Future Prospects of Colorectal Cancer Screening: Characterizing Interval Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
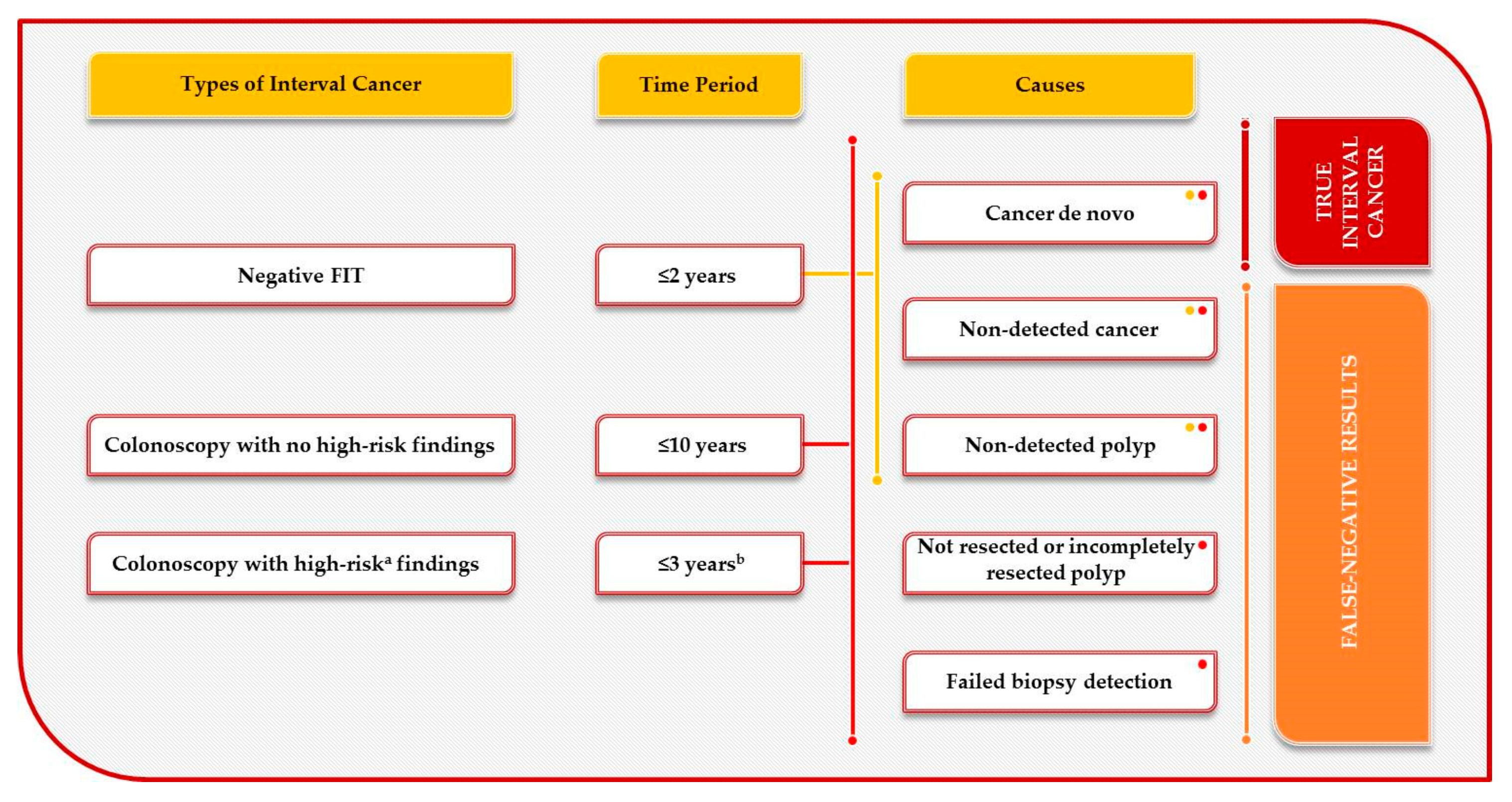
1.1. Interval Colorectal Cancer Definition
1.2. Diagnostic Accuracy of Faecal Immunochemical Tests in CRC Screening
1.3. Episode Sensitivity in an FIT Screening Programme
2. Methods
Search Strategy and Selection Criteria
3. Results
3.1. Interval CRC Proportion and Epidemiological and Clinical Characteristics of Interval CRC
3.2. Molecular Characterization of Interval CRC
3.2.1. Pathways of Tumorigenesis and Consensus Molecular Subtype (CMS) Classification
3.2.2. Studies of Molecular Characterization of Interval CRC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hewitson, P.; Glasziou, P.; Watson, E.; Towler, B.; Irwig, L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): An update. Am. J. Gastroenterol. 2008, 103, 1541–1549. [Google Scholar] [CrossRef]
- IARC. Colorectal Cancer Screening; IARC Working Group on the Evaluation of Cancer-Preventive Interventions: Lyon, France, 2019; Volume 17. [Google Scholar]
- Ponti, A.; Anttila, A.; Ronco, G.; Senore, C. Report on the Implementation of the Council Recommendation on Cancer Screening; International Agency for Research on Cancer: Lyon, France, 2017; Available online: https://screening.iarc.fr/EUreport.php (accessed on 1 July 2020).
