Research on Anal Squamous Cell Carcinoma: Systemic Therapy Strategies for Anal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Chemotherapy for Locoregional Disease
3. Role of Induction or Maintenance Chemotherapy
4. Systemic Therapy for Metastatic Disease
5. The Evolving Role of Immune Checkpoint Inhibitors
6. Role of Human Papillomavirus in Treatment Strategies
7. Precision Medicine and Targeted Therapy
7.1. Epidermal Growth Factor Receptor Blockade
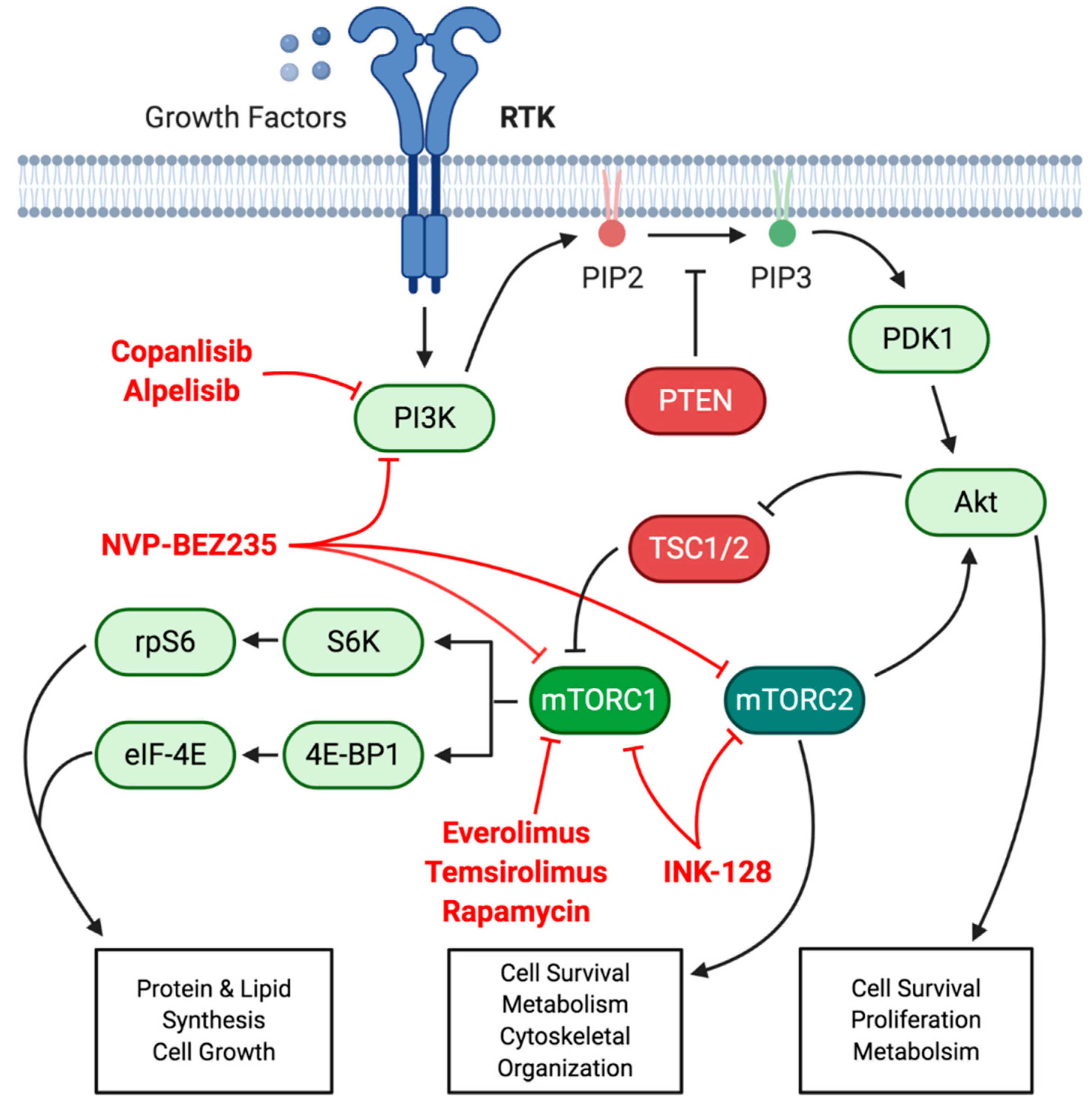
7.2. PI3K/Akt/mTOR Signaling Axis
7.3. Other Opportunities for Targeted Therapy
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Skibber, J.; Rodriguez-Bigas, M.A.; Gordon, P.H. Surgical consideration in anal cancer. Surg. Oncol. Clin. N. Am. 2004, 13, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Serup-Hansen, E.; Linnemann, D.; Skovrider-Ruminski, W.; Hogdall, E.; Geertsen, P.F.; Havsteen, H. Human papillomavirus genotyping and p16 expression as prognostic factors for patients with American Joint Committee on Cancer stages I to III carcinoma of the anal canal. J. Clin. Oncol. 2014, 32, 1812–1817. [Google Scholar] [CrossRef] [Green Version]
- Shin, M.-K.; Payne, S.; Bilger, A.; Matkowskyj, K.A.; Carchman, E.; Meyer, D.S.; Bentires-Alj, M.; Deming, D.A.; Lambert, P.F. Activating mutations in Pik3ca contribute to anal carcinogenesis in the presence or absence of HPV-16 oncogenes. Clin. Cancer Res. 2019, 25, 1889–1900. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Greenall, M.J.; Quan, S.H.; DeCosse, J.J. Epidermoid cancer of the anus. Br. J. Surg. 1985, 72, S97–S103. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, B.G.; Evans, H.L. Carcinoma of the anal canal: A study of 79 cases. Am. J. Clin. Pathol. 1985, 83, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Nigro, N.D.; Vaitkevicius, V.K.; Considine, B. Combined therapy for cancer of the anal canal: A preliminary report. Dis. Colon Rectum 1974, 17, 354–356. [Google Scholar] [CrossRef]
- Cummings, B.; Keane, T.; O’Sullivan, B.; Wong, C.; Catton, C. Epidermoid anal cancer: Treatment by radiation alone or by radiation and 5-fluorouracil with and without mitomycin C. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 1115–1125. [Google Scholar] [CrossRef]
- Bartelink, H.; Roelofsen, F.; Eschwege, F.; Rougier, P.; Bosset, J.F.; Gonzalez, D.G.; Peiffert, D.; Van Glabbeke, M.; Pierart, M. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: Results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J. Clin. Oncol. 1997, 15, 2040–2049. [Google Scholar]
