Scholar] [CrossRef]
- Bentzen, A.G.; Balteskard, L.; Wanderås, E.H.; Frykholm, G.; Wilsgaard, T.; Dahl, O.; Guren, M.G. Impaired health-related quality of life after chemoradiotherapy for anal cancer: Late effects in a national cohort of 128 survivors. Acta Oncol. Madr. 2013, 52, 736–744. [Google Scholar] [CrossRef] [Green Version]
- Brændengen, M.; Tveit, K.M.; Bruheim, K.; Cvancarova, M.; Berglund, K.; Glimelius, B. Late patient-reported toxicity after preoperative radiotherapy or chemoradiotherapy in nonresectable rectal cancer: Results from a randomized phase III study. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1017–1024. [Google Scholar] [CrossRef]
- Kachnic, L.A.; Tsai, H.K.; Coen, J.J.; Blaszkowsky, L.S.; Hartshorn, K.; Kwak, E.L.; Willins, J.D.; Ryan, D.P.; Hong, T.S. Dose-painted intensity-modulated radiation therapy for anal cancer: A multi-institutional report of acute toxicity and response to therapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 6, 413–421. [Google Scholar] [CrossRef]
- Muirhead, R.; Partridge, M.; Hawkins, M.A. A tumor control probability model for anal squamous cell carcinoma. Radiother. Oncol. 2015, 116, 192–196. [Google Scholar] [CrossRef]
- Milano, M.T.; Jani, A.B.; Farrey, K.J.; Rash, C.; Heimann, R.; Chmura, S.J. Intensity-modulated radiation therapy (IMRT) in the treatment of anal cancer: Toxicity and clinical outcome. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 354–361. [Google Scholar] [CrossRef]
- Franco, P.; Mistrangelo, M.; Arcadipane, F.; Munoz, F.; Sciacero, P.; Spadi, R.; Migliaccio, F.; Angelini, V.; Bombaci, S.; Rondi, N.; et al. Intensity-modulated radiation therapy with simultaneous integrated boost combined with concurrent chemotherapy for the treatment of anal cancer patients: 4-year results of a consecutive case series. Cancer Invest. 2015, 33, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Yates, A.; Carroll, S.; Kneebone, A.; Tse, R.; Horvath, L.; Byrne, C.; Solomon, M.; Hruby, G. Implementing intensity-modulated radiotherapy with simultaneous integrated boost for anal cancer: 3 year outcomes at two Sydney institutions. Clin. Oncol. 2015, 27, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Call, J.A.; Prendergast, B.M.; Jensen, L.G.; Ord, C.B.; Goodman, K.A.; Jacob, R.; Mell, L.K.; Thomas, C.R.; Jabbour, S.K.; Miller, R.C. Intensity-modulated radiation therapy for anal cancer results from a multi-institutional retrospective cohort study. Am. J. Clin. Oncol. Cancer Clin. Trials 2016, 39, 8–12. [Google Scholar]
- Shridhar, R.; Shibata, D.; Chan, E.; Thomas, C.R. Anal cancer: Current standards in care and recent changes in practice. CA. Cancer J. Clin. 2015, 65, 139–162. [Google Scholar] [CrossRef]
- Pepek, J.M.; Willett, C.G.; Wu, Q.J.; Yoo, S.; Clough, R.W.; Czito, B.G. Intensity-modulated radiation therapy for anal malignancies: A preliminary toxicity and disease outcomes analysis. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- DeFoe, S.G.; Beriwal, S.; Jones, H.; Rakfal, S.; Heron, D.E.; Kabolizadeh, P.; Smith, R.P.; Lalonde, R. Concurrent Chemotherapy and Intensity-modulated Radiation Therapy for Anal—Clinical Outcomes in a Large National Cancer Institute-designated Integrated Cancer Centre Network. Clin. Oncol. 2012, 24, 424–431. [Google Scholar] [CrossRef]
- Vieillot, S.; Fenoglietto, P.; Lemanski, C.; Moscardo, C.L.; Gourgou, S.; Dubois, J.B.; Aillères, N.; Azria, D. IMRT for locally advanced anal cancer: Clinical experience of the Montpellier Cancer Center. Radiat. Oncol. 2012, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Han, K.; Cummings, B.J.; Lindsay, P.; Skliarenko, J.; Craig, T.; Le, L.W.; Brierley, J.; Wong, R.; Dinniwell, R.; Bayley, A.J.; et al. Prospective evaluation of acute toxicity and quality of life after IMRT and concurrent chemotherapy for anal canal and perianal cancer. Int. J. Radiat. Oncol. 2014, 90, 587–594. [Google Scholar] [CrossRef]
- Janssen, S.; Glanzmann, C.; Bauerfeind, P.; Stieb, S.; Studer, G.; Brown, M.; Riesterer, O. Clinical experience of SIB-IMRT in anal cancer and selective literature review. Radiat. Oncol. 2014, 9, 199. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.P.; Abboud, M.; Crane, C.H.; Eng, C.; Chang, G.J.; Rodriguez-Bigas, M.A.; Skibber, J.M.; You, Y.N.; Beddar, S.; Krishnan, S.; et al. Intensity-modulated radiation therapy (IMRT) with concurrent chemotherapy for anal cancer: A large single-institution experience. J. Clin. Oncol. 2012, 30, 661. [Google Scholar] [CrossRef]
- Belgioia, L.; Vagge, S.; Agnese, D.; Garelli, S.; Murialdo, R.; Fornarini, G.; Chiara, S.; Gallo, F.; Bacigalupo, A.; Corvò, R. Intensified intensity-modulated radiotherapy in anal cancer with prevalent HPV p16 positivity. World J. Gastroenterol. 2015, 21, 10688–10696. [Google Scholar] [CrossRef]
- Saarilahti, K.; Arponen, P.; Vaalavirta, L.; Tenhunen, M. The effect of intensity-modulated radiotherapy and high dose rate brachytherapy on acute and late radiotherapy-related adverse events following chemoradiotherapy of anal cancer. Radiother. Oncol. 2008, 87, 383–390. [Google Scholar] [CrossRef]