- Rutter, M.D.; Beintaris, I.; Valori, R.; Chiu, H.M.; Corley, D.A.; Cuatrecasas, M.; Dekker, E.; Forsberg, A.; Gore-Booth, J.; Haug, U.; et al. World Endoscopy Organization Consensus Statements on Post-Colonoscopy and Post-Imaging Colorectal Cancer. Gastroenterology 2018, 155, 909–925.e3. [Google Scholar] [CrossRef] [Green Version]
- Rutter, M.D.; East, J.; Rees, C.J.; Cripps, N.; Docherty, J.; Dolwani, S.; Kaye, P.V.; Monahan, K.J.; Novelli, M.R.; Plumb, A.; et al. British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post-polypectomy and post-colorectal cancer resection surveillance guidelines. Gut 2020, 69, 201–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, C.; Antonelli, G.; Dumonceau, J.M.; Regula, J.; Bretthauer, M.; Chaussade, S.; Dekker, E.; Ferlitsch, M.; Gimeno-Garcia, A.; Jover, R.; et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline-Update 2020. Endoscopy 2020, 52, 687–700. [Google Scholar] [CrossRef]
- Gupta, S.; Lieberman, D.; Anderson, J.C.; Burke, C.A.; Dominitz, J.A.; Kaltenbach, T.; Robertson, D.J.; Shaukat, A.; Syngal, S.; Rex, D.K. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020, 158, 463–485.e5. [Google Scholar] [CrossRef]
- Atkin, W.; Wooldrage, K.; Brenner, A.; Martin, J.; Shah, U.; Perera, S.; Lucas, F.; Brown, J.P.; Kralj-Hans, I.; Greliak, P.; et al. Adenoma surveillance and colorectal cancer incidence: A retrospective, multicentre, cohort study. Lancet. Oncol. 2017, 18, 823–834. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.K.; Jensen, C.D.; Levin, T.R.; Doubeni, C.A.; Zauber, A.G.; Chubak, J.; Kamineni, A.S.; Schottinger, J.E.; Ghai, N.R.; Udaltsova, N.; et al. Long-term Risk of Colorectal Cancer and Related Death After Adenoma Removal in a Large, Community-based Population. Gastroenterology 2020, 158, 884–894 e5. [Google Scholar] [CrossRef]
- Wieszczy, P.; Kaminski, M.F.; Franczyk, R.; Loberg, M.; Kobiela, J.; Rupinska, M.; Kocot, B.; Rupinski, M.; Holme, O.; Wojciechowska, U.; et al. Colorectal Cancer Incidence and Mortality After Removal of Adenomas During Screening Colonoscopies. Gastroenterology 2020, 158, 875–883.e5. [Google Scholar] [CrossRef] [PubMed]
- Cottet, V.; Jooste, V.; Fournel, I.; Bouvier, A.M.; Faivre, J.; Bonithon-Kopp, C. Long-term risk of colorectal cancer after adenoma removal: A population-based cohort study. Gut 2012, 61, 1180–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, A.J.; Robbins, E.C.; Pack, K.; Stenson, I.; Kirby, P.L.; Patel, B.; Rutter, M.D.; Veitch, A.M.; Saunders, B.P.; Duffy, S.W.; et al. Long-term colorectal cancer incidence after adenoma removal and the effects of surveillance on incidence: A multicentre, retrospective, cohort study. Gut 2020, 69, 1645–1658. [Google Scholar] [CrossRef] [Green Version]
- Rivero-Sanchez, L.; Grau, J.; Auge, J.M.; Moreno, L.; Pozo, A.; Serradesanferm, A.; Diaz, M.; Carballal, S.; Sanchez, A.; Moreira, L.; et al. Colorectal cancer after negative colonoscopy in fecal immunochemical test-positive participants from a colorectal cancer screening program. Endosc. Int. Open 2018, 6, E1140–E1148. [Google Scholar] [CrossRef] [Green Version]
- Heisser, T.; Peng, L.; Weigl, K.; Hoffmeister, M.; Brenner, H. Outcomes at follow-up of negative colonoscopy in average risk population: Systematic review and meta-analysis. BMJ 2019, 367, el6109. [Google Scholar] [CrossRef] [Green Version]
- Stoffel, E.M.; Erichsen, R.; Froslev, T.; Pedersen, L.; Vyberg, M.; Koeppe, E.; Crockett, S.D.; Hamilton, S.R.; Sorensen, H.T.; Baron, J.A. Clinical and Molecular Characteristics of Post-Colonoscopy Colorectal Cancer: A Population-based Study. Gastroenterology 2016, 151, 870–873.e3. [Google Scholar] [CrossRef] [Green Version]
- Meklin, J.; SyrjAnen, K.; Eskelinen, M. Fecal Occult Blood Tests in Colorectal Cancer Screening: Systematic Review and Meta-analysis of Traditional and New-generation Fecal Immunochemical Tests. Anticancer Res. 2020, 40, 3591–3604. [Google Scholar] [CrossRef]
- Lee, J.K.; Liles, E.G.; Bent, S.; Levin, T.R.; Corley, D.A. Accuracy of fecal immunochemical tests for colorectal cancer: Systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, e171. [Google Scholar] [CrossRef]
- Imperiale, T.F.; Gruber, R.N.; Stump, T.E.; Emmett, T.W.; Monahan, P.O. Performance Characteristics of Fecal Immunochemical Tests for Colorectal Cancer and Advanced Adenomatous Polyps: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2019, 170, 319–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, H.M.; Lee, Y.C.; Tu, C.H.; Chen, C.C.; Tseng, P.H.; Liang, J.T.; Shun, C.T.; Lin, J.T.; Wu, M.S. Association between early stage colon neoplasms and false-negative results from the fecal immunochemical test. Clin. Gastroenterol. Hepatol.: Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2013, 11, 832–838.e2. [Google Scholar] [CrossRef] [PubMed]
- van Doorn, S.C.; Stegeman, I.; Stroobants, A.K.; Mundt, M.W.; de Wijkerslooth, T.R.; Fockens, P.; Kuipers, E.J.; Bossuyt, P.M.; Dekker, E. Fecal immunochemical testing results and characteristics of colonic lesions. Endoscopy 2015, 47, 1011–1017. [Google Scholar] [CrossRef] [Green Version]
