Impact of Pancreatic Resection on Survival in Locally Advanced Resectable Gastric Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
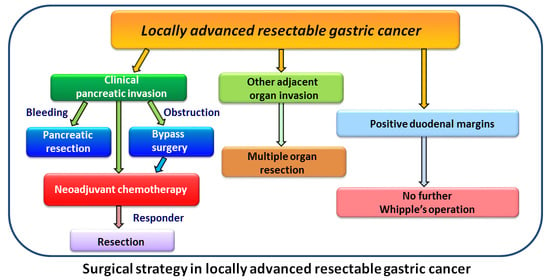
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Data Collection
2.4. Postoperative Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Demographics and Clinicopathological Features in G1 and G2 Patients
3.2. Predictors of Disease-Free and Overall Survival in G1 and G2 Patients
3.3. Demographics and Clinicopathological Features in G3 and G4 Patients
3.4. Predictors of Disease-Free and Overall Survival in G3 and G4 Patients
3.5. Recurrence Rates and Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.Y.; Yuan, S.Q.; Nie, R.C.; Li, S.M.; Yang, L.R.; Duan, J.L.; Chen, Y.B.; Zhang, X.S. Prognostic Factors and Recurrence Patterns in T4 Gastric Cancer Patients after Curative Resection. J. Cancer 2019, 10, 1181–1188. [Google Scholar] [CrossRef]
- Mita, K.; Ito, H.; Fukumoto, M.; Murabayashi, R.; Koizumi, K.; Hayashi, T.; Kikuchi, H. Surgical outcomes and survival after extended multiorgan resection for T4 gastric cancer. Am. J. Surg. 2012, 203, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.T.; Tsai, C.Y.; Hsu, J.T.; Vinayak, R.; Liu, K.H.; Yeh, C.N.; Yeh, T.S.; Hwang, T.S.; Jan, Y.Y. Aggressive surgical approach for patients with T4 gastric carcinoma: Promise or myth? Ann. Surg. Oncol. 2011, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Li, D.B.; You, J.; Wang, S.J.; Zhou, Y.M. Pancreaticoduodenectomy for locally advanced gastric cancer: Results from a pooled analysis. Asian J. Surg. 2019, 42, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, D.J.; Lee, K.U. Pancreaticoduodenectomy for locally advanced gastric cancer. Hepatogastroenterology 2007, 54, 977–980. [Google Scholar]
- Chan, W.H.; Cheow, P.C.; Chung, A.Y.; Ong, H.S.; Koong, H.N.; Wong, W.K. Pancreaticoduodenectomy for locally advanced stomach cancer: Preliminary results. ANZ J. Surg. 2008, 78, 767–770. [Google Scholar] [CrossRef]
- Martin, R.C., 2nd; Jaques, D.P.; Brennan, M.F.; Karph, M. Achieving R0 resection for locally advanced gastric cancer: Is it worth the risk of multiorgan resection? J. Am. Coll. Surg. 2002, 194, 568–577. [Google Scholar] [CrossRef]
- Nanthakumaran, S.; Fernandes, E.; Thompson, A.M.; Rapson, T.; Gilbert, F.J.; Park, K.G.M. Morbidity and mortality rates following gastric cancer surgery and contiguous organ removal, a population based study. Eur. J. Surg. Oncol. 2005, 31, 1141–1144. [Google Scholar] [CrossRef]
