Tertiary Prevention of HCC in Chronic Hepatitis B or C Infected Patients
Abstract
:Simple Summary
Abstract
1. Introduction
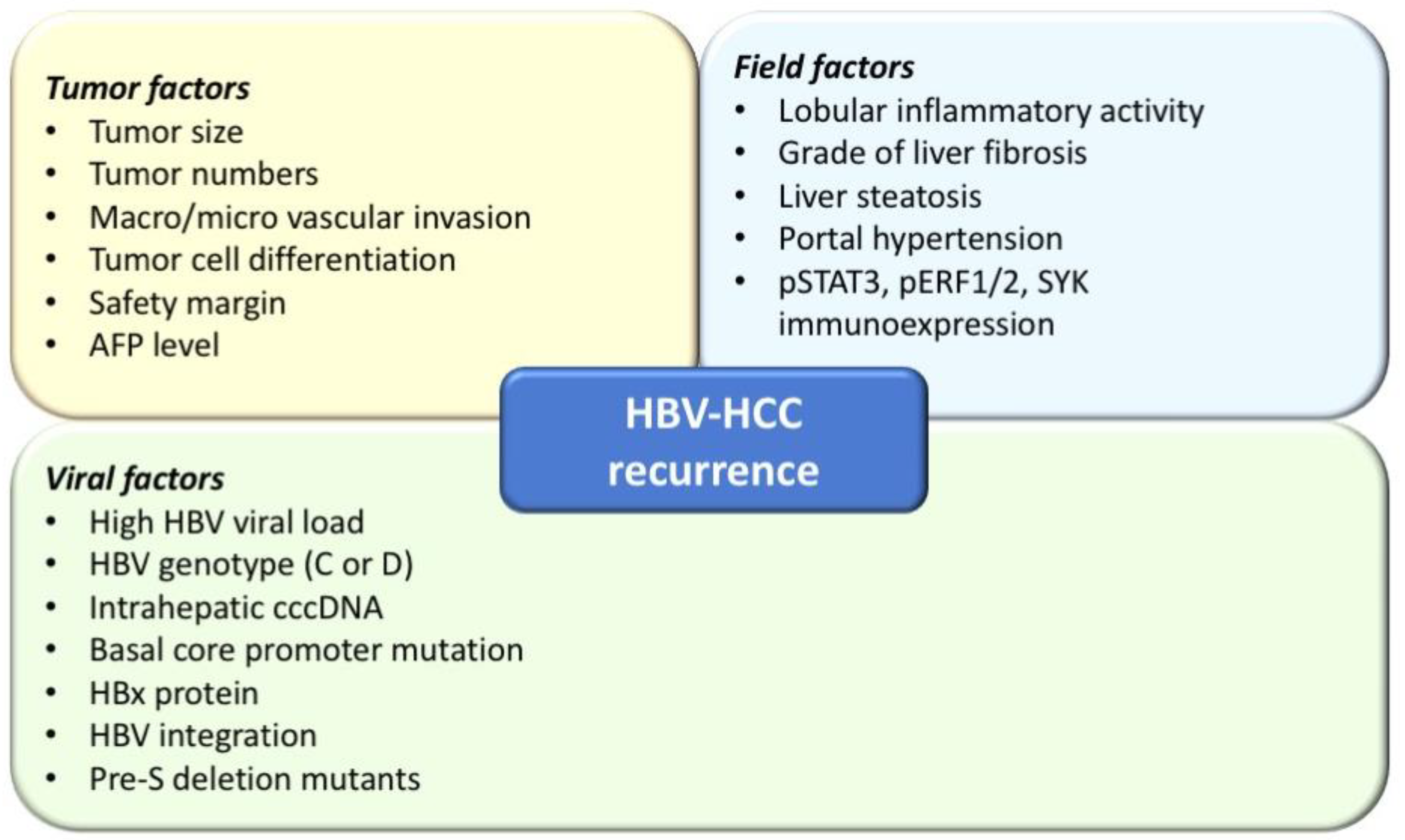
1.1. Viral Factors Associated with HCC Recurrence: HBV
1.2. Tertiary Prevention for Patients with HBV-Related HCC Following Curative Therapy: Interferon-Based Therapy
1.3. Tertiary Prevention for Patients with HBV-Related HCC Following Curative Therapy: Nuc Therapy
1.4. Carcinogenesis in Chronic Hepatitis C Patients
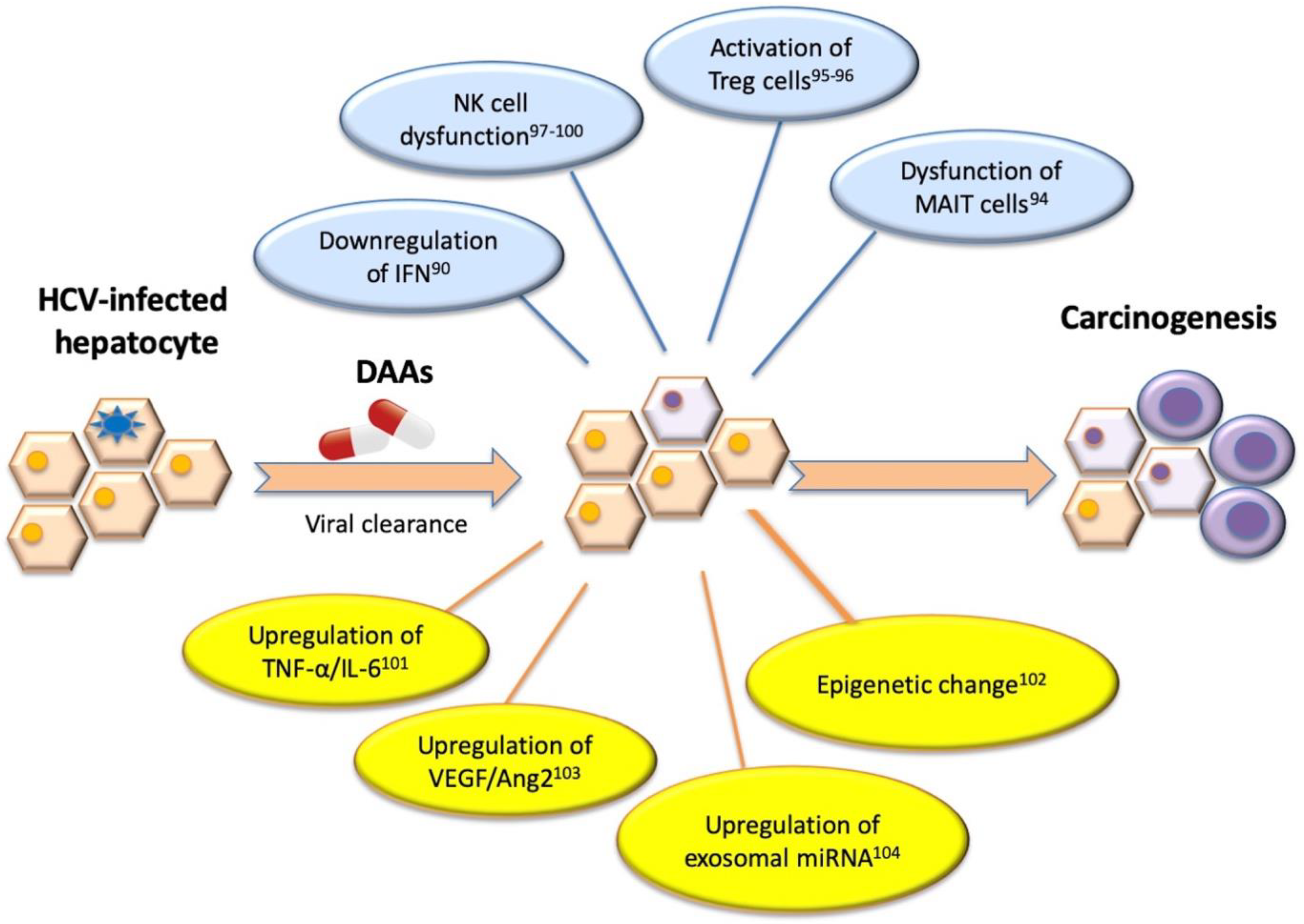
1.5. The Alteration of Host Immune System by Antiviral Therapy Differs between IFN and DAA Treatment in CHC-HCC Patients
1.6. The Tertiary Prevention for Patients with HCV-Related HCC Following Curative Therapy: Interferon-Based Therapy
1.7. The Tertiary Prevention Efficacy for Patients with HCV-Related HCC Following Curative Therapy: DAA
1.8. DAA Versus Untreated
1.9. DAA Versus IFN-Based Therapy
1.10. The Optimal Timing of Adjuvant DAA Treatment for Tertiary Prevention of CHC-HCC
1.11. The Role of Immune Checkpoint Inhibitors in HBV and HCV-Related HCC Patients
2. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADV | adefovir |
| AFP | Alpha-fetoprotein |
| BCLC | Barcelona Clinic Liver Cancer classification |
| CHB | chronic hepatitis B |
| CHC | chronic hepatitis C |
| CI | confidence interval |
| CTLA-4 | cytotoxic T-Lymphocyte antigen 4 |
| DAA | direct acting antiviral agents |
| DFS | disease free survival |
| EMT | epithelial mesenchymal transition |
| ER | endoplasmic reticulum |
| ERK | extracellular signal-regulated kinase |
| ETV | entecavir |
| FXR | Farnesoid X receptor |
| HBsAg | hepatitis B surface antigen |
| HBV | hepatitis B virus |
| HBV-HCC | HBV related HCC |
| HCC | hepatocellular carcinoma |
| HCV | hepatitis C virus |
| HCV-HCC | HCV related HCC |
| HR | hazard ratio |
| IFN | interferon |
| LAM | lamivudine |
| MAIT | mucosal associated invariant T |
| NK | natural killer cell |
| Nuc | nucleos(t)ide analogues |
| OS | overall survival |
| PD-1 | programmed cell death protein 1 |
| Peg-IFN | pegylated interferon |
| RCT | randomized control trial |
| RFA | radiofrequency ablation |
| RFS | recurrence-free survival |
| ROS | reactive oxygen species |
| SVR | sustained virologic response |
| TAF | tenofovir alafenamide |
| TDF | tenofovir disoproxil fumarate |
| TERT | telomerase reverse transcriptase |
| TDF-β | transforming growth factor |
| Treg | regulatory T cell. |
References
- WHO. Latest Global Cancer Data: Cancer Burden Rises to 19.1 Million New Cases and 9.6 Million Cancer Deaths in 2018. Available online: https://www.who.int/cancer/PRGlobocanFinal.pdf (accessed on 12 September 2018).
