Supplemental 18F-FDG-PET/CT for Detection of Malignant Transformation of IPMN—A Model-Based Cost-Effectiveness Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Economic Modeling
2.1.1. Decision Model
2.1.2. Markov Model Structure
2.2. Input Parameters
2.2.1. Diagnostic Efficacy Parameters
2.2.2. Utilities and Costs
2.2.3. Transition Probabilities
| Variable | Estimation | Source |
|---|---|---|
| Pre-test probability of malignant IPMN | 52% | Sugimoto et al., 2017 [26]/Wilson et al., 2017 [27] |
| Average age at 18F-FDG-PET examination | 64.3 | Hong et al., 2010 [24]/Sperti et al., 2007 [25] |
| Assumed WTP | $100,000 | Sanders et al., 2016 [42] |
| Discount rate | 3.00% | Sanders et al., 2016 [42] |
| Diagnostic test performances | ||
| CT/MRI sensitivity (for risk factors predictive of malignancy) | 80.9% | Sultana et al., 2015 [15] |
| CT/MRI specificity (for risk factors predictive of malignancy) | 76.2% | Sultana et al., 2015 [15] |
| 18F-FDG-PET sensitivity (for risk factors predictive of malignancy) | 96.8% | Sultana et al., 2015 [15] |
| 18F-FDG-PET specificity (for risk factors predictive of malignancy) | 91.1% | Sultana et al., 2015 [15] |
| Costs | ||
| Contrast-enhanced MRI | $492 | Medicare CPT code 74183 |
| 18F-FDG-PET | $1551 | Medicare CPT code 78814 |
| Open pancreatoduodenectomy | $28,623 | Gerber et al., 2017 [33] |
| Distal pancreatic resection | $13,900 | Rutz et al., 2014 [34] |
| Proportion of pancreatic head resection vs. distal pancreatic resection | 78%/21% | Mimura et al., 2010 [36] |
| Cost of recurrent disease | $78,630 | Tramontano et al. [37] |
| Mean cost of readmissions | $1930 | Kent et al., 2011 [35] |
| Utilities | ||
| QOL of patients with IPMN | 1.00 | Assumption |
| QOL of patients receiving IPMN resection | 0.818 | Adapted from Ljungman et al., 2011 [29] |
| QOL of patients with recurrence | 0.65 | Adapted from Müller-Nordhorn et al., 2006 [28] |
| Long-term QOL of patients after IPMN resection | 0.896 | Adapted from Ljungman et al., 2011 [29] |
| Death | 0.00 | Assumption |
| Transition probabilities | ||
| Risk of death without malignant IPMN | age-adjusted | US Life Tables 2017 [38] |
| Risk of malignant transformation | 2.23% | Choi et al., 2017 [39] |
| Risk of death due to malignant IPMN | 2.7% | Chari et al., 2002 [41] |
| Risk of death due to recurrent malignant IPMN | 28.3% | Chari et al., 2002 [41] |
| Perioperative mortality in pancreatic surgery | 4.6% | Huang et al., 2010 [40] |
| Probability of recurrence of malignant IPMN | 16.7 | Chari et al., 2002 [41] |
| Reduction in risk of recurrence due to early detection by PET | 10% | Assumption |
2.3. Economic Analysis
2.3.1. Cost-Effectiveness Analysis
2.3.2. Sensitivity Analysis
2.4. Data Availability
3. Results
3.1. Cost-Effectiveness Analysis
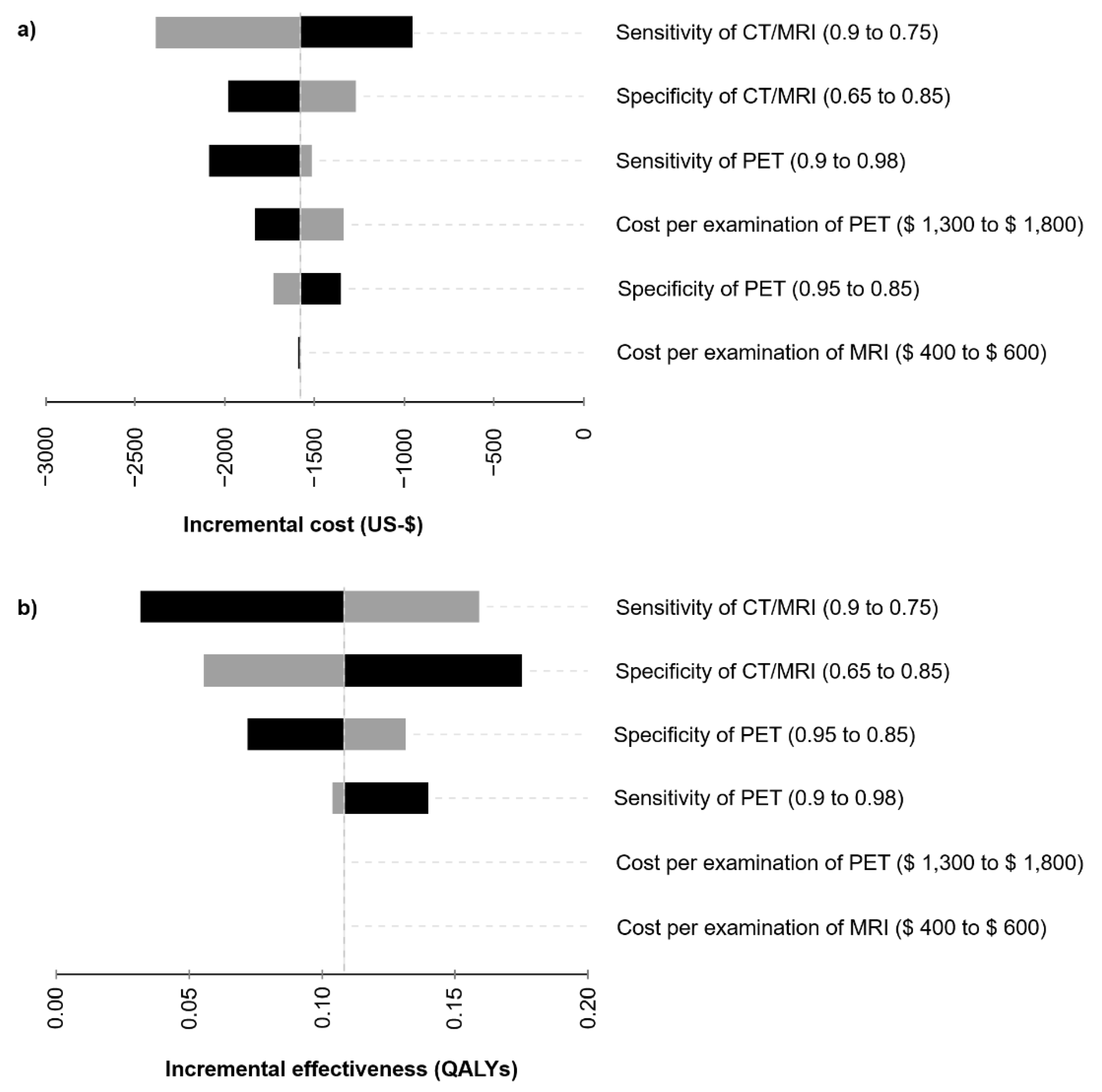