- Werf, L.R.; Eshuis, W.J.; Draaisma, W.A.; Etten, B.; Gisbertz, S.S.; Harst, E.; Leim, M.S.L.; Lemmens, V.E.P.P.; Wijnhoven, B.P.L.; Besselink, M.G.; et al. Nationwide Outcome of Gastrectomy with En-Bloc Partial Pancreatectomy for Gastric Cancer. J. Gastrointest. Surg. 2019, 23, 2327–2337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Chou, H.H.; Kuo, C.J.; Hsu, J.T.; Chen, T.S.; Lin, C.J.; Tseng, J.H.; Yeh, T.S.; Hwang, T.L.; Jan, Y.Y. Clinicopathologic study of node-negative advanced gastric cancer and analysis of factors predicting its recurrence and prognosis. Am. J. Surg. 2013, 205, 623–630. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Wang, Q.; Li, J.; Bai, B.; Li, Z.; Wu, X.; Yu, P.; Li, X.; Yin, J. Postoperative complications and prognosis after radical gastrectomy for gastric cancer: A systematic review and meta-analysis of observational studies. World J. Surg. Oncol. 2019, 17, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, P.; Wu, Z.; Li, Z.; Bu, Z.; Wu, A.; Wu, X.; Zhang, L.; Shi, J.; Ji, J. Impact of postoperative major complications on long-term survival after radical resection of gastric cancer. BMC Cancer 2019, 19, 883. [Google Scholar] [CrossRef] [Green Version]
- Tokunaga, M.; Tanizawa, Y.; Bando, E.; Kawamura, T.; Terashima, M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann. Surg. Oncol. 2013, 20, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Hiki, N.; Sano, T.; Nomura, S.; Nunobe, S.; Kumagai, K.; Aiko, S.; Watanabe, R.; Kosuga, T.; Yamaguchi, T. Prognostic significance of complications after curative surgery for gastric cancer. Ann. Surg. Oncol. 2014, 21, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Galata, C.; Blank, S.; Weiss, C.; Ronellenfitsch, U.; Reissfelder, C.; Hardt, J. Role of postoperative complications in overall survival after radical resection for gastric cancer: A retrospective single-center analysis of 1107 patients. Cancers 2019, 11, 1890. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Goldfarb, Y.; Sorski, L.; Benish, M.; Levi, B.; Melamed, R.; Ben-Eliyahu, S. Improving postoperative immune status and resistance to cancer metastasis: A combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann. Surg. 2011, 253, 798–810. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Sakuramoto, S.; Sasako, M.; Yamaguchi, T.; Kinoshita, T.; Fujii, M.; Nashimoto, A.; Furukawa, H.; Nakajima, T.; Ohashi, Y.; Imamura, H.; et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N. Engl. J. Med. 2007, 357, 1810–1820. [Google Scholar] [CrossRef]
- Bang, Y.J.; Kim, Y.W.; Yang, H.K.; Chung, H.C.; Park, Y.K.; Lee, K.H.; Lee, K.W.; Kim, Y.H.; Noh, S.; Cho, J.Y.; et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet 2012, 379, 315–321. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompsm, J.N.; Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Iveson, T.J.; Smith, D.B.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar]