- Singal, A.G.; Lok, A.S.; Feng, Z.; Kanwal, F.; Parikh, N.D. Conceptual Model for the Hepatocellular Carcinoma Screening Continuum: Current Status and Research Agenda. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.I.; Yeh, S.H.; Chen, P.J.; Iloeje, U.H.; Jen, C.L.; Su, J.; Wang, L.Y.; Lu, S.N.; You, S.L.; Chen, D.S.; et al. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J. Natl. Cancer Inst. 2008, 100, 1134–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.X.; Mehta, N. Does Hepatocellular Carcinoma Surveillance Increase Survival in At-Risk Populations? Patient Selection, Biomarkers, and Barriers. Dig. Dis. Sci. 2020, 65, 3456–3462. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Bru, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Predictive factors for long term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan. The Liver Cancer Study Group of Japan. Cancer 1994, 74, 2772–2780. [CrossRef]
- Balsells, J.; Charco, R.; Lazaro, J.L.; Murio, E.; Vargas, V.; Allende, E.; Margarit, C. Resection of hepatocellular carcinoma in patients with cirrhosis. Br. J. Surg. 1996, 83, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.N.; Chen, M.F.; Lee, W.C.; Jeng, L.B. Prognostic factors of hepatic resection for hepatocellular carcinoma with cirrhosis: Univariate and multivariate analysis. J. Surg. Oncol. 2002, 81, 195–202. [Google Scholar] [CrossRef]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Tiong, L.; Maddern, G.J. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br. J. Surg. 2011, 98, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Su, C.W.; Lei, H.J.; Chau, G.Y.; Hung, H.H.; Wu, J.C.; Hsia, C.Y.; Lui, W.Y.; Su, Y.H.; Wu, C.W.; Lee, S.D. The effect of age on the long-term prognosis of patients with hepatocellular carcinoma after resection surgery: A propensity score matching analysis. Arch. Surg. 2012, 147, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Roayaie, S.; Jibara, G.; Tabrizian, P.; Park, J.W.; Yang, J.; Yan, L.; Schwartz, M.; Han, G.; Izzo, F.; Chen, M.; et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology 2015, 62, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.H.; Zhong, J.; Berhane, S.; Toyoda, H.; Cucchetti, A.; Shi, K.; Tada, T.; Chong, C.C.N.; Xiang, B.-D.; Li, L.-Q.; et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J. Hepatol. 2018, 69, 1284–1293. [Google Scholar] [CrossRef] [Green Version]
- Vibert, E.; Schwartz, M.; Olthoff, K.M. Advances in resection and transplantation for hepatocellular carcinoma. J. Hepatol. 2020, 72, 262–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiina, S.; Tateishi, R.; Arano, T.; Uchino, K.; Enooku, K.; Nakagawa, H.; Asaoka, Y.; Sato, T.; Masuzaki, R.; Kondo, Y.; et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am. J. Gastroenterol. 2012, 107, 569–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, W.Y.; Su, C.W.; Chiou, Y.Y.; Chiu, N.C.; Liu, C.A.; Fang, K.C.; Huo, T.I.; Huang, Y.H.; Chang, C.C.; Hou, M.C.; et al. Hepatocellular Carcinoma: Nomograms Based on the Albumin-Bilirubin Grade to Assess the Outcomes of Radiofrequency Ablation. Radiology 2017, 285, 670–680. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.C.; Huang, Y.H.; Chau, G.Y.; Su, C.W.; Lai, C.R.; Lee, P.C.; Huo, T.I.; Sheen, I.J.; Lee, S.D.; Lui, W.Y. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J. Hepatol. 2009, 51, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Yeh, S.H.; Chen, J.T.; Wu, C.C.; Hsu, M.T.; Tsai, S.F.; Chen, P.J.; Lin, C.H. Chromosomal changes and clonality relationship between primary and recurrent hepatocellular carcinoma. Gastroenterology 2000, 119, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Hung, I.F.; Poon, R.T.; Lai, C.L.; Fung, J.; Fan, S.T.; Yuen, M.F. Recurrence of hepatitis B-related hepatocellular carcinoma is associated with high viral load at the time of resection. Am. J. Gastroenterol. 2008, 103, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Shirai, H.; Yao, S.; Yagi, S.; Kamo, N.; Seo, S.; Taura, K.; et al. Preoperative Visceral Adiposity and Muscularity Predict Poor Outcomes after Hepatectomy for Hepatocellular Carcinoma. Liver Cancer 2019, 8, 92–109. [Google Scholar] [CrossRef]
- Su, C.W.; Chiou, Y.W.; Tsai, Y.H.; Teng, R.D.; Chau, G.Y.; Lei, H.J.; Hung, H.H.; Huo, T.I.; Wu, J.C. The Influence of Hepatitis B Viral Load and Pre-S Deletion Mutations on Post-Operative Recurrence of Hepatocellular Carcinoma and the Tertiary Preventive Effects by Anti-Viral Therapy. PLoS ONE 2013, 8, e66457. [Google Scholar] [CrossRef] [Green Version]
- Su, C.W.; Chau, G.Y.; Hung, H.H.; Yeh, Y.C.; Lei, H.J.; Hsia, C.Y.; Lai, C.R.; Lin, H.C.; Wu, J.C. Impact of Steatosis on Prognosis of Patients with Early-Stage Hepatocellular Carcinoma After Hepatic Resection. Ann. Surg. Oncol. 2015, 22, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Colecchia, A.; Colli, A.; Ravaioli, F.; Casazza, G.; Bacchi Reggiani, M.L.; Cucchetti, A.; Cescon, M.; Festi, D. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J. Hepatol. 2019, 70, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Hsieh, W.Y.; Chau, G.Y.; Chen, P.H.; Su, C.W.; Hou, M.C.; Lei, H.J.; Huo, T.I.; Huang, Y.H.; Lin, H.C.; et al. Esophageal varices are not predictive of patient prognosis after surgical resection of hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.R.; Tian, M.X.; Jin, L.; Yang, L.X.; Ding, Z.B.; Shen, Y.H.; Peng, Y.F.; Zhou, J.; Qiu, S.J.; Dai, Z.; et al. High levels of hepatitis B surface antigen are associated with poorer survival and early recurrence of hepatocellular carcinoma in patients with low hepatitis B viral loads. Ann. Surg. Oncol. 2015, 22, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Sohn, W.; Paik, Y.H.; Kim, J.M.; Kwon, C.H.; Joh, J.W.; Cho, J.Y.; Gwak, G.Y.; Choi, M.S.; Lee, J.H.; Koh, K.C.; et al. HBV DNA and HBsAg levels as risk predictors of early and late recurrence after curative resection of HBV-related hepatocellular carcinoma. Ann. Surg. Oncol. 2014, 21, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Nahm, J.H.; Lee, H.S.; Kim, H.; Yim, S.Y.; Shin, J.H.; Yoo, J.E.; Ahn, S.H.; Choi, J.S.; Lee, J.S.; Park, Y.N. Pathologic Predictive Factors for Late Recurrence of Hepatocellular Carcinoma in Chronic Liver Disease. Liver Int. 2021. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Changchien, C.S.; Lee, C.M.; Hung, C.H.; Hu, T.H.; Wang, J.H.; Wang, J.C.; Lu, S.N. Combined mutations in pre-s/surface and core promoter/precore regions of hepatitis B virus increase the risk of hepatocellular carcinoma: A case-control study. J. Infect. Dis. 2008, 198, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.H.; Liu, C.J.; Jow, G.M.; Chen, P.J.; Chen, D.S.; Chen, B.F. Fine mapping of hepatitis B virus pre-S deletion and its association with hepatocellular carcinoma. Liver Int. 2012, 32, 1373–1381. [Google Scholar] [CrossRef]