3.2. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanaka, M.; Castillo, C.F.-D.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef]
- Klöppel, G.; Solcia, E.; Sobin, L.H.; Longnecker, D.S.; Capella, C. Histological Typing of Tumours of the Exocrine Pancreas; Springer International Publishing: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Moris, M.; Bridges, M.D.; Pooley, R.A.; Raimondo, M.; Woodward, T.A.; Stauffer, J.A.; Asbun, H.J.; Wallace, M.B. Association Between Advances in High-Resolution Cross-Section Imaging Technologies and Increase in Prevalence of Pancreatic Cysts from 2005 to 2014. Clin. Gastroenterol. Hepatol. 2016, 14, 585–593.e3. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Ngamruengphong, S.; Nagendra, S.; Chak, A. Asymptomatic pancreatic cystic neoplasm: A cost-effectiveness analysis of different strategies of management. Gastrointest. Endosc. 2009, 70, 690–699.e6. [Google Scholar] [CrossRef]
- El Khoury, R.; Kabir, C.; Maker, V.K.; Banulescu, M.; Wasserman, M.; Maker, A.V. What is the Incidence of Malignancy in Resected Intraductal Papillary Mucinous Neoplasms? An Analysis of Over 100 US Institutions in a Single Year. Ann. Surg. Oncol. 2018, 25, 1746–1751. [Google Scholar] [CrossRef]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Gillen, S.; Schuster, T.; Büschenfelde, C.M.Z.; Friess, H.; Kleeff, J. Preoperative/Neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta-analysis of Response and Resection Percentages. PLoS Med. 2010, 7, e1000267. [Google Scholar] [CrossRef] [Green Version]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef]
- The European Study Group on Cystic Tumours of the Pancreas European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [CrossRef]
- Roch, A.M.; Ceppa, E.P.; Al-Haddad, M.A.; DeWitt, J.M.; House, M.G.; Zyromski, N.J.; Nakeeb, A.; Schmidt, C.M. The Natural History of Main Duct–Involved, Mixed-Type Intraductal Papillary Mucinous Neoplasm. Ann. Surg. 2014, 260, 680–690. [Google Scholar] [CrossRef]
- Best, L.M.; Rawji, V.; Pereira, S.P.; Davidson, B.R.; Gurusamy, K.S. Imaging modalities for characterising focal pancreatic lesions. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.-M.; Yin, S.; Siddiqui, A.A.; Salem, R.R.; Schrope, B.; Sethi, A.; Poneros, J.M.; Gress, F.G.; Genkinger, J.M.; Do, C.; et al. Comparison of the diagnostic accuracy of three current guidelines for the evaluation of asymptomatic pancreatic cystic neoplasms. Medicine 2017, 96, e7900. [Google Scholar] [CrossRef] [PubMed]