- Arnott, S.; Cunningham, J.; Gallagher, J. UK Co-ordinating Committee on Cancer Research Anal Canal Trial Working Party. Epidermoid anal cancer: Results from the UKCCCR randomized trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. Lancet 1996, 348, 1049–1054. [Google Scholar]
- Northover, J.; Glynne-Jones, R.; Sebag-Montefiore, D.; James, R.; Meadows, H.; Wan, S.; Jitlal, M.; Ledermann, J. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br. J. Cancer 2010, 102, 1123–1128. [Google Scholar] [CrossRef] [Green Version]
- Flam, M.; John, M.; Pajak, T.F.; Petrelli, N.; Myerson, R.; Doggett, S.; Quivey, J.; Rotman, M.; Kerman, H.; Coia, L.; et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: Results of a phase III randomized intergroup study. J. Clin. Oncol. 1996, 14, 2527–2539. [Google Scholar] [CrossRef] [PubMed]
- Buckstein, M.; Arens, Y.; Wisnivesky, J.; Gaisa, M.; Goldstone, S.; Sigel, K. A Population-Based Cohort Analysis of Chemoradiation Versus Radiation Alone for Definitive Treatment of Stage I Anal Cancer in Older Patients. Dis. Colon Rectum 2018, 61, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Starling, N.; Rao, S.; Iveson, T.; Nicholson, M.; Coxon, F.; Middleton, G.; Daniel, F.; Oates, J.; Norman, A.R.; et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 2008, 358, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Twelves, C.; Scheithauer, W.; McKendrick, J.; Seitz, J.-F.; Van Hazel, G.; Wong, A.; Diaz-Rubio, E.; Gilberg, F.; Cassidy, J. Capecitabine versus 5-fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: Final results from the X-ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann. Oncol. 2012, 23, 1190–1197. [Google Scholar] [CrossRef]
- Goodman, K.A.; Rothenstein, D.; Lajhem, C.; Wu, A.; Cercek, A.; Saltz, L.B. Capecitabine plus mitomycin in patients undergoing definitive chemoradiation for anal squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, S32–S33. [Google Scholar] [CrossRef]
- Meulendijks, D.; Dewit, L.G.H.; Tomasoa, N.B.; Van Tinteren, H.; Beijnen, J.H.; Schellens, J.H.M.; Cats, A. Chemoradiotherapy with capecitabine for locally advanced anal carcinoma: An alternative treatment option. Br. J. Cancer 2014, 111, 1726–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thind, G.; Johal, B.; Follwell, M.; Kennecke, H.F. Chemoradiation with capecitabine and mitomycin-C for stage I-III anal squamous cell carcinoma. Radiat. Oncol. 2014, 9, 124. [Google Scholar] [CrossRef] [Green Version]