- Jin, J.; Jung, H.Y.; Lee, K.H.; Yi, N.J.; Suh, K.S.; Jang, J.J.; Lee, K.B. Nuclear Expression of Hepatitis B Virus X Protein Is Associated with Recurrence of Early-Stage Hepatocellular Carcinomas: Role of Viral Protein in Tumor Recurrence. J. Pathol. Transl. Med. 2016, 50, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Li, C.L.; Ho, M.C.; Lin, Y.Y.; Tzeng, S.T.; Chen, Y.J.; Pai, H.Y.; Wang, Y.C.; Chen, C.L.; Lee, Y.H.; Chen, D.S.; et al. Cell-Free Virus-Host Chimera DNA From Hepatitis B Virus Integration Sites as a Circulating Biomarker of Hepatocellular Cancer. Hepatology 2020. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.A.; Lee, C. Hepatitis B virus X gene and hepatocarcinogenesis. J. Gastroenterol. 2011, 46, 974–990. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Chen, L.; Wu, M.; Huang, W.; Wu, X.; Huang, D.; Xie, Y.; Shi, G. Partial abrogation of FXR-KNG1 signaling by carboxyl-terminal truncated HBx-C30 in hepatitis B virus-associated hepatocellular carcinoma. Virus Res. 2021, 293, 198264. [Google Scholar] [CrossRef]
- Wang, L.H.; Huang, W.; Lai, M.D.; Su, I.J. Aberrant cyclin A expression and centrosome overduplication induced by hepatitis B virus pre-S2 mutants and its implication in hepatocarcinogenesis. Carcinogenesis 2012, 33, 466–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Y.C.; Neo, J.C.; Wu, J.C.; Chen, Y.F.; Kao, C.H.; Tsai, T.F. Expression of a hepatitis B virus pre-S2 deletion mutant in the liver results in hepatomegaly and hepatocellular carcinoma in mice. J. Pathol. 2017, 241, 463–474. [Google Scholar] [CrossRef]
- Yen, C.J.; Ai, Y.L.; Tsai, H.W.; Chan, S.H.; Yen, C.S.; Cheng, K.H.; Lee, Y.P.; Kao, C.W.; Wang, Y.C.; Chen, Y.L.; et al. Hepatitis B virus surface gene pre-S-2 mutant as a high-risk serum marker for hepatoma recurrence after curative hepatic resection. Hepatology 2018, 68, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.W.; Chu, Y.D.; Lai, M.W.; Lin, C.L.; Liang, K.H.; Lin, Y.H.; Yeh, C.T. Hepatitis B Virus Covalently Closed Circular DNA Predicts Postoperative Liver Cancer Metastasis Independent of Virological Suppression. Cancers 2021, 13, 538. [Google Scholar] [CrossRef] [PubMed]
- Su, C.W.; Yang, Y.Y.; Lin, H.C. Impact of etiological treatment on prognosis. Hepatol. Int. 2018, 12, 56–67. [Google Scholar] [CrossRef]
- Marcellin, P.; Lau, G.K.; Bonino, F.; Farci, P.; Hadziyannis, S.; Jin, R.; Lu, Z.M.; Piratvisuth, T.; Germanidis, G.; Yurdaydin, C.; et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 2004, 351, 1206–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.M.; Lin, C.J.; Hsu, C.W.; Tai, D.I.; Sheen, I.S.; Lin, D.Y.; Liaw, Y.F. Prospective randomized controlled study of interferon-alpha in preventing hepatocellular carcinoma recurrence after medical ablation therapy for primary tumors. Cancer 2004, 100, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.S.; Jin, F.; Huang, X.W.; Shen, X.Z. Interferon-alpha therapy after curative resection prevents early recurrence and improves survival in patients with hepatitis B virus-related hepatocellular carcinoma. J. Surg. Oncol. 2010, 102, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.C.; Tang, Z.Y.; Wang, L.; Qin, L.X.; Ma, Z.C.; Ye, Q.H.; Zhang, B.H.; Qian, Y.B.; Wu, Z.Q.; Fan, J.; et al. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: A randomized clinical trial. J. Cancer Res. Clin. Oncol. 2006, 132, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.M.; Liu, C.L.; Chan, S.C.; Lam, C.M.; Poon, R.T.P.; Ng, I.O.L.; Fan, S.T.; Wong, J. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann. Surg. 2007, 245, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.T.; Chen, M.F.; Li, L.A.; Lee, P.H.; Jeng, L.B.; Lin, D.Y.; Wu, C.C.; Mok, K.T.; Chen, C.L.; Lee, W.C.; et al. Long-term results of a randomized, observation-controlled, phase III trial of adjuvant interferon Alfa-2b in hepatocellular carcinoma after curative resection. Ann. Surg. 2012, 255, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Yang, X.; He, R.Q.; Hu, Q.G.; Song, Z.F.; Xiong, J.; Zheng, Q.C. Antiviral therapy after curative treatment of hepatitis B/C virus-related hepatocellular carcinoma: A systematic review of randomized trials. Hepatol. Res. 2014, 44, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhang, Q.; Xu, Y.; Wang, X.; Yu, F.; Zhang, Y.; Zhao, P.; Guo, H.; Zhou, C.; Wang, Z.; et al. Peg-interferon and nucleos(t)ide analogue combination at inception of antiviral therapy improves both anti-HBV efficacy and long-term survival among HBV DNA-positive hepatocellular carcinoma patients after hepatectomy/ablation. J. Viral Hepat. 2020, 27, 387–396. [Google Scholar] [CrossRef]
- Surveillance Group; Diagnosis Group; Staging Group; Surgery Group; Local Ablation Group; TACE/TARE/HAI Group; Target Therapy/Systemic Therapy Group; Radiotherapy Group; Prevention Group; Drafting Group. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J. Formos. Med Assoc. 2018, 117, 381–403. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, P.; Gane, E.; Buti, M.; Afdhal, N.; Sievert, W.; Jacobson, I.M.; Washington, M.K.; Germanidis, G.; Flaherty, J.F.; Schall, R.A.; et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet 2013, 381, 468–475. [Google Scholar] [CrossRef]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Gill, U.S.; Kennedy, P.T.F. The impact of currently licensed therapies on viral and immune responses in chronic hepatitis B: Considerations for future novel therapeutics. J. Viral Hepat. 2019, 26, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Liaw, Y.F.; Sung, J.J.Y.; Chow, W.C.; Farrell, G.; Lee, C.Z.; Yuen, H.; Tanwandee, T.; Tao, Q.M.; Shue, K.; Keene, O.N.; et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N. Engl. J. Med. 2004, 351, 1521–1531. [Google Scholar] [CrossRef]
- Su, T.H.; Hu, T.H.; Chen, C.Y.; Huang, Y.H.; Chuang, W.L.; Lin, C.C.; Wang, C.C.; Su, W.W.; Chen, M.Y.; Peng, C.Y.; et al. Four-year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver Int. 2016, 36, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Lin, J.T.; Ho, H.J.; Su, C.W.; Lee, T.Y.; Wang, S.Y.; Wu, C.; Wu, J.C. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: A nationwide cohort study. Gastroenterology 2014, 147, 143–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Yang, H.I.; Nguyen, N.; Hoang, J.; Kim, Y.; Vu, V.; Le, A.; Chaung, K.; Nguyen, V.; Trinh, H.; et al. Reduction of chronic hepatitis B-related hepatocellular carcinoma with anti-viral therapy, including low risk patients. Aliment. Pharmacol. Ther. 2016, 44, 846–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, G.L.; Tse, Y.K.; Chan, H.L.; Yip, T.C.; Tsoi, K.K.; Wong, V.W. Oral nucleos(t)ide analogues reduce recurrence and death in chronic hepatitis B-related hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2016, 43, 802–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Tokumoto, Y.; Joko, K.; Michitaka, K.; Mashiba, T.; Hiraoka, A.; Ochi, H.; Koizumi, Y.; Tada, F.; Hirooka, M.; et al. Effects of long-term entecavir treatment on the incidence of hepatocellular carcinoma in chronic hepatitis B patients. Hepatol. Int. 2016, 10, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.C.; Chok, K.S.; Yuen, W.K.; Chan, S.C.; Poon, R.T.; Lo, C.M.; Fan, S.T. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch. Surg. 2011, 146, 675–681. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.Y.; Chen, Y.J.; Ho, H.J.; Hsu, Y.C.; Kuo, K.N.; Wu, M.S.; Lin, J.T. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA 2012, 308, 1906–1914. [Google Scholar] [CrossRef] [Green Version]
