CHPF Regulates the Aggressive Phenotypes of Hepatocellular Carcinoma Cells via the Modulation of the Decorin and TGF-β Pathways
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
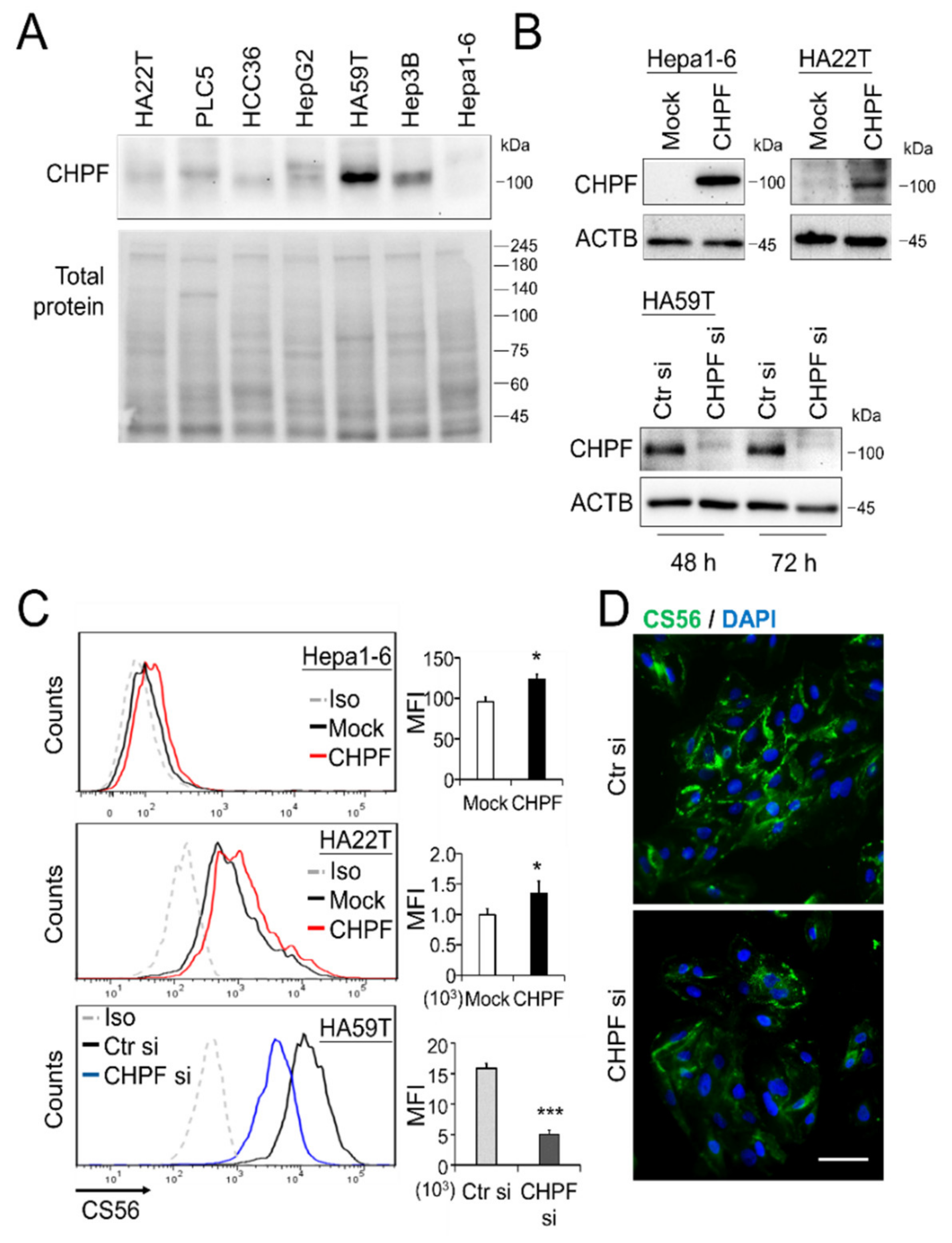
2.1. Downregulation of CHPF in HCC
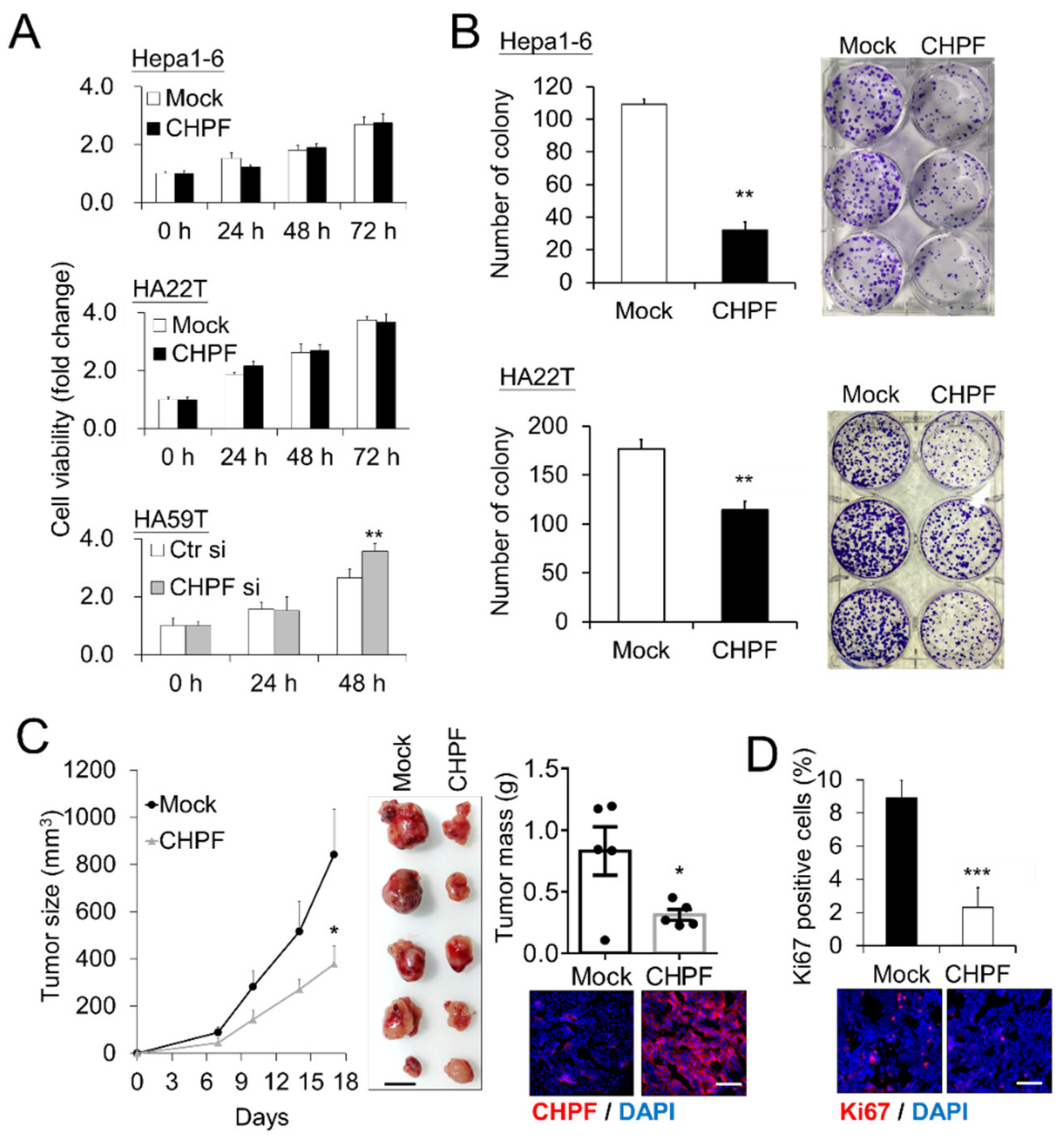
2.2. CHPF Affects HCC Cell Growth In Vitro and In Vivo
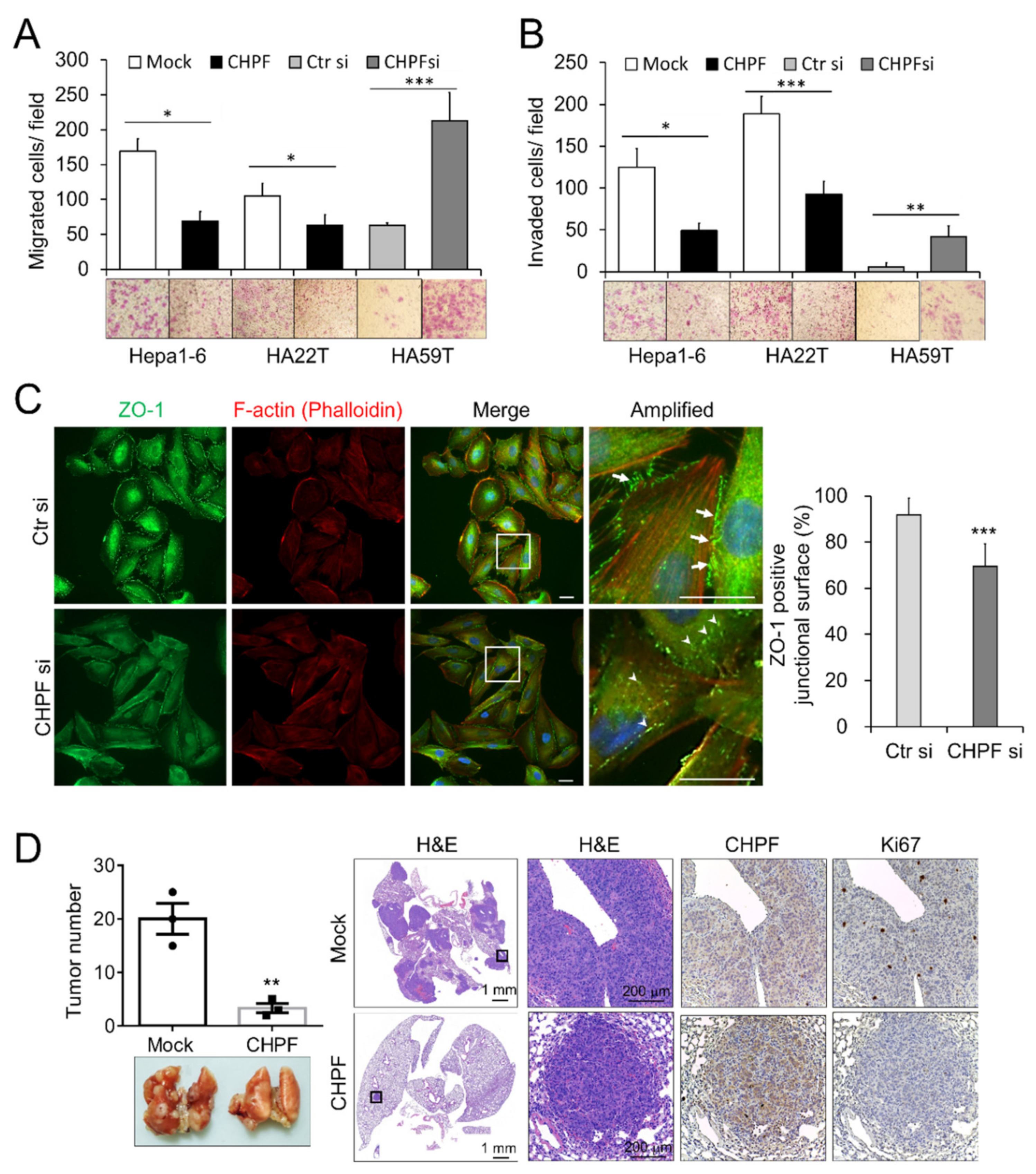
2.3. CHPF Regulates the Migration, Invasion, and Metastasis of HCC Cells
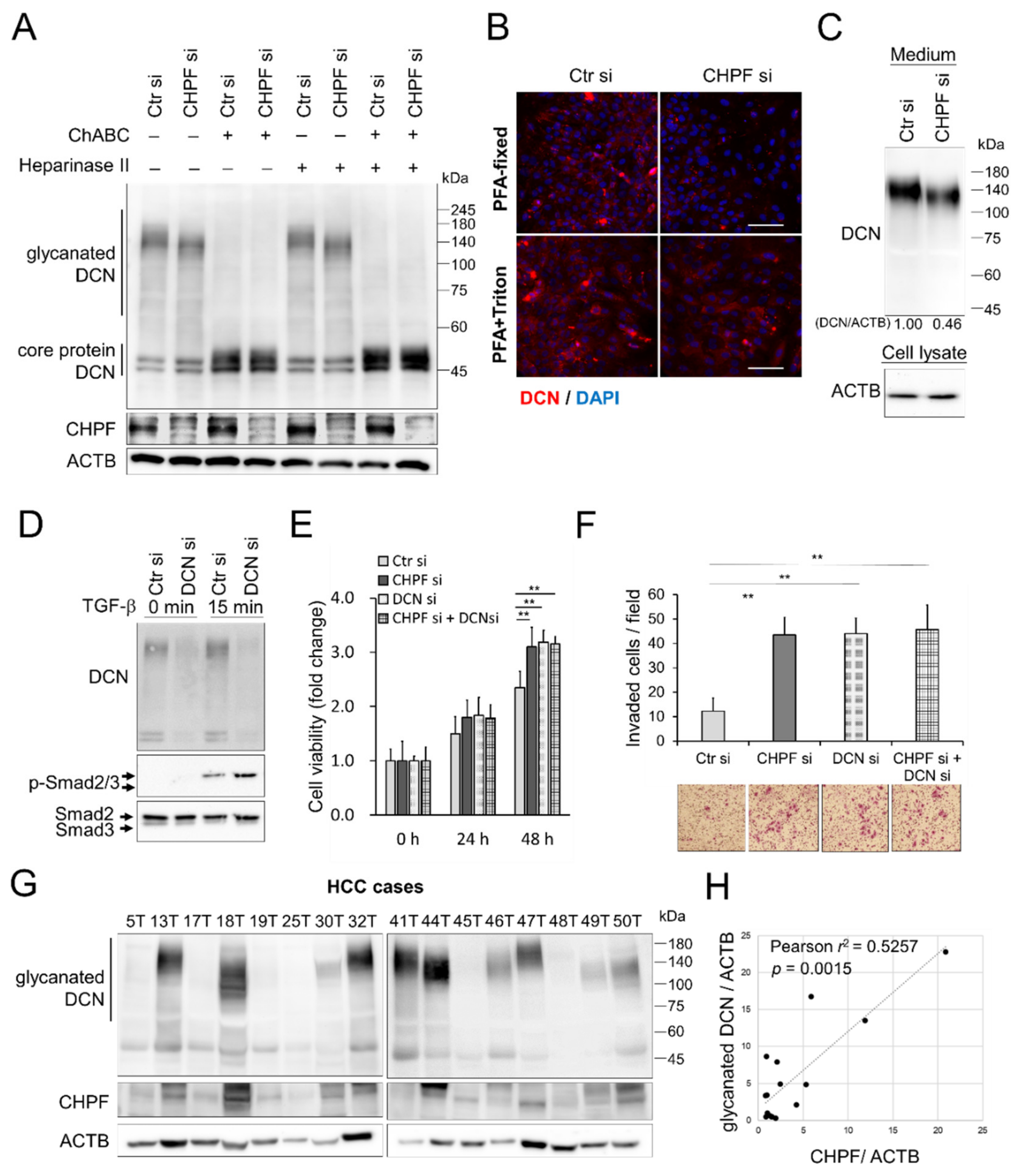
2.4. CHPF Modulates TGF-β Signaling and Modifies the CS Chains of Decorin (DCN)
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Human Tissue Samples
4.3. Immunohistochemistry
4.4. Cell Culture
4.5. Transfection and RNA Interference
4.6. Cell Invasion and Migration Assay
4.7. Cell Proliferation and Colony Formation
4.8. Immunofluorescence
4.9. Western Blotting
4.10. Metastasis and Tumor Growth Mice Models
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHPF | Chondroitin polymerizing factor |
| CS | chondroitin sulfate |
| DCN | decorin |
| GAGs | Glycosaminoglycans |
| GalNAc | N-acetylgalactosamine |
| HCC | hepatocellular carcinoma |
| PGs | proteoglycans |
| TME | tumor microenvironment |
References
- Laursen, L. A preventable cancer. Nature 2014, 516, S2–S3. [Google Scholar] [CrossRef]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Shen, F.; Lau, W.Y. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2017, 33, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Q.; Lou, Y.; Fu, Q.; Chen, Q.; Wei, T.; Yang, J.; Tang, J.; Wang, J.; Chen, Y.; et al. Hypoxia-inducible factor-1α/interleukin-1β signaling enhances hepatoma epithelial-mesenchymal transition through macrophages in a hypoxic-inflammatory microenvironment. Hepatology 2018, 67, 1872–1889. [Google Scholar] [CrossRef]