- Serafini, S.; Sperti, C.; Brazzale, A.R.; Cecchin, D.; Zucchetta, P.; Pierobon, E.S.; Ponzoni, A.; Valmasoni, M.; Moletta, L. The Role of Positron Emission Tomography in Clinical Management of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Cancers 2020, 12, 807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, N.; Koh, Y.-X.; Goh, B.K. Systematic review of the utility of 18-FDG PET in the preoperative evaluation of IPMNs and cystic lesions of the pancreas. Surgery 2019, 165, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Jackson, R.; Tim, G.; Bostock, E.; Psarelli, E.E.; Cox, T.F.; Sutton, R.; Ghaneh, P.; Raraty, M.G.T.; Neoptolemos, J.P.; et al. What Is the Best Way to Identify Malignant Transformation Within Pancreatic IPMN: A Systematic Review and Meta-Analyses. Clin. Transl. Gastroenterol. 2015, 6, e130. [Google Scholar] [CrossRef]
- Weber, W.A.; Schwaiger, M.; Avril, N. Quantitative assessment of tumor metabolism using FDG-PET imaging. Nucl. Med. Biol. 2000, 27, 683–687. [Google Scholar] [CrossRef]
- Bertagna, F.; Treglia, G.; Baiocchi, G.L.; Giubbini, R. F18-FDG-PET/CT for evaluation of intraductal papillary mucinous neoplasms (IPMN): A review of the literature. Jpn. J. Radiol. 2013, 31, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Regenet, N.; Sauvanet, A.; Muscari, F.; Meunier, B.; Mariette, C.; Adham, M.; Moutardier, V.; Delpero, J.-R.; Regimbeau, J.-M.; Pessaux, P.; et al. The value of 18F-FDG positron emission tomography to differentiate benign from malignant intraductal papillary mucinous neoplasms: A prospective multicenter study. J. Visc. Surg. 2020, 157, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cui, Y.; Shao, J.; Shao, Z.; Su, F.; Li, Y. The diagnostic role of CT, MRI/MRCP, PET/CT, EUS and DWI in the differentiation of benign and malignant IPMN: A meta-analysis. Clin. Imaging 2021, 72, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Sharib, J.; Esserman, L.; Koay, E.J.; Maitra, A.; Shen, Y.; Kirkwood, K.S.; Ozanne, E.M. Cost-effectiveness of consensus guideline based management of pancreatic cysts: The sensitivity and specificity required for guidelines to be cost-effective. Surgery 2020, 168, 601–609. [Google Scholar] [CrossRef]
- Aronsson, L.; Ansari, D.; Andersson, B.; Persson, U.; Fridhammar, A.; Andersson, R. Intraductal papillary mucinous neoplasms of the pancreas—A cost-effectiveness analysis of management strategies for the branch-duct subtype. HPB 2018, 20, 1206–1214. [Google Scholar] [CrossRef] [Green Version]
- Kadom, N.; Itri, J.N.; Trofimova, A.; Otero, H.J.; Horný, M. Cost-Effectiveness Analysis: An Overview of Key Concepts, Recommendations, Controversies, and Pitfalls. Acad. Radiol. 2019, 26, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Iragorri, N.; Spackman, E. Assessing the value of screening tools: Reviewing the challenges and opportunities of cost-effectiveness analysis. Public Health Rev. 2018, 39, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, H.-S.; Yun, M.; Cho, A.; Choi, J.-Y.; Kim, M.