- Ke, Y.; Ma, L.; You, X.M.; Huang, S.X.; Liang, Y.R.; Xiang, B.D.; Li, L.Q.; Zhong, J.H. Antiviral therapy for hepatitis B virus-related hepatocellular carcinoma after radical hepatectomy. Cancer Biol. Med. 2013, 10, 158–164. [Google Scholar] [CrossRef]
- Yin, J.; Li, N.; Han, Y.; Xue, J.; Deng, Y.; Shi, J.; Guo, W.; Zhang, H.; Wang, H.; Cheng, S.; et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: A two-stage longitudinal clinical study. J. Clin. Oncol. 2013, 31, 3647–3655. [Google Scholar] [CrossRef]
- Sun, P.; Dong, X.; Cheng, X.; Hu, Q.; Zheng, Q. Nucleot(s)ide analogues for hepatitis B virus-related hepatocellular carcinoma after curative treatment: A systematic review and meta-analysis. PLoS ONE 2014, 9, e102761. [Google Scholar] [CrossRef]
- Huang, G.; Lau, W.Y.; Wang, Z.G.; Pan, Z.Y.; Yuan, S.X.; Shen, F.; Zhou, W.P.; Wu, M.C. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: A randomized controlled trial. Ann. Surg 2015, 261, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.C.; Wong, G.L.; Wong, V.W.; Ip, P.C.; Cheung, Y.S.; Wong, J.; Lee, K.F.; Lai, P.B.; Chan, H.L. Antiviral therapy improves post-hepatectomy survival in patients with hepatitis B virus-related hepatocellular carcinoma: A prospective-retrospective study. Aliment. Pharmacol. Ther. 2015, 41, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Lin, J.T.; Zeng, Y.S.; Chen, Y.J.; Wu, M.S.; Wu, C.Y. Association between nucleos(t)ide analog and tumor recurrence in hepatitis B virus-related hepatocellular carcinoma after radiofrequency ablation. Hepatology 2016, 63, 1517–1527. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.X.; Cheng, J.W.; Huang, A.; Zhang, X.; Wang, J.; Fan, J.; Zhou, J.; Yang, X.R. The effect of antiviral therapy on patients with hepatitis B virus-related hepatocellular carcinoma after curative resection: A systematic review and meta-analysis. OncoTargets Ther. 2017, 10, 5363–5375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, V.W.; Chan, S.L.; Wong, V.W.; Liang, L.Y.; Yip, T.C.; Lai, J.C.; Yuen, B.W.; Luk, H.W.; Tse, Y.K.; Lee, H.W.; et al. Increasing antiviral treatment uptake improves survival in patients with HBV-related HCC. JHEP Rep. 2020, 2, 100152. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Ahn, H.; Lee, D.H.; Lee, J.H.; Jung, Y.J.; Chang, Y.; Nam, J.Y.; Cho, Y.Y.; Lee, D.H.; Cho, E.J.; et al. Entecavir and tenofovir reduce hepatitis B virus-related hepatocellular carcinoma recurrence more effectively than other antivirals. J. Viral Hepat. 2018, 25, 707–717. [Google Scholar] [CrossRef]
- Choi, J.; Jo, C.; Lim, Y.S. Tenofovir Versus Entecavir on Recurrence of Hepatitis B Virus-Related Hepatocellular Carcinoma After Surgical Resection. Hepatology 2020. [Google Scholar] [CrossRef]
- Chien, R.N.; Kao, J.H.; Peng, C.Y.; Chen, C.H.; Liu, C.J.; Huang, Y.H.; Hu, T.H.; Yang, H.I.; Lu, S.N.; Ni, Y.H.; et al. Taiwan consensus statement on the management of chronic hepatitis B. J. Formos. Med. Assoc. 2019, 118, 7–38. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.T.; Chen, T.; Hsu, C.W.; Chen, Y.C.; Lai, M.W.; Liang, K.H.; Chen, T.C. Emergence of the rtA181T/sW172* mutant increased the risk of hepatoma occurrence in patients with lamivudine-resistant chronic hepatitis B. BMC Cancer 2011, 11, 398. [Google Scholar] [CrossRef] [Green Version]
- Yip, T.C.; Wong, G.L.; Chan, H.L.; Tse, Y.K.; Lam, K.L.; Lui, G.C.; Wong, V.W. HBsAg seroclearance further reduces hepatocellular carcinoma risk after complete viral suppression with nucleos(t)ide analogues. J. Hepatol. 2019, 70, 361–370. [Google Scholar] [CrossRef]
- Feld, J.J.; Krassenburg, L.A.P. What Comes First: Treatment of Viral Hepatitis or Liver Cancer? Dig. Dis. Sci. 2019, 64, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.A.; Lake, A.J.; Scott, S.; Sherman, R.L.; Noone, A.M.; Howlader, N.; Henley, S.J.; Anderson, R.N.; Firth, A.U.; Ma, J.; et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018, 124, 2785–2800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thrift, A.P.; El-Serag, H.B.; Kanwal, F. Global epidemiology and burden of HCV infection and HCV-related disease. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.B.; Wittenborn, J.S.; Weinbaum, C.M.; Sabin, M.; Smith, B.D.; Lesesne, S.B. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig. Liver Dis. 2011, 43, 66–72. [Google Scholar] [CrossRef]
- Hoshida, Y.; Fuchs, B.C.; Bardeesy, N.; Baumert, T.F.; Chung, R.T. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J. Hepatol. 2014, 61, S79–S90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesri, E.A.; Feitelson, M.A.; Munger, K. Human viral oncogenesis: A cancer hallmarks analysis. Cell Host Microbe 2014, 15, 266–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkari, L.; Gregoire, D.; Floc’h, N.; Moreau, M.; Hernandez, C.; Simonin, Y.; Rosenberg, A.R.; Lassus, P.; Hibner, U. Hepatitis C viral protein NS5A induces EMT and participates in oncogenic transformation of primary hepatocyte precursors. J. Hepatol. 2012, 57, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Zucman-Rossi, J. TERT promoter mutations in primary liver tumors. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 9–14. [Google Scholar] [CrossRef]
- Friedman, R.M.; Contente, S. Treatment of hepatitis C infections with interferon: A historical perspective. Hepat. Res. Treat. 2010, 2010, 323926. [Google Scholar] [CrossRef] [Green Version]
- Manns, M.P.; McHutchison, J.G.; Gordon, S.C.; Rustgi, V.K.; Shiffman, M.; Reindollar, R.; Goodman, Z.D.; Koury, K.; Ling, M.-H.; Albrecht, J.K. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet 2001, 358, 958–965. [Google Scholar] [CrossRef]
- Fried, M.W.; Shiffman, M.L.; Reddy, K.R.; Smith, C.; Marinos, G.; Gonçales Jr, F.L.; Häussinger, D.; Diago, M.; Carosi, G.; Dhumeaux, D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 2002, 347, 975–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feld, J.J.; Hoofnagle, J.H. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature 2005, 436, 967. [Google Scholar] [CrossRef] [PubMed]
- Stark, G.R.; Kerr, I.M.; Williams, B.R.; Silverman, R.H.; Schreiber, R.D. How cells respond to interferons. Annu. Rev. Biochem. 1998, 67, 227–264. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Erickson, S.; Szeps, M.; Gruber, A.; Sangfelt, O.; Einhorn, S.; Pisa, P.; Grandér, D. Interferon α down-regulates telomerase reverse transcriptase and telomerase activity in human malignant and nonmalignant hematopoietic cells. Blood 2000, 96, 4313–4318. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Okano, J.-I.; Murawaki, Y. Differential effects of interferon alpha-2b and beta on the signaling pathways in human liver cancer cells. J. Gastroenterol. 2005, 40, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Negrier, S.; Escudier, B.; Lasset, C.; Douillard, J.-Y.; Savary, J.; Chevreau, C.; Ravaud, A.; Mercatello, A.; Peny, J.; Mousseau, M. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. N. Engl. J. Med. 1998, 338, 1272–1278. [Google Scholar] [CrossRef]
