Cost-Effectiveness of Digital Breast Tomosynthesis vs. Abbreviated Breast MRI for Screening Women with Intermediate Risk of Breast Cancer—How Low-Cost Must MRI Be?
Abstract
Simple Summary
Abstract
1. Introduction
- Compared to DBT, is AB-MRI a cost-effective modality in screening women with dense breasts for breast cancer?
- Considering the cost benefits by abbreviating breast MRI: How cheap do we have to abbreviate MRI in order to compensate for the diagnostic difference?
2. Results
2.1. Cost-Effectiveness Analysis
2.2. Sensitivity Analysis
2.2.1. Deterministic Sensitivity Analysis
2.2.2. Costs of AB-MRI
2.2.3. Probabilistic Sensitivity Analysis
3. Discussion
4. Materials and Methods
4.1. Screening Collective
4.2. Cost-Effectiveness Modelling
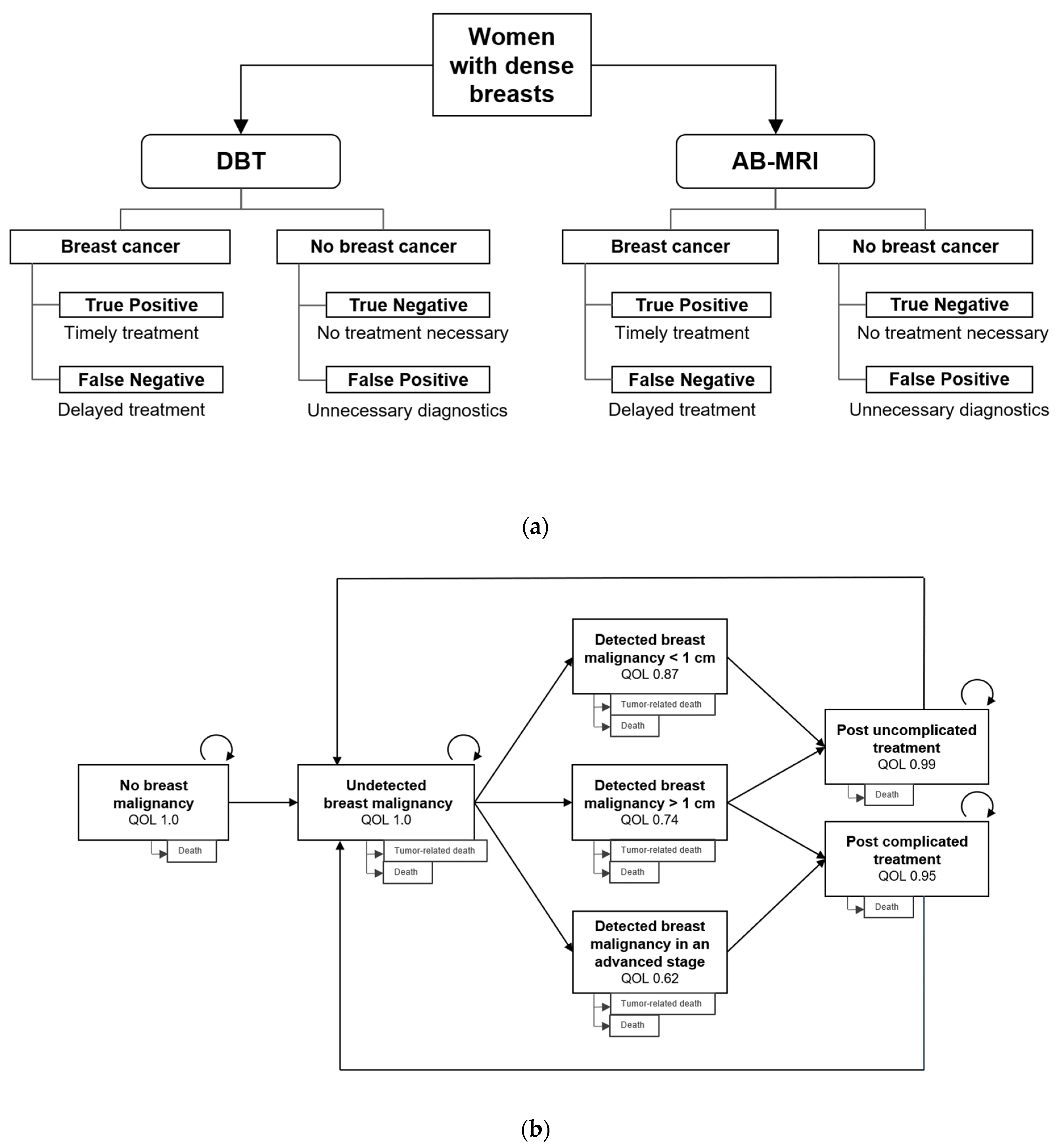
4.2.1. Decision Model
4.2.2. Markov Model
4.3. Input Parameters
4.3.1. Diagnostic Efficacy Parameters
4.3.2. Utilities
4.3.3. Cost Estimates
4.3.4. Transition Probabilities
4.4. Economic Analysis
4.4.1. Cost-Effectiveness Analysis
4.4.2. Sensitivity Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AB-MRI | Abbreviated breast MRI |
| DBT | Digital breast tomosynthesis |
| ICER | Incremental cost-effectiveness ratio |
| MRM | MR-mammography |
| MRI | Magnetic resonance imaging |
| PSA | Probabilistic sensitivity analysis |
| QALY | Quality-adjusted life-year |
| QOL | Quality of life |
| WTP | Willingness to pay |
References
- Sprague, B.L.; Gangnon, R.E.; Burt, V.; Trentham-Dietz, A.; Hampton, J.M.; Wellman, R.D.; Kerlikowske, K.; Miglioretti, D.L. Prevalence of Mammographically Dense Breasts in the United States. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef]
- Pisano, E.D.; Hendrick, R.E.; Yaffe, M.J.; Baum, J.K.; Acharyya, S.; Cormack, J.B.; Hanna, L.A.; Conant, E.F.; Fajardo, L.L.; Bassett, L.W.; et al. Diagnostic Accuracy of Digital versus Film Mammography: Exploratory Analysis of Selected Population Subgroups in DMIST. Radiology 2008, 246, 376–383. [Google Scholar] [CrossRef]
- Boyd, N.F.; Guo, H.; Martin, L.J.; Sun, L.; Stone, J.; Fishell, E.; Jong, R.A.; Hislop, G.; Chiarelli, A.; Minkin, S.; et al. Mammographic Density and the Risk and Detection of Breast Cancer. N. Engl. J. Med. 2007, 356, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.F.; De Lange, S.V.; Pijnappel, R.M.; Mann, R.M.; Peeters, P.H.; Monninkhof, E.M.; Emaus, M.J.; Loo, C.E.; Bisschops, R.H.; Lobbes, M.B.; et al. Supplemental MRI Screening for Women with Extremely Dense Breast Tissue. N. Engl. J. Med. 2019, 381, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, S.M.; Rafferty, E.A.; Rose, S.L.; Durand, M.A.; Plecha, D.M.; Greenberg, J.S.; Hayes, M.K.; Copit, D.S.; Carlson, K.L.; Cink, T.M.; et al. Breast Cancer Screening Using Tomosynthesis in Combination with Digital Mammography. JAMA 2014, 311, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Franken, E.A.; Garg, M.; Fajardo, L.L.; Niklason, L.T. Breast Tomosynthesis: Present Considerations and Future Applications. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2007, 27, 231–240. [Google Scholar] [CrossRef]
- Kaiser, C.G.; Reich, C.; Dietzel, M.; Baltzer, P.A.T.; Krammer, J.; Wasser, K.; Schoenberg, S.O.; Kaiser, W.A. DCE-MRI of the breast in a stand-alone setting outside a complementary strategy—Results of the TK-study. Eur. Radiol. 2015, 25, 1793–1800. [Google Scholar] [CrossRef]
- Bennani-Baiti, B.; Bennani-Baiti, N.; Baltzer, P.A. Diagnostic Performance of Breast Magnetic Resonance Imaging in Non-Calcified Equivocal Breast Findings: Results from a Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0160346. [Google Scholar] [CrossRef]
- Kuhl, C.; Weigel, S.; Schrading, S.; Arand, B.; Bieling, H.B.; König, R.; Tombach, B.; Leutner, C.; Rieber-Brambs, A.; Nordhoff, D.; et al. Prospective Multicenter Cohort Study to Refine Management Recommendations for Women at Elevated Familial Risk of Breast Cancer: The EVA Trial. J. Clin. Oncol. 2010, 28, 1450–1457. [Google Scholar] [CrossRef]
- Sardanelli, F.; Podo, F.; Santoro, F.; Manoukian, S.; Bergonzi, S.; Trecate, G.; Vergnaghi, D.; Federico, M.; Cortesi, L.; Corcione, S.; et al. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the high breast cancer risk italian 1 study): Final results. Investig. Radiol. 2011, 46, 94–105. [Google Scholar] [CrossRef]
- Riedl, C.C.; Luft, N.; Bernhart, C.; Weber, M.; Bernathova, M.; Tea, M.-K.M.; Rudas, M.; Singer, C.F.; Helbich, T.H. Triple-Modality Screening Trial for Familial Breast Cancer Underlines the Importance of Magnetic Resonance Imaging and Questions the Role of Mammography and Ultrasound Regardless of Patient Mutation Status, Age, and Breast Density. J. Clin. Oncol. 2015, 33, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Sardanelli, F.; Aase, H.S.; Álvarez, M.; Azavedo, E.; Baarslag, H.J.; Balleyguier, C.; Baltzer, P.A.; Beslagic, V.; Bick, U.; Bogdanovic-Stojanovic, D.; et al. Position paper on screening for breast cancer by the European Society of Breast Imaging (EUSOBI) and 30 national breast radiology bodies from Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Israel, Lithuania, Moldova, The Netherlands, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Spain, Sweden, Switzerland and Turkey. Eur. Radiol. 