- Bazan, J.G.; Hara, W.; Hsu, A.; Kunz, P.A.; Ford, J.; Fisher, G.A.; Welton, M.L.; Shelton, A.; Kapp, D.S.; Koong, A.C.; et al. Intensity-modulated radiation therapy versus conventional radiation therapy for squamous cell carcinoma of the anal canal. Cancer 2011, 117, 3342–3351. [Google Scholar] [CrossRef]
- Dewas, C.V.; Maingon, P.; Dalban, C.; Petitfils, A.; Peignaux, K.; Truc, G.; Martin, E.; Khoury, C.; Dewas, S.; Créhange, G. Does gap-free intensity modulated chemoradiation therapy provide a greater clinical benefit than 3D conformal chemoradiation in patients with anal cancer? Radiat. Oncol. 2012, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chuong, M.D.; Freilich, J.M.; Hoffe, S.E.; Fulp, W.; Weber, J.M.; Almhanna, K.; Dinwoodie, W.; Rao, N.; Meredith, K.L.; Shridhar, R. Intensity-modulated radiation therapy vs. 3D conformal radiation therapy for squamous cell carcinoma of the anal canal. Gastrointest. Cancer Res. 2013, 6, 39–45. [Google Scholar] [PubMed]
- Koerber, S.A.; Slynko, A.; Haefner, M.F.; Krug, D.; Schoneweg, C.; Kessel, K.; Kopp-Schneider, A.; Herfarth, K.; Debus, J.; Sterzing, F. Efficacy and toxicity of chemoradiation in patients with anal cancer—A retrospective analysis. Radiat. Oncol. 2014, 9, 113. [Google Scholar] [CrossRef] [Green Version]
- Zagar, T.M.; Willett, C.G.; Czito, B.G. Intensity-modulated radiation therapy for anal cancer: Toxicity versus Outcomes. Oncology 2010, 9, 113. [Google Scholar]
- Mitra, D.; Hong, T.S.; Horick, N.; Rose, B.; Drapek, L.N.; Blaszkowsky, L.S.; Allen, J.N.; Kwak, E.L.; Murphy, J.E.; Clark, J.W.; et al. Long-term outcomes and toxicities of a large cohort of anal cancer patients treated with dose-painted IMRT per RTOG 0529. Adv. Radiat. Oncol. 2017, 2, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Arcadipane, F.; Franco, P.; Ceccarelli, M.; Furfaro, G.; Rondi, N.; Trino, E.; Martini, S.; Iorio, G.C.; Mistrangelo, M.; Cassoni, P.; et al. Image-guided IMRT with simultaneous integrated boost as per RTOG 0529 for the treatment of anal cancer. Asia. Pac. J. Clin. Oncol. 2018, 14, 217–223. [Google Scholar] [CrossRef]
- Franco, P.; De Bari, B.; Arcadipane, F.; Lepinoy, A.; Ceccarelli, M.; Furfaro, G.; Mistrangelo, M.; Cassoni, P.; Valgiusti, M.; Passardi, A.; et al. Comparing simultaneous integrated boost vs sequential boost in anal cancer patients: Results of a retrospective observational study. Radiat. Oncol. 2018, 13, 172. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, T.H.; Kim, D.Y.; Cho, K.H.; Kim, J.Y.; Park, S.Y.; Kim, D.H.; Lim, S.B.; Choi, H.S.; Chang, H.J. The Effect of Belly Board Location in Rectal Cancer Patients Treated with Preoperative Radiotherapy. Clin. Oncol. 2006, 18, 441–446. [Google Scholar] [CrossRef]
- Tae, H.K.; Eui, K.C.; Dae, Y.K.; Sung, Y.P.; Kwan, H.C.; Kyung, H.J.; Young, H.K.; Dae, K.S.; Jeong, S.Y.; Park, J.G. Comparison of the belly board device method and the distended bladder method for reducing irradiated small bowel volumes in preoperative radiotherapy of rectal cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2005, 64, 769–775. [Google Scholar]
- Briere, T.M.; Crane, C.H.; Beddar, S.; Bhosale, P.; Mok, H.; Delclos, M.E.; Krishnan, S.; Das, P. Reproducibility and genital sparing with a vaginal dilator used for female anal cancer patients. Radiother. Oncol. 2012, 104, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Joseph, K.; Rose, B.; Warkentin, H.; Yun, J.; Ghosh, S.; Tankel, K. Peri-anal surface dose in anal canal VMAT radiotherapy. J. Med. Imaging Radiat. Oncol. 2018, 62, 734–738. [Google Scholar] [CrossRef]
- Das, I.J.; Lanciano, R.M.; Movsas, B.; Kagawa, K.; Barnes, S.J. Efficacy of a belly board device with CT-simulation in reducing small bowel volume within pelvic irradiation fields. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 67–76. [Google Scholar] [CrossRef]
- Anderson, C.; Koshy, M.; Staley, C.; Esiashvili, N.; Ghavidel, S.; Fowler, Z.; Fox, T.; Esteves, F.; Landry, J.; Godette, K. PET-CT Fusion in Radiation Management of Patients with Anorectal Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 155–162. [Google Scholar] [CrossRef]
- Myerson, R.J.; Garofalo, M.C.; El Naqa, I.; Abrams, R.A.; Apte, A.; Bosch, W.R.; Das, P.; Gunderson, L.L.; Hong, T.S.; Kim, J.J.J.; et al. Elective Clinical Target Volumes for Conformal Therapy in Anorectal Cancer: A Radiation Therapy Oncology Group Consensus Panel Contouring Atlas. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 824–830. [Google Scholar] [CrossRef] [Green Version]
- Lengelé, B.; Scalliet, P. Anatomical bases for the radiological delineation of lymph node areas. Part III: Pelvis and lower limbs. Radiother. Oncol. 2009, 92, 22–33. [Google Scholar] [CrossRef]
