Total Laryngectomy—Still Cutting-Edge?
Abstract
Simple Summary
Abstract
1. Background
2. Diagnostic Work-Up
2.1. Indication for Total Laryngectomy
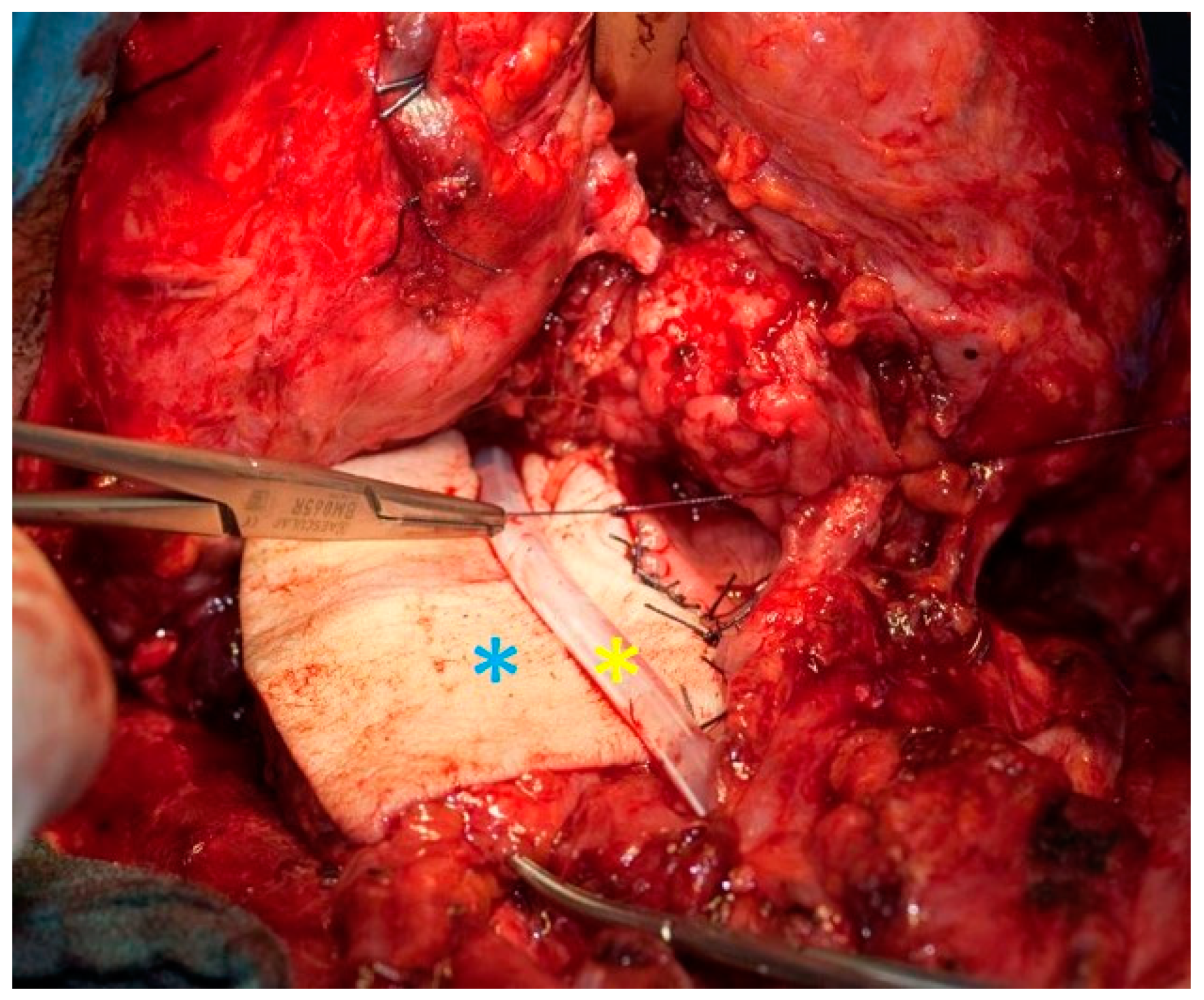
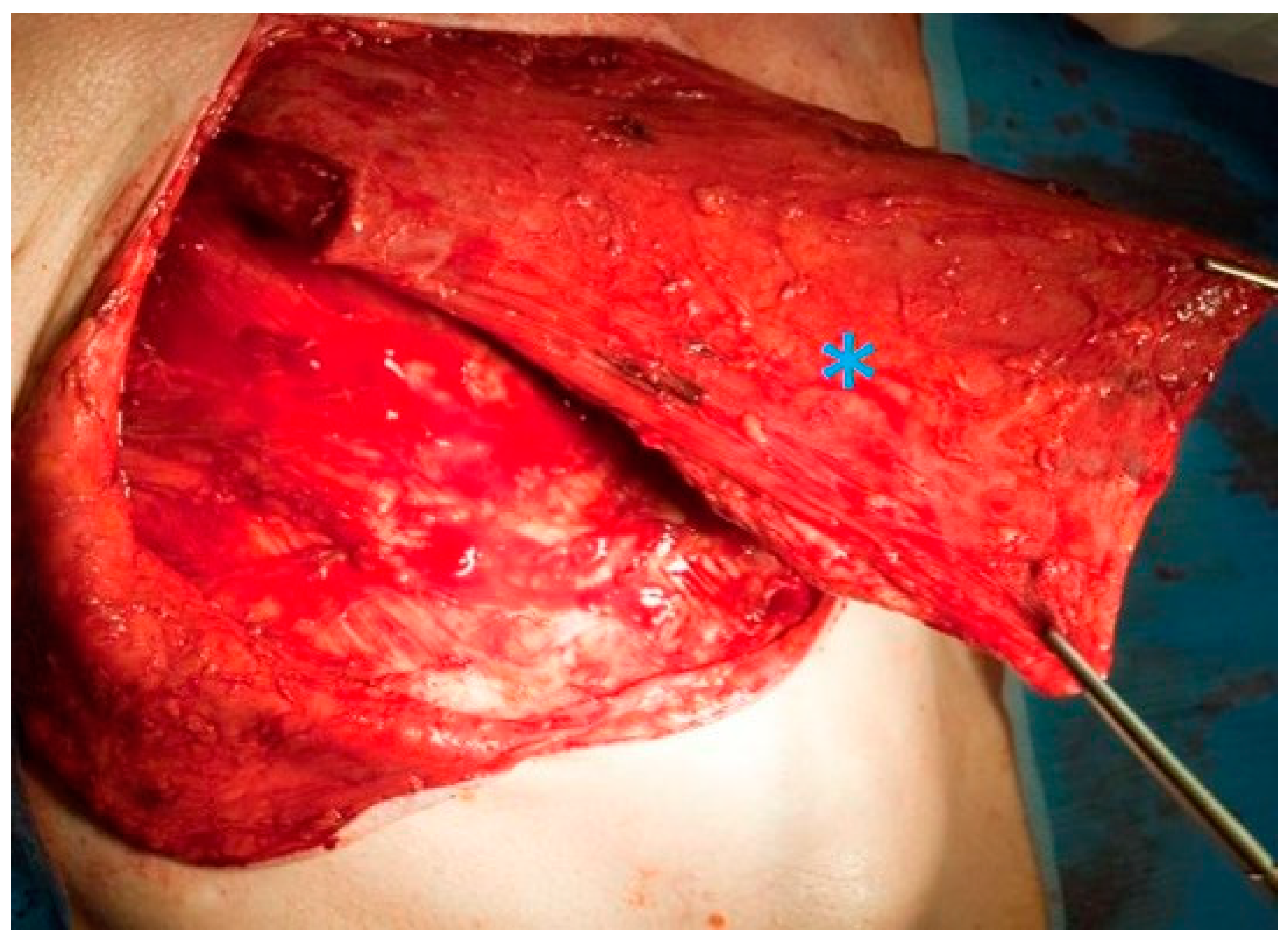
2.2. Surgical Technique for Total Laryngectomy
2.3. Transoral Robotic Laryngectomy