- Brenner, H.; Hoffmeister, M.; Birkner, B.; Stock, C. Which adenomas are detected by fecal occult blood testing? A state-wide analysis from Bavaria, Germany. Int. J. Cancer 2015, 136, 1672–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carot, L.; Castells, A.; Hernandez, C.; Alvarez-Urturi, C.; Balaguer, F.; Lanas, A.; Cubiella, J.; Tasende, J.D.; Jover, R.; Hernandez, V.; et al. Detection of serrated lesions in proximal colon by simulated sigmoidoscopy vs. faecal immunochemical testing in a multicentre, pragmatic, randomised controlled trial. United Eur. Gastroenterol. J. 2018, 6, 1527–1537. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.C.; Shun, C.T.; Hsu, W.F.; Tu, C.H.; Tsai, P.Y.; Lin, B.R.; Liang, J.T.; Wu, M.S.; Chiu, H.M. Fecal Immunochemical Test Detects Sessile Serrated Adenomas and Polyps With a Low Level of Sensitivity. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2017, 15, 872–879.e1. [Google Scholar] [CrossRef] [Green Version]
- Bosch, L.J.W.; Melotte, V.; Mongera, S.; Daenen, K.L.J.; Coupe, V.M.H.; van Turenhout, S.T.; Stoop, E.M.; de Wijkerslooth, T.R.; Mulder, C.J.J.; Rausch, C.; et al. Multitarget Stool DNA Test Performance in an Average-Risk Colorectal Cancer Screening Population. Am. J. Gastroenterol. 2019, 114, 1909–1918. [Google Scholar] [CrossRef] [Green Version]
- Rozen, P.; Levi, Z.; Hazazi, R.; Waked, A.; Vilkin, A.; Maoz, E.; Birkenfeld, S.; Leshno, M.; Niv, Y. Identification of colorectal adenomas by a quantitative immunochemical faecal occult blood screening test depends on adenoma characteristics, development threshold used and number of tests performed. Aliment. Pharmacol. Ther. 2009, 29, 906–917. [Google Scholar] [CrossRef]
- Cubiella, J.; Castro, I.; Hernandez, V.; Gonzalez-Mao, C.; Rivera, C.; Iglesias, F.; Cid, L.; Soto, S.; de-Castro, L.; Vega, P.; et al. Characteristics of adenomas detected by fecal immunochemical test in colorectal cancer screening. Cancer Epidemiol. Biomark. Prev.: A Publ. Am. Assoc. Cancer Res. Cosponsored By Am. Soc. Prev. Oncol. 2014, 23, 1884–1892. [Google Scholar] [CrossRef] [Green Version]
- Morikawa, T.; Kato, J.; Yamaji, Y.; Wada, R.; Mitsushima, T.; Sakaguchi, K.; Shiratori, Y. Sensitivity of immunochemical fecal occult blood test to small colorectal adenomas. Am. J. Gastroenterol. 2007, 102, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Nakama, H.; Zhang, B.; Kamijo, N. Sensitivity of immunochemical fecal occult blood test for colorectal flat adenomas. Hepato-Gastroenterology 2004, 51, 1333–1336. [Google Scholar]
- Lu, M.; Luo, X.; Li, N.; Chen, H.; Dai, M. Diagnostic Accuracy Of Fecal Occult Blood Tests For Detecting Proximal Versus Distal Colorectal Neoplasia: A Systematic Review And Meta-Analysis. Clin. Epidemiol. 2019, 11, 943–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirai, H.W.; Tsoi, K.K.; Chan, J.Y.; Wong, S.H.; Ching, J.Y.; Wong, M.C.; Wu, J.C.; Chan, F.K.; Sung, J.J.; Ng, S.C. Systematic review with meta-analysis: Faecal occult blood tests show lower colorectal cancer detection rates in the proximal colon in colonoscopy-verified diagnostic studies. Aliment. Pharmacol. Ther. 2016, 43, 755–764. [Google Scholar] [CrossRef]
- Zorzi, M.; Hassan, C.; Capodaglio, G.; Narne, E.; Turrin, A.; Baracco, M.; Dal Cin, A.; Fiore, A.; Martin, G.; Repici, A.; et al. Divergent Long-Term Detection Rates of Proximal and Distal Advanced Neoplasia in Fecal Immunochemical Test Screening Programs: A Retrospective Cohort Study. Ann. Intern. Med. 2018, 169, 602–609. [Google Scholar] [CrossRef]
- Cubiella, J.; Salve, M.; Diaz-Ondina, M.; Vega, P.; Alves, M.T.; Iglesias, F.; Sanchez, E.; Macia, P.; Blanco, I.; Bujanda, L.; et al. Diagnostic accuracy of the faecal immunochemical test for colorectal cancer in symptomatic patients: Comparison with NICE and SIGN referral criteria. Colorectal Dis.: Off. J. Assoc. Coloproctology Great Br. Irel. 2014, 16, O273–O282. [Google Scholar] [CrossRef]
- von Karsa, L.; Patnick, J.; Segnan, N.; Atkin, W.; Halloran, S.; Lansdorp-Vogelaar, I.; Malila, N.; Minozzi, S.; Moss, S.; Quirke, P.; et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis: Overview and introduction to the full supplement publication. Endoscopy 2013, 45, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.; Shea, B.; O’connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2010. [Google Scholar]
- Wieten, E.; Schreuders, E.H.; Grobbee, E.J.; Nieboer, D.; Bramer, W.M.; Lansdorp-Vogelaar, I.; Bruno, M.J.; Kuipers, E.J.; Spaander, M.C.W. Incidence of faecal occult blood test interval cancers in population-based colorectal cancer screening: A systematic review and meta-analysis. Gut 2019, 68, 873–881. [Google Scholar] [CrossRef]
- Chiang, T.H.; Chuang, S.L.; Chen, S.L.; Chiu, H.M.; Yen, A.M.; Chiu, S.Y.; Fann, J.C.; Chou, C.K.; Lee, Y.C.; Wu, M.S.; et al. Difference in performance of fecal immunochemical tests with the same hemoglobin cutoff concentration in a nationwide colorectal cancer screening program. Gastroenterology 2014, 147, 1317–1326. [Google Scholar] [CrossRef] [Green Version]
- Parente, F.; Boemo, C.; Ardizzoia, A.; Costa, M.; Carzaniga, P.; Ilardo, A.; Moretti, R.; Cremaschini, M.; Parente, E.M.; Pirola, M.E. Outcomes and cost evaluation of the first two rounds of a colorectal cancer screening program based on immunochemical fecal occult blood test in northern Italy. Endoscopy 2013, 45, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Shin, A.; Choi, K.S.; Jun, J.K.; Noh, D.K.; Suh, M.; Jung, K.W.; Kim, B.C.; Oh, J.H.; Park, E.C. Validity of fecal occult blood test in the national cancer screening program, Korea. PLoS ONE 2013, 8, e79292. [Google Scholar] [CrossRef] [Green Version]