2017, 27, 2737–2743. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.K.; Strobel, K.; Bieling, H.; Leutner, C.; Schild, H.H.; Schrading, S. Supplemental Breast MR Imaging Screening of Women with Average Risk of Breast Cancer. Radiology 2017, 283, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Comstock, C.E.; Gatsonis, C.; Newstead, G.M.; Snyder, B.S.; Gareen, I.F.; Bergin, J.T.; Rahbar, H.; Sung, J.S.; Jacobs, C.; Harvey, J.A.; et al. Comparison of Abbreviated Breast MRI vs Digital Breast Tomosynthesis for Breast Cancer Detection Among Women With Dense Breasts Undergoing Screening. JAMA 2020, 323, 746–756. [Google Scholar] [CrossRef]
- Froelich, M.F.; Kaiser, C.G. Cost-effectiveness of MR-mammography as a solitary imaging technique in women with dense breasts: An economic evaluation of the prospective TK-Study. Eur. Radiol. 2021, 31, 967–974. [Google Scholar] [CrossRef]
- Kaiser, C.G.; Dietzel, M.; Vag, T.; Froelich, M.F. Cost-effectiveness of MR-mammography vs. conventional mammography in screening patients at intermediate risk of breast cancer—A model-based economic evaluation. Eur. J. Radiol. 2021, 136, 109355. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Schrading, S.; Strobel, K.; Schild, H.H.; Hilgers, R.-D.; Bieling, H.B. Abbreviated Breast Magnetic Resonance Imaging (MRI): First Postcontrast Subtracted Images and Maximum-Intensity Projection—A Novel Approach to Breast Cancer Screening With MRI. J. Clin. Oncol. 2014, 32, 2304–2310. [Google Scholar] [CrossRef]
- Kul, S.; Metin, Y.; Kul, M.; Metin, N.; Eyuboglu, I.; Ozdemir, O. Assessment of breast mass morphology with diffusion-weighted MRI: Beyond apparent diffusion coefficient. J. Magn. Reson. Imaging 2018, 48, 1668–1677. [Google Scholar] [CrossRef]
- Yamada, T.; Kanemaki, Y.; Okamoto, S.; Nakajima, Y. Comparison of detectability of breast cancer by abbreviated breast MRI based on diffusion-weighted images and postcontrast MRI. Jpn. J. Radiol. 2018, 36, 331–339. [Google Scholar] [CrossRef]
- Goto, M.; Sakai, K.; Yokota, H.; Kiba, M.; Yoshida, M.; Imai, H.; Weiland, E.; Yokota, I.; Yamada, K. Diagnostic performance of initial enhancement analysis using ultra-fast dynamic contrast-enhanced MRI for breast lesions. Eur. Radiol. 2018, 29, 1164–1174. [Google Scholar] [CrossRef]
- Grimm, L.J.; Soo, M.S.; Yoon, S.; Kim, C.; Ghate, S.V.; Johnson, K.S. Abbreviated screening protocol for breast MRI: A feasibility study. Acad Radiol. 2015, 22, 1157–1162. [Google Scholar] [CrossRef]