- Giannelli, G.; Koudelkova, P.; Dituri, F.; Mikulits, W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J. Hepatol. 2016, 65, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Mereiter, S.; Balmaña, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell 2019, 36, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.; Relvas-Santos, M.; Azevedo, R.; Santos, L.L.; Ferreira, J.A. Protein Glycosylation and Tumor Microenvironment Alterations Driving Cancer Hallmarks. Front. Oncol. 2019, 9, 380. [Google Scholar] [CrossRef]
- Lv, H.; Yu, G.; Sun, L.; Zhang, Z.; Zhao, X.; Chai, W. Elevate Level of Glycosaminoglycans and Altered Sulfation Pattern of Chondroitin Sulfate Are Associated with Differentiation Status and Histological Type of Human Primary Hepatic Carcinoma. Oncology 2007, 72, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Salanti, A.; Clausen, T.M.; Agerbæk, M.Ø.; Al Nakouzi, N.; Dahlbäck, M.; Oo, H.Z.; Lee, S.; Gustavsson, T.; Rich, J.R.; Hedberg, B.J.; et al. Targeting Human Cancer by a Glycosaminoglycan Binding Malaria Protein. Cancer Cell 2015, 28, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.-L.; Li, S.-Y.; Dang, S.-S.; Cheng, Y.-A.; Zhang, X.; Wang, W.-J.; Hughes, C.E.; Caterson, B. Increased expression of chondroitin sulphate proteoglycans in rat hepatocellular carcinoma tissues. World J. Gastroenterol. 2012, 18, 3962–3976. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Mikami, T.; Uyama, T.; Mizuguchi, S.; Nomura, K.; Kitagawa, H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 2003, 13, 612–620. [Google Scholar] [CrossRef]
- Akita, K.; Von Holst, A.; Furukawa, Y.; Mikami, T.; Sugahara, K.; Faissner, A. Expression of Multiple Chondroitin/Dermatan Sulfotransferases in the Neurogenic Regions of the Embryonic and Adult Central Nervous System Implies that Complex Chondroitin Sulfates Have a Role in Neural Stem Cell Maintenance. Stem Cells 2008, 26, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S. Potential Therapeutic Application of Chondroitin Sulfate/Dermatan Sulfate. Curr. Drug Discov. Technol. 