- Chiu, H.M.; Chen, S.L.; Yen, A.M.; Chiu, S.Y.; Fann, J.C.; Lee, Y.C.; Pan, S.L.; Wu, M.S.; Liao, C.S.; Chen, H.H.; et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the One Million Taiwanese Screening Program. Cancer 2015, 121, 3221–3229. [Google Scholar] [CrossRef] [Green Version]
- Jensen, C.D.; Corley, D.A.; Quinn, V.P.; Doubeni, C.A.; Zauber, A.G.; Lee, J.K.; Zhao, W.K.; Marks, A.R.; Schottinger, J.E.; Ghai, N.R.; et al. Fecal Immunochemical Test Program Performance Over 4 Rounds of Annual Screening: A Retrospective Cohort Study. Ann. Intern. Med. 2016, 164, 456–463. [Google Scholar] [CrossRef]
- Giorgi Rossi, P.; Carretta, E.; Mangone, L.; Baracco, S.; Serraino, D.; Zorzi, M.; Colorectal Cancer Screening, I.W.G. Incidence of interval cancers in faecal immunochemical test colorectal screening programmes in Italy. J. Med. Screen. 2018, 25, 32–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portillo, I.; Arana-Arri, E.; Idigoras, I.; Bilbao, I.; Martinez-Indart, L.; Bujanda, L.; Gutierrez-Ibarluzea, I. Colorectal and interval cancers of the Colorectal Cancer Screening Program in the Basque Country (Spain). World J. Gastroenterol. 2017, 23, 2731–2742. [Google Scholar] [CrossRef]
- van der Vlugt, M.; Grobbee, E.J.; Bossuyt, P.M.M.; Bos, A.; Bongers, E.; Spijker, W.; Kuipers, E.J.; Lansdorp-Vogelaar, I.; Spaander, M.C.W.; Dekker, E. Interval Colorectal Cancer Incidence Among Subjects Undergoing Multiple Rounds of Fecal Immunochemical Testing. Gastroenterology 2017, 153, 439–447.e2. [Google Scholar] [CrossRef] [Green Version]
- Buron, A.; Macia, F.; Andreu, M.; Pellise, M.; Castells, X.; Grau, J. Population-based colorectal cancer screening: Interval cancers and relationship with the quantitative faecal immunological for hemoglobin. Med. Clin. 2019, 152, 303–306. [Google Scholar] [CrossRef]
- Mlakar, D.N.; Bric, T.K.; Skrjanec, A.L.; Krajc, M. Interval cancers after negative immunochemical test compared to screen and non-responders’ detected cancers in Slovenian colorectal cancer screening programme. Radiol. Oncol. 2018, 52, 413–421. [Google Scholar] [CrossRef] [Green Version]
- van de Veerdonk, W.; Hoeck, S.; Peeters, M.; Van Hal, G.; Francart, J.; De Brabander, I. Occurrence and characteristics of faecal immunochemical screen-detected cancers vs. non-screen-detected cancers: Results from a Flemish colorectal cancer screening programme. United Eur. Gastroenterol. J. 2020, 8, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Toes-Zoutendijk, E.; Kooyker, A.I.; Dekker, E.; Spaander, M.C.W.; Opstal-van Winden, A.W.J.; Ramakers, C.; Buskermolen, M.; van Vuuren, A.J.; Kuipers, E.J.; van Kemenade, F.J.; et al. Incidence of Interval Colorectal Cancer After Negative Results From First-Round Fecal Immunochemical Screening Tests, by Cutoff Value and Participant Sex and Age. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020, 18, 1493–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorzi, M.; Hassan, C.; Senore, C.; Capodaglio, G.; Turrin, A.; Narne, E.; Mussato, A.; Rizzato, S.; Chinellato, E.; Zamberlan, S.; et al. Interval colorectal cancers after negative faecal immunochemical test in a 13-year screening programme. J. Med. Screen. 2020. [Google Scholar] [CrossRef]
- Walsh, E.; Rees, C.J.; Gill, M.; Parker, C.E.; Bevan, R.; Perry, S.L.; Bury, Y.; Mills, S.; Bradburn, D.M.; Bramble, M.; et al. Are there biological differences between screen-detected and interval colorectal cancers in the English Bowel Cancer Screening Programme? Br. J. Cancer 2016, 115, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Haug, U.; Hundt, S. Sex differences in performance of fecal occult blood testing. Am. J. Gastroenterol. 2010, 105, 2457–2464. [Google Scholar] [CrossRef]
- van Turenhout, S.T.; Oort, F.A.; van der Hulst, R.W.; Visscher, A.P.; Terhaar sive Droste, J.S.; Scholten, P.; Bouman, A.A.; Meijer, G.A.; Mulder, C.J.; van Rossum, L.G.; et al. Prospective cross-sectional study on faecal immunochemical tests: Sex specific cut-off values to obtain equal sensitivity for colorectal cancer? BMC Gastroenterol. 2014, 14, e217. [Google Scholar] [CrossRef] [Green Version]
- Benedix, F.; Kube, R.; Meyer, F.; Schmidt, U.; Gastinger, I.; Lippert, H.; Colon/Rectum Carcinomas Study, G. Comparison of 17,641 patients with right- and left-sided colon cancer: Differences in epidemiology, perioperative course, histology, and survival. Dis. Colon Rectum 2010, 53, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.O.; Jess, P. Possible better long-term survival in left versus right-sided colon cancer-a systematic review. Dan. Med. J. 2012, 59, A4444. [Google Scholar] [PubMed]
- Cheng, L.; Eng, C.; Nieman, L.Z.; Kapadia, A.S.; Du, X.L. Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am. J. Clin. Oncol. 2011, 34, 573–580. [Google Scholar] [CrossRef]
- Cai, Y.; Rattray, N.J.W.; Zhang, Q.; Mironova, V.; Santos-Neto, A.; Hsu, K.S.; Rattray, Z.; Cross, J.R.; Zhang, Y.; Paty, P.B.; et al. Sex Differences in Colon Cancer Metabolism Reveal A Novel Subphenotype. Sci. Rep. 2020, 10, e4905. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Chen, Y.; Xie, L.; Meng, Y.; Zhu, L.; Chu, H.; Gu, D.; Zhang, Z.; Du, M.; Wang, M. Sex hormones and genetic variants in hormone metabolic pathways associated with the risk of colorectal cancer. Environ. Int. 2020, 137, e105543. [Google Scholar] [CrossRef]