- Yano, H.; Iemura, A.; Haramaki, M.; Ogasawara, S.; Takayama, A.; Akiba, J.; Kojiro, M. Interferon alfa receptor expression and growth inhibition by interferon alfa in human liver cancer cell lines. Hepatology 1999, 29, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Klaus, A.; Birchmeier, W. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer 2008, 8, 387. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, S.; Mocellin, S. The anticancer face of interferon alpha (IFN-alpha): From biology to clinical results, with a focus on melanoma. Curr. Med. Chem. 2010, 17, 3327–3336. [Google Scholar] [CrossRef] [PubMed]
- Villani, R.; Vendemiale, G.; Serviddio, G. Molecular Mechanisms Involved in HCC Recurrence after Direct-Acting Antiviral Therapy. Int. J. Mol. Sci. 2018, 20, 49. [Google Scholar] [CrossRef] [Green Version]
- Burchill, M.A.; Golden-Mason, L.; Wind-Rotolo, M.; Rosen, H.R. Memory re-differentiation and reduced lymphocyte activation in chronic HCV-infected patients receiving direct-acting antivirals. J. Viral Hepat. 2015, 22, 983–991. [Google Scholar] [CrossRef]
- Martin, B.; Hennecke, N.; Lohmann, V.; Kayser, A.; Neumann-Haefelin, C.; Kukolj, G.; Böcher, W.-O.; Thimme, R. Restoration of HCV-specific CD8+ T cell function by interferon-free therapy. J. Hepatol. 2014, 61, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Hengst, J.; Strunz, B.; Deterding, K.; Ljunggren, H.G.; Leeansyah, E.; Manns, M.P.; Cornberg, M.; Sandberg, J.K.; Wedemeyer, H.; Bjorkstrom, N.K. Nonreversible MAIT cell-dysfunction in chronic hepatitis C virus infection despite successful interferon-free therapy. Eur. J. Immunol. 2016, 46, 2204–2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langhans, B.; Nischalke, H.D.; Kramer, B.; Hausen, A.; Dold, L.; van Heteren, P.; Huneburg, R.; Nattermann, J.; Strassburg, C.P.; Spengler, U. Increased peripheral CD4(+) regulatory T cells persist after successful direct-acting antiviral treatment of chronic hepatitis C. J. Hepatol. 2017, 66, 888–896. [Google Scholar] [CrossRef]
- Dilek, N.; Poirier, N.; Hulin, P.; Coulon, F.; Mary, C.; Ville, S.; Vie, H.; Clemenceau, B.; Blancho, G.; Vanhove, B. Targeting CD28, CTLA-4 and PD-L1 costimulation differentially controls immune synapses and function of human regulatory and conventional T-cells. PLoS ONE 2013, 8, e83139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serti, E.; Chepa-Lotrea, X.; Kim, Y.J.; Keane, M.; Fryzek, N.; Liang, T.J.; Ghany, M.; Rehermann, B. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology 2015, 149, 190–200.e192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pahl, J.; Cerwenka, A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiology 2017, 222, 11–20. [Google Scholar] [CrossRef]
- Chu, P.S.; Nakamoto, N.; Taniki, N.; Ojiro, K.; Amiya, T.; Makita, Y.; Murata, H.; Yamaguchi, A.; Shiba, S.; Miyake, R.; et al. On-treatment decrease of NKG2D correlates to early emergence of clinically evident hepatocellular carcinoma after interferon-free therapy for chronic hepatitis C. PLoS ONE 2017, 12, e0179096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehermann, B. Natural Killer Cells in Viral Hepatitis. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 578–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debes, J.D.; van Tilborg, M.; Groothuismink, Z.M.A.; Hansen, B.E.; Schulze Zur Wiesch, J.; von Felden, J.; de Knegt, R.J.; Boonstra, A. Levels of Cytokines in Serum Associate With Development of Hepatocellular Carcinoma in Patients With HCV Infection Treated With Direct-Acting Antivirals. Gastroenterology 2018, 154, 515–517.e513. [Google Scholar] [CrossRef] [PubMed]
- Hamdane, N.; Juhling, F.; Crouchet, E.; El Saghire, H.; Thumann, C.; Oudot, M.A.; Bandiera, S.; Saviano, A.; Ponsolles, C.; Roca Suarez, A.A.; et al. HCV-Induced Epigenetic Changes Associated With Liver Cancer Risk Persist After Sustained Virologic Response. Gastroenterology 2019, 156, 2313–2329.e2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faillaci, F.; Marzi, L.; Critelli, R.; Milosa, F.; Schepis, F.; Turola, E.; Andreani, S.; Vandelli, G.; Bernabucci, V.; Lei, B.; et al. Liver Angiopoietin-2 Is a Key Predictor of De Novo or Recurrent Hepatocellular Cancer After Hepatitis C Virus Direct-Acting Antivirals. Hepatology 2018, 68, 1010–1024. [Google Scholar] [CrossRef] [Green Version]
- Itami-Matsumoto, S.; Hayakawa, M.; Uchida-Kobayashi, S.; Enomoto, M.; Tamori, A.; Mizuno, K.; Toyoda, H.; Tamura, T.; Akutsu, T.; Ochiya, T.; et al. Circulating Exosomal miRNA Profiles Predict the Occurrence and Recurrence of Hepatocellular Carcinoma in Patients with Direct-Acting Antiviral-Induced Sustained Viral Response. Biomedicines 2019, 7, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poynard, T.; McHutchison, J.; Manns, M.; Trepo, C.; Lindsay, K.; Goodman, Z.; Ling, M.H.; Albrecht, J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology 2002, 122, 1303–1313. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.C.; Ho, H.J.; Wu, M.S.; Lin, J.T.; Wu, C.Y. Postoperative peg-interferon plus ribavirin is associated with reduced recurrence of hepatitis C virus-related hepatocellular carcinoma. Hepatology 2013, 58, 150–157. [Google Scholar] [CrossRef]
- Chen, Y.C.; Teng, W.; Hsieh, Y.C.; Chen, W.T.; Jeng, W.J.; Huang, C.H.; Lin, C.C.; Chen, Y.C.; Lin, S.M.; Lin, C.Y.; et al. Timely eradication of HCV viremia by PegIFN/RBV is crucial in prevention of post RFA recurrence in CHC-HCC patients. J. Formos. Med. Assoc. 2019, 118, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.; Hsieh, Y.C.; Lui, K.W.; Chen, W.T.; Hung, C.F.; Huang, C.H.; Chen, Y.C.; Jeng, W.J.; Lin, C.C.; Lin, C.Y.; et al. Eradication of hepatitis C virus profoundly prolongs survival in hepatocellular carcinoma patients receiving transarterial chemoembolization. J. Viral Hepat. 2017, 24, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.F.; Yeh, M.L.; Yu, M.L.; Dai, C.Y.; Huang, C.F.; Huang, C.I.; Tsai, P.C.; Lin, P.C.; Chen, Y.L.; Chang, W.T.; et al. The tertiary prevention of hepatocellular carcinoma in chronic hepatitis C patients. J. Gastroenterol. Hepatol. 2015, 30, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Zeng, X.; Yang, Z.; Meng, Z. Effect and safety of interferon for hepatocellular carcinoma: A systematic review and meta-analysis. PLoS ONE 2013, 8, e61361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manthravadi, S.; Paleti, S.; Pandya, P. Impact of sustained viral response postcurative therapy of hepatitis C-related hepatocellular carcinoma: A systematic review and meta-analysis. Int. J. Cancer 2017, 140, 1042–1049. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Romito, R.; Schiavo, M.; Mariani, L.; Camerini, T.; Bhoori, S.; Capussotti, L.; Calise, F.; Pellicci, R.; Belli, G. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology 2006, 44, 1543–1554. [Google Scholar] [CrossRef]