2008, 5, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, S.; Yamada, S.; Sugahara, K. Molecular interactions between chondroitin–dermatan sulfate and growth factors/receptors/matrix proteins. Curr. Opin. Struct. Biol. 2015, 34, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Djerbal, L.; Lortat-Jacob, H.; Kwok, J. Chondroitin sulfates and their binding molecules in the central nervous system. Glycoconj. J. 2017, 34, 363–376. [Google Scholar] [CrossRef]
- Silagi, E.S.; Shapiro, I.M.; Risbud, M.V. Glycosaminoglycan synthesis in the nucleus pulposus: Dysregulation and the pathogenesis of disc degeneration. Matrix Biol. 2018, 71, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Narimatsu, Y.; Clausen, T.M.; Gomes, C.; Karlsson, R.; Steentoft, C.; Spliid, C.B.; Gustavsson, T.; Salanti, A.; Persson, A.; et al. The GAGOme: A cell-based library of displayed glycosaminoglycans. Nat. Methods 2018, 15, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Izumikawa, T.; Koike, T.; Shiozawa, S.; Sugahara, K.; Tamura, J.-I.; Kitagawa, H. Identification of Chondroitin Sulfate Glucuronyltransferase as Chondroitin Synthase-3 Involved in Chondroitin Polymerization. J. Biol. Chem. 2008, 283, 11396–11406. [Google Scholar] [CrossRef] [PubMed]
- Izumikawa, T.; Uyama, T.; Okuura, Y.; Sugahara, K.; Kitagawa, H. Involvement of chondroitin sulfate synthase-3 (chondroitin synthase-2) in chondroitin polymerization through its interaction with chondroitin synthase-1 or chondroitin-polymerizing factor. Biochem. J. 2007, 403, 545–552. [Google Scholar] [CrossRef]
- Liu, C.-H.; Lan, C.-T.; Chou, J.-F.; Tseng, T.-J.; Liao, W.-C. CHSY1 promotes aggressive phenotypes of hepatocellular carcinoma cells via activation of the hedgehog signaling pathway. Cancer Lett. 2017, 403, 280–288. [Google Scholar] [CrossRef]
- Liao, W.-C.; Liao, C.-K.; Tseng, T.-J.; Ho, Y.-J.; Chen, Y.-R.; Lin, K.-H.; Lai, T.-J.; Lan, C.-T.; Wei, K.-C.; Liu, C.-H. Chondroitin sulfate synthase 1 enhances proliferation of glioblastoma by modulating PDGFRA stability. Oncogenesis 2020, 9, 1–11. [Google Scholar] [CrossRef]