- Sun, Y.; Mironova, V.; Chen, Y.; Lundh, E.P.F.; Zhang, Q.; Cai, Y.; Vasiliou, V.; Zhang, Y.; Garcia-Milian, R.; Khan, S.A.; et al. Molecular Pathway Analysis Indicates a Distinct Metabolic Phenotype in Women With Right-Sided Colon Cancer. Transl. Oncol. 2020, 13, 42–56. [Google Scholar] [CrossRef] [PubMed]
- McGivern, A.; Wynter, C.V.; Whitehall, V.L.; Kambara, T.; Spring, K.J.; Walsh, M.D.; Barker, M.A.; Arnold, S.; Simms, L.A.; Leggett, B.A.; et al. Promoter hypermethylation frequency and BRAF mutations distinguish hereditary non-polyposis colon cancer from sporadic MSI-H colon cancer. Fam. Cancer 2004, 3, 101–107. [Google Scholar] [CrossRef]
- Sun, B.L. Current Microsatellite Instability Testing in Management of Colorectal Cancer. Clin. Colorectal Cancer 2020. [Google Scholar] [CrossRef]
- Sadik, R.; Abrahamsson, H.; Stotzer, P.O. Gender differences in gut transit shown with a newly developed radiological procedure. Scand. J. Gastroenterol. 2003, 38, 36–42. [Google Scholar] [CrossRef]
- Degen, L.P.; Phillips, S.F. Variability of gastrointestinal transit in healthy women and men. Gut 1996, 39, 299–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.S.; Kuo, B.; McCallum, R.W.; Chey, W.D.; DiBaise, J.K.; Hasler, W.L.; Koch, K.L.; Lackner, J.M.; Miller, C.; Saad, R.; et al. Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2009, 7, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Hoffmeister, M.; Stegmaier, C.; Brenner, G.; Altenhofen, L.; Haug, U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: Estimates based on 840,149 screening colonoscopies. Gut 2007, 56, 1585–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieten, E.; Schreuders, E.H.; Nieuwenburg, S.A.; Hansen, B.E.; Lansdorp-Vogelaar, I.; Kuipers, E.J.; Bruno, M.J.; Spaander, M.C. Effects of Increasing Screening Age and Fecal Hemoglobin Cutoff Concentrations in a Colorectal Cancer Screening Program. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2016, 14, 1771–1777. [Google Scholar] [CrossRef]
- McCashland, T.M.; Brand, R.; Lyden, E.; de Garmo, P.; Project, C.R. Gender differences in colorectal polyps and tumors. Am. J. Gastroenterol. 2001, 96, 882–886. [Google Scholar] [CrossRef]
- Oines, M.; Helsingen, L.M.; Bretthauer, M.; Emilsson, L. Epidemiology and risk factors of colorectal polyps. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 419–424. [Google Scholar] [CrossRef]
- Meester, R.G.S.; van Herk, M.; Lansdorp-Vogelaar, I.; Ladabaum, U. Prevalence and Clinical Features of Sessile Serrated Polyps: A Systematic Review. Gastroenterology 2020, 159, 105–118.e5. [Google Scholar] [CrossRef]
- Idigoras Rubio, I.; Arana-Arri, E.; Portillo Villares, I.; Bilbao Iturribarrria, I.; Martinez-Indart, L.; Imaz-Ayo, N.; de la Cruz, M.; de Castro, V.; Lopez de Munain, A.; Torrejon Perez, I.; et al. Participation in a population-based screening for colorectal cancer using the faecal immunochemical test decreases mortality in 5 years. Eur. J. Gastroenterol. Hepatol. 2019, 31, 197–204. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Chuang, S.L.; Chen, S.L.; Yen, A.M.; Fann, J.C.; Chang, D.C.; Lee, Y.C.; Wu, M.S.; Chou, C.K.; Hsu, W.F.; et al. Faecal haemoglobin concentration influences risk prediction of interval cancers resulting from inadequate colonoscopy quality: Analysis of the Taiwanese Nationwide Colorectal Cancer Screening Program. Gut 2017, 66, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Buron, A.; Roman, M.; Auge, J.M.; Macia, F.; Grau, J.; Sala, M.; Louro, J.; Martinez-Alonso, M.; Alvarez-Urturi, C.; Andreu, M.; et al. Changes in FIT values below the threshold of positivity and short-term risk of advanced colorectal neoplasia: Results from a population-based cancer screening program. Eur. J. Cancer 2019, 107, 53–59. [Google Scholar] [CrossRef]
- Digby, J.; Fraser, C.G.; Carey, F.A.; Steele, R.J.C. Can the performance of a quantitative FIT-based colorectal cancer screening programme be enhanced by lowering the threshold and increasing the interval? Gut 2018, 67, 993–994. [Google Scholar] [CrossRef] [Green Version]
- Barnett, K.N.; Weller, D.; Smith, S.; Steele, R.J.; Vedsted, P.; Orbell, S.; Moss, S.M.; Melia, J.W.; Patnick, J.; Campbell, C. The contribution of a negative colorectal screening test result to symptom appraisal and help-seeking behaviour among patients subsequently diagnosed with an interval colorectal cancer. Health Expect. Int. J. Public Particip. Health Care Health Policy 2018, 21, 764–773. [Google Scholar] [CrossRef] [Green Version]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Smeby, J.; Sveen, A.; Merok, M.A.; Danielsen, S.A.; Eilertsen, I.A.; Guren, M.G.; Dienstmann, R.; Nesbakken, A.; Lothe, R.A. CMS-dependent prognostic impact of KRAS and BRAFV600E mutations in primary colorectal cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1227–1234. [Google Scholar] [CrossRef] [Green Version]
- Nishihara, R.; Wu, K.; Lochhead, P.; Morikawa, T.; Liao, X.; Qian, Z.R.; Inamura, K.; Kim, S.A.; Kuchiba, A.; Yamauchi, M.; et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N. Engl. J. Med. 2013, 369, 1095–1105. [Google Scholar] [CrossRef] [Green Version]
- Sawhney, M.S.; Farrar, W.D.; Gudiseva, S.; Nelson, D.B.; Lederle, F.A.; Rector, T.S.; Bond, J.H. Microsatellite instability in interval colon cancers. Gastroenterology 2006, 131, 1700–1705. [Google Scholar] [CrossRef]