- Kudo, M.; Sakaguchi, Y.; Chung, H.; Hatanaka, K.; Hagiwara, S.; Ishikawa, E.; Takahashi, S.; Kitai, S.; Inoue, T.; Minami, Y. Long-term interferon maintenance therapy improves survival in patients with HCV-related hepatocellular carcinoma after curative radiofrequency ablation. Oncology 2007, 72, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.C.; Aikata, H.; Katamura, Y.; Azakami, T.; Kawaoka, T.; Saneto, H.; Uka, K.; Mori, N.; Takaki, S.; Kodama, H. Effects of a 24-week course of interferon-α therapy after curative treatment of hepatitis C virus-associated hepatocellular carcinoma. World, J. Gastroenterol. WJG 2007, 13, 5343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanogawa, N.; Ogasawara, S.; Chiba, T.; Saito, T.; Motoyama, T.; Suzuki, E.; Ooka, Y.; Tawada, A.; Kanda, T.; Mikami, S. Sustained virologic response achieved after curative treatment of hepatitis C virus-related hepatocellular carcinoma as an independent prognostic factor. J. Gastroenterol. Hepatol. 2015, 30, 1197–1204. [Google Scholar] [CrossRef] [Green Version]
- Petta, S.; Cabibbo, G.; Barbara, M.; Attardo, S.; Bucci, L.; Farinati, F.; Giannini, E.G.; Tovoli, F.; Ciccarese, F.; Rapaccini, G.L.; et al. Hepatocellular carcinoma recurrence in patients with curative resection or ablation: Impact of HCV eradication does not depend on the use of interferon. Aliment. Pharmacol. Ther. 2017, 45, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Baumert, T.F.; Berg, T.; Lim, J.K.; Nelson, D.R. Status of Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection and Remaining Challenges. Gastroenterology 2019, 156, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Knop, V.; Hoppe, D.; Welzel, T.; Vermehren, J.; Herrmann, E.; Vermehren, A.; Friedrich-Rust, M.; Sarrazin, C.; Zeuzem, S.; Welker, M.W. Regression of fibrosis and portal hypertension in HCV-associated cirrhosis and sustained virologic response after interferon-free antiviral therapy. J. Viral Hepat. 2016, 23, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.R.; Irving, W.L.; Cheung, M.C.; Walker, A.J.; Hudson, B.E.; Verma, S.; McLauchlan, J.; Mutimer, D.J.; Brown, A.; Gelson, W.T.; et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J. Hepatol. 2016, 64, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Lim, J.K.; Kuo, A.; Di Bisceglie, A.M.; Galati, J.S.; Morelli, G.; Everson, G.T.; Kwo, P.Y.; Brown, R.S., Jr.; Sulkowski, M.S.; et al. All-oral direct-acting antiviral therapy in HCV-advanced liver disease is effective in real-world practice: Observations through HCV-TARGET database. Aliment. Pharmacol. Ther. 2017, 45, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Marino, Z.; Perello, C.; Inarrairaegui, M.; Ribeiro, A.; Lens, S.; Diaz, A.; Vilana, R.; Darnell, A.; Varela, M.; et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 2016, 65, 719–726. [Google Scholar] [CrossRef] [Green Version]
- Conti, F.; Buonfiglioli, F.; Scuteri, A.; Crespi, C.; Bolondi, L.; Caraceni, P.; Foschi, F.G.; Lenzi, M.; Mazzella, G.; Verucchi, G.; et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J. Hepatol. 2016, 65, 727–733. [Google Scholar] [CrossRef]
- Warzyszynska, K.; Jonas, M.; Wasiak, D.; Kosieradzki, M.; Malkowski, P. Accelerated hepatocellular carcinoma recurrence rate after postoperative direct-acting antivirals treatment—preliminary report. Clin. Exp. Hepatol. 2017, 3, 194–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabibbo, G.; Petta, S.; Calvaruso, V.; Cacciola, I.; Cannavo, M.R.; Madonia, S.; Distefano, M.; Larocca, L.; Prestileo, T.; Tine, F.; et al. Is early recurrence of hepatocellular carcinoma in HCV cirrhotic patients affected by treatment with direct-acting antivirals? A prospective multicentre study. Aliment. Pharmacol. Ther. 2017, 46, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, E.; Furusyo, N.; Nomura, H.; Dohmen, K.; Higashi, N.; Takahashi, K.; Kawano, A.; Azuma, K.; Satoh, T.; Nakamuta, M.; et al. Short-term risk of hepatocellular carcinoma after hepatitis C virus eradication following direct-acting anti-viral treatment. Aliment. Pharmacol. Ther. 2018, 47, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Calleja, J.L.; Crespo, J.; Rincon, D.; Ruiz-Antoran, B.; Fernandez, I.; Perello, C.; Gea, F.; Lens, S.; Garcia-Samaniego, J.; Sacristan, B.; et al. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: Results from a Spanish real-world cohort. J. Hepatol. 2017, 66, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Koga, H.; Ide, T.; Kuromatsu, R.; Hashimoto, S.; Yatsuhashi, H.; Seike, M.; Higuchi, N.; Nakamuta, M.; Shakado, S.; et al. Predictors of hepatocellular carcinoma recurrence associated with the use of direct-acting antiviral agent therapy for hepatitis C virus after curative treatment: A prospective multicenter cohort study. Cancer Med. 2019, 8, 2646–2653. [Google Scholar] [CrossRef]
- Bielen, R.; Moreno, C.; Van Vlierberghe, H.; Bourgeois, S.; Mulkay, J.P.; Vanwolleghem, T.; Verlinden, W.; Brixco, C.; Decaestecker, J.; de Galocsy, C.; et al. The risk of early occurrence and recurrence of hepatocellular carcinoma in hepatitis C-infected patients treated with direct-acting antivirals with and without pegylated interferon: A Belgian experience. J. Viral Hepat. 2017, 24, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.C.M.; Walker, A.J.; Hudson, B.E.; Verma, S.; McLauchlan, J.; Mutimer, D.J.; Brown, A.; Gelson, W.T.H.; MacDonald, D.C.; Agarwal, K.; et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J. Hepatol. 2016, 65, 741–747. [Google Scholar] [CrossRef] [Green Version]
- Yoshimasu, Y.; Furuichi, Y.; Kasai, Y.; Takeuchi, H.; Sugimoto, K.; Nakamura, I.; Itoi, T. Predictive factors for hepatocellular carcinoma occurrence or recurrence after direct-acting antiviral agents in patients with chronic hepatitis C. J. Gastrointestin. Liver Dis. 2019, 28, 63–71. [Google Scholar] [CrossRef]
- The ANRS Collaborative Study Group on Hepatocellular Carcinoma. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J. Hepatol. 2016, 65, 734–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolly, P.; Waidmann, O.; Vermehren, J.; Moreno, C.; Vögeli, I.; Berg, T.; Semela, D.; Zeuzem, S.; Dufour, J.-F. Hepatocellular carcinoma recurrence after direct antiviral agent treatment: A European multicentre study. J. Hepatol. 2017, 67, 876–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virlogeux, V.; Pradat, P.; Hartig-Lavie, K.; Bailly, F.; Maynard, M.; Ouziel, G.; Poinsot, D.; Lebosse, F.; Ecochard, M.; Radenne, S.; et al. Direct-acting antiviral therapy decreases hepatocellular carcinoma recurrence rate in cirrhotic patients with chronic hepatitis C. Liver Int. 2017, 37, 1122–1127. [Google Scholar] [CrossRef]
- Zavaglia, C.; Okolicsanyi, S.; Cesarini, L.; Mazzarelli, C.; Pontecorvi, V.; Ciaccio, A.; Strazzabosco, M.; Belli, L.S. Is the risk of neoplastic recurrence increased after prescribing direct-acting antivirals for HCV patients whose HCC was previously cured? J. Hepatol. 2017, 66, 236–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, K.; Kawamura, Y.; Kobayashi, M.; Kominami, Y.; Fujiyama, S.; Sezaki, H.; Hosaka, T.; Akuta, N.; Saitoh, S.; Suzuki, F.; et al. Direct-Acting Antivirals Decreased Tumor Recurrence After Initial Treatment of Hepatitis C Virus-Related Hepatocellular Carcinoma. Dig. Dis. Sci. 2017, 62, 2932–2942. [Google Scholar] [CrossRef] [PubMed]