- Liao, W.-C.; Liao, C.-K.; Tsai, Y.-H.; Tseng, T.-J.; Chuang, L.-C.; Lan, C.-T.; Chang, H.-M.; Liu, C.-H. DSE promotes aggressive glioma cell phenotypes by enhancing HB-EGF/ErbB signaling. PLoS ONE 2018, 13, e0198364. [Google Scholar] [CrossRef]
- Liao, W.-C.; Yen, H.-R.; Liao, C.-K.; Tseng, T.-J.; Lan, C.-T.; Liu, C.-H. DSE regulates the malignant characters of hepatocellular carcinoma cells by modulating CCL5/CCR1 axis. Am. J. Cancer Res. 2019, 9, 347–362. [Google Scholar] [PubMed]
- Fan, Y.-H.; Xiao, B.; Lv, S.-G.; Ye, M.-H.; Zhu, X.-G.; Wu, M.-J. Lentivirus-mediated knockdown of chondroitin polymerizing factor inhibits glioma cell growth in�vitro. Oncol. Rep. 2017, 38, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.-M.; Zhang, T.; Da, Z.; Wu, X.-A. CHPF promotes lung adenocarcinoma proliferation and anti-apoptosis via the MAPK pathway. Pathol. Res. Pr. 2019, 215, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhao, F.; Xu, Y.; Huang, K.; Guo, X.; Zheng, B.; Liu, X.; Luo, Z.; Kong, Y.; Xu, M.; et al. Chondroitin polymerizing factor (CHPF) promotes development of malignant melanoma through regulation of CDK1. Cell Death Dis. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Ito, Y.; Hikino, M.; Yajima, Y.; Mikami, T.; Sirko, S.; Von Holst, A.; Faissner, A.; Fukui, S.; Sugahara, K. Structural characterization of the epitopes of the monoclonal antibodies 473HD, CS-56, and MO-225 specific for chondroitin sulfate D-type using the oligosaccharide library. Glycobiology 2004, 15, 593–603. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Orbán, E.; Szabó, E.; Lotz, G.; Kupcsulik, P.; Páska, C.; Schaff, Z.; Kiss, A. Different Expression of Occludin and ZO-1 in Primary and Metastatic Liver Tumors. Pathol. Oncol. Res. 2008, 14, 299–306. [Google Scholar] [CrossRef]