- Arain, M.A.; Sawhney, M.; Sheikh, S.; Anway, R.; Thyagarajan, B.; Bond, J.H.; Shaukat, A. CIMP status of interval colon cancers: Another piece to the puzzle. Am. J. Gastroenterol. 2010, 105, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Arain, M.; Thaygarajan, B.; Bond, J.H.; Sawhney, M. Is BRAF mutation associated with interval colorectal cancers? Dig. Dis. Sci. 2010, 55, 2352–2356. [Google Scholar] [CrossRef]
- Shaukat, A.; Arain, M.; Anway, R.; Manaktala, S.; Pohlman, L.; Thyagarajan, B. Is KRAS mutation associated with interval colorectal cancers? Dig. Dis. Sci. 2012, 57, 913–917. [Google Scholar] [CrossRef]
- Richter, J.M.; Pino, M.S.; Austin, T.R.; Campbell, E.; Szymonifka, J.; Russo, A.L.; Hong, T.S.; Borger, D.; Iafrate, A.J.; Chung, D.C. Genetic mechanisms in interval colon cancers. Dig. Dis. Sci. 2014, 59, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Cisyk, A.L.; Penner-Goeke, S.; Lichtensztejn, Z.; Nugent, Z.; Wightman, R.H.; Singh, H.; McManus, K.J. Characterizing the prevalence of chromosome instability in interval colorectal cancer. Neoplasia 2015, 17, 306–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cisyk, A.L.; Nugent, Z.; Wightman, R.H.; Singh, H.; McManus, K.J. Characterizing Microsatellite Instability and Chromosome Instability in Interval Colorectal Cancers. Neoplasia 2018, 20, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Park, S.K.; Yang, H.J.; Jung, Y.S.; Choi, K.Y.; Kim, K.E.; Jung, K.U.; Kim, H.O.; Kim, H.; Chun, H.K.; et al. Microsatellite Instability Status of Interval Colorectal Cancers in a Korean Population. Gut Liver 2016, 10, 781–785. [Google Scholar] [CrossRef] [Green Version]
- Samadder, N.J.; Neklason, D.; Snow, A.; Samowitz, W.; Cessna, M.H.; Rowe, K.; Sandhu, I.; Boucher, K.; Pappas, L.; Smith, K.R.; et al. Clinical and Molecular Features of Post-Colonoscopy Colorectal Cancers. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019, 17, 2731–2739.e2. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Urabe, Y.; Oka, S.; Shimohara, Y.; Nishimura, T.; Inagaki, K.; Okamoto, Y.; Matsumoto, K.; Yamashita, K.; Sumimoto, K.; et al. Clinical Features and Genomic Characterization of Post-Colonoscopy Colorectal Cancer. Clin. Transl. Gastroenterol. 2020, 11, e00246. [Google Scholar] [CrossRef]
- Jass, J.R.; Stewart, S.M.; Stewart, J.; Lane, M.R. Hereditary non-polyposis colorectal cancer--morphologies, genes and mutations. Mutat. Res. 1994, 310, 125–133. [Google Scholar] [CrossRef]
- Rijcken, F.E.; Hollema, H.; Kleibeuker, J.H. Proximal adenomas in hereditary non-polyposis colorectal cancer are prone to rapid malignant transformation. Gut 2002, 50, 382–386. [Google Scholar] [CrossRef]
- Dienstmann, R.; Vermeulen, L.; Guinney, J.; Kopetz, S.; Tejpar, S.; Tabernero, J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat. Rev. Cancer 2017, 17, 79–92. [Google Scholar] [CrossRef]
- Iacopetta, B. Are there two sides to colorectal cancer? Int. J. Cancer 2002, 101, 403–408. [Google Scholar] [CrossRef]
- Kaminski, M.F.; Regula, J.; Kraszewska, E.; Polkowski, M.; Wojciechowska, U.; Didkowska, J.; Zwierko, M.; Rupinski, M.; Nowacki, M.P.; Butruk, E. Quality indicators for colonoscopy and the risk of interval cancer. N. Engl. J. Med. 2010, 362, 1795–1803. [Google Scholar] [CrossRef] [Green Version]
- Samadder, N.J.; Curtin, K.; Tuohy, T.M.; Pappas, L.; Boucher, K.; Provenzale, D.; Rowe, K.G.; Mineau, G.P.; Smith, K.; Pimentel, R.; et al. Characteristics of missed or interval colorectal cancer and patient survival: A population-based study. Gastroenterology 2014, 146, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.J.; Lieberman, D.A.; Winawer, S.J.; Ahnen, D.J.; Baron, J.A.; Schatzkin, A.; Cross, A.J.; Zauber, A.G.; Church, T.R.; Lance, P.; et al. Colorectal cancers soon after colonoscopy: A pooled multicohort analysis. Gut 2014, 63, 949–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, D.J.; Lee, J.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Lieberman, D.; Levin, T.R.; Rex, D.K. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017, 152, 1217–1237.e3. [Google Scholar] [CrossRef] [Green Version]
- Crockett, S.D.; Nagtegaal, I.D. Terminology, Molecular Features, Epidemiology, and Management of Serrated Colorectal Neoplasia. Gastroenterology 2019, 157, 949–966.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashida, H. Endoscopic diagnosis of sessile serrated polyp: A systematic review. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2019, 31, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Bailie, L.; Loughrey, M.B.; Coleman, H.G. Lifestyle Risk Factors for Serrated Colorectal Polyps: A Systematic Review and Meta-analysis. Gastroenterology 2017, 152, 92–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botteri, E.; Borroni, E.; Sloan, E.K.; Bagnardi, V.; Bosetti, C.; Peveri, G.; Santucci, C.; Specchia, C.; van den Brandt, P.; Gallus, S.; et al. Smoking and Colorectal Cancer Risk, Overall and by Molecular Subtypes: A Meta-Analysis. Am. J. Gastroenterol. 2020, 115, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Buikhuisen, J.Y.; Torang, A.; Medema, J.P. Exploring and modelling colon cancer inter-tumor heterogeneity: Opportunities and challenges. Oncogenesis 2020, 9, e66. [Google Scholar] [CrossRef]