- Moschetta, M.; Telegrafo, M.; Rella, L.; Ianora, A.A.S.; Angelelli, G. Abbreviated Combined MR Protocol: A New Faster Strategy for Characterizing Breast Lesions. Clin. Breast Cancer 2016, 16, 207–211. [Google Scholar] [CrossRef]
- Strahle, D.A.; Pathak, D.R.; Sierra, A.; Saha, S.; Strahle, C.; Devisetty, K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res. Treat. 2017, 162, 283–295. [Google Scholar] [CrossRef]
- Fleming, M.M.; Hughes, D.R.; Golding, L.P.; McGinty, G.B.; Macfarlane, D.; Duszak, R. Digital Breast Tomosynthesis Implementation: Considerations for Emerging Breast Cancer Screening Bundled Payment Models. J. Am. Coll. Radiol. 2019, 16, 902–907. [Google Scholar] [CrossRef]
- Chong, A.; Weinstein, S.P.; McDonald, E.S.; Conant, E.F. Digital Breast Tomosynthesis: Concepts and Clinical Practice. Radiology 2019, 292, 1–14. [Google Scholar] [CrossRef]
- Sanders, G.D.; Neumann, P.J.; Basu, A.; Brock, D.W.; Feeny, D.; Krahn, M.; Kuntz, K.M.; Meltzer, D.O.; Owens, D.K.; Prosser, L.A.; et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016, 316, 1093–1103. [Google Scholar] [CrossRef]
- Ahern, C.H.; Shih, Y.-C.T.; Dong, W.; Parmigiani, G.; Shen, Y. Cost-effectiveness of alternative strategies for integrating MRI into breast cancer screening for women at high risk. Br. J. Cancer 2014, 111, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.J.; Cella, D.F.; Mo, F.E.; Bonomi, A.; Tulsky, D.S.; Lloyd, S.R.; Deasy, S.; Cobleigh, M.; Shiomoto, G. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J. Clin. Oncol. 1997, 15, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Polsky, D.; Mandelblatt, J.S.; Weeks, J.C.; Venditti, L.; Hwang, Y.-T.; Glick, H.A.; Hadley, J.; Schulman, K.A. Economic Evaluation of Breast Cancer Treatment: Considering the Value of Patient Choice. J. Clin. Oncol. 2003, 21, 1139–1146. [Google Scholar] [CrossRef]
- Hunter, S.A.; Morris, C.; Nelson, K.; Snyder, B.J.; Poulton, T.B. Digital Breast Tomosynthesis: Cost-Effectiveness of Using Private and Medicare Insurance in Community-Based Health Care Facilities. Am. J. Roentgenol. 2017, 208, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Blumen, H.; Fitch, K.; Polkus, V. Comparison of Treatment Costs for Breast Cancer, by Tumor Stage and Type of Service. Am. Heal. Drug Benefits 2016, 9, 23–32. [Google Scholar]