- Ng, M.; Leong, T.; Chander, S.; Chu, J.; Kneebone, A.; Carroll, S.; Wiltshire, K.; Ngan, S.; Kachnic, L. Australasian Gastrointestinal Trials Group (AGITG) contouring atlas and planning guidelines for intensity-modulated radiotherapy in anal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1455–1462. [Google Scholar] [CrossRef]
- Gay, H.A.; Barthold, H.J.; O’Meara, E.; Bosch, W.R.; El Naqa, I.; Al-Lozi, R.; Rosenthal, S.A.; Lawton, C.; Lee, W.R.; Sandler, H.; et al. Pelvic normal tissue contouring guidelines for radiation therapy: A radiation therapy oncology group consensus panel atlas. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e353–e362. [Google Scholar] [CrossRef] [Green Version]
- Eng, C.; Chang, G.J.; Das, P.; Rodriguez-Bigas, M.; Skibber, J.M.; Qiao, W.; Rosner, G.L.; Ukegbu, L.T.; Wolff, R.A.; Crane, C.H. Phase II study of capecitabine and oxaliplatin with concurrent radiation therapy (XELOX-XRT) for squamous cell carcinoma of the anal canal. J. Clin. Oncol. 2009, 27, 4116. [Google Scholar] [CrossRef]
- Ugurluer, G.; Ballerini, G.; Moeckli, R.; Matzinger, O.; Bourhis, J.; Ozsahin, M. Helical tomotherapy for the treatment of anal canal cancer: A dosimetric comparison with 3D conformal radiotherapy. Tumori 2015, 101, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Vieillot, S.; Azria, D.; Lemanski, C.; Moscardo, C.L.; Gourgou, S.; Dubois, J.B.; Aillères, N.; Fenoglietto, P. Plan comparison of volumetric-modulated arc therapy (RapidArc) and conventional intensity-modulated radiation therapy (IMRT) in anal canal cancer. Radiat. Oncol. 2010, 5, 92. [Google Scholar] [CrossRef] [Green Version]
- Cendales, R.; Vásquez, J.; Arbelaez, J.; Bobadilla, I.; Torres, F.; Gaitan, A. IMRT, Rapidarc® and conformal radiotherapy in the treatment of tumours of the anal canal. Ecancermedicalscience 2014, 8, 469. [Google Scholar]
- Tozzi, A.; Cozzi, L.; Iftode, C.; Ascolese, A.; Campisi, M.C.; Clerici, E.; Comito, T.; De Rose, F.D.; Fogliata, A.; Franzese, C.; et al. Radiation therapy of anal canal cancer: From conformal therapy to volumetric modulated arc therapy. BMC Cancer 2014, 14, 833. [Google Scholar] [CrossRef] [Green Version]
- Rose, B.; Mitra, D.; Hong, T.S.; Jee, K.W.; Niemierko, A.; Drapek, L.N.; Blaszkowsky, L.S.; Allen, J.N.; Murphy, J.E.; Clark, J.W.; et al. Irradiation of anatomically defined pelvic subsites and acute hematologic toxicity in anal cancer patients undergoing chemoradiation. Pract. Radiat. Oncol. 2017, 7, e291–e297. [Google Scholar] [CrossRef] [PubMed]
- Bazan, J.G.; Luxton, G.; Mok, E.C.; Koong, A.C.; Chang, D.T. Normal tissue complication probability modeling of acute hematologic toxicity in patients treated with intensity-modulated radiation therapy for squamous cell carcinoma of the anal canal. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 700–706. [Google Scholar] [CrossRef]
- Lee, A.Y.; Golden, D.W.; Bazan, J.G.; Kopec, M.; Pelizzari, C.A.; Aggarwal, S.; Chang, D.T.; Liauw, S.L. Hematologic Nadirs During Chemoradiation for Anal Cancer: Temporal Characterization and Dosimetric Predictors. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; Fiandra, C.; Arcadipane, F.; Trino, E.; Giglioli, F.R.; Ragona, R.; Ricardi, U. Incorporating 18FDG-PET-defined pelvic active bone marrow in the automatic treatment planning process of anal cancer patients undergoing chemo-radiation. BMC Cancer 2017, 17, 710. [Google Scholar] [CrossRef] [Green Version]
- Arcadipane, F.; Silvetti, P.; Olivero, F.; Gastino, A.; De Luca, V.; Mistrangelo, M.; Cassoni, P.; Racca, P.; Gallio, E.; Lesca, A.; et al. Bone marrow-sparing IMRT in anal cancer patients undergoing concurrent chemo-radiation: Results of the first phase of a prospective phase II trial. Cancers 2020, 12, 3306. [Google Scholar] [CrossRef]
- Mirabeau-Beale, K.; Hong, T.S.; Niemierko, A.; Ancukiewicz, M.; Blaszkowsky, L.S.; Crowley, E.M.; Cusack, J.C.; Drapek, L.C.; Kovalchuk, N.; Markowski, M.; et al. Clinical and treatment factors associated with vaginal stenosis after definitive chemoradiation for anal canal cancer. Pract. Radiat. Oncol. 2015, 5, e113–e118. [Google Scholar] [CrossRef]
- Marshall, D.C.; Ghiassi-Nejad, Z.; Powers, A.R.; Argiriadi, P.; Reidenberg, J.S.; Ru, M.; Dumane, V.A.; Buckstein, M.; Goodman, K.A.; Schnur, J.; et al. A Dosimetric Comparison Of A Novel Clitoris-Sparing Intensity Modulated Radiotherapy (IMRT) Approach vs. Standard IMRT for Treatment of Female Patients with Anal Cancer. Int. J. Radiat. Oncol. 2020, 108, e636. [Google Scholar] [CrossRef]
- Arians, N.; Häfner, M.; Krisam, J.; Lang, K.; Wark, A.; Koerber, S.A.; Hommertgen, A.; Debus, J. Intrafractional vaginal dilation in anal cancer patients undergoing pelvic radiotherapy (DILANA)- A prospective, randomized, 2-armed phase-II-trial. BMC Cancer 2020, 20, 52–58. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Sebag-Montefiore, D.; Meadows, H.M.; Cunningham, D.; Begum, R.; Adab, F.; Benstead, K.; Harte, R.J.; Stewart, J.; Beare, S.; et al. Best time to assess complete clinical response after chemoradiotherapy in squamous cell carcinoma of the anus (ACT II): A post-hoc analysis of randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Cummings, B.J. Concomitant radiotherapy and chemotherapy for anal cancer. Semin.Oncol. 1992, 19, 102–108. [Google Scholar]