2.4. Tracheostomy before Total Laryngectomy
2.5. Percutaneous Endoscopic Gastrostomy
2.6. Neck Dissection
3. Adjuvants
3.1. Radio(chemo)therapy as an Alternative
3.2. Surgery or Radiochemotherapy?
3.3. Salvage Laryngectomy
4. Complications of Total Laryngectomy
5. Prognosis after Laryngectomy
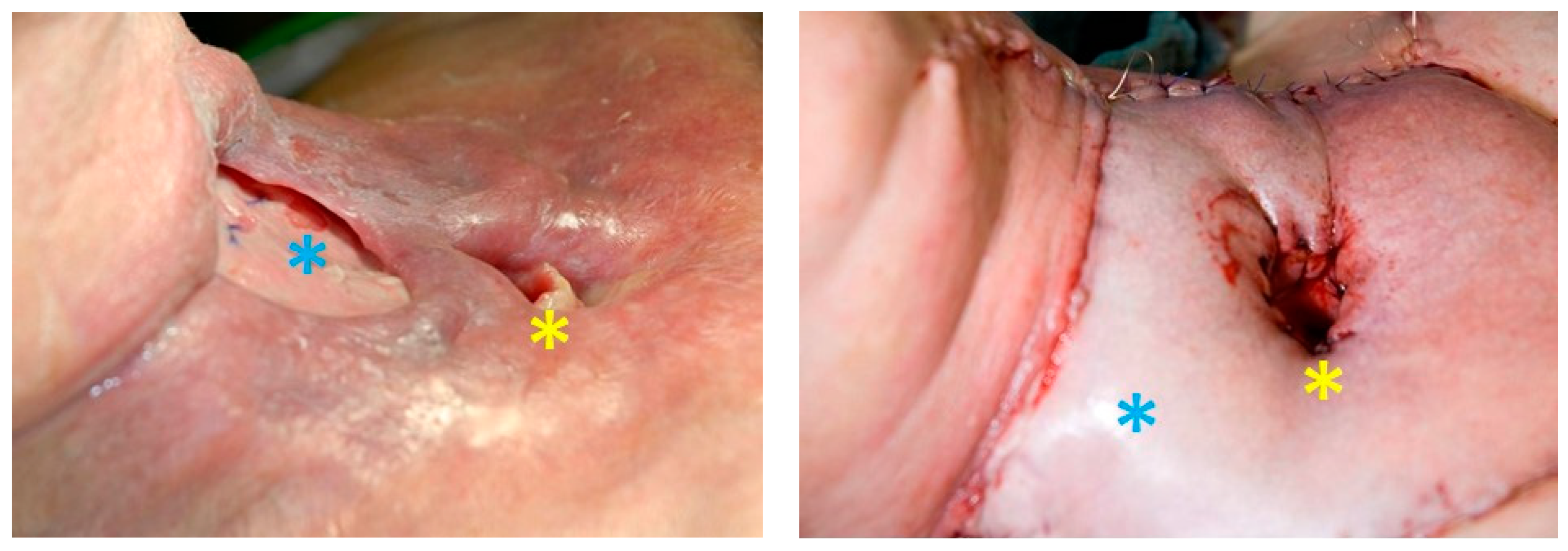
6. Rehabilitation
7. Quality of Life after Total Laryngectomy
Funding
Conflicts of Interest
References
- Kramp, B.; Dommerich, S. Kanülen und Stimmprothesen. Laryngo-Rhino-Otologie 2009, 88, S95–S118. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://gco.iarc.fr/today/online-analysis-table (accessed on 19 March 2021).