- Saraiya, N.; Yopp, A.C.; Rich, N.E.; Odewole, M.; Parikh, N.D.; Singal, A.G. Systematic review with meta-analysis: Recurrence of hepatocellular carcinoma following direct-acting antiviral therapy. Aliment. Pharmacol. Ther. 2018, 48, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nautiyal, A.; Loke, Y.K. Oral direct-acting antivirals and the incidence or recurrence of hepatocellular carcinoma: A systematic review and meta-analysis. Frontline Gastroenterol. 2018, 9, 262–270. [Google Scholar] [CrossRef]
- Huang, A.C.; Mehta, N.; Dodge, J.L.; Yao, F.Y.; Terrault, N.A. Direct-acting antivirals do not increase the risk of hepatocellular carcinoma recurrence after local-regional therapy or liver transplant waitlist dropout. Hepatology 2018, 68, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Rich, N.E.; Mehta, N.; Branch, A.; Pillai, A.; Hoteit, M.; Volk, M.; Odewole, M.; Scaglione, S.; Guy, J.; et al. Direct-Acting Antiviral Therapy Not Associated With Recurrence of Hepatocellular Carcinoma in a Multicenter North American Cohort Study. Gastroenterology 2019. [Google Scholar] [CrossRef] [PubMed]
- Cabibbo, G.; Celsa, C.; Calvaruso, V.; Petta, S.; Cacciola, I.; Cannavò, M.R.; Madonia, S.; Rossi, M.; Magro, B.; Rini, F.; et al. Direct acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J. Hepatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Tateishi, R.; Nakagomi, R.; Fujiwara, N.; Sato, M.; Enooku, K.; Nakagawa, H.; Asaoka, Y.; Kondo, Y.; Shiina, S.; et al. The impact of direct-acting antivirals on early tumor recurrence after radiofrequency ablation in hepatitis C-related hepatocellular carcinoma. J. Hepatol. 2016, 65, 1272–1273. [Google Scholar] [CrossRef] [Green Version]
- Nishibatake Kinoshita, M.; Minami, T.; Tateishi, R.; Wake, T.; Nakagomi, R.; Fujiwara, N.; Sato, M.; Uchino, K.; Enooku, K.; Nakagawa, H.; et al. Impact of direct-acting antivirals on early recurrence of HCV-related HCC: Comparison with interferon-based therapy. J. Hepatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nagata, H.; Nakagawa, M.; Asahina, Y.; Sato, A.; Asano, Y.; Tsunoda, T.; Miyoshi, M.; Kaneko, S.; Otani, S.; Kawai-Kitahata, F.; et al. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J. Hepatol. 2017, 67, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Nagaoki, Y.; Imamura, M.; Nisihda, Y.; Daijo, K.; Teraoka, Y.; Honda, F.; Nakamura, Y.; Morio, K.; Fujino, H.; Nakahara, T.; et al. The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma; comparison with interferon-based therapy. J. Med. Virol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mashiba, T.; Joko, K.; Kurosaki, M.; Ochi, H.; Osaki, Y.; Kojima, Y.; Nakata, R.; Goto, T.; Takehiro, A.; Kimura, H.; et al. Does interferon-free direct-acting antiviral therapy for hepatitis C after curative treatment for hepatocellular carcinoma lead to unexpected recurrences of HCC? A multicenter study by the Japanese Red Cross Hospital Liver Study Group. PLoS ONE 2018, 13, e0194704. [Google Scholar] [CrossRef]
- El Kassas, M.; Funk, A.L.; Salaheldin, M.; Shimakawa, Y.; Eltabbakh, M.; Jean, K.; El Tahan, A.; Sweedy, A.T.; Afify, S.; Youssef, N.F.; et al. Increased recurrence rates of hepatocellular carcinoma after DAA therapy in a hepatitis C-infected Egyptian cohort: A comparative analysis. J. Viral Hepat. 2018, 25, 623–630. [Google Scholar] [CrossRef]
- Waziry, R.; Hajarizadeh, B.; Grebely, J.; Amin, J.; Law, M.; Danta, M.; George, J.; Dore, G.J. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J. Hepatol. 2017, 67, 1204–1212. [Google Scholar] [CrossRef]
- Teng, W.; Jeng, W.J.; Yang, H.I.; Chen, W.T.; Hsieh, Y.C.; Huang, C.H.; Lin, C.C.; Lin, C.Y.; Lin, S.M.; Sheen, I.S. Interferon Is Superior to Direct Acting Antiviral Therapy in Tertiary Prevention of Early Recurrence of Hepatocellular Carcinoma. Cancers 2019, 12, 23. [Google Scholar] [CrossRef] [Green Version]
- Cabibbo, G.; Petta, S.; Barbara, M.; Attardo, S.; Bucci, L.; Farinati, F.; Giannini, E.G.; Negrini, G.; Ciccarese, F.; Rapaccini, G.L.; et al. Hepatic decompensation is the major driver of death in HCV-infected cirrhotic patients with successfully treated early hepatocellular carcinoma. J. Hepatol. 2017, 67, 65–71. [Google Scholar] [CrossRef]
- Ogawa, E.; Furusyo, N.; Kajiwara, E.; Takahashi, K.; Nomura, H.; Maruyama, T.; Tanabe, Y.; Satoh, T.; Nakamuta, M.; Kotoh, K.; et al. Efficacy of pegylated interferon alpha-2b and ribavirin treatment on the risk of hepatocellular carcinoma in patients with chronic hepatitis C: A prospective, multicenter study. J. Hepatol. 2013, 58, 495–501. [Google Scholar] [CrossRef]
- Roberts, L.R.; Sirlin, C.B.; Zaiem, F.; Almasri, J.; Prokop, L.J.; Heimbach, J.K.; Murad, M.H.; Mohammed, K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology 2018, 67, 401–421. [Google Scholar] [CrossRef] [Green Version]
- Singal, A.G.; Lim, J.K.; Kanwal, F. AGA Clinical Practice Update on Interaction Between Oral Direct-Acting Antivirals for Chronic Hepatitis C Infection and Hepatocellular Carcinoma: Expert Review. Gastroenterology 2019, 156, 2149–2157. [Google Scholar] [CrossRef] [Green Version]
- Prenner, S.B.; VanWagner, L.B.; Flamm, S.L.; Salem, R.; Lewandowski, R.J.; Kulik, L. Hepatocellular carcinoma decreases the chance of successful hepatitis C virus therapy with direct-acting antivirals. J. Hepatol. 2017, 66, 1173–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beste, L.A.; Green, P.K.; Berry, K.; Kogut, M.J.; Allison, S.K.; Ioannou, G.N. Effectiveness of hepatitis C antiviral treatment in a USA cohort of veteran patients with hepatocellular carcinoma. J. Hepatol. 2017, 67, 32–39. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-C.; Chao, Y.; Chen, M.-H.; Lan, K.-H.; Lee, I.C.; Hou, M.-C.; Huang, Y.-H. Risk of HBV reactivation in patients with immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Wong, G.L.; Wong, V.W.; Hui, V.W.; Yip, T.C.; Tse, Y.K.; Liang, L.Y.; Lui, R.N.; Mok, T.S.; Chan, H.L.; Chan, S.L. Hepatitis Flare During Immunotherapy in Patients With Current or Past Hepatitis B Virus Infection. Am. J. Gastroenterol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Wilson, M.; Pruckner, C.; Kirkwood, J.M. PD-1 Blockade in Advanced Melanoma in Patients with Hepatitis C and/or HIV. Case Rep. Oncol. Med. 2015, 2015, 737389. [Google Scholar] [CrossRef] [Green Version]
- Pertejo-Fernandez, A.; Ricciuti, B.; Hammond, S.P.; Marty, F.M.; Recondo, G.; Rangachari, D.; Costa, D.B.; Awad, M.M. Safety and efficacy of immune checkpoint inhibitors in patients with non-small cell lung cancer and hepatitis B or hepatitis C infection. Lung Cancer 2020, 145, 181–185. [Google Scholar] [CrossRef] [PubMed]
| Author/Year | Patients No. | HCC Tx | F/U(Median) | HCC Recurrence Rate | HR | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| IFN vs. Control | ||||||||
| IFN | Control | IFN | Control | |||||
| Sun 2006 (RCT) | 118 | 118 | Resection | 36.5 months | Median RFS: 31.2 (95% CI: 14.8–47.7) months | Median RFS: 17.7 (95% CI: 9.2–26.3) months | p = 0.1425 | [42] |