- Mackowski, A.; Levitt, M.; Makin, G.; Salama, P.; Tan, P.; Penter, C.; Platell, C. Anal squamous cell carcinoma: Are we improving outcomes? ANZ J. Surg. 2018, 88, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Dee, E.C.; Eyler, C.E.; Sanford, N.N.; Wo, J.Y. Local Therapy Options for Recurrent Rectal and Anal Cancer: Current Strategies and New Directions. Curr. Colorectal Cancer Rep. 2019, 15, 157–169. [Google Scholar] [CrossRef]
- Hallemeier, C.L.; You, Y.N.; Larson, D.W.; Dozois, E.J.; Nelson, H.; Klein, K.A.; Miller, R.C.; Haddock, M.G. Multimodality therapy including salvage surgical resection and intraoperative radiotherapy for patients with squamous-cell carcinoma of the anus with residual or recurrent disease after primary chemoradiotherapy. Dis. Colon Rectum 2014, 57, 442–448. [Google Scholar] [CrossRef]
- Renehan, A.G.; O’Dwyer, S.T. Management of Local Disease Relapse. Color. Dis. 2011, 13, 44–52. [Google Scholar] [CrossRef]
- Bignell, M.; Chave, H.; Branagan, G. Outcome of surgery for recurrent anal cancer: Results from a tertiary referral centre. Color. Dis. 2018, 20, 771–777. [Google Scholar] [CrossRef]
- Pesi, B.; Scaringi, S.; Di Martino, C.; Batignani, G.; Giudici, F.; Bisogni, D.; Tonelli, F.; Bechi, P. Results of Surgical Salvage Treatment for Anal Canal Cancer: A Retrospective Analysis with Overview of the Literature. Dig. Surg. 2017, 34, 380–386. [Google Scholar] [CrossRef]
- Akbari, R.P.; Paty, P.B.; Guillem, J.G.; Weiser, M.R.; Temple, L.K.; Minsky, B.D.; Saltz, L.; Wong, W.D. Oncologic outcomes of salvage surgery for epidermoid carcinoma of the anus initially managed with combined modality therapy. Dis. Colon Rectum 2004, 47, 1136–1144. [Google Scholar] [CrossRef]
- Schiller, D.E.; Cummings, B.J.; Rai, S.; Le, L.W.; Last, L.; Davey, P.; Easson, A.; Smith, A.J.; Swallow, C.J. Outcomes of salvage surgery for squamous cell carcinoma of the anal canal. Ann. Surg. Oncol. 2007, 14, 2780–2789. [Google Scholar] [CrossRef] [PubMed]
- Mullen, J.T.; Rodriguez-Bigas, M.A.; Chang, G.J.; Barcenas, C.H.; Crane, C.H.; Skibber, J.M.; Feig, B.W. Results of surgical salvage after failed chemoradiation therapy for epidermoid carcinoma of the anal canal. Ann. Surg. Oncol. 2007, 14, 478–483. [Google Scholar] [CrossRef]
- Hannes, S.; Reinisch, A.; Bechstein, W.O.; Habbe, N. Salvage abdominoperineal excisions in recurrent anal cancer—impact of different reconstruction techniques on outcome, morbidity, and complication rates. Int. J. Colorectal Dis. 2016, 31, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Ellenhorn, J.D.I.; Enker, W.E.; Quan, S.H.Q. Salvage abdominoperineal resection following combined chemotherapy and radiotherapy for epidermoid carcinoma of the anus. Ann. Surg. Oncol. 1994, 1, 105–110. [Google Scholar] [CrossRef]
- Hagemans, J.A.W.; Blinde, S.E.; Nuyttens, J.J.; Morshuis, W.G.; Mureau, M.A.M.; Rothbarth, J.; Verhoef, C.; Burger, J.W.A. Salvage Abdominoperineal Resection for Squamous Cell Anal Cancer: A 30-Year Single-Institution Experience. Ann. Surg. Oncol. 2018, 25, 1970–1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, P.J.; Svensson, C.; Goldman, S.; Glimelius, B. Salvage abdominoperineal resection in anal epidermoid cancer. Br. J. Surg. 2002, 89, 1425–1429. [Google Scholar] [CrossRef]
- Severino, N.P.; Chadi, S.A.; Rosen, L.; Coiro, S.; Choman, E.; Berho, M.; Wexner, S.D. Survival following salvage abdominoperineal resection for persistent and recurrent squamous cell carcinoma of the anus: Do these disease categories affect survival? Color. Dis. 2016, 18, 959–966. [Google Scholar] [CrossRef]
- Singh, M.; Kinsley, S.; Huang, A.; Ricci, J.A.; Clancy, T.E.; Irani, J.; Goldberg, J.; Breen, E.; Bleday, R.; Talbot, S.G. Gracilis Flap Reconstruction of the Perineum: An Outcomes Analysis. J. Am. Coll. Surg. 2016, 223, 602–610. [Google Scholar] [CrossRef]
- Chessin, D.B.; Hartley, J.; Cohen, A.M.; Mazumdar, M.; Cordeiro, P.; Disa, J.; Mehrara, B.; Minsky, B.D.; Paty, P.; Weiser, M.; et al. Rectus flap reconstruction decreases perineal wound complications after pelvic chemoradiation and surgery: A cohort study. Ann. Surg. Oncol. 2005, 12, 104–110. [Google Scholar] [CrossRef]
- Eng, C.; Chang, G.J.; You, Y.N.; Das, P.; Rodriguez-Bigas, M.; Xing, Y.; Vauthey, J.-N.; Rogers, J.E.; Ohinata, A.; Pathak, P.; et al. The role of systemic chemotherapy and multidisciplinary management in improving the overall survival of patients with metastatic squamous cell carcinoma of the anal canal. Oncotarget 2015, 5, 11133–11142. [Google Scholar] [CrossRef] [Green Version]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F. Anal Carcinoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. Available online: https://jnccn.org/view/journals/jnccn/16/7/article-p852.xml (accessed on 9 March 2021).