- Glynne-Jones, R.; Meadows, H.; Wan, S.; Gollins, S.; Leslie, M.; Levine, E.; McDonald, A.C.; Myint, S.; Samuel, L.; Sebag-Montefiore, D. EXTRA-a multicenter phase II study of chemoradiation using a 5 day per week oral regimen of capecitabine and intravenous mitomycin C in anal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.C.R.; Moniz, C.M.V.; Riechelmann, R.P.; Alex, A.K.; Braghirolli, M.I.; Bariani, G.; Nahas, C.S.R.; Hoff, P.M.G. Phase II study of capecitabine in substitution of 5-FU in the chemoradiotherapy regimen for patients with localized squamous cell carcinoma of the anal canal. J. Gastrointest Cancer 2016, 47, 75–81. [Google Scholar] [CrossRef]
- James, R.D.; Glynne-Jones, R.; Meadows, H.M.; Cunningham, D.; Myint, A.S.; Saunders, M.P.; Maughan, T.; McDonald, A.; Essapen, S.; Leslie, M.; et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): A randomized, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol. 2013, 14, 516–524. [Google Scholar] [CrossRef]
- Sebag-Montefiore, D.; Meadows, H.M.; Cunningham, D.; Plowman, P.N.; Hurman, D.C.; Davidson, N.; Grieve, R.; Levine, E.; Glynne-Jones, R. Three cytotoxic drugs combined with pelvic radiation and as maintenance chemotherapy for patients with squamous cell carcinoma of the anus (SCCA): Long-term follow-up of a phase II pilot study using 5-fluorouracil, mitomycin C and cisplatin. Radiother. Oncol. 2012, 104, 155–160. [Google Scholar] [CrossRef]
- Gunderson, L.L.; Winter, K.A.; Ajani, J.A.; Pedersen, J.E.; Moughan, J.; Benson, A.B.; Jr, C.R.T.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; et al. Long-term update of US GI Intergroup RTOG 98-11 phase III trial for anal carcinoma: Survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J. Clin. Oncol. 2012, 30, 4344–4351. [Google Scholar] [CrossRef]
- Spithoff, K.; Cummings, B.; Jonker, D.; Biagi, J. Chemoradiotherapy for squamous cell cancer of the anal canal: A systematic review. Clin. Oncol. R Coll Radiol. 2014, 26, 473–487. [Google Scholar] [CrossRef]
- Peiffert, D.; Tournier-Rangeard, L.; Gérard, J.-P.; Lemanski, C.; François, E.; Giovannini, M.; Cvitkovic, F.; Mirabel, X.; Bouché, O.; Luporsi, E.; et al. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: Final analysis of the randomized UNICANCER ACCORD 03 trial. J. Clin. Oncol. 2012, 30, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Horner, M.; Ries, L.A.G.; Krapcho, M.; Neyman, N.; Aminou, R.; Howlader, N.; Altekruse, S.F.; Feuer, E.J.; Huang, L.; Mariotto, A.; et al. SEER Cancer Statistics Review, 1975–2006; National Cancer Institute: Bethesda, MD, USA, 2009. [Google Scholar]
- National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Available online: https://seer.cancer.gov/ (accessed on 25 January 2021).