- Macdonald, J.S.; Smalley, S.R.; Benedetti, J.; Hundahl, S.A.; Estes, N.C.; Stemmermann, G.N.; Haller, D.G.; Ajani, J.A.; Gunderson, L.L.; Jessup, M.; et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N. Engl. J. Med. 2001, 345, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Yook, J.H.; Park, Y.K.; Kim, Y.; Kim, J.; Ryu, M.; Rha, S.Y.; Chung, Y.; Kim, I.; Oh, S.C.; et al. Phase III randomized study of neoadjuvant chemotherapy with docetaxel, oxaliplatin and S-1 followed by surgery and adjuvant S-1, vs surgery and adjuvant S-1, for resectable advanced gastric cancer. Ann. Oncol. 2019, 30 (Suppl. 5), V876–V877. [Google Scholar] [CrossRef]
- Boku, N.; Ryu, M.H.; Kato, K.; Chung, H.C.; Minashi, K.; Lee, K.W.; Cho, H.; Kang, W.K.; Komatsu, Y.; Tsuda, M.; et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: Interim results of a randomized, phase II trial (ATTRACTION-4). Ann. Oncol. 2019, 30, 250–258. [Google Scholar]
- Bang, Y.J.; Kang, Y.K.; Catenacci, D.V.; Muro, K.; Fuchs, C.S.; Geva, R.; Hara, H.; Golan, T.; Garrido, M.; Jalal, S.I.; et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: Results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer 2019, 22, 828–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, E.C.; Wotherspoon, A.; Peckitt, C.; Gonzalez, D.; Hulkki-Wilson, S.; Eltahir, Z.; Fassan, M.; Rugge, M.; Valeri, N.; Okines, A.; et al. Mismatch repair deficiency, microsatellite instability, and survival. JAMA Oncol. 2017, 3, 1197–1203. [Google Scholar] [CrossRef] [Green Version]
- Wagner, A.D.; Grabsch, H.I.; Mauer, M.; Marreaud, S.; Caballero, C.; Thuss-Patience, P.; Mueller, L.; Elme, A.; Moehler, M.H.; Martens, U.; et al. EORTC-1203-GITCG-the “INNOVATION”-trial: Effect of chemotherapy alone versus chemotherapy plus trastuzumab, versus chemotherapy plus trastuzumab plus pertuzumab, in the perioperative treatment of HER2 positive, gastric and gastroesophageal junction adenocarcinoma on pathologic response rate: A randomized phase II-intergroup trial of the EORTC-Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI-Cancer group. BMC Cancer 2019, 19, 494. [Google Scholar]



| Variables | Group 1 | Group 2 | p Value |
|---|---|---|---|
| No. of patients | 50 | 94 | |
| Age (years), mean ± SD | 62.4 ± 14.0 | 63.2 ± 12.2 | 0.731 |
| Gender | 0.722 | ||
| Male | 36 (72.0) | 65 (69.1) | |
| Female | 14 (28.0) | 29 (30.9) | |
| Tumor size (cm), mean ± SD | 7.4 ± 2.8 | 7.1 ± 3.3 | 0.599 |
| Tumor location | 0.074 | ||
| Upper | 12 (24.0) | 33 (35.1) | |
| Middle | 9 (18.0) | 5 (5.3) | |
| Lower | 25 (50.0) | 46 (48.9) | |
| Whole | 4 (8.0) | 10 (10.6) | |
| Type of gastrectomy | 0.521 | ||
| Total | 32 (64.0) | 55 (58.5) | |
| Subtotal | 18 (36.0) | 39 (41.5) | |
| Nodal status | 0.129 | ||
| N0 | 12 (24.0) | 10 (10.6) | |
| N1 | 4 (8.0) | 7 (7.4) | |
| N2 | 10 (20.0) | 23 (24.5) | |
| N3a | 9 (18.0) | 31 (33.0) | |
| N3b | 15 (30.0) | 23 (24.5) | |
| Stage | 0.105 | ||
| IIIA | 12 (24.0) | 10 (10.6) | |
| IIIB | 14 (28.0) | 30 (31.9) | |
| IIIC | 24 (48.0) | 54 (57.5) | |
| No. of lymph node retrieval, mean ± SD | 36.8 ± 20.3 | 37.7 ± 18.0 | 0.777 |
| LNR, mean ± SD | 0.32 ± 0.31 | 0.32 ± 0.27 | 0.968 |