- Sclafani, F.; Morano, F.; Cunningham, D.; Baratelli, C.; Kalaitzaki, E.; Watkins, D.; Starling, N.; Chau, I.; Rao, S. Platinum-Fluoropyrimidine and Paclitaxel-Based Chemotherapy in the Treatment of Advanced Anal Cancer Patients. Oncologist 2017, 22, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.; Sclafani, F.; Eng, C.; Adams, R.A.; Guren, M.G.; Sebag-Montefiore, D.; Benson, A.; Bryant, A.; Peckitt, C.; Segelov, E.; et al. International rare cancers initiative multicenter randomized phase II trial of cisplatin and fluorouracil versus carboplatin and paclitaxel in advanced anal cancer: InterAAct. J. Clin. Oncol. 2020, 38, 2510–2518. [Google Scholar] [CrossRef]
- Kim, S.; François, E.; André, T.; Samalin, E.; Jary, M.; El Hajbi, F.; Baba-Hamed, N.; Pernot, S.; Kaminsky, M.C.; Bouché, O.; et al. Docetaxel, cisplatin, and fluorouracil chemotherapy for metastatic or unresectable locally recurrent anal squamous cell carcinoma (Epitopes-HPV02): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 1094–1106. [Google Scholar] [CrossRef]
- Strauss, J.; Gatti-Mays, M.E.; Redman, J.; Madan, R.A.; Lamping, E.; Manu, M.; Burmeister, A.; Marte, J.L.; Cordes, L.M.; Ojalvo, L.; et al. Safety and activity of M7824, a bifunctional fusion protein targeting PD-L1 and TGF-β, in patients with HPV associated cancers. J. Clin. Oncol. 2018, 36, 3007. [Google Scholar] [CrossRef]
- Ott, P.A.; Piha-Paul, S.A.; Munster, P.; Pishvaian, M.J.; van Brummelen, E.M.J.; Cohen, R.B.; Gomez-Roca, C.; Ejadi, S.; Stein, M.; Chan, E.; et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1036–1041. [Google Scholar] [CrossRef]
- Osborne, E.M.; Eng, C.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Chang, G.J.; Nancy You, Y.Q.; Bednarski, B.K.; Minsky, B.D.; Delclos, M.E.; Koay, E.; et al. Hyperfractionated accelerated reirradiation for patients with recurrent anal cancer previously treated with definitive chemoradiation. Am. J. Clin. Oncol. Cancer Clin. Trials 2018, 41, 632–637. [Google Scholar] [CrossRef]
- Wright, J.L.; Gollub, M.J.; Weiser, M.R.; Saltz, L.B.; Wong, W.D.; Paty, P.B.; Temple, L.K.; Guillem, J.G.; Minsky, B.D.; Goodman, K.A. Surgery and high-dose-rate intraoperative radiation therapy for recurrent squamous-cell carcinoma of the anal canal. Dis. Colon Rectum 2011, 54, 1090–1097. [Google Scholar] [CrossRef]
- Ojerholm, E.; Kirk, M.L.; Thompson, R.F.; Zhai, H.; Metz, J.M.; Both, S.; Ben-Josef, E.; Plastaras, J.P. Pencil-beam scanning proton therapy for anal cancer: A dosimetric comparison with intensity-modulated radiotherapy. Acta Oncol. 2015, 54, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Czito, B.; Yin, F.F.; Willett, C. Advanced radiation therapy technologies in the treatment of rectal and anal cancer: Intensity-modulated photon therapy and proton therapy. Clin. Colorectal Cancer 2007, 6, 348–356. [Google Scholar] [CrossRef]
- Anand, A.; Bues, M.; Rule, W.G.; Keole, S.R.; Beltran, C.J.; Yin, J.; Haddock, M.G.; Hallemeier, C.L.; Miller, R.C.; Ashman, J.B. Scanning proton beam therapy reduces normal tissue exposure in pelvic radiotherapy for anal cancer. Radiother. Oncol. 2015, 117, 505–508. [Google Scholar] [CrossRef]
- Wo, J.Y.; Ben-Josef, E.; Yeap, B.Y.; Jiang, W.; DeLaney, T.F.; Ryan, D.P.; Metz, J.M.; Drapek, L.C.; Allen, J.N.; Clark, J.W.; et al. A Pilot Feasibility Study of Definitive Concurrent Chemoradiation with Pencil Beam Scanning Proton Beam in Combination with 5-fluorouracil and Mitomycin-c for Carcinoma of the Anal Canal. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, S63. [Google Scholar] [CrossRef]
- Colaco, R.J.; Nichols, R.C.; Huh, S.; Getman, N.; Ho, M.W.; Li, Z.; Morris, C.G.; Mendenhall, W.M.; Mendenhall, N.P.; Hoppe, B.S. Protons offer reduced bone marrow, small bowel, and urinary bladder exposure for patients receiving neoadjuvant radiotherapy for resectable rectal cancer. J. Gastrointest. Oncol. 2014, 5, 3–8. [Google Scholar] [PubMed]
- Sebag-Montefiore, D.; Adams, R.; Bell, S.; Berkman, L.; Gilbert, D.C.; Glynne-Jones, R.; Goh, V.; Gregory, W.; Harrison, M.; Kachnic, L.A.; et al. The Development of an Umbrella Trial (PLATO) to Address Radiation Therapy Dose Questions in the Locoregional Management of Squamous Cell Carcinoma of the Anus. Int. J. Radiat. Oncol. 2016, 96, E164–E165. [Google Scholar] [CrossRef] [Green Version]