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Anal Carcinoma (Version 2). Available online: Https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 25 January 2021).
- Glynne-Jones, R.; Nilsson, P.J.; Aschele, C.; Goh, V.; Peiffert, D.; Cervantes, A.; Arnold, D. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Radiother. Oncol. 2014, 111, 330–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsunaga, M.; Miwa, K.; Oka, Y.; Nagasu, S.; Sakaue, T.; Fukahori, M.; Ushijima, T.; Akagi, Y. Successful Treatment of Metastatic Anal Canal Adenocarcinoma with mFOLFOX6 + Bevacizumab. Case Rep. Oncol. 2016, 9, 249–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondaca, S.; Chatila, W.K.; Bates, D.; Hechtman, J.F.; Cercek, A.; Segal, N.H.; Stadler, Z.K.; Varghese, A.M.; Kundra, R.; Capanu, M.; et al. FOLFCIS treatment and genomic correlates of response in advanced anal squamous cell cancer. Clin. Colorectal Cancer 2019, 18, e39–e52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; François, E.; André, T.; Samalin, E.; Jary, M.; El Hajbi, F.; Baba-Hamed, N.; Pernot, S.; Kaminsky, M.-C.; Bouché, O.; et al. Docetaxel, cisplatin, and fluorouracil chemotherapy for metastatic or unresectable locally recurrent anal squamous cell carcinoma (Epitopes-HPV02): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 1094–1106. [Google Scholar] [CrossRef]
- Rao, S.; Sclafani, F.; Eng, C.; Adams, R.A.; Guren, M.G.; Sebag-Montefiore, D.; Benson, A.; Bryant, A.; Peckitt, C.; Segelov, E.; et al. International Rare Concerns Initiative multicenter randomized phase II trial of cisplatin and fluorouracil versus carboplatin and paclitaxel in advanced anal cancer: InterAAct. J. Clin. Oncol. 2020, 38, 2510–2518. [Google Scholar] [CrossRef]
- Ott, P.A.; Piha-Paul, S.A.; Munster, P.; Pishvaian, M.J.; van Brummelen, E.M.J.; Cohen, R.B.; Gomez-Roca, C.; Ejadi, S.; Stein, M.; Chan, E.; et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann. Oncol. 2017, 28, 1036–1041. [Google Scholar] [CrossRef]
- Morris, V.K.; E Salem, M.; Nimeiri, H.; Iqbal, S.; Singh, P.; Ciombor, K.; Polite, B.; Deming, D.; Chan, E.; Wade, J.L.; et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Salem, M.E.; Puccini, A.; Grothey, A.; Raghavan, D.; Goldberg, R.M.; Xiu, J.; Korn, W.M.; Weinberg, B.A.; Hwang, J.J.; Shields, A.F.; et al. Landscape of tumor mutation load, mismatch repair deficiency, and PD-L1 expression in a large patient cohort of gastrointestinal cancers. Mol. Cancer Res. 2018, 16, 805–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.; Park, H.-C.; Kim, M.S.; Han, M.-R.; Lee, S.H.; Chung, Y.-J. Whole-exome sequencing identified mutational profiles of squamous cell carcinomas of the anus. Hum. Pathol. 2018, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Seiwert, T.Y.; Gupta, S.; Weiss, J.; Gluck, I.; Eder, J.P.; Burtness, B.; Tahara, M.; Keam, B.; Kang, H.; et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: Pooled analyses after long-term follow-up in KEYNOTE-012. Br. J. Cancer 2018, 119, 153–159. [Google Scholar] [CrossRef]
- Wai, K.C.; Strohl, M.P.; van Zante, A.; Ha, P.K. Molecular diagnostics in human papillomavirus-related head and neck squamous cell carcinoma. Cells 2020, 9, 500. [Google Scholar] [CrossRef] [Green Version]