- Cha, J.M.; Suh, M.; Kwak, M.S.; Sung, N.Y.; Choi, K.S.; Park, B.; Jun, J.K.; Hwang, S.H.; Lee, D.H.; Kim, B.C.; et al. Risk of Interval Cancer in Fecal Immunochemical Test Screening Significantly Higher During the Summer Months: Results from the National Cancer Screening Program in Korea. Am. J. Gastroenterol. 2018, 113, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Doubeni, C.A.; Jensen, C.D.; Fedewa, S.A.; Quinn, V.P.; Zauber, A.G.; Schottinger, J.E.; Corley, D.A.; Levin, T.R. Fecal Immunochemical Test (FIT) for Colon Cancer Screening: Variable Performance with Ambient Temperature. J. Am. Board Fam. Med. Jabfm 2016, 29, 672–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Urturi, C.; Andreu, M.; Hernandez, C.; Perez-Riquelme, F.; Carballo, F.; Ono, A.; Cruzado, J.; Cubiella, J.; Hernandez, V.; Mao, C.G.; et al. Impact of age- and gender-specific cut-off values for the fecal immunochemical test for hemoglobin in colorectal cancer screening. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2016, 48, 542–551. [Google Scholar] [CrossRef]

| Author and Year | Country | Study Period | Number of Screening Sounds | FIT Cut-off, μg Hb/g Faeces | Number of Participants with a Negative FIT | Total Number of Screen-Detected CRCs | Total Number of iCRCs | iCRC, % (n) | FIT Sensitivity (%) | iCRC Characteristics | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Samples | Men | Women | Interval Cancer Proportion (%) a | Proportional Interval Cancer Rate b (95% CI c) | ||||||||
| Parente et al., 2013 [37] | Italy | 2005–2007 | 1 (Prevalent) | 20 | 78,226 | 165 | 8 | ND | ND | Total: 4.6 | 11.0 (5,22) d | ND |
| 1 | Aged 50–69 y | |||||||||||
| Shin et al., 2013 [38] | Korea | 2004–2007 | 2 (Prevalent and incident) | 10 | 8,134,104 | 2961 | 2047 | 60.5 (1233) | 39.4 (805) | Total: 59.1 | 25.0 (24,26) d | Female |
| 1st round: 59.7 | Proximal location | |||||||||||
| 1 | Aged ≥ 50 y | Subsequent: 56.1 | ||||||||||
| Chiu et al., 2015 [39] | Taiwan | 2004–2009 | 3 (Prevalent and incident) | 20 | 1,113,932 | 2728 | 968 | ND | ND | Total: 26.2 | 14.0 (14,15) d | Female |
| 1 | Aged 50–69 y | Advanced stages | ||||||||||
| Jensen et al., 2016 [40] | USA | 2007–2012 | 4 (Prevalent and incident) | 20 | 780,577 | 958 | 242 | ND | ND | Total: 20.2 | 9.0 (8–11) d | Advanced stages |
| 1 | Aged 50–70 y | Proximal location | ||||||||||
| Giorgi Rossi et al., 2017 [41] | Italy | 2000–2008 | 1 (Prevalent) | 20 | 805,914 e | ND | 172 | 52.9 (91) | 47.1 (81) | ND | 21.0 (18,25) d | Female |
| 1 | Aged 50–69 y | Proximal location | ||||||||||
| Portillo et al., 2017 [42] | Spain | 2009–2015 | 3 (Prevalent and incident) | 17–20 | 769,124 | 2518 | 186 | 67.2 (125) | 32.8 (61) | Total: 6.9 | 4.0 (3,5) d | Proximal location |
| 1 | Aged 50–69 y | 1st round: 8.1 | Advanced stages | |||||||||
| Subsequent: range 1–5 | Worse prognosis | |||||||||||
| van der Vlugt et al., 2017 [43] | Netherlands | 2006–2015 | 3 (Prevalent and incident) | 10 | 15,711 | 116 | 27 | 59.0 (16) | 41.0 (11) | Total: 22.3 | 24.0 (17,35) d | Advanced stages |
| 1 | Aged 50–74 y | |||||||||||
| Burón et al., 2018 [44] | Spain | 2010–2013 | 2 (Prevalent and incident) | 20 | 161,691 | 415 | 92 | 17.6 (54) | 18.8 (38) | Total: 18.0 | ND | Advanced stages |
| 1 | Aged 50–69 y | 1st round: 16.0 | ||||||||||
| Proximal location | ||||||||||||
| 2nd round: 22.0 | Rectum | |||||||||||
| Novak Mlakar et al., 2018 [45] | Slovenia | 2011–2012 | 1 (Incident) | 20 | 236,801 | 493 | 79 | 63.3 (50) | 36.7 (29) | Total: 13.8 | 11.6 | Proximal location |
| 2 | Aged 50–69 y | Advanced stages | ||||||||||
| van der Veerdonk et al., 2019 [46] | Belgium | 2013–2017 | 2 (Prevalent and incident) | 15 | 1,123,479 | 4094 | 772 | 54.0 (417) | 46.0 (355) | Total: 18.9 | ND | Female |
| 1 | Aged 56–74 y | 1st round: ND f | Older age | |||||||||
| 2nd round: 5.4 | Proximal location | |||||||||||
| Toes-Zoutendijk et al., 2020 [47] | Netherlands | 2014–2018 | 1 (Prevalent) | 15 and 47 | 111,800 and 373,174 g | 1102 and 2108 g | 126 and 418 g | 42.1 and 50.0 g (73 and 209 g) | 57.9 and 50.0 g (53 and 209 g) | Total: 10.3 and 16.5 g | 9.5 and 13.8 g | Female |
| 1 | Aged 55–75 y | Older age | ||||||||||
| Proximal location | ||||||||||||
| Zorzi et al., 2020 [48] | Italy | 2002–2015 | 6 (Prevalent and incident) | 20 | 423,539 | 781 | 150 | 48.0 (72) | 52.0 (78) | Total: 16.1 | 13.7 (12–16) | Female |
| 1st round: 11 | 1st round: 18.5 (14–24) | |||||||||||
| 1 | Aged 50–69 y | Subsequent range: 7–27 | Subsequent range: 8–16 | Proximal location | ||||||||
| Author and Year | Setting (Country) | Definition of iCRC | N | Exclusion for | Matching | Interval Cancer Characteristics Compared with Detected Cancers | Other iCRC Characteristics | |||
|---|---|---|---|---|---|---|---|---|---|---|
| MSI | CIMP | CNI | Mutations | |||||||
| Sawhney et al., 2006 [76] | Minneapolis Veterans Administration Population (USA) | PCCRC: CRC within 60 months of a colonoscopy | 51 PCCRCs, 112 detected | IBD, FAP | Matched 2:1 by sex and age | ↑ 30% PCCRCs vs. 10% DCRCs; ORa: 3.7; 95% CI, 1.5–9.1 | NA | NA | NA | PCCRCs were more proximal, smaller and had mucinous histology. No differences for histologic grade and TNM stage. |
| Arain et al., 2010 [77] | 52 PCCRCs, 103 detected (including patients from 2006 study) | ↑ 29% PCCRCs vs. 11% DCRCs; OR b: 2.7; 95% CI, 1.1–6.8 | ↑ 57% PCCRCs vs. 33% DCRCs; OR b: 2.4; 95% CI, 1.2–4.9 | NA | NA | |||||
| Shaukat et al., 2010 [78] | DCRC: CRC on index colonoscopy | 63 PCCRCs, 131 detected (including patients from 2006 and 2010 study) | ↑ 29% PCCRCs vs. 11% DCRCs; OR b: 2.6; 95% CI 1.1–6.7 | NA | NA | KRAS ↓ 13% PCCRCs vs. 29% DCRCs; OR b: 0.4; 95% CI, 0.2–0.9; BRAF = 28% PCCRCs vs. 19% DCRCs; OR b: 0.9; 95% CI, 0.4–2.4 | ||||