- Nagai, T.; Arao, T.; Nishio, K.; Matsumoto, K.; Hagiwara, S.; Sakurai, T.; Minami, Y.; Ida, H.; Ueshima, K.; Nishida, N.; et al. Impact of Tight Junction Protein ZO-1 and TWIST Expression on Postoperative Survival of Patients with Hepatocellular Carcinoma. Dig. Dis. 2016, 34, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, X.-J.; Sun, X.; Xia, T.-S.; Li, X.-X.; Shi, L.; Zhu, L.; Zhou, W.-B.; Wei, J.-F.; Ding, Q. RBM38 is involved in TGF-β-induced epithelial-to-mesenchymal transition by stabilising zonula occludens-1 mRNA in breast cancer. Br. J. Cancer 2017, 117, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Viloria-Petit, A.M.; David, L.; Jia, J.Y.; Erdemir, T.; Bane, A.L.; Pinnaduwage, D.; Roncari, L.; Narimatsu, M.; Bose, R.; Moffat, J.; et al. A role for the TGF-Par6 polarity pathway in breast cancer progression. Proc. Natl. Acad. Sci. USA 2009, 106, 14028–14033. [Google Scholar] [CrossRef]
- Huang, J.; Qiu, M.; Wan, L.; Wang, G.; Huang, T.; Chen, Z.; Jiang, S.; Li, X.; Xie, L.; Cai, L. TGF-β1 Promotes Hepatocellular Carcinoma Invasion and Metastasis via ERK Pathway-Mediated FGFR4 Expression. Cell. Physiol. Biochem. 2018, 45, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Karamanos, N.K. Proteoglycans remodeling in cancer: Underlying molecular mechanisms. Matrix Biol. 2019, 75, 220–259. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Wei, V.M.; Iozzo, R.; Höök, M.; Grande-Allen, K.J. Decorin-transforming Growth Factor-β Interaction Regulates Matrix Organization and Mechanical Characteristics of Three-dimensional Collagen Matrices. J. Biol. Chem. 2007, 282, 35887–35898. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, A.; Romarís, M.; Rasmussen, L.M.; Heinegård, D.; Twardzik, D.R.; Border, W.A.; Ruoslahti, E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem. J. 1994, 302, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Wang, Z.; Ju, W.; Wang, D. Decorin inhibits the proliferation of HepG2 cells by elevating the expression of transforming growth factor-β receptor II. Exp. Ther. Med. 2016, 12, 2191–2195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ricciardelli, C.; Brooks, J.H.; Suwiwat, S.; Sakko, A.J.; Mayne, K.; Raymond, W.A.; Seshadri, R.; LeBaron, R.G.; Horsfall, D.J. Regulation