| Differentiation | 0.387 | ||
| Yes | 13 (26.0) | 31 (33.0) | |
| No | 37 (74.0) | 63 (67.0) | |
| Lymphatic invasion | 0.290 | ||
| Yes | 36 (72.0) | 75 (79.8) | |
| No | 14 (28.0) | 19 (20.2) | |
| Vascular invasion | 0.387 | ||
| Yes | 14 (28.0) | 33 (35.1) | |
| No | 36 (72.0) | 61 (64.9) | |
| Perineural invasion | <0.001 | ||
| Yes | 25 (50.0) | 75 (79.8) | |
| No | 25 (50.0) | 19 (20.2) | |
| Complication | 11 (22.0) | 35 (37.2) | 0.062 |
| Hospital mortality | 1 (2.0) | 9(9.6) | 0.165 |
| Adjuvant chemotherapy | 36 (72.0) | 61 (64.9) | 0.457 |
| Intervals between surgery to chemotherapy (months), mean ± SD | 1.9 ± 1.3 | 1.7 ± 1.1 | 0.304 |
| Chemotherapy cycles, mean ± SD | 8.8 ± 8.4 | 7.2 ± 5.3 | 0.287 |
| Factors | Median (Months) | 95% CI | p Value | Hazard Ratios | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Age | 0.668 | |||||
| ≤65 (n = 74) | 12.0 | 6.2–17.8 | ||||
| >65 (n = 60) | 9.6 | 6.5–12.6 | ||||
| Gender | 0.057 | |||||
| Male (n = 94) | 13.0 | 6.8–19.1 | ||||
| Female (n = 40) | 7.9 | 4.3–11.4 | ||||
| Tumor size (cm) | 0.458 | |||||
| ≤6.5 (n = 71) | 11.7 | 8.0–15.5 | ||||
| >6.5 (n = 63) | 9.2 | 5.5–13.0 | ||||
| Location | 0.903 | |||||
| Upper (n = 40) | 10.0 | 3.9–16.0 | ||||
| Middle (n = 13) | 9.5 | 0.1–19.7 | ||||
| Lower (n = 68) | 11.4 | 7.1–15.6 | ||||
| Whole (n = 13) | 9.2 | 5.0–13.5 | ||||
| Type of gastrectomy | 0.206 | |||||
| Total (n = 78) | 9.2 | 6.6–11.9 | ||||
| Subtotal (n = 56) | 12.0 | 7.7–16.2 | ||||
| Pancreatic resection | 0.003 | |||||
| No, Group 1 (n = 49) | 19.3 | 3.2–35.4 | 1 | |||
| Yes, Group 2 (n = 85) | 9.3 | 5.3–13.2 | 1.740 | 1.116–2.713 | 0.015 | |
| Nodal status | <0.0001 | |||||
| N0 (n = 20) | 41.0 | 9.7–72.4 | ||||
| N1 (n = 10) | 7.9 | 3.0–12.7 | ||||
| N2 (n = 30) | 14.9 | 6.6–23.3 | ||||
| N3a (n = 39) | 12.6 | 4.3–20.9 | ||||
| N3b (n = 35) | 6.1 | 5.2–77.0 | ||||
| Stage | <0.001 | |||||
| IIIA (n = 20) | 41.0 | 9.7–72.4 | 1 | |||
| IIIB (n = 40) | 10.8 | 7.6–14.0 | 1.542 | 0.363–6.551 | 0.558 | |
| IIIC (n = 74) | 7.1 | 4.0–10.2 | 1.803 | 0.330–9.854 | 0.497 | |
| LNR | <0.0001 | |||||
| ≤0.04 (n = 25) | 41.0 | 12.8–69.3 | 1 | |||
| >0.04, ≤0.41 (n = 67) | 10.8 | 7.3–14.3 | 1.546 | 0.440–5.432 | 0.497 | |
| >0.41 (n = 42) | 6.3 | 5.2–7.3 | 3.109 | 0.792–12.198 | 0.104 | |
| Differentiation | 0.352 | |||||
| No (n = 42) | 11.7 | 6.2–17.2 | ||||
| Yes (n = 92) | 10.4 | 7.8–13.0 | ||||
| Lymphatic invasion | 0.020 | |||||
| No (n = 30) | 20.9 | 14.3–27.5 | 1 | |||
| Yes (n = 104) | 9.3 | 6.3–12.3 | 0.743 | 0.359–1.538 | 0.424 | |
| Vascular invasion | 0.117 | |||||
| No (n = 91) | 11.4 | 7.1–15.6 | ||||
| Yes (n = 43) | 7.9 | 3.0–12.8 | ||||
| Perineural invasion | 0.003 | |||||
| No (n = 42) | 13.3 | 2.3–24.2 | 1 | |||
| Yes (n = 92) | 9.3 | 6.2–12.3 | 1.256 | 0.772–2.043 | 0.359 | |
| Complication | 0.850 | |||||
| No (n = 95) | 11.0 | 8.1–13.9 | ||||