- Doran, S.L.; Stevanovic, S.; Adhikary, S.; Gartner, J.J.; Jia, L.; Kwong, M.L.M.; Faquin, W.C.; Hewitt, S.M.; Sherry, R.M.; Yang, J.C.; et al. T-cell receptor gene therapy for human papillomavirus-associated epithelial cancers: A first-in-human, phase I/II study. J. Clin. Oncol. 2019, 37, 2759–2768. [Google Scholar] [CrossRef]
- Levovitz, C.; Chen, D.; Ivansson, E.; Gyllensten, U.; Finnigan, J.P.; Alshawish, S.; Zhang, W.; Schadt, E.E.; Posner, M.R.; Genden, E.M.; et al. TGFB receptor 1: An immune susceptibility gene in HPV-associated cancer. Cancer Res. 2014, 74, 6833–6844. [Google Scholar] [CrossRef] [Green Version]
- Paliga, A.; Onerheim, R.; Gologan, A.; Chong, G.; Spatz, A.; Niazi, T.; Garant, A.; Macheto, D.; Alcindor, T.; Vuong, T. EGFR and K-ras gene mutation status in squamous cell anal carcinoma: A role for concurrent radiation and EGFR inhibitors? Br. J. Cancer 2012, 107, 1864–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, F.; Abramowitz, L.; Benabderrahmane, D.; Duval, X.; Descatoire, V.; Henin, D.; Lehy, T.; Aparicio, T. Growth factor receptor expression in anal squamous lesions: Modifications associated with oncogenic human papillomavirus and human immunodeficiency virus. Hum. Pathol. 2009, 40, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Zanellato, E.; Franzetti-Pellanda, A.; Molinari, F.; Movilia, A.; Paganotti, A.; Deantonio, L.; De Dosso, S.; Assi, A.; Crippa, S.; et al. EGFR, KRAS, BRAF and PIK3CA characterization in squamous cell anal cancer. Histol. Histopathol. 2014, 29, 513–521. [Google Scholar] [PubMed]
- Morris, V.; Rao, X.; Pickering, C.; Foo, W.C.; Rashid, A.; Eterovic, K.; Kim, T.; Chen, K.; Wang, J.; Shaw, K.; et al. Comprehensive genomic profiling of metastatic squamous cell carcinoma of the anal canal. Mol. Cancer Res. 2017, 15, 1542–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivatto, L.O.; Vieira, F.M.; Pereira, B.V.; Victorino, A.P.; Bezerra, M.; Araújo, C.M.; Erlich, F.; Faroni, L.; Castro, L.; Lusis, E.C.; et al. Phase 1 study of cetuximab in combination with 5-fluorouracil, displatin, and radiotherapy in patients with locally advanced anal canal carcinoma. Cancer 2013, 119, 2973–2980. [Google Scholar] [CrossRef]
- Deutsch, E.; Lemanski, C.; Pignon, J.P.; Levy, A.; Delarochefordiere, A.; Martel-Lafay, I.; Rio, E.; Malka, D.; Conroy, T.; Miglianico, L.; et al. Unexpected toxicity of cetuximab combined with conventional chemoradiotherapy in patients with locally advanced anal cancer. Results of the UNICANCER ACCORD 16 phase II trial. Ann. Oncol. 2013, 24, 2837–2838. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.K.; Zhao, F.; Sparano, J.A.; Palefsky, J.; Whittington, R.; Mitchell, E.P.; Mulcahy, M.F.; Armstrong, K.I.; Nabbout, N.H.; Kalnicki, S.; et al. Cetuximab plus chemoradiotherapy in immunocompetent patients with anal carcinoma: A phase II Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group trial (E3205). J. Clin. Oncol. 2017, 35, 718–726. [Google Scholar] [CrossRef]
- Sparano, J.A.; Lee, J.Y.; Palefsky, J.; Henry, D.H.; Wachsman, W.; Rajdev, L.; Aboulafia, D.; Ratner, L.; Fitzgerald, T.J.; Kachnic, L.; et al. Cetuximab plus chemoradiotherapy for HIV-associated anal carcinoma: A phase II AIDS Malignancy Consortium trial. J. Clin. Oncol. 2016, 35, 727–733. [Google Scholar] [CrossRef] [Green Version]