| Lo 2007 (RCT) | 40 (35 HBV) | 40 (37 HBV) | Resection | 5 years | 5-year recurrence: 21/40 (52.5%) | 5-year recurrence: 22/40 (55%) | p = 0.311 | [43] |
| Qu 2010 | 101 | 467 | Resection | 53.3 months | year RFS: 86.1% 5-year RFS: 37.5% | year RFS: 73.9% 5-year RFS: 33.5% | 0.786 (95% CI: 0.597–1.035), p = 0.086 | [41] |
| Chen 2012 (RCT) | 133 (106 HBV) | 135 (109 HBV) | Resection | 63.8 months | year RFS: 71.7% 5-year RFS: 41.7% | year RFS: 75.1% 5-year RFS: 41.3% | p = 0.766 | [44] |
| Sun 2014 (meta-analysis) | 264 | 267 | Resection | NR | NR | NR | 0.87 (95% CI: 0.70–1.09), p = 0.23 | [45] |
| NA vs. Control | ||||||||
| NA | Control | NA | Control | |||||
| Chan 2011 | 42 (38: LAM;4: ETV) | 94 | Resection | NR | year RFS: 66.5% 5-year RFS: 51.4% | year RFS: 48.9% 5-year RFS: 33.8% | p = 0.05 | [57] |
| Wu 2012 (PSM) | 518 | 4051 | Resection | NA: 2.18 (IQR 1.21–3.69) years Control: 1.57 (IQR:0.77–3.15) years | 6-year recurrence rate: 45.6% (95%CI: 36.5–54.6%) | 6-year recurrence rate: 54.6% (95% CI: 52.5–56.6%) | 0.66 (95% CI: 0.55–0.81), p < 0.001 | [58] |
| Ke 2013 (PSM) | 141 (LAM) | 337 | Resection | 24 months | year RFS: 73.1% 5-year RFS: 44.5% | year RFS: 68.8% 5-year RFS: 43.0% | p = 0.503 | [59] |
| Yin 2013 (RCT) | 81 (LAM) | 82 | Resection | 39.9 (IQR: 27.3–47.8) months | 2-year RFS: 55.6% 4-year RFS: 37.3% | 2-year RFS: 19.5% 4-year RFS: 12.1% | p < 0.001 | [60] |
| Su 2013 | 62 | 271 | Resection | 45.9 (IQR: 22.4–78.9) months | year RFS: 90.2% 5-year RFS: 57.5% | year RFS :63.6% 5-year RFS :34.1% | 2.296 (95%CI:1.451–3.632), p < 0.001 | [21] |
| Sun 2014 (meta-analysis) | 1194 (LAM) | 5052 | Resection; RFA | NR | NR | NR | 0.66 (95% CI: 0.54–0.80), p < 0.0001 | [61] |
| Huang 2015 (RCT) | 100 (ADV) | 100 | Resection | 60 (range: 4–70) months | year RFS: 85.0% 5-year RFS: 46.1% | year RFS: 84.0% 5-year RFS: 27.1% | 0.651 (95% CI: 0.451–0.938), p = 0.021 | [62] |
| Chong 2015 | 254 (ETV: 61.0%; LAM: 30.3%; ADV: 5.5%) | 150 | Resection | 52.4 months | year RFS: 74.8% 5-year RFS: 44.7% | 11-year RFS: 61.1% 5-year RFS: 38.1% | p = 0.166 | [63] |
| Lee 2016 (PSM) | 133 | 266 | RFA | 0.69 (95% CI: 0.50–0.95), p = 0.02 | 2-year recurrence rate: 41.8% (95%CI: 32.9–50.6%) | 2-year recurrence rate: 54.3% (95%CI: 48.0–60.6%) | [64] | |
| Wong 2016 | 968 | 1230 | Resection; RFA; TACE; | 2.8 (IQR: 1.4–4.9) years | Incidence of recurrence: 10.7 (95% CI: 9.3–12.2) per 100 person-years | Incidence of recurrence: 16.6 (95% CI: 15.1–18.2) per 100 person-years | 0.63 (95% CI: 0.49–0.80), p < 0.001 | [55] |
| Chen 2017 (meta-analysis) | 2546 | 6463 | Resection | NR | NR | NR | 0.68 (95% CI: 0.51–0.67, p< 0.001) | [65] |
| NA vs. NA | ||||||||
| NA | NA | NA | NA | |||||
| Cho 2018 (IPDW) | High-potency NA: 256 | Low-potency NA: 90 | Resection; RFA | 53.6 months | High-potency NA Median RFS: 88.2 (IQR: 27.0–103.6) months | Low-potency NA Median RFS: 25.1 (IQR: 9.7–61.5) months | 0.470 (95% CI: 0.338–0.652), p < 0.001 | [67] |
| Choi 2020 (PSM) | ETV: 567 | TDF: 567 | Resection | ETV: 4.4 years TDF: 2.6 years | ETV 3-year RFS: 64.1% | TDF 3-year RFS: 73.2% | 0.82 (95% CI: 0.68–0.98), p = 0.03 | [68] |
| Author/Year | Patients No. | HCC Tx | Start of F/U, Median F/U | HCC Recurrence Rate | HR | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| DAA Alone | |||||||||
| Reig 2016 | 58 | Resection RFA TACE | HCV Tx, 3.5 months | 27.6% | - | [121] | |||
| Conti 2016 | 59 | Resection RFA TACE | HCV Tx, 5.5 months | 28.8% | - | [122] | |||
| Cabibbo 2017 | 143 | Resection RFA TACE | HCV Tx, 8.7 months | 1 year: 26.6% | - | [124] | |||
| Bielen 2017 | 41 | Resection RFA TACE | HCV Tx, 6 months | 0.5 year: 14.6% | - | [128] | |||
| Ogawa 2018 | 152 | Resection RFA TACE RT | HCV Tx, 17 months | 1 year: Non-cirrhosis: 6.5% Cirrhosis: 23.1% | - | [125] | |||
| Calleja 2017 | 70 | NR | HCV Tx, 12 months | 1 year: 30.0% | - | [126] | |||
| Cheung 2016 | 29 | NR | HCV Tx, 15 months | 0.5 year:6.9% | - | [129] | |||
| Yoshimasu 2019 | 23 | NR | HCV Tx, 21 months | 1 year: 13% | - | [130] | |||
| Nakano 2019 | 459 | Resection RFA | HCV Tx, 29.4 months | 1 year: 27.1% | - | [127] | |||
| DAA vs. Untreated | |||||||||
| DAA | Untreated | DAA | Untreated | ||||||
| ANRS 2016 (CO22 HEPATHER) | 189 | 78 | NR | HCV Tx DAA: 20 months Untreated: 26 months | 0.73 vs. 0.66/100 person-months (aHR: 1.04, 95% CI: 0.53–2.07) | 0.88 | [131] | ||
| ANRS 2016 (CO12 CirVir) | 13 | 66 | Resection RFA | HCV Tx, 21.3 months | 1.11 vs. 1.73/100 person-months (aHR: 0.40, 95% CI: 0.05–3.03) | 0.75 | [131] | ||
| Kassas 2017 (IPTW) | 53 | 63 | Resection RFA | HCC Tx, DAA: 16 months Untreated: 23 months | 37.7% | 25.4% | <0.01 | [146] | |
| Virlogeux 2017 | 23 | 45 | Resection RFA TACE | HCC Tx, DAA: 17 months Untreated: 10 months | 1.7 vs. 4.2/100 person-months (aHR: 0.24, 95% CI: 0.10–0.55) | 0.01 | [133] | ||
| Ikeda 2017 (PSM) | 89 | 89 | Resection RFA TACE | HCV Tx, 20.7 months | 2 years: 21.8% | 2 years: 46.5% | <0.01 | [135] | |
| Huang 2018 (IPTW) | 62 | 87 | Resection RFA TACE | HCC Tx DAA: 31 months Untreated: 22 months | 1 year: 47.0% | 1 year: 49.8% | 0.93 | [138] | |
| Singal 2019 (PSM) | 304 | 489 | Resection RFA TACE TARE/RT | HCC Tx, 10.4 months | aHR: 0.91, 95%CI: 0.69–1.19 | >0.05 | [139] | ||
| Cabibbo 2019 (PSM) | 102 | 102 | Resection RFA | HCV Tx, DAA: 21 months Untreated: 18 months | 1 year: 15% 2 years: 27% | 1 year: 20% 2 years: 40% | 0.15 | [140] | |
| IFN vs. DAA | |||||||||
| IFN | DAA | IFN | DAA | ||||||
| Waziry 2017 (meta-analysis) | 1485 | 867 | NR | HCC Tx, IFN: 60 months DAA: 15.6 months | 9.2 vs. 12.2/100 person-years (RR 0.62; 95%CI 0.11–3.45) | 0.56 | [147] | ||
| Petta 2017 | 57 | 58 | Resection RFA | HCC Tx, IFN: 34 months DAA: 18 months | 2 years:15.2% | 2 years: 26.3% | 0.49 | [116] | |
| Nagata 2017* (PSM) | 22 | 22 | Resection RFA | HCC Tx, IFN: 74 months DAA: 27 months | 5 years: 54.2% | 5 years: 45.1% | 0.54 | [143] | |
| Mashiba 2018* (PSM) | 56 | 56 | NR | HCV Tx, IFN: 25.5 months DAA: 7.7 months | NR | NR | 0.21 | [145] | |
| Kinoshita 2018* (PSM) | 61 | 61 | RFA | HCV Tx, IFN: 86.4 months DAA: 21.6 months | 2 years: 61% | 2 years: 60% | 0.43 | [142] | |
| Nagaoki 2018 (PSM) | 32 | 32 | Resection RFA RT | HCC Tx, IFN: 63.6 months DAA: 33.6 months | 1 year: 0% 3 years: 34% | 1 year: 5% 3 years: 26% | 0.36 | [144] | |
| Teng 2019 (PSM) | 50 | 50 | Resection RFA TACE | HCV Tx, IFN: 74.4 months DAA: 30.0 months | 1 year: 22% 2 years: 48% | 1 year: 48% 2 years: 58% | 0.04 | [148] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, W.; Liu, Y.-C.; Jeng, W.-J.; Su, C.-W. Tertiary Prevention of HCC in Chronic Hepatitis B or C Infected Patients. Cancers 2021, 13, 1729. https://doi.org/10.3390/cancers13071729
Teng W, Liu Y-C, Jeng W-J, Su C-W. Tertiary Prevention of HCC in Chronic Hepatitis B or C Infected Patients. Cancers. 2021; 13(7):1729. https://doi.org/10.3390/cancers13071729
Chicago/Turabian StyleTeng, Wei, Yen-Chun Liu, Wen-Juei Jeng, and Chien-Wei Su. 2021. "Tertiary Prevention of HCC in Chronic Hepatitis B or C Infected Patients" Cancers 13, no. 7: 1729. https://doi.org/10.3390/cancers13071729