| Yes (n = 39) | 9.2 | 4.6–13.9 | ||||
| Adjuvant chemotherapy | 0.160 | |||||
| No (n = 41) | 8.5 | 5.0–12.0 | ||||
| Yes (n = 93) | 11.4 | 8.3–14.5 | ||||
| Factors | Median (Months) | 95% CI | p Value | Hazard Ratios | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Age | 0.247 | |||||
| ≤65 (n = 74) | 22.7 | 15.1–30.2 | ||||
| >65 (n = 60) | 16.6 | 12.9–20.2 | ||||
| Gender | 0.257 | |||||
| Male (n = 94) | 20.4 | 15.1–25.7 | ||||
| Female (n = 40) | 16.0 | 9.9–22.2 | ||||
| Tumor size (cm) | 0.529 | |||||
| ≤6.5 (n = 71) | 17.8 | 10.4–25.2 | ||||
| >6.5 (n = 63) | 18.0 | 15.2–20.8 | ||||
| Location | 0.774 | |||||
| Upper (n = 40) | 13.5 | 6.7–20.2 | ||||
| Middle (n = 13) | 15.8 | 0.1–33.2 | ||||
| Lower (n = 68) | 18.3 | 12.6–24.0 | ||||
| Whole (n = 13) | 18.4 | 14.0–22.7 | ||||
| Type of gastrectomy | 0.123 | |||||
| Total (n = 78) | 17.4 | 12.4–22.3 | ||||
| Subtotal (n = 56) | 21.9 | 15.2–28.6 | ||||
| Pancreatic resection | 0.004 | |||||
| No, Group 1 (n = 49) | 27.1 | 14.2–40.1 | 1 | |||
| Yes, Group 2 (n = 85) | 16.0 | 12.1–20.0 | 1.897 | 1.210–2.974 | 0.005 | |
| Nodal status | <0.0001 | |||||
| N0 (n = 20) | 61.1 | 0.1–143.3 | ||||
| N1 (n = 10) | 12.6 | 8.8–16.4 | ||||
| N2 (n = 30) | 23.1 | 13.3–32.9 | ||||
| N3a (n = 39) | 16.6 | 10.8–22.4 | ||||
| N3b (n = 35) | 12.7 | 10.5–15.0 | ||||
| Stage | <0.001 | |||||
| IIIA (n = 20) | 61.1 | 0.1–143.3 | 1 | |||
| IIIB (n = 40) | 22.7 | 15.3–30.1 | 1.225 | 0.296–5.072 | 0.779 | |
| IIIC (n = 74) | 13.6 | 10.8–16.4 | 1.368 | 0.262–7.140 | 0.710 | |
| LNR | <0.0001 | |||||
| ≤0.04 (n = 25) | 51.8 | 0.1–107.6 | 1 | |||
| >0.04, ≤0.41 (n = 67) | 17.8 | 11.6–24.0 | 1.622 | 0.464–5.665 | 0.449 | |
| >0.41 (n = 42) | 12.7 | 10.7–14.8 | 3.720 | 0.957–14.462 | 0.058 | |
| Differentiation | 0.053 | |||||
| No (n = 42) | 21.4 | 16.1–26.7 | ||||
| Yes (n = 92) | 15.8 | 10.7–20.8 | ||||
| Lymphatic invasion | 0.009 | |||||
| No (n = 30) | 28.4 | 19.8–37.1 | 1 | |||
| Yes (n = 104) | 15.8 | 12.4–19.2 | 0.931 | 0.455–1.902 | 0.844 | |
| Vascular invasion | 0.053 | |||||
| No (n = 91) | 21.4 | 16.1–26.7 | ||||
| Yes (n = 43) | 15.8 | 10.7–20.8 | ||||
| Perineural invasion | 0.004 | |||||
| No (n = 42) | 23.2 | 14.3–2.1 | 1 | |||
| Yes (n = 92) | 17.2 | 13.1–21.3 | 1.262 | 0.774–2.056 | 0.351 | |
| Complication | 0.981 | |||||
| No (n = 95) | 17.7 | 11.9–23.5 | ||||
| Yes (n = 39) | 18.3 | 10.36–26.3 | ||||
| Adjuvant chemotherapy | 0.111 | |||||
| No (n = 41) | 13.1 | 6.6–19.5 | ||||
| Yes (n = 93) | 20.4 | 15.2–25.7 | ||||
| Variables | Group 3 | Group 4 | p Value |
|---|---|---|---|
| No. of patients | 98 | 46 | |
| Age (years), mean ± SD | 63.7 ± 12.6 | 61.7 ± 13.3 | 0.381 |
| Gender | 0.523 | ||
| Male | 65 (66.3) | 28 (60.9) | |
| Female | 33 (33.7) | 18 (39.1) | |
| Tumor size (cm), mean ± SD | 6.5 ± 3.0 | 6.6 ± 2.8 | 0.834 |
| Tumor location | 0.189 | ||
| Upper | 7 (7.1) | 1 (2.2) | |