- Casadei Gardini, A.; Capelli, L.; Ulivi, P.; Giannini, M.; Freier, E.; Tamberi, S.; Scarpi, E.; Passandi, A.; Zoli, W.; Ragazzini, A.; et al. KRAS, BRAF and PIK3CA status in squamous cell anal carcinoma (SCAC). PLoS ONE 2014, 9, e92071. [Google Scholar] [CrossRef]
- Cacheux, W.; Rouleau, E.; Briaux, A.; Tsantoulis, P.; Mariani, P.; Richard-Molard, M.; Buecher, B.; Dangles-Marie, V.; Richon, S.; Lazartigues, J.; et al. Mutational analysis of anal cancers demonstrates frequent PIK3CA mutations associated with poor outcome after salvage abdominoperineal resection. Br. J. Cancer 2016, 114, 1387–1394. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.H.; Sanford, E.; Johnson, A.; Klempner, S.J.; Schrock, A.B.; Palma, N.A.; Erlich, R.L.; Frampton, G.M.; Chalmers, Z.R.; Vergilio, J.; et al. Comprehensive genomic profiling of anal squamous cell carcinoima reveals distinct genomically defined classes. Ann. Oncol. 2016, 27, 1336–1341. [Google Scholar] [CrossRef]
- Trilla-Fuertes, L.; Ghanem, I.; Maurel, J.; G.-Pastrian, L.; Mendiola, M.; Pena, C.; Lopez-Vacas, R.; Prado-Vazquez, G.; Lopez-Camach, E.; Zapater-Moros, A.; et al. Comprehensive characterization of the mutational landscape in localized anal squamous cell carcinoma. Transl. Oncol 2020, 13, 100778. [Google Scholar] [CrossRef]
- Patel, H.; Polanco-Echeverry, G.; Segditas, S.; Volikos, E.; McCart, A.; Lai, C.; Guenther, T.; Zaitoun, A.; Sieber, O.; Ilyas, M.; et al. Activation of AKT and nuclear accumulation of wild type TP53 and MDM2 in anal squamous cell carcinoma. Int. J. Cancer 2007, 121, 2668–2673. [Google Scholar] [CrossRef] [PubMed]
- Cacheux, W.; Dangles-Marie, V.; Rouleau, E.; Lazartigue, J.; Girard, E.; Briaux, A.; Mariani, P.; Richon, S.; Vacher, S.; Buecher, B.; et al. Exome sequencing reveals aberrant signaling pathways as hallmark of treatment-naïve anal squamous cell cancer. Oncotarget 2017, 9, 464–476. [Google Scholar] [CrossRef] [Green Version]
- Cacheux, W.; Tsantoulis, P.; Briaux, A.; Vacher, S.; Mariani, P.; Richard-Molard, M.; Buecher, B.; Richon, S.; Jeannot, E.; Lazartigues, J.; et al. Array comparative genomic hybridization identifies high level of PI3K/Akt/mTOR pathway alterations in anal cancer recurrences. Cancer Med. 2018, 7, 3213–3225. [Google Scholar] [CrossRef]
- Stelzer, M.K.; Pitot, H.C.; Liem, A.; Lee, D.; Kennedy, G.D.; Lambert, P.F. Rapamycin inhibits anal carcinogenesis in two preclinical animal models. Cancer Prev. Res. 2010, 3, 1542–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.-J.; Zhang, L.; Zhang, W.; Hall, B.; Bian, Y.; Kulkami, A.B. Inhibition of mTOR reduces anal carcinogenesis in transgenic mouse model. PLoS ONE 2013, 8, e74888. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Angulo, A.M.; Dejan, J.; Guillem, A.; Schellens, J.H.M.; Burris, H.A.; Berlin, J.; Middleton, M.R.; Schuler, M.H.; Van Geel, R.; Helgason, T.; et al. Safety, pharmacokinetics, and preliminary activity of the a-specific PI3K inhibitor BYL719: Results form the first-in-human study. J. Clin. Oncol. 2013, 31, 2531. [Google Scholar] [CrossRef]