- Richardson, L.C.; Henley, S.J.; Miller, J.W.; Massetti, G.; Thomas, C.C. Patterns and Trends in Age-Specific Black-White Differences in Breast Cancer Incidence and Mortality—United States, 1999–2014. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 1093–1098. [Google Scholar] [CrossRef]
- Arias, E. United States Life Tables, 2017. Natl. Vital. Stat. Rep. Cent. Dis. Control. Prev. 2019, 68, 1–66. [Google Scholar]
- Wishart, G.C.; Azzato, E.M.; Greenberg, D.C.; Rashbass, J.; Kearins, O.; Lawrence, G.; Caldas, C.; Pharoah, P.D. Predict: A new UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res. BCR. 2010, 12, 1–10. [Google Scholar] [CrossRef]
- Heil, J.; Rauch, G.; Szabo, A.Z.; Garcia-Etienne, C.A.; Golatta, M.; Domschke, C.; Badiian, M.; Kern, P.; Schuetz, F.; Wallwiener, M.; et al. Breast Cancer Mastectomy Trends Between 2006 and 2010: Association with Magnetic Resonance Imaging, Immediate Breast Reconstruction, and Hospital Volume. Ann. Surg. Oncol. 2013, 20, 3839–3846. [Google Scholar] [CrossRef]
- Lombardi, A.; Pastore, E.; Maggi, S.; Stanzani, G.; Vitale, V.; Romano, C.; Bersigotti, L.; Vecchione, A.; Amanti, C. Positive margins (R1) risk factors in breast cancer conservative surgery. Breast Cancer Targets Ther. 2019, 11, 243–248. [Google Scholar] [CrossRef]
- Woods, B.; Revill, P.; Sculpher, M.; Claxton, K. Country-Level Cost-Effectiveness Thresholds: Initial Estimates and the Need for Further Research. Value Heal. 2016, 19, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.J.; Cohen, J.T.; Weinstein, M.C. Updating Cost-Effectiveness—The Curious Resilience of the $50,000-per-QALY Threshold. N. Engl. J. Med. 2014, 371, 796–797. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.; Ubels, J.; Norström, F. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: A systematic review. Glob. Health Action 2018, 11, 1447828. [Google Scholar] [CrossRef] [PubMed]



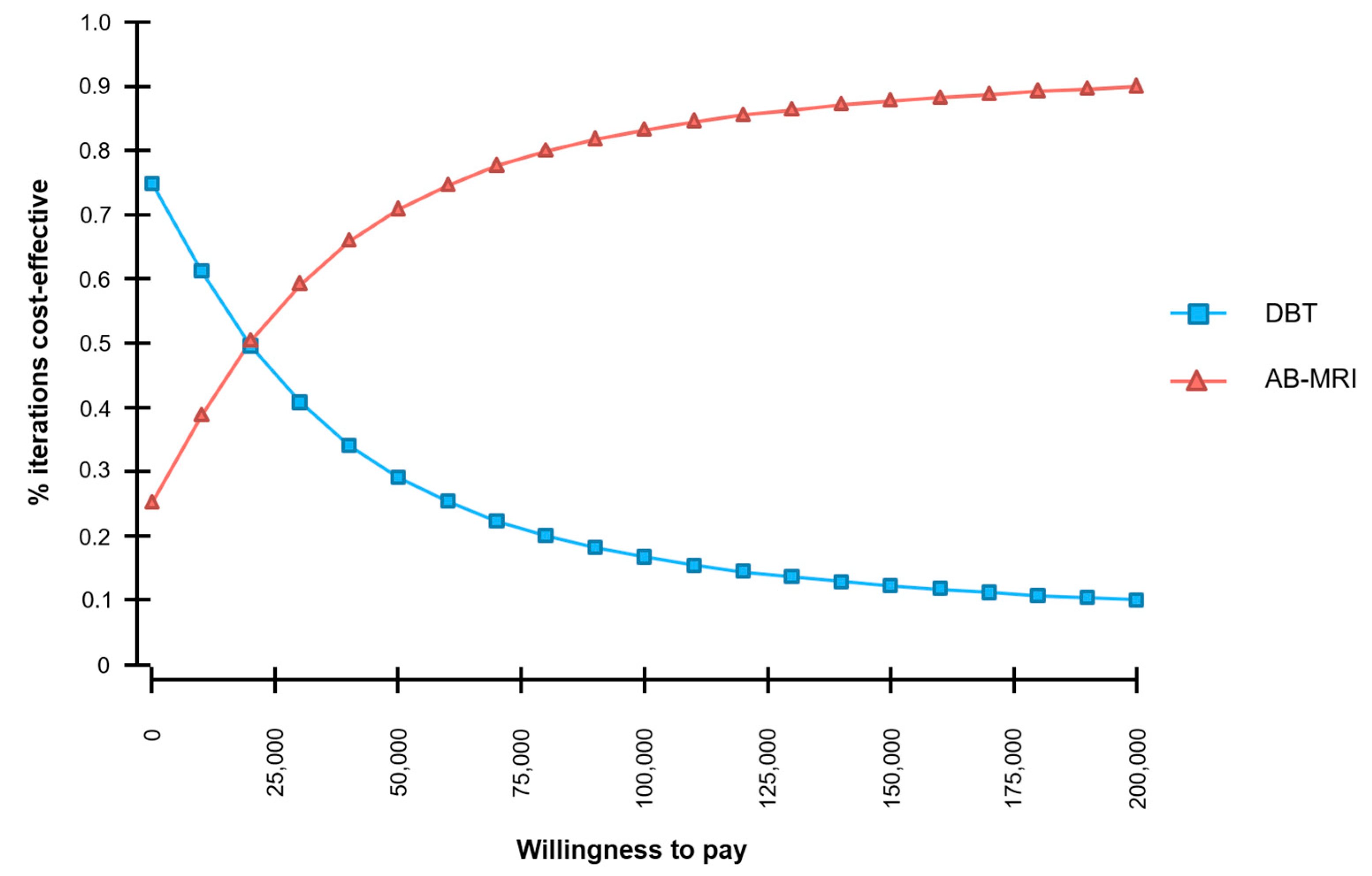
| Strategy | Cumulative Costs (US-$) | Incremental Costs (US-$) | Cumulative Effects (QALYs) | Incremental Effects (QALYs) | ICER (US-$/QALY) |
|---|---|---|---|---|---|
| DBT | 8798.47 | n/a | 19.23 | n/a | n/a |
| AB-MRI | 9504.77 | 706.30 | 19.27 | 0.03 | 20,807.04 |
| Variable | Estimation | Source | Distribution |
|---|---|---|---|
| Pre-test probability of malignant lesion | 1.59% | Comstock et al. 2020 | β |
| Average age at screening | 54.9 | Comstock et al. 2020 | normal |
| Screening interval | two years | Assumption | |
| Incidence of breast cancer in women with dense breasts | 0.40% | Richardson et al. 2016 | β |
| Assumed WTP | $100,000 | Sanders et al. 2016 | |
| Discount rate | 3.00% | Sanders et al. 2016 | |