| Middle | 6 (6.1) | 0 | |
| Lower | 78 (79.6) | 43 (93.5) | |
| Whole | 7 (7.1) | 2 (4.3) | |
| Type of gastrectomy | 0.535 | ||
| Total | 25 (25.5) | 14 (30.4) | |
| Subtotal | 73 (74.5) | 32 (69.6) | |
| T status | <0.0001 | ||
| T3 | 7 (7.1) | 1 (2.2) | |
| T4a | 79 (80.7) | 13 (28.3) | |
| T4b | 12 (12.2) | 32 (69.6) | |
| Nodal status | 0.017 | ||
| N0 | 8 (8.2) | 8 (17.4) | |
| N1 | 6 (6.1) | 6 (13.0) | |
| N2 | 14 (14.3) | 9 (19.6) | |
| N3a | 30 (30.6) | 16 (34.8) | |
| N3b | 40 (40.8) | 7 (15.2) | |
| Stage | 0.940 | ||
| IIB | 7 (7.1) | 2 (4.3) | |
| IIIA | 19 (19.4) | 10 (21.7) | |
| IIIB | 28 (28.6) | 14 (30.4) | |
| IIIC | 44 (44.9) | 20 (43.5) | |
| No. of lymph node retrieval, mean ± SD | 31.5 ± 13.8 | 38.3 ± 16.0 | 0.009 |
| LNR, mean ± SD | 0.48 ± 0.32 | 0.24 ± 0.22 | <0.0001 |
| Differentiation | 0.574 | ||
| Yes | 21 (21.4) | 8 (25.0) | |
| No | 77 (78.6) | 24 (75.0) | |
| Lymphatic invasion | 0.052 | ||
| Yes | 82 (83.7) | 32 (69.6) | |
| No | 16 (16.3) | 14 (30.4) | |
| Vascular invasion | 0.969 | ||
| Yes | 28 (28.6) | 13 (28.3) | |
| No | 70 (71.4) | 33 (71.7) | |
| Perineural invasion | 0.536 | ||
| Yes | 75 (76.5) | 33 (71.7) | |
| No | 23 (23.5) | 13 (28.3) | |
| Complication | 25 (25.5) | 21 (45.7) | 0.016 |
| Hospital mortality | 7 (7.1) | 4 (8.7) | 0.744 |
| Adjuvant chemotherapy | 63 (64.3) | 30 (65.2) | 0.913 |
| Intervals between surgery to chemotherapy (months), mean ± SD | 1.5 ± 1.0 | 1.8 ± 0.8 | 0.132 |
| Chemotherapy cycles, mean ± SD | 7.9 ± 9.5 | 8.5 ± 5.9 | 0.792 |
| Factors | Median (Months) | 95% CI | p Value | Hazard Ratios | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Age | 0.953 | |||||
| ≤65 (n = 67) | 12.0 | 8.1–15.8 | ||||
| >65 (n = 66) | 11.4 | 9.1–13.6 | ||||
| Gender | 0.678 | |||||
| Male (n = 86) | 11.7 | 9.2–14.2 | ||||
| Female (n = 47) | 11.4 | 8.6–14.2 | ||||
| Tumor size (cm) | 0.522 | |||||
| ≤6.0 (n = 72) | 11.7 | 9.1–14.3 | ||||
| >6.0 (n = 61) | 11.4 | 8.2–14.6 | ||||
| Location | 0.711 | |||||
| Upper (n = 7) | 12.6 | 0.1–28.9 | ||||
| Middle (n = 6) | 12.6 | 0.1–25.1 | ||||
| Lower (n = 113) | 11.4 | 9.5–13.2 | ||||
| Whole (n = 7) | 15.5 | 10.0–20.9 | ||||
| Type of gastrectomy | 0.150 | |||||
| Total (n = 34) | 10.4 | 5.4–15.4 | ||||
| Subtotal (n = 99) | 12.0 | 9.5–14.4 | ||||
| Duodenal margins | 0.809 | |||||
| Positive, Group 3 (n = 91) | 12.0 | 9.9–14.1 | ||||
| Negative, Group 4 (n = 42) | 11.4 | 9.3–13.5 | ||||
| T status | 0.074 | |||||
| T3 (n = 8) | 11.2 | 6.8–15.7 | ||||
| T4a (n = 86) | 12.6 | 10.2–14.9 | ||||
| T4b (n = 39) | 7.9 | 4.4–11.4 | ||||
| Nodal status | ||||||
| N0 (n = 15) | NA | <0.001 | ||||
| N1 (n = 11) | 11.8 | 6.3–17.3 | ||||
| N2 (n = 22) | 11.7 | 8.4–15.0 | ||||
| N3a (n = 44) | 12.0 | 9.3–14.6 | ||||
| N3b (n = 41) | 7.1 | 3.3–10.9 | ||||
| Stage | ||||||
| II (n = 9) | NA | <0.0001 | 1 | |||
| IIIA (n = 28) | 14.3 | 9.8–18.8 | 7.177 | 1.569–32.828 | 0.011 | |