-J.; Kim, K.W.; Choi, Y.J.; Lee, J.D. The Utility of F-18 FDG PET/CT in the Evaluation of Pancreatic Intraductal Papillary Mucinous Neoplasm. Clin. Nucl. Med. 2010, 35, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Sperti, C.; Bissoli, S.; Pasquali, C.; Frison, L.; Liessi, G.; Chierichetti, F.; Pedrazzoli, S. 18-Fluorodeoxyglucose Positron Emission Tomography Enhances Computed Tomography Diagnosis of Malignant Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann. Surg. 2007, 246, 932–939. [Google Scholar] [CrossRef]
- Sugimoto, M.; Elliott, I.A.; Nguyen, A.H.; Kim, S.; Muthusamy, V.R.; Watson, R.; Hines, O.J.; Dawson, D.W.; Reber, H.A.; Donahue, T.R. Assessment of a Revised Management Strategy for Patients With Intraductal Papillary Mucinous Neoplasms Involving the Main Pancreatic Duct. JAMA Surg. 2017, 152, e163349. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.C.; Maithel, S.K.; Bentrem, D.; Abbott, D.E.; Weber, S.; Cho, C.; Martin, R.C.; Scoggins, C.R.; Kim, H.J.; Merchant, N.B.; et al. Are the Current Guidelines for the Surgical Management of Intraductal Papillary Mucinous Neoplasms of the Pancreas Adequate? A Multi-Institutional Study. J. Am. Coll. Surg. 2017, 224, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Müller-Nordhorn, J.; Roll, S.; Böhmig, M.; Nocon, M.; Reich, A.; Braun, C.; Noesselt, L.; Wiedenmann, B.; Willich, S.; Brüggenjürgen, B. Health-Related Quality of Life in Patients with Pancreatic Cancer. Digestion 2006, 74, 118–125. [Google Scholar] [CrossRef]
- Ljungman, D.; Lundholm, K.; Hyltander, A. Cost-Utility Estimation of Surgical Treatment of Pancreatic Carcinoma Aimed at Cure. World J. Surg. 2011, 35, 662–670. [Google Scholar] [CrossRef]
- Weinberg, B.M.; Spiegel, B.M.; Tomlinson, J.S.; Farrell, J.J. Asymptomatic Pancreatic Cystic Neoplasms: Maximizing Survival and Quality of Life Using Markov-Based Clinical Nomograms. Gastroenterology 2010, 138, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Billings, B.; Christein, J.; Harmsen, W.; Harrington, J.; Chari, S.; Que, F.; Farnell, M.; Nagorney, D.; Sarr, M. Quality-of-Life after Total Pancreatectomy: Is It Really That Bad on Long-term Follow-up? J. Gastrointest. Surg. 2005, 9, 1059–1067. [Google Scholar] [CrossRef]
- Epelboym, I.; Winner, M.; DiNorcia, J.; Lee, M.K.; Lee, J.A.; Schrope, B.; Chabot, J.A.; Allendorf, J.D. Quality of life in patients after total pancreatectomy is comparable with quality of life in patients who undergo a partial pancreatic resection. J. Surg. Res. 2014, 187, 189–196. [Google Scholar] [CrossRef]
- Gerber, M.H.; Delitto, D.; Crippen, C.J.; George, T.J.; Behrns, K.E.; Trevino, J.G.; Cioffi, J.L.; Hughes, S.J. Analysis of the Cost Effectiveness of Laparoscopic Pancreatoduodenectomy. J. Gastrointest. Surg. 2017, 21, 1404–1410. [Google Scholar] [CrossRef]