| Shaukat et al., 2012 [79] | ||||||||||
| Nishihara et al., 2013 [75] | Nurses’ Health Study/Health Professionals Follow-up Study (USA) | PCCRC: CRC within 60 months of a colonoscopy | 62 PCCRCs, 606 detected | IBD, FAP | No | ↑ 25% PCCRCs vs. 14% DCRCs; OR c: 2.1; 95% CI, 1.1–4.0 | ↑ 30% PCCRCs vs. 15% DCRCs; OR c: 2.2; 95% CI, 1.1–4.2 | NA | BRAF = 22% PCCRCs vs. 14% DCRCs; OR b: 1.8; 95% CI, 0.3–3.6; KRAS = 23% PCCRCs vs. 36% DCRCs; OR b: 0.6; 95% CI, 0.3–1.1 | High-level long interspersed nucleotide element-1 (LINE-1) methylation (OR c: 3.21; 95% CI, 1.3–8.0). PIK3CA mutation was not associated. |
| Opportunistic screening with colonoscopy | DCRC: CRC >60 months of a colonoscopy or without prior endoscopy | |||||||||
| Richter et al., 2014 [80] | Massachusetts General Hospital (USA) | PCCRC: CRC within 12–60 months of a colonoscopy | 42 PCCRCs (42 by MMR status and genomic analysis) | IBD; FAP | No | 41% of PCCRCs | NA | NA | BRAF = 17% PCCRCs vs. 10% DCRCs d; KRAS = 29% PCCRCs vs. 37% DCRCs d | NRAS,PIK3CA,APC, CTNNB1, EGF, PTEN, and TP53 mutations were not associated. |
| DCRC: CRC on index colonoscopy | 226 detected (226 with only genomic analysis) | |||||||||
| Cisyk et al., 2015 [81] | Winnipeg Population-based (Canada) | PCCRC: CRC within 6–36 months of a colonoscopy | 46 PCCRC (46 analysed) | IBD < 50 years | Matched 2:1 by sex, age and tumor location | NA | = 82% PCCRCs vs. 85% DCRCs d | NA | No differences in location | |
| 95 detected (95 analysed) | ||||||||||
| Cisyk et al., 2018 [82] | DCRC: CRC within 1 month of a colonoscopy | 46 PCCRCs (45 analysed) | ↑ MSI~1.5x higher d 27% PCCRCs vs. 17% DCRCs | NA | = 14% PCCRCs vs. 12% DCRCs CIN+/MSI+d | NA | ||||
| 95 detected (90 analysed) | ||||||||||
| Lee et al., 2016 [83] | Kangbuk Samsung Hospital (Korea) | PCCRC: CRC within 12–60 months of a colonoscopy | Only CRC removed surgically | IBD; Hereditary cancer | No | ↑ 32% PCCRCs vs. 8% DCRCs; OR e: 3.9; 95% CI: 1.3–11.0 | NA | NA | NA | No differences in age, sex, location or TNM. |
| DCRC: CRC index colonoscopy or >60 months of a colonoscopy | 25 PCCRCs, 261 detected | |||||||||
| Stoffel et al., 2016 [15] | Denmark Population-based (69% of CRC nationwide) (Denmark) | PCCRC: CRC within ≥ 6 months of a colonoscopy | 725 PCCRCs (725 by MMR status and 85 by genomic analysis) | None | No | 30% PCCRCs vs. 17% DCRCs; OR: 1.4; 95% CI: 0.9–2.1 | NA | NA | 19% BRAF mutations in PCCRCs; 27% KRAS/ NRAS mutations in PCCRC | PCCRCs were associated with older patients, earlier stage, IBD and proximal tumors. 19% P1K3CA mutations in PCCRCs. |
| DCRC: CRC within 6 months of a colonoscopy | 9640 detected (8337 by MMR status and no genomic analysis) | |||||||||
| Walsh et al., 2016 [49] | The Northern Region Colorectal Cancer Audit Group cohort (United Kingdom) Stool-based CRC screening programme | iCRC: after a negative gFOBT and before next invitation | 28 iCRCs | IBD; Hereditary cancer | Matched 1:2–3 by tumor location, Dukes’ stage, and histological differentiation grade | = 14% iCRCs vs. 5% DCRCs d | NA | NA | NA | iCRCs were larger and more likely to exhibit histological venous invasion. |
| Screen-DCRC: after a positive gFOBT | 43 screen-DCRCs | |||||||||
| Samadder et al., 2019 [84] | Utah Population-based (USA) | PCCRC: CRC within 6–60 months of a colonoscopy | Clinical data: 159 PCCRCs (84 analysed); | None | Matched by age, sex, andhospital site | ↑ 32% PCCRCs vs. 13% DCRC; OR f: 4.2; 95% CI: 1.5–11.1 | = 17% PCCRCs vs. 21% DCRC; OR f: 1.4; 95% CI: 0.6–3.2 | NA | BRAF = 24% PCCRCs vs. 24% DCRCs; OR f: 1.2; 95% CI: 0.6–2.4; KRAS = 30% PCCRCs vs. 29% DCRCs; OR f: 1.1; 95% CI: 0.5–2.2 | PCCRCs were associated with proximal colon and early stage. |
| DCRC: CRC within 6 months of a colonoscopy | 2500 DCRC (84 analysed) | |||||||||
| Tanaka et al., 2020 [85] | Single-centre Hiroshima University Hospital (Japan) | PCCRC: CRC or pTis within 6–60 months of colonoscopy | 34 PCCRCs (33 by MMR status and 23 by genomic analysis. Note: 18 of 33 were pTis) | Hereditary Cancer; IBD | No | 21% of PCCRCs | NA | NA | 13% BRAF mutations in PCCRCs; 52% KRAS/NRAS mutations in PCCRCs | 22% P1K3CA mutations in PCCRCs. |
| DCRC: CRC within 6 months of a colonoscopy. | 1698 DCRCs (not analysed) | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibáñez-Sanz, G.; Sanz-Pamplona, R.; Garcia, M.; on behalf of the MSIC-SC Research Group. Future Prospects of Colorectal Cancer Screening: Characterizing Interval Cancers. Cancers 2021, 13, 1328. https://doi.org/10.3390/cancers13061328
Ibáñez-Sanz G, Sanz-Pamplona R, Garcia M, on behalf of the MSIC-SC Research Group. Future Prospects of Colorectal Cancer Screening: Characterizing Interval Cancers. Cancers. 2021; 13(6):1328. https://doi.org/10.3390/cancers13061328
Chicago/Turabian StyleIbáñez-Sanz, Gemma, Rebeca Sanz-Pamplona, Montse Garcia, and on behalf of the MSIC-SC Research Group. 2021. "Future Prospects of Colorectal Cancer Screening: Characterizing Interval Cancers" Cancers 13, no. 6: 1328. https://doi.org/10.3390/cancers13061328
APA StyleIbáñez-Sanz, G., Sanz-Pamplona, R., Garcia, M., & on behalf of the MSIC-SC Research Group. (2021). Future Prospects of Colorectal Cancer Screening: Characterizing Interval Cancers. Cancers, 13(6), 1328. https://doi.org/10.3390/cancers13061328