| Diagnostic test performances | |||
| DBT sensitivity | 39.1% | Comstock et al. 2020 | β |
| DBT specificity | 97.4% | Comstock et al. 2020 | β |
| AB-MRI sensitivity | 95.7% | Comstock et al. 2020 | β |
| AB-MRI specificity | 86.7% | Comstock et al. 2020 | β |
| Full protocol MR mammography specificity | 94.0% | Benndorf et al. 2010 | β |
| Costs (Short Term) | |||
| DBT costs | $214.20 | Fleming et al. 2018/ Hunter et al. 2017 | γ |
| MRM costs | $314.00 | Medicare (CPT code 77047) | γ |
| No further action (true negative) | $0.00 | Assumption | |
| Biopsy | $1536.00 | Medicare (CPT code 19083) | γ |
| Costs (Long Term) | |||
| Yearly costs without tumor | $0.00 | Assumption | |
| Cost of treatment for tumor < 1 cm | $60,637 | Blumen et al. 2015 | γ |
| Cost of treatment for tumor > 1 cm | $82,121 | Blumen et al. 2015 | γ |
| Cost of treatment for advanced stage breast malignancy | $129,387 | Blumen et al. 2015 | γ |
| Utilities | |||
| QOL of patients without detected tumor | 1.00 | Assumption | |
| QOL of patients with detected tumor < 1 cm | 0.87 | Brady et al. 1997 | β |
| QOL of patients with detected tumor > 1 cm | 0.74 | Ahern et al. 2014 | β |
| QOL of patients with detected regional breast cancer in an advanced stage | 0.62 | Polsky et al. 2003 | β |
| QOL of patients post simple treatment | 0.99 | Assumption | β |
| QOL of patients post intensive treatment | 0.95 | Assumption | β |
| Death | 0.00 | Assumption | |
| Transition probabilities | |||
| Risk of death without tumor (yearly) | age-adjusted | US Life Tables 2017, women of all ethnicities, Arias et al. 2019 | β |
| Risk of death with undetected tumor | 10.00% in 10 years | Assumption | β |
| Risk of death with detected < 1 cm tumor | 0.11% | NHS Predict | β |
| Risk of death with detected > 1 cm tumor | 0.78% | NHS Predict | β |
| Risk of death with detected tumor in advanced stage | 1.81% | NHS Predict | β |
| Probability of initial R0 resection < 1 cm | 100.00% | Assumption | |
| Probability of initial R0 resection ≥ 1 cm | 90.00% | Lombardi et al. 2019 | β |
| Proportion of N+ in < 1 cm tumors | 0.00% | Assumption | |
| Proportion of N+ in > 1 cm tumors | 40.00% | Heil et al. 2013 | β |
| Proportion of successfully treated tumors < 1 cm if detected within 1 screening interval | 100.00% | Assumption |
| AB-MRI (US-$) | DBT (US-$) | ||||
|---|---|---|---|---|---|
| 180 | 200 | 220 | 240 | 260 | |
| 210 | 8468.90, 8501.48, 959.55 | 8661.63, 8501.48, cost-saving | 8854.36, 8501.48, cost-saving | 9047.09, 8501.48, cost-saving | 9239.82, 8501.48, cost-saving |