- Rutz, D.R.; Squires, M.H.; Maithel, S.K.; Sarmiento, J.M.; Etra, J.W.; Perez, S.D.; Knechtle, W.; Cardona, K.; Russell, M.C.; Staley, C.A.; et al. Cost comparison analysis of open versus laparoscopic distal pancreatectomy. HPB 2014, 16, 907–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kent, T.S.; Sachs, T.E.; Callery, M.P.; Vollmer, C.M. Readmission after Major Pancreatic Resection: A Necessary Evil? J. Am. Coll. Surg. 2011, 213, 515–523. [Google Scholar] [CrossRef]
- Mimura, T.; Masuda, A.; Matsumoto, I.; Shiomi, H.; Yoshida, S.; Sugimoto, M.; Sanuki, T.; Yoshida, M.; Fujita, T.; Kutsumi, H.; et al. Predictors of Malignant Intraductal Papillary Mucinous Neoplasm of the Pancreas. J. Clin. Gastroenterol. 2010, 44, e224–e229. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, A.C.; Chen, Y.; Watson, T.R.; Eckel, A.; Sheehan, D.F.; Peters, M.L.B.; Pandharipande, P.V.; Hur, C.; Kong, C.Y. Pancreatic cancer treatment costs, including patient liability, by phase of care and treatment modality, 2000–2013. Medicine 2019, 98, e18082. [Google Scholar] [CrossRef] [PubMed]
- Arias, E. United States Life Tables, 2017. Natl. Vital Stat. Rep. Cent. Dis. Control Prev. Natl. Cent. Health Stat. Natl. Vital Stat. Syst. 2019, 59, 1–60. [Google Scholar]
- Choi, S.-Y.; Kim, J.H.; Yu, M.H.; Eun, H.W.; Lee, H.K.; Han, J.K. Diagnostic performance and imaging features for predicting the malignant potential of intraductal papillary mucinous neoplasm of the pancreas: A comparison of EUS, contrast-enhanced CT and MRI. Abdom. Radiol. 2017, 42, 1449–1458. [Google Scholar] [CrossRef]
- Huang, E.S.; Gazelle, G.S.; Hur, C. Consensus Guidelines in the Management of Branch Duct Intraductal Papillary Mucinous Neoplasm: A Cost-Effectiveness Analysis. Dig. Dis. Sci. 2009, 55, 852–860. [Google Scholar] [CrossRef]
- Chari, S.T.; Yadav, D.; Smyrk, T.C.; DiMagno, E.P.; Miller, L.J.; Raimondo, M.; Clain, J.E.; Norton, I.A.; Pearson, R.K.; Petersen, B.T.; et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology 2002, 123, 1500–1507. [Google Scholar] [CrossRef]
- Sanders, G.D.; Neumann, P.J.; Basu, A.; Brock, D.W.; Feeny, D.; Krahn, M.; Kuntz, K.M.; Meltzer, D.O.; Owens, D.K.; Prosser, L.A.; et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses. JAMA 2016, 316, 1093–1103. [Google Scholar] [CrossRef]
- Scholten, L.; Stoop, T.F.; Del Chiaro, M.; Busch, O.R.; Van Eijck, C.; Molenaar, I.Q.; De Vries, J.H.; Besselink, M.G. Systematic review of functional outcome and quality of life after total pancreatectomy. BJS 2019, 106, 1735–1746. [Google Scholar] [CrossRef]
- Levink, I.; Bruno, M.J.; Cahen, D.L. Management of Intraductal Papillary Mucinous Neoplasms: Controversies in Guidelines and Future Perspectives. Curr. Treat. Options Gastroenterol. 2018, 16, 316–332. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, R.; Thosani, N.; Annangi, S.; Guha, S.; Bhutani, M.S. Diagnostic yield of EUS-FNA-based cytology distinguishing malignant and benign IPMNs: A systematic review and meta-analysis. Pancreatology 2014, 14, 380–384. [Google Scholar] [CrossRef]