- Doll, C.M.; Moughan, J.; Klimowicz, A.; Ho, C.K.; Kornaga, E.N.; Lees-Miller, S.P.; Ajani, J.A.; Crane, C.H.; Kachnic, L.A.; Okawara, G.S.; et al. Significance of Co-expression of Epidermal Growth Factor Receptor and Ki67 on Clinical Outcome in Patients With Anal Cancer Treated With Chemoradiotherapy: An Analysis of NRG Oncology RTOG 9811. Int. J. Radiat. Oncol. 2017, 97, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Garg, M.K.; Zhao, F.; Sparano, J.A.; Palefsky, J.; Whittington, R.; Mitchell, E.P.; Mulcahy, M.F.; Armstrong, K.I.; Nabbout, N.H.; Kalnicki, S.; et al. Cetuximab plus chemoradiotherapy in immunocompetent patients with anal carcinoma: A phase II Eastern cooperative oncology group-American college of radiology imaging network cancer research group trial (E3205). J. Clin. Oncol. 2017, 35, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.A.; Lee, J.Y.; Palefsky, J.; Henry, D.H.; Wachsman, W.; Rajdev, L.; Aboulafia, D.; Ratner, L.; Fitzgerald, T.J.; Kachnic, L.; et al. Cetuximab plus chemoradiotherapy for HIV-associated anal carcinoma: A phase II AIDS malignancy consortium trial. J. Clin. Oncol. 2017, 35, 727–733. [Google Scholar] [CrossRef] [Green Version]
- Safran, H.; Leonard, K.L.; Perez, K.; Vrees, M.; Klipfel, A.; Schechter, S.; Oldenburg, N.; Roth, L.; Shah, N.; Rosati, K.; et al. Tolerability of ADXS11-001 Lm-LLO Listeria-Based Immunotherapy With Mitomycin, Fluorouracil, and Radiation for Anal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 1175–1178. [Google Scholar] [CrossRef]
- Safran, H.; Leonard, K.L.; DiPetrillo, T.A.; Klipfel, A.; Schechter, S.; Oldenburg, N.; Vrees, M.; Roth, L.; Shah, N.; Mantripragada, K.C.; et al. ADXS11-001 Lm-LLO Immunotherapy, Mitomycin, 5-fluorouracil (5-FU) and Intensity-modulated radiation therapy (IMRT) for Anal Cancer. J. Clin. Oncol. 2017, 35, e15072. [Google Scholar] [CrossRef]
- Das, P.; Bhatia, S.; Eng, C.; Ajani, J.A.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Chang, G.J.; Bhosale, P.; Delclos, M.E.; Krishnan, S.; et al. Predictors and Patterns of Recurrence After Definitive Chemoradiation for Anal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Eng, C. Anal cancer: Current and future methodology. Cancer Investig. 2006, 24, 535–544. [Google Scholar] [CrossRef]
- Ajani, J.A.; Carrasco, C.H.; Jackson, D.E.; Wallace, S. Combination of cisplatin plus fluoropyrimidine chemotherapy effective against liver metastases from carcinoma of the anal canal. Am. J. Med. 1989, 878, 221–224. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [Green Version]

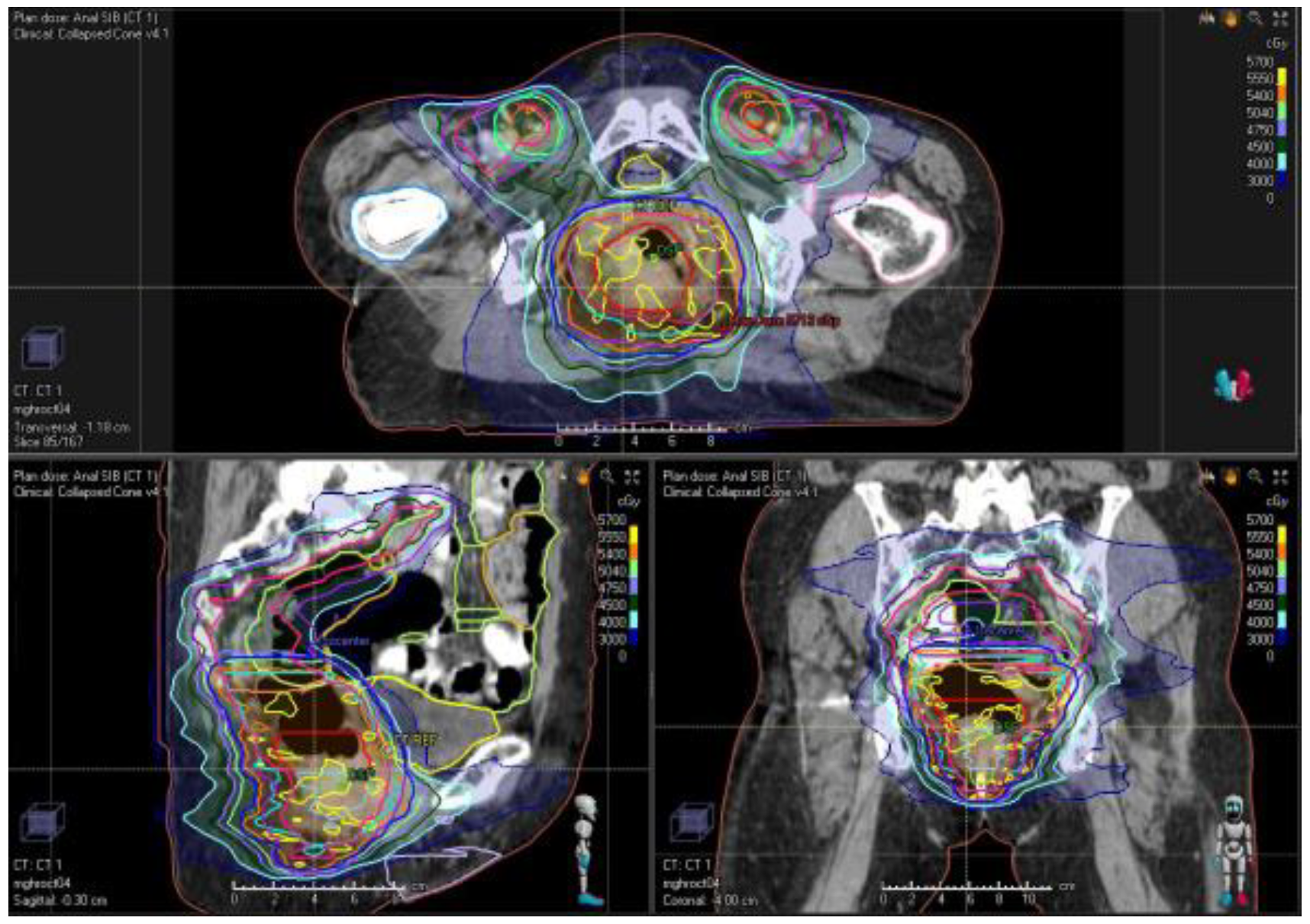
| Trial | Inclusion | Design | Treatments | Results |
|---|---|---|---|---|
| Radiotherapy vs. chemoradiotherapy | ||||
| UKCCCR Anal Cancer Trial (ACT I) [44,45] | Localized and metastatic, barring exclusion criteria (e.g., previous treatment, cancer at another site, or tumor considered suitable for local excision only [T1 N0]) | 585 patients; 295 in the CRT arm and 290 in the RT-only arm | 45 Gy EBRT and 15 Gy EBRT boost or 25 Gy brachytherapy boost, with vs. without concurrent MMC and 5FU | 42-month follow-up: CRT vs. RT alone locoregional recurrence relative risk 0.54 (95% CI 0.42–0.69, p < 0.0001) Anal cancer-specific mortality relative risk 0.71 (95%CI 0.53–0.95, p = 0.02) 12-year follow-up: for every 100 patients treated with CRT, 25.3 fewer patients experienced locoregional recurrence and 12.5 fewer experienced anal cancer-specific death compared to the RT-only cohort |
| EORTC [46] | T3-4N0-3 or T1-2N1-3 anal cancer | 110 randomized, 103 eligible, 51 to CRT and 52 to RT alone | 45 Gy EBRT with 15 Gy or 30 Gy EBRT boost with vs. without concurrent 5FU and MMC | 5-year local control greater for CRT vs. RT alone (68 vs. 51%, p = 0.02) 3-year overall survival similar between groups (65 vs. 72%, p = 0.17) |
| Omission of MMC | ||||
| RTOG 87-04/ECOG 1289 [48] | Patients with any epidermoid malignancy of the anal canal in which the primary tumor was measurable (any T or N stage) | 310 randomized, 295 eligible, 145 to EBRT + 5FU, 146 EBRT + 5FU + MMC | 45–50.4 Gy EBRT with 5FU with vs. without MMC | MMC associated with greater colostomy-free survival (71% for 5FU and MMC vs. 59% for 5FU alone; p = 0.014) and improved disease-free survival (73% for 5FU and MMC vs. 51% for 5FU alone; p = 0.0003) No OS difference and greater toxicity with MMC |