- Mouw, K.W.; Cleary, J.M.; Reardon, B.; Pike, J.; Braunstein, L.Z.; Kim, J.; Amin-Mansour, A.; Miao, D.; Damish, A.; Chin, J.; et al. Genomic evolution after chemoradiotherapy in anal squamous cell carcinoma. Clin. Cancer Res. 2017, 23, 3214–3222. [Google Scholar] [CrossRef] [Green Version]
- Meulendijks, D.; Tomasoa, N.B.; Dewit, L.; Smits, P.H.M.; Bakker, R.; van Velthuysen, M.-L.F.; Rosenberg, E.H.; Beijnen, J.H.; Schellens, J.H.M.; Cats, A. HPV-negative squamous cell carcinoma of the anal canal is unresponsive to standard treatment and frequently carries disruptive mutations in TP53. Br. J. Cancer 2015, 112, 1358–1366. [Google Scholar] [CrossRef]
- Soares, P.C.; Abdelhay, E.S.; Thuler, L.C.S.; Soares, B.M.; Demachki, S.; Rocha Ferro, G.V.; Assumpcao, P.P.; Lamarao, L.M.; Ribeiro Pinto, L.F.; Rodriguez Burbano, R.M. HPV positive, wild type TP53, and p16 overexpression correlate with the absence of residual tumors after chemoradiotherapy in anal squamous cell carcinoma. BMC Gastroenterol. 2018, 18, 30. [Google Scholar] [CrossRef]
- Wessely, A.; Heppt, M.V.; Kammerbauer, C.; Steeb, T.; Kirchner, T.; Flaig, M.J.; French, L.E.; Berking, C.; Schmoeckel, E.; Reinholz, M. Evaluation of PD-L1 expression and HPV genotyping in anal squamous cell carcinoma. Cancers 2020, 12, 2516. [Google Scholar] [CrossRef] [PubMed]
- Kon, N.; Ou, Y.; Wang, S.-J.; Li, H.; Rustgi, A.K.; Gu, W. mTOR inhibition acts as an unexpected checkpoint in p53-mediated tumor suppression. Genes Dev. 2020, 35, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N. Engl. J. Med. 2020, 383, 1207–1217. [Google Scholar] [CrossRef]
- Nassar, A.H.; Adib, E.; Kwiatkowski, D.J. Distribution of KRASG12C somatic mutations across race, sex and cancer type. N. Engl. J. Med. 2021, 384, 185–187. [Google Scholar] [CrossRef]
- Guster, J.D.; Weissleder, S.V.; Busch, C.-J.; Kriegs, M.; Peterson, C.; Knecht, R.; Dikomey, E.; Rieckmann, T. The inhibition of PARP but not EGFR results in the radiosensitization of HPV/p16-positive HNSCC cell lines. Radiother. Oncol. 2014, 113, 345–351. [Google Scholar] [CrossRef]
- Yasukawa, M.; Fujihara, H.; Fujimori, H.; Kawaguchi, K.; Yamada, H.; Nakayama, R.; Yamamoto, N.; Kishi, Y.; Hamada, Y.; Masutani, M. Synergetic effects of PARP inhibitor AZD2281 and cisplatin in oral squamous cell carcinoma in vitro and in vivo. Int. J. Mol. Sci. 2016, 17, 272. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, A.L.; Young, C.D.; Bian, L.; Weigel, K.; Nolan, K.; Frederick, B.; Han, G.; He, G.; Trahan, G.D.; Rudolph, M.C.; et al. PARP inhibition enhances radiotherapy of SMAD4-deficient human head and neck squamous cell carcinomas in experimental models. Clin. Cancer Res. 2020, 26, 3058–3070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, L.; Boggs, D.H.; Xing, C.; Zhang, Z.; Anderson, J.C.; Wajapeyee, N.; Veale, C.; Bredel, M.; Shi, L.Z.; Bonner, J.A.; et al. Combining PARP and DNA-PK inhibitors with irradiation inhibits HPV-negative head and neck cancer squamous cacinoma growth. Front. Genet. 2020, 11, 1036. [Google Scholar] [CrossRef]
- Jelinek, M.J.; Foster, N.R.; Zoroufy, A.J.; Schwartz, G.K.; Munster, P.N.; Seiwert, T.Y.; de Souza, J.A.; Vokes, E.E. A phase I trial adding poly(ADP-ribose) polymerase inhibitor veliparib to induction carboplatin-paclitaxel in patients with head and neck squamous cell carcinoma: Alliance A091101. Oral Oncol. 2021, 114, 105171. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Roberts, C.W.M. The SWI/SNF copmlex in cancer—Biology, biomarkers and therapy. Nat. Rev. Clin. Oncol. 2020, 17, 435–448. [Google Scholar] [CrossRef]