- Wiegand, S. Evidenz und Evidenzlücken zur Chirurgie des Larynxkarzinoms. Laryngo-Rhino-Otologie 2016, 95. [Google Scholar] [CrossRef] [PubMed]
- Talamini, R.; Bosetti, C.; La Vecchia, C.; Maso, L.D.; Levi, F.; Bidoli, E.; Negri, E.; Pasche, C.; Vaccarella, S.; Barzan, L.; et al. Combined effect of tobacco and alcohol on laryngeal cancer risk: A case–control study. Cancer Causes Control. 2002, 13, 957–964. [Google Scholar] [CrossRef]
- Michel, O. Berufskrankheit: Kehlkopfkarzinom. HNO 2016, 64, 268–270. [Google Scholar] [CrossRef]
- Castellsagué, X.; Alemany, L.; Quer, M.; Halec, G.; Quirós, B.; Tous, S.; Clavero, O.; Alòs, L.; Biegner, T.; Szafarowski, T.; et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J. Natl. Cancer Inst. 2016, 108, djv403. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Ko, H.C.; Harari, P.M.; Wieland, A.M.; Chen, S.; Baschnagel, A.M.; Kimple, R.J.; Witek, M.E. Impact of HPV Status on the Prognostic Potential of the AJCC Staging System for Larynx Cancer. Otolaryngol. Neck Surg. 2018, 159, 456–465. [Google Scholar] [CrossRef]
- Bootz Singer, H. Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Diagnostik, Therapie und Nachsorge des Laryngeal Carzinomas, Langversion 1.0, 2019, AWMF-Registernummer: 017/076OL. 2019. Available online: http://www.leitlinienprogramm-onkologie.de/leitlinien/larynxkarzinom/ (accessed on 27 April 2019).
- Güneş, S.; Başaran, B.; Aslan, İ. Surgical treatment of hypopharyngeal cancer. Turk. J. Ear Nose Throat 2018, 28, 105–111. [Google Scholar] [CrossRef]
- Petersen, J.F.; Timmermans, A.J.; Van Dijk, B.A.C.; Overbeek, L.I.H.; Smit, L.A.; Hilgers, F.J.M.; Stuiver, M.M.; Brekel, M.W.M.V.D. Trends in treatment, incidence and survival of hypopharynx cancer: A 20-year population-based study in the Netherlands. Eur. Arch. Oto-Rhino-Laryngology 2018, 275, 181–189. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hsiao, J.-R.; Lee, W.-T.; Lee, Y.-C.; Ou, C.-Y.; Chang, C.-C.; Lu, Y.-C.; Huang, J.-S.; Wong, T.-Y.; Chen, K.-C.; et al. Investigating the Association between Alcohol and Risk of Head and Neck Cancer in Taiwan. Sci. Rep. 2017, 7, 9701. [Google Scholar] [CrossRef]
- Menvielle, G.; Luce, D.; Goldberg, P.; Bugel, I.; Leclerc, A. Smoking, alcohol drinking and cancer risk for various sites of the larynx and hypopharynx. A case–control study in France. Eur. J. Cancer Prev. 2004, 13, 165–172. [Google Scholar] [CrossRef]
- Löhler, J.; Gerstner, A.O.H.; Bootz, F.; Walther, L.E. Incidence and localization of abnormal mucosa findings in patients consulting ENT outpatient clinics and data analysis of a cancer registry. Eur. Arch. Oto-Rhino-Laryngology 2013, 271, 1289–1297. [Google Scholar] [CrossRef]
- Steuer, C.E.; El-Deiry, M.; Parks, J.R.; Higgins, K.A.; Saba, N.F. An update on larynx cancer. CA Cancer J. Clin. 2017, 67, 31–50. [Google Scholar] [CrossRef]
- Kostev, K.; Jacob, L.E.; Kalder, M.; Sesterhenn, A.M.; Seidel, D.U. Association of laryngeal cancer with vocal cord leukoplakia and associated risk factors in 1,184 patients diagnosed in otorhinolaryngology practices in Germany. Mol. Clin. Oncol. 2018, 8, 689–693. [Google Scholar] [CrossRef]
- Network NCC. NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers; Version 2.2018; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2018. [Google Scholar]
- Haerle, S.K.; Schmid, D.; Ahmad, N.; Hany, T.; Stoeckli, S. The value of 18F-FDG PET/CT for the detection of distant metastases in high-risk patients with head and neck squamous cell carcinoma. Oral Oncol. 2011, 47, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Grosse, J.; Hellwig, D. Update: PET/CT und PET/MRT bei Tumoren des Kopf-Hals-Bereiches. Der Nukl. 2018, 41, 222–231. [Google Scholar] [CrossRef]