| Cisplatin vs. MMC | ||||
| RTOG 98-11 [7,49] | T2-4NanyM0 (T1 or M1 excluded) | 682 randomized, 649 eligible, 325 to RT + 5FU/MMC and 324 to RT + 5FU/cisplatin | 45 Gy with allowance for 10–14 Gy boost with 5FU + MMC vs. RT + 5FU + cisplatin | 5-year colostomy-free survival improved in MMC arm (72 vs. 65%, p = 0.05); DFS improved in MMC arm (67.8 vs. 57.8%, p = 0.006); OS improved in MMC arm (78.3 vs. 70.7%, p = 0.026) |
| ACT II [50] | Any T, any N, no distant metastases | 940 randomized, 472 in RT + 5FU/MMC cohort and 468 in RT + 5FU/cisplatin cohort | 50.4 Gy with continuous 5FU, with bolus cisplatin or MMC | 3-year colostomy-free similar (68% in MMC arm, 67% in cisplatin arm, p = 0.94); DFS similar (69% in both arms, p = 0.63); OS similar (79% in MMC arm, 77% in cisplatin arm, p = 0.7) |
| RT dose escalation vs. de-escalation | ||||
| RTOG 92-08 [36,48,51] | Any except T1N0 | Single-arm phase II study with 47 patients: standard chemotherapy (5FU/MMC) + high dose RT | 2 weeks of RT, then mandatory gap, total RT dose 59.4 Gy | Median follow-up duration 12 years, estimated 5-year DFS 53%; estimated 5-year colostomy-free survival 58%; estimated 5-year OS 85% |
| ACCORD-03 [52] | Patients with tumors ≥ 40 mm, or <40 mm and N1-3M0 | 2 × 2 factorial randomization: neoadjuvant chemotherapy and CRT (5FU/cisplatin) +/− high-dose RT; 283 of 307 achieved full treatment | 45 Gy/25 fractions with standard dose boost (15 Gy) vs. high-dose boost (20–25 Gy) with EBRT or brachytherapy | Similar colostomy-free survival (the primary endpoint) for standard vs. escalated boost dose (78 vs. 74%, p = 0.067); nonsignificant improvement in 5-year local control rate for escalated boost dose (83.1%) vs. standard-boost (78.2%) |
| Intensity-modulated radiotherapy (IMRT) | ||||
| RTOG 05-29 [53] | T2N0, T3-4N0-3 | Phase II trial evaluating CRT with concurrent 5FU/MMC and dose-painted IMRT | T2N0: 42 Gy elective nodal and 50.4 Gy anal tumor PTVs in 28 fractions T3-4N0-3: 45 Gy elective nodal and 50.4 Gy < 3 cm or 54 Gy > 3 cm metastatic nodal and 54 Gy anal tumor PTVs in 30 fractions | Dose-painted IMRT associated with reduced grade 3+ genitourinary and gastrointestinal toxicity (22 vs. 36%, p = 0.014), and grade 3+ dermatologic toxicity (20 vs. 47%, p < 0.001) when compared to the historical RTOG 98-11 MMC arm |
| Pencil beam scanning proton beam radiotherapy (PBS-PT) | ||||
| MGH prospective series [54] | T1-4, N0-3 disease | 25 patients, of whom 23 completed treatment per protocol | PBS-PT per RTOG 0529 dose schema and concurrent 5-FU/MMC | Grade 3+ radiation dermatitis rate 24%; overall rate of clinical complete response was 88%; 2-year local failure rate 12%, colostomy-free survival 72%, progression-free survival 80%, and overall survival 84% |
| Immunooncology | ||||
| Multicenter US prospective series [55] | Patients with anal cancer (squamous cell only, adenocarcinoma excluded) and at least one previous systemic therapy for surgically unresectable or metastatic disease | Phase II study of 37 patients with metastatic disease | Nivolumab IV every 2 weeks (3 mg/kg) | Of 37 patients who received at least one dose of nivolumab, 9 (24%) demonstrated tumor response, of whom 2 experienced a complete response |
| Trial/NCT ID | Inclusion | Design | Treatments |
|---|---|---|---|
| RT dose escalation vs. de-escalation | |||
| ECOG-DECREASE [75] | T1-2N0M0 | Randomized phase II: standard-dose CRT vs. de-intensified CRT | 28 fractions vs. de-intensified 20–23 fractions of IMRT with MMC and 5FU or capecitabine Standard: T1-T2 N0: 50.4 Gy to primary tumor with 42 Gy to elective nodal regions, all in 28 fractions De-intensified: T1 N0: 36 Gy to primary tumor with 32 Gy to elective nodal regions, all in 20 fractions T2 N0: 41.4 Gy to primary tumor with 34.5 Gy to elective nodal regions, all in 23 fractions |
| ACT III [76,157] | T1N0 | Single-arm phase II: dose reduced CRT | No RT for >1 mm margin; for <1 mm margin, 41.4 Gy in 23 fractions |
| ACT IV [76,157] | T1-2, N0 | Randomized phase II: standard chemotherapy (5FU/MMC) and standard vs. de-intensified RT | Standard RT arm of 50.4 Gy in 28 fractions or de-intensified radiation arm of 41.4 Gy in 23 fractions |
| ACT V [76,157] | T3-4, N0-X | Randomized phase II/III: Standard chemotherapy (5FU/MMC) with standard vs. 2 escalated radiation doses | 53.2 Gy, 58.8 Gy, or 61.6 Gy all in 28 fractions with standard concurrent chemo. One of the dose-escalation arms will proceed to phase III |