| 230 | 8468.90, 8694.42, 6643.46 | 8661.63, 8694.42, 965.78 | 8854.36, 8694.42, cost-saving | 9047.09, 8694.42, cost-saving | 9239.82, 8694.42, cost-saving |
| 250 | 8468.90, 8887.36, 12,327.37 | 8661.63, 8887.36, 6649.69 | 8854.36, 8887.36, 972.01 | 9047.09, 8887.36, cost-saving | 9239.82, 8887.36, cost-saving |
| 270 | 8468.90, 9080.30, 18,011.27 | 8661.63, 9080.30, 12,333.59 | 8854.36, 9080.30, 6655.92 | 9047.09, 9080.30, 978.24 | 9239.82, 9080.30, cost-saving |
| 290 | 8468.90, 9273.24, 23,695.18 | 8661.63, 9273.24, 18,017.50 | 8854.36, 9273.24, 12,339.82 | 9047.09, 9273.24, 6662.15 | 9239.82, 9273.24, 984.47 |
| 310 | 8468.90, 9466.18, 29,379.08 | 8661.63, 9466.18, 23,701.41 | 8854.36, 9466.18, 18,023.73 | 9047.09, 9466.18, 12,346.05 | 9239.82, 9466.18, 6668.38 |
| 330 | 8468.90, 9659.12, 35,062.99 | 8661.63, 9659.12, 29,385.31 | 8854.36, 9659.12, 23,707.64 | 9047.09, 9659.12, 18,029.96 | 9239.82, 9659.12, 12,352.28 |
| 350 | 8468.90, 9852.06, 40,746.89 | 8661.63, 9852.06, 35,069.22 | 8854.36, 9852.06, 29,391.54 | 9047.09, 9852.06, 23,713.86 | 9239.82, 9852.06, 18,036.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tollens, F.; Baltzer, P.A.T.; Dietzel, M.; Rübenthaler, J.; Froelich, M.F.; Kaiser, C.G. Cost-Effectiveness of Digital Breast Tomosynthesis vs. Abbreviated Breast MRI for Screening Women with Intermediate Risk of Breast Cancer—How Low-Cost Must MRI Be? Cancers 2021, 13, 1241. https://doi.org/10.3390/cancers13061241
Tollens F, Baltzer PAT, Dietzel M, Rübenthaler J, Froelich MF, Kaiser CG. Cost-Effectiveness of Digital Breast Tomosynthesis vs. Abbreviated Breast MRI for Screening Women with Intermediate Risk of Breast Cancer—How Low-Cost Must MRI Be? Cancers. 2021; 13(6):1241. https://doi.org/10.3390/cancers13061241
Chicago/Turabian StyleTollens, Fabian, Pascal A.T. Baltzer, Matthias Dietzel, Johannes Rübenthaler, Matthias F. Froelich, and Clemens G. Kaiser. 2021. "Cost-Effectiveness of Digital Breast Tomosynthesis vs. Abbreviated Breast MRI for Screening Women with Intermediate Risk of Breast Cancer—How Low-Cost Must MRI Be?" Cancers 13, no. 6: 1241. https://doi.org/10.3390/cancers13061241
APA StyleTollens, F., Baltzer, P. A. T., Dietzel, M., Rübenthaler, J., Froelich, M. F., & Kaiser, C. G. (2021). Cost-Effectiveness of Digital Breast Tomosynthesis vs. Abbreviated Breast MRI for Screening Women with Intermediate Risk of Breast Cancer—How Low-Cost Must MRI Be? Cancers, 13(6), 1241. https://doi.org/10.3390/cancers13061241