- Goh, B.K. International guidelines for the management of pancreatic intraductal papillary mucinous neoplasms. World J. Gastroenterol. 2015, 21, 9833–9837. [Google Scholar] [CrossRef] [PubMed]
- Pulvirenti, A.; Margonis, G.A.; Morales-Oyarvide, V.; McIntyre, C.A.; Lawrence, S.A.; Goldman, D.A.; Gonen, M.; Weiss, M.J.; Ferrone, C.R.; He, J.; et al. Intraductal Papillary Mucinous Neoplasms. Ann. Surg. 2019. [Google Scholar] [CrossRef] [PubMed]
- Nagamachi, S.; Nishii, R.; Wakamatsu, H.; Mizutani, Y.; Kiyohara, S.; Fujita, S.; Futami, S.; Sakae, T.; Furukoji, E.; Tamura, S.; et al. The usefulness of 18F-FDG PET/MRI fusion image in diagnosing pancreatic tumor: Comparison with 18F-FDG PET/CT. Ann. Nucl. Med. 2013, 27, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, M.; Isohashi, K.; Onishi, H.; Hori, M.; Kim, T.; Higuchi, I.; Inoue, A.; Shimosegawa, E.; Takeda, Y.; Hatazawa, J. 18F-FDG PET/MRI fusion in characterizing pancreatic tumors: Comparison to PET/CT. Int. J. Clin. Oncol. 2011, 16, 408–415. [Google Scholar] [CrossRef]
- Huo, L.; Feng, F.; Liao, Q.; Jin, Z.; Li, F.; Zhao, Y. Intraductal Papillary Mucinous Neoplasm of the Pancreas With High Malignant Potential on FDG PET/MRI. Clin. Nucl. Med. 2016, 41, 989–990. [Google Scholar] [CrossRef]


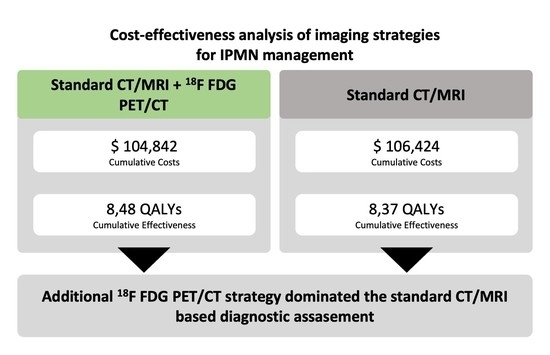
| Strategy | Cumulative Discounted Costs (US-$) | Incremental Costs (US-$) | Cumulative Discounted Effectiveness (QALYs) | Incremental Effectiveness (QALYs) | Net Monetary Benefit (US-$) |
|---|---|---|---|---|---|
| Add. 18F-FDG- PET/CT | $104,842 | n/a | 8.48 | n/a | $742,697 |
| CT/MRI | $106,424 | $1581 | 8.37 | −0.11 | $730,272 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bicu, F.; Rink, J.S.; Froelich, M.F.; Cyran, C.C.; Rübenthaler, J.; Birgin, E.; Röhrich, M.; Tollens, F. Supplemental 18F-FDG-PET/CT for Detection of Malignant Transformation of IPMN—A Model-Based Cost-Effectiveness Analysis. Cancers 2021, 13, 1365. https://doi.org/10.3390/cancers13061365
Bicu F, Rink JS, Froelich MF, Cyran CC, Rübenthaler J, Birgin E, Röhrich M, Tollens F. Supplemental 18F-FDG-PET/CT for Detection of Malignant Transformation of IPMN—A Model-Based Cost-Effectiveness Analysis. Cancers. 2021; 13(6):1365. https://doi.org/10.3390/cancers13061365
Chicago/Turabian StyleBicu, Felix, Johann S. Rink, Matthias F. Froelich, Clemens C. Cyran, Johannes Rübenthaler, Emrullah Birgin, Manuel Röhrich, and Fabian Tollens. 2021. "Supplemental 18F-FDG-PET/CT for Detection of Malignant Transformation of IPMN—A Model-Based Cost-Effectiveness Analysis" Cancers 13, no. 6: 1365. https://doi.org/10.3390/cancers13061365