| IIIB (n = 38) | 12.0 | 10.2–13.8 | 12.507 | 2.439–64.141 | 0.002 | |
| IIIC (n = 58) | 7.4 | 3.8–11.0 | 18.754 | 3.467–101.458 | 0.001 | |
| LNR | <0.0001 | |||||
| ≤0.10 (n = 28) | 22.3 | 8.0–36.5 | 1 | |||
| >0.10 (n = 105) | 10.4 | 7.6–13.1 | 1.070 | 0.506–2.266 | 0.859 | |
| Differentiation | 0.879 | |||||
| No (n = 98) | 12.0 | 9.7–14.3 | ||||
| Yes (n = 35) | 10.7 | 7.9–13.4 | ||||
| Lymphatic invasion | 0.002 | |||||
| No (n = 28) | 10.7 | 8.3–13.1 | 1 | |||
| Yes (n = 105) | 22.3 | 10.6–34.0 | 0.778 | 0.415–1.458 | 0.433 | |
| Vascular invasion | 0.112 | - | ||||
| No (n = 95) | 12.0 | 10.4–13.6 | ||||
| Yes (n = 38) | 7.9 | 4.2–11.5 | ||||
| Perineural invasion | 0.023 | |||||
| No (n = 34) | 12.0 | 10.1–13.9 | 1 | |||
| Yes (n = 99) | 11.4 | 8.0–14.8 | 1.068 | 0.635–1.796 | 0.803 | |
| Complication | 0.997 | |||||
| No (n = 97) | 11.4 | 9.9–12.8 | ||||
| Yes (n = 36) | 12.6 | 7.7–17.4 | ||||
| Adjuvant chemotherapy | 0.046 | |||||
| No (n = 40) | 9.1 | 4.6–13.7 | 1.904 | 1.188–3.053 | 0.007 | |
| Yes (n = 93) | 12.6 | 9.9–15.3 | 1 | |||
| Factors | Median (Months) | 95% CI | p Value | Hazard Ratios | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Age | 0.824 | |||||
| ≤65 (n = 67) | 21.9 | 14.8–28.9 | ||||
| >65 (n = 66) | 16.6 | 12.8–20.3 | ||||
| Gender | 0.781 | |||||
| Male (n = 86) | 20.4 | 15.7–25.2 | ||||
| Female (n = 47) | 18.2 | 16.5–19.8 | ||||
| Tumor size (cm) | 0.617 | |||||
| ≤6.0 (n = 72) | 17.8 | 14.0–21.6 | ||||
| >6.0 (n = 61) | 18.8 | 14.1–23.5 | ||||
| Location | 0.669 | |||||
| Upper (n = 7) | 27.4 | 6.1–48.8 | ||||
| Middle (n = 6) | 18.8 | 16.4–21.2 | ||||
| Lower (n = 113) | 18.2 | 14.5–21.8 | ||||
| Whole (n = 7) | 20.4 | 9.0–31.8 | ||||
| Type of gastrectomy | 0.084 | |||||
| Total (n = 34) | 13.9 | 7.3–20.6 | ||||
| Subtotal (n = 99) | 20.6 | 16.2–24.9 | ||||
| Duodenal margins | 0.964 | |||||
| Positive, Group 3 (n = 91) | 18.8 | 14.8–22.8 | ||||
| Negative, Group 4 (n = 42) | 17.8 | 15.5–20.1 | ||||
| T status | 0.109 | |||||
| T3 (n = 8) | 20.6 | 9.3–31.8 | ||||
| T4a (n = 86) | 20.7 | 14.7–26.7 | ||||
| T4b (n = 39) | 16.6 | 10.4–22.8 | ||||
| Nodal status | <0.001 | |||||
| N0 (n = 15) | - | |||||
| N1 (n = 11) | 15.1 | 0.6–29.6 | ||||
| N2 (n = 22) | 17.2 | 9.9–24.5 | ||||
| N3a (n = 44) | 17.8 | 13.3–22.3 | ||||
| N3b (n = 41) | 13.9 | 6.1–21.8 | ||||
| Stage | <0.0001 | |||||
| II (n = 9) | NA | 1 | ||||
| IIIA (n = 28) | 20.4 | 15.5–25.3 | 7.074 | 1.532–32.667 | 0.012 | |
| IIIB (n = 38) | 18.2 | 12.4–23.9 | 8.942 | 1.712–46.704 | 0.009 | |
| IIIC (n = 58) | 13.9 | 8.1–19.7 | 12.450 | 2.265–68.439 | 0.004 | |
| LNR | <0.0001 | |||||
| ≤0.10 (n = 28) | 26.3 | 16.3–36.3 | 1 | |||
| >0.10 (n = 105) | 17.2 | 14.6–19.8 | 1.409 | 0.673–2.950 | 0.363 | |
| Differentiation | 0.358 | |||||
| No (n = 98) | 18.2 | 15.0–21.3 | ||||
| Yes (n = 35) | 22.1 | 11.3–32.8 | ||||