of stromal versican expression by breast cancer cells and importance to relapse-free survival in patients with node-negative primary breast cancer. Clin. Cancer Res. 2002, 8, 1054–1060. [Google Scholar]
- Ricciardelli, C.; Russell, D.L.; Ween, M.P.; Mayne, K.; Suwiwat, S.; Byers, S.; Marshall, V.R.; Tilley, W.D.; Horsfall, D.J. Formation of Hyaluronan and Versican-rich Pericellular Matrix by Prostate Cancer Cells Promotes Cell Motility. J. Biol. Chem. 2007, 282, 10814–10825. [Google Scholar] [CrossRef]
- Ricciardelli, C.; Mayne, K.; Sykes, P.J.; Raymond, W.A.; McCaul, K.; Marshall, V.R.; Horsfall, D.J. Elevated levels of versican but not decorin predict disease progression in early-stage prostate cancer. Clin. Cancer Res. 1998, 4, 963–971. [Google Scholar] [PubMed]
- Zhangyuan, G.; Wang, F.; Zhang, H.; Jiang, R.; Tao, X.; Yu, D.; Jin, K.; Yu, W.; Liu, Y.; Yin, Y.; et al. VersicanV1 promotes proliferation and metastasis of hepatocellular carcinoma through the activation of EGFR–PI3K–AKT pathway. Oncogene 2019, 39, 1213–1230. [Google Scholar] [CrossRef] [PubMed]
- Dituri, F.; Scialpi, R.; Schmidt, T.A.; Frusciante, M.; Mancarella, S.; Lupo, L.G.; Villa, E.; Giannelli, G. Proteoglycan-4 is correlated with longer survival in HCC patients and enhances sorafenib and regorafenib effectiveness via CD44 in vitro. Cell Death Dis. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Reszegi, A.; Horváth, Z.; Fehér, H.; Wichmann, B.; Tátrai, P.; Kovalszky, I.; Baghy, K. Protective Role of Decorin in Primary Hepatocellular Carcinoma. Front. Oncol. 2020, 10, 645. [Google Scholar] [CrossRef]