| Proton therapy | |||
| NCT03690921 (MDACC) | Non-metastatic disease | Single-arm phase II trial assessing adverse effects of proton RT and standard chemotherapy (cisplatin and 5FU) | Linear energy transfer (LET)-optimized intensity-modulated proton therapy (IMPT) |
| NCT03018418 (Cincinnati) | T2-4 disease with any N | Prospective pilot study evaluating the feasibility of intensity-modulated proton therapy in reducing RT toxicity | Primary target volume 50.4–54 CGE in 28–30 fractions; nodal volumes 42–54 CGE in 28–30 fractions, with 5FU and MMC |
| NCT04462042 (Umeå University/Sweden) | T2 (>4 cm)-4, N0-1c, M0 | Open label, multi-center, randomised phase II study, comparing proton to photon RT | Photon: primary tumor and nodal metastases >2 cm 57.5 Gy in 27 fractions (VMAT/IMRT/tomotherapy); nodal metastases up to 2 cm will receive 50.5 Gy in 27 fractions; elective nodes will receive 41.6 Gy Proton: spot scanning, total dose to the primary tumor target and node metastases >2 cm is 57.5 Gy(RBE) in 27 fractions; nodal metastases up to 2 cm will receive 50.5 Gy(RBE) in 27 fractions; elective nodes will receive 41.6 Gy(RBE) |
| Immuno-oncology | |||
| NCT03233711 | stage IIB (T3N0M0 only), IIIA (T2N1M0), IIIB (T4N0M0), or IIIC (T3N1M0, T4N1M0) invasive squamous cell carcinoma of the anus or anorectum | Randomized phase III trial of nivolumab after combined modality therapy | Up to 6 months of nivolumab IV vs. up to 6 months of observation |
| NCT02919969 | Metastatic anal cancer with no limitations to prior treatment | Phase II study of pembrolizumab | Pembrolizumab 200 mg IV infusion every 3 weeks |
| NCT03519295 | Unresectable locally advanced, recurrent, or metastatic squamous cell anal carcinoma | mDCF (docetaxel, cisplatin, 5FU) with vs. without atezolizumab | 8 cycles of mDCF with vs. without MPDL3280A (atezolizumab) for 12 months |
| NCT04230759 (RADIANCE trial) | Locally advanced disease (IIB: T3N0M0; IIIA: T1-2N1M0; IIIB: T4N0M0; IIIC: T3-4N1M0; T2 > 4 cm Nany) | Phase II trial assessing the efficacy of durvalumab in combination with CRT with MMC + 5FU | 53.2–58.9 Gy with nodal and elective nodal irradiation, with MMC + 5FU, with vs. without 12 doses of durvalumab |
| NCT03944252 | Progression on or after first-line systemic therapy for surgically unresectable or metastatic disease | Randomized Phase II trial of cetuximab and avelumab or avelumab alone for unresectable, locally advanced, or metastatic anal cancer progressed after at least one line of systemic therapy | Avelumab IV with vs. without cetuximab, given until progression of disease |
| NCT04444921 | Inoperable, recurrent, or metastatic disease | Randomized phase III trial of nivolumab with chemotherapy in treatment-naive metastatic anal cancer | Carboplatin and paclitaxel with vs. without nivolumab |
| NCT01285778 | T2-4, any N | Phase II assessing the efficacy and toxicity of radiotherapy with 5FU, MMC, and panitumumab | Radiation therapy will be administered concurrent with chemotherapy and panitumumab treatment (IV over 8 weeks) |
| NCT04472429 (POD1UM-303/InterAACT 2) | Inoperable locally recurrent or metastatic SCAC with no prior systemic therapy other than chemotherapy administered with radiotherapy as a radiosensitizer | Phase III double-blind randomized trial of carboplatin-paclitaxel with Retifanlimab or placebo in patients with inoperable locally recurrent or metastatic disease with no prior systemic chemotherapy | Carboplatin, paclitaxel, and either placebo or retifanlimab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dee, E.C.; Byrne, J.D.; Wo, J.Y. Evolution of the Role of Radiotherapy for Anal Cancer. Cancers 2021, 13, 1208. https://doi.org/10.3390/cancers13061208
Dee EC, Byrne JD, Wo JY. Evolution of the Role of Radiotherapy for Anal Cancer. Cancers. 2021; 13(6):1208. https://doi.org/10.3390/cancers13061208
Chicago/Turabian StyleDee, Edward Christopher, James D. Byrne, and Jennifer Y. Wo. 2021. "Evolution of the Role of Radiotherapy for Anal Cancer" Cancers 13, no. 6: 1208. https://doi.org/10.3390/cancers13061208
APA StyleDee, E. C., Byrne, J. D., & Wo, J. Y. (2021). Evolution of the Role of Radiotherapy for Anal Cancer. Cancers, 13(6), 1208. https://doi.org/10.3390/cancers13061208