| Lymphatic invasion | 0.003 | |||||
| No (n = 28) | 26.3 | 16.3–36.3 | 1 | |||
| Yes (n = 105) | 17.2 | 15.7–18.7 | 0.723 | 0.383–1.364 | 0.316 | |
| Vascular invasion | 0.187 | |||||
| No (n = 95) | 18.8 | 15.7–21.9 | ||||
| Yes (n = 38) | 15.1 | 9.1–21.2 | ||||
| Perineural invasion | 0.006 | |||||
| No (n = 34) | 22.1 | 14.39–29.2 | 1 | |||
| Yes (n = 99) | 17.3 | 12.6–21.9 | 1.206 | 0.724–2.007 | 0.472 | |
| Complication | 0.656 | |||||
| No (n = 97) | 18.8 | 15.5–22.2 | ||||
| Yes (n = 36) | 18.3 | 13.9–22.7 | ||||
| Adjuvant chemotherapy | 0.060 | |||||
| No (n = 40) | 12.7 | 0.1–25.9 | ||||
| Yes (n = 93) | 20.6 | 16.2–25.0 | ||||
| Variables | Group 1 (n = 49) | Group 2 (n = 85) | p Value | Group 3 (n = 91) | Group 4 (n = 42) | p Value |
|---|---|---|---|---|---|---|
| Recurrence | 0.026 | 0.408 | ||||
| Yes | 32 (65.3) | 70 (82.4) | 71 (78.0) | 30 (71.4) | ||
| No | 17 (34.7) | 15 (17.6) | 20 (22.0) | 12 (28.6) | ||
| Recurrence pattern | ||||||
| Local/regional (L) | 13 (26.5) | 32 (37.6) | 0.189 | 23 (25.3) | 20 (47.6) | 0.010 |
| Hematogenous (H) | 18 (36.7) | 30 (35.3) | 0.867 | 28 (30.8) | 10 (23.8) | 0.409 |
| Peritoneal (P) | 13 (26.5) | 26 (30.6) | 0.618 | 35 (38.5) | 16 (38.1) | 0.968 |
| Recurrence pattern | 0.122 | 0.008 | ||||
| None | 17 (34.7) | 15 (17.6) | 20 (22.0) | 12 (28.6) | ||
| L | 4 (8.2) | 17 (20.0) | 13 (14.3) | 7 (16.7) | ||
| L + H | 7 (14.3) | 7 (8.2) | 5 (5.5) | 5 (11.9) | ||
| H | 8 (16.3) | 20 (23.5) | 18 (19.8) | 2 (4.8) | ||
| H + P | 3 (6.1) | 2 (2.4) | 4 (4.4) | 3 (7.1) | ||
| L + P | 2 (4.1) | 7 (8.2) | 4 (4.4) | 8 (19.0) | ||
| P | 8 (16.3) | 16 (18.8) | 26 (28.6) | 5 (11.9) | ||
| L + H + P | 0 | 1 (1.2) | 1 (1.0) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.-C.; Tang, C.-M.; Le, P.-H.; Kuo, C.-J.; Chen, T.-H.; Wang, S.-Y.; Chou, W.-C.; Chen, T.-C.; Yeh, T.-S.; Hsu, J.-T. Impact of Pancreatic Resection on Survival in Locally Advanced Resectable Gastric Cancer. Cancers 2021, 13, 1289. https://doi.org/10.3390/cancers13061289
Chang S-C, Tang C-M, Le P-H, Kuo C-J, Chen T-H, Wang S-Y, Chou W-C, Chen T-C, Yeh T-S, Hsu J-T. Impact of Pancreatic Resection on Survival in Locally Advanced Resectable Gastric Cancer. Cancers. 2021; 13(6):1289. https://doi.org/10.3390/cancers13061289
Chicago/Turabian StyleChang, Shih-Chun, Chi-Ming Tang, Puo-Hsien Le, Chia-Jung Kuo, Tsung-Hsing Chen, Shang-Yu Wang, Wen-Chi Chou, Tse-Ching Chen, Ta-Sen Yeh, and Jun-Te Hsu. 2021. "Impact of Pancreatic Resection on Survival in Locally Advanced Resectable Gastric Cancer" Cancers 13, no. 6: 1289. https://doi.org/10.3390/cancers13061289
APA StyleChang, S.-C., Tang, C.-M., Le, P.-H., Kuo, C.-J., Chen, T.-H., Wang, S.-Y., Chou, W.-C., Chen, T.-C., Yeh, T.-S., & Hsu, J.-T. (2021). Impact of Pancreatic Resection on Survival in Locally Advanced Resectable Gastric Cancer. Cancers, 13(6), 1289. https://doi.org/10.3390/cancers13061289