- Xu, Q.; Lin, W.; Tao, C.; Huang, X.; Li, J. Chondroitin polymerizing factor (CHPF) contributes to malignant proliferation and migration of hepatocellular carcinoma cells. Biochem. Cell Biol. 2020, 98, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.G.; Phamluong, K.; Lin, W.Y.; Barck, K.; Carano, R.A.; Diehl, L.; Peterson, A.S.; Martin, F.; Solloway, M.J. Chondroitin sulfate synthase 1 (Chsy1) is required for bone development and digit patterning. Dev. Biol. 2012, 363, 413–425. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Chakrani, F.; Perrotti, D.; McQuillan, D.J.; Skorski, T.; Calabretta, B.; Eichstetter, I. Cooperative action of germ-line mutations in decorin and p53 accelerates lymphoma tumorigenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 3092–3097. [Google Scholar] [CrossRef]
- Mao, L.; Yang, J.; Yue, J.; Chen, Y.; Zhou, H.; Fan, D.; Zhang, Q.; Buraschi, S.; Iozzo, R.V.; Bi, X. Decorin deficiency promotes epithelial-mesenchymal transition and colon cancer metastasis. Matrix Biol. 2021, 95, 1–14. [Google Scholar] [CrossRef]
- Bi, X.; Tong, C.; Dockendorff, A.; Bancroft, L.; Gallagher, L.; Guzman, G.; Iozzo, R.V.; Augenlicht, L.H.; Yang, W. Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis 2008, 29, 1435–1440. [Google Scholar] [CrossRef]
- Järvinen, T.A.H.; Prince, S. Decorin: A Growth Factor Antagonist for Tumor Growth Inhibition. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Horváth, Z.; Kovalszky, I.; Fullár, A.; Kiss, K.; Schaff, Z.; Iozzo, R.V.; Baghy, K. Decorin deficiency promotes hepatic carcinogenesis. Matrix Biol. 2014, 35, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Markmann, A.; Hausser, H.; Schönherr, E.; Kresse, H. Influence of decorin expression on transforming growth factor-β-mediated collagen gel retraction and biglycan induction. Matrix Biol. 2000, 19, 631–636. [Google Scholar] [CrossRef]
- Chan, W.L.; Steiner, M.; Witkos, T.; Egerer, J.; Busse, B.; Mizumoto, S.; Pestka, J.M.; Zhang, H.; Hausser, I.; Khayal, L.A.; et al. Impaired proteoglycan glycosylation, elevated TGF-β signaling, and abnormal osteoblast differentiation as the basis for bone fragility in a mouse model for gerodermia osteodysplastica. PLoS Genet. 2018, 14, e1007242. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Xu, C.; Cao, P.; Tian, Y.; Zhang, Y.; Shi, C.; Xu, J.; Yuan, W.; Chen, H. TGF-β Stimulates Expression of Chondroitin Polymerizing Factor in Nucleus Pulposus Cells Through the Smad3, RhoA/ROCK1, and MAPK Signaling Pathways. J. Cell. Biochem. 2018, 119, 566–579. [Google Scholar] [CrossRef] [PubMed]


| Factor | Feature | CHPF Expression | p Value (Two-Sided Fisher’s Exact Test) | |
|---|---|---|---|---|
| Low | High | |||
| Tissue types | Non-tumor | 15 | 34 | 0.0275 * |
| Tumor | 40 | 38 | ||
| Sex | Male | 31 | 29 | 1.00 |
| Female | 9 | 9 | ||
| Age | <55 years | 10 | 7 | 0.5869 |
| ≥55 years | 30 | 31 | ||
| Tumor stage | T1 + T2 | 20 | 29 | 0.0202 * |
| T3 + T3 | 20 | 9 | ||
| Metastasis | No | 1 | 0 | 1.00 |
| Yes | 39 | 38 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-H.; Wu, B.-R.; Ho, Y.-J.; Chu, Y.-H.; Hsu, W.-C.; Tseng, T.-J.; Li, J.-P.; Liao, W.-C. CHPF Regulates the Aggressive Phenotypes of Hepatocellular Carcinoma Cells via the Modulation of the Decorin and TGF-β Pathways. Cancers 2021, 13, 1261. https://doi.org/10.3390/cancers13061261
Liu C-H, Wu B-R, Ho Y-J, Chu Y-H, Hsu W-C, Tseng T-J, Li J-P, Liao W-C. CHPF Regulates the Aggressive Phenotypes of Hepatocellular Carcinoma Cells via the Modulation of the Decorin and TGF-β Pathways. Cancers. 2021; 13(6):1261. https://doi.org/10.3390/cancers13061261
Chicago/Turabian StyleLiu, Chiung-Hui, Bo-Rui Wu, Ying-Jui Ho, Yin-Hung Chu, Wei-Cheng Hsu, To-Jung Tseng, Ju-Pi Li, and Wen-Chieh Liao. 2021. "CHPF Regulates the Aggressive Phenotypes of Hepatocellular Carcinoma Cells via the Modulation of the Decorin and TGF-β Pathways" Cancers 13, no. 6: 1261. https://doi.org/10.3390/cancers13061261
APA StyleLiu, C.-H., Wu, B.-R., Ho, Y.-J., Chu, Y.-H., Hsu, W.-C., Tseng, T.-J., Li, J.-P., & Liao, W.-C. (2021). CHPF Regulates the Aggressive Phenotypes of Hepatocellular Carcinoma Cells via the Modulation of the Decorin and TGF-β Pathways. Cancers, 13(6), 1261. https://doi.org/10.3390/cancers13061261