- Margueron, R.; Reinberg, D. The polycomb complex PRC2 and its mark in life. Nature 2011, 469, 343–349. [Google Scholar] [CrossRef] [Green Version]


| Trial | N | Treatment Arms | Outcomes |
|---|---|---|---|
| EORTC 22861 [9] | 110 | Randomized phase III study comparing 5-FU + mitomycin with radiation vs. radiation alone |
|
| ACT I [11] | 500 | Randomized phase III study comparing 5-FU + mitomycin with radiation vs. radiation alone |
|
| RTOG 87-04/ECOG 1289 [12] | 310 | Randomized phase III study comparing chemoradiation with 5-FU + mitomycin vs. 5-FU alone |
|
| EXTRA [19] | 31 | Single-arm phase II study using capecitabine + mitomycin chemoradiation |
|
| [20] | 43 | Single-arm phase II study using capecitabine-based chemoradiation |
|
| ACT II [21] | 940 | Randomized phase III, 2 × 2 factorial design, comparing chemoradiation with mitomycin + 5-FU vs. cisplatin + 5-FU with or without maintenance chemo | Comparing mitomycin + 5-FU and cisplatin + 5-FU
|
| [22] | 19 | Phase II pilot study treating with 5-FU + mitomycin + cisplatin chemoradiation |
|
| RTOG 98-11 [23] | 649 | Randomized phase III study comparing chemoradiation with 5-FU and mitomycin vs. 5-FU and cisplatin |
|
| ACCORD 03 [24] | 307 | Randomized phase III study comparing chemoradiation with or without induction 5-FU and cisplatin |
|
| Trial | N | Treatment Arms | Outcomes |
|---|---|---|---|
| Epitopes-HPV02 [32] | 66 | Nonrandomized, single-arm phase II treating with either DCF or mDCF with allocation determined by age and PS |
|
| InterAAct [33] | 91 | Randomized phase II study comparing carboplatin + paclitaxel vs. cisplatin + 5-FU |
|
| KEYNOTE-028 [34] | 25 | Single-arm phase Ib study of pembrolizumab in second line |
|
| NCI9673 [35] | 37 | Single-arm phase II study of nivolumab in second line |
|
| Trial | N | Treatment Arms | Outcomes |
|---|---|---|---|
| [46] | 21 | Single-arm phase I study with chemoradiation with 5-FU, cisplatin and cetuximab |
|
| ACCORD 16 [47] | 16 | Single-arm phase II study with chemoradiation with 5-FU, cisplatin and cetuximab |
|
| E3205 [48] | 61 | Single-arm phase II study of pembrolizumab in second line |
|
| AMC045 [49] | 37 | Single-arm phase II study of nivolumab in second line |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carr, R.M.; Jin, Z.; Hubbard, J. Research on Anal Squamous Cell Carcinoma: Systemic Therapy Strategies for Anal Cancer. Cancers 2021, 13, 2180. https://doi.org/10.3390/cancers13092180
Carr RM, Jin Z, Hubbard J. Research on Anal Squamous Cell Carcinoma: Systemic Therapy Strategies for Anal Cancer. Cancers. 2021; 13(9):2180. https://doi.org/10.3390/cancers13092180
Chicago/Turabian StyleCarr, Ryan M., Zhaohui Jin, and Joleen Hubbard. 2021. "Research on Anal Squamous Cell Carcinoma: Systemic Therapy Strategies for Anal Cancer" Cancers 13, no. 9: 2180. https://doi.org/10.3390/cancers13092180
APA StyleCarr, R. M., Jin, Z., & Hubbard, J. (2021). Research on Anal Squamous Cell Carcinoma: Systemic Therapy Strategies for Anal Cancer. Cancers, 13(9), 2180. https://doi.org/10.3390/cancers13092180