- Mehanna, H.; McConkey, C.C.; Rahman, J.K.; Wong, W.-L.; Smith, A.F.; Nutting, C.; Hartley, A.G.; Hall, P.; Hulme, C.; Patel, D.K.; et al. PET-NECK: A multicentre randomised Phase III non-inferiority trial comparing a positron emission tomography–computerised tomography-guided watch-and-wait policy with planned neck dissection in the management of locally advanced (N2/N3) nodal metastases in patients with squamous cell head and neck cancer. Heal. Technol. Assess. 2017, 21, 1–122. [Google Scholar] [CrossRef]
- Mehanna, H.; Wong, W.-L.; McConkey, C.C.; Rahman, J.K.; Robinson, M.; Hartley, A.G.J.; Nutting, C.; Powell, N.; Al-Booz, H.; Robinson, M.; et al. PET-CT surveillance versus neck dissection in advanced head and neck cancer. N. Engl. J. Med. 2016, 374, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.F.; John, M.A.S.; Lawson, G.; Grogan, T.; Elashoff, D.; Mendelsohn, A.H. Diagnostic value of sentinel lymph node biopsy in head and neck cancer: A meta-analysis. Eur. Arch. Oto-Rhino-Laryngol. 2012, 270, 2115–2122. [Google Scholar] [CrossRef]
- Tomifuji, M.; Shiotani, A.; Fujii, H.; Araki, K.; Saito, K.; Inagaki, K.; Mukai, M.; Kitagawa, Y.; Ogawa, K. Sentinel Node Concept in Clinically N0 Laryngeal and Hypopharyngeal Cancer. Ann. Surg. Oncol. 2008, 15, 2568–2575. [Google Scholar] [CrossRef]
- Werner, J.A.; Dünne, A.-A.; Ramaswamy, A.; Folz, B.J.; Lippert, B.M.; Moll, R.; Behr, T. Sentinel node detection in N0 cancer of the pharynx and larynx. Br. J. Cancer 2002, 87, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.G.; Schoeff, S.S.; Watts, C.A.; Reibel, J.F.; Levine, P.A.; Shonka, D.C.; Jameson, M.J. Utility of abdominal imaging to assess for liver metastasis in patients with head and neck cancer and abnormal liver function tests. Am. J. Otolaryngol. 2014, 35, 137–140. [Google Scholar] [CrossRef]
- Poon, C.; Stenson, K. Overview of The diagnosis and Staging of Head and Neck Cancer. Available online: https://www.uptodate.com/contents/overview-of-the-diagnosis-and-staging-of-head-and-neck-cancer (accessed on 19 March 2021).
- Kaanders, J.H.A.M.; Lenarz, T.; Pop, L.A.M.; Schmoll, H.J.; de Mulder, P.H.M.; Marres, H.A.M. Larynxkarzinom. In Kompendium Internistische Onkologie; Springer: Berlin/Heidelberg, Germany, 2006; pp. 3397–3420. [Google Scholar]
- Werner, J.A. Totale Laryngektomie. In HNO-Operationslehre; Rettinger, G., Hosemann, W.G., Hüttenbrink, K.-B., Werner, J.A., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 2017. [Google Scholar]
- Agrawal, N.; Goldenberg, D. Primary and Salvage Total Laryngectomy. Otolaryngol. Clin. N. Am. 2008, 41, 771–780. [Google Scholar] [CrossRef]
- Vahl, J.; Hoffmann, T. Neck dissection-Surgical treatment of cervical lymphatic drainage pathways. HNO 2019, 67, 61–76. [Google Scholar] [CrossRef]
- Alicandri-Ciufelli, M.; Bonali, M.; Piccinini, A.; Marra, L.; Ghidini, A.; Cunsolo, E.M.; Maiorana, A.; Presutti, L.; Conte, P.F. Surgical margins in head and neck squamous cell carcinoma: What is ‘close’? Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 2603–2609. [Google Scholar] [CrossRef]
- Schlag, P.M.; Hartmann, J.T.; Budach, V. Weichgewebetumoren; Springer International Publishing: Heidelberg, Germany, 2011. [Google Scholar]
- Bonkowsky, V.; Strutz, J. Laryngektomie. In Praxis der HNO-Heilkunde, Kopf- und Halschirurgie; Strutz, J., Mann, W.J., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 2017. [Google Scholar]
- Fagan, J. Total Laryngectomy. Open Access Atlas Otolaryngol. Head Neck Oper. Surg. 2016, 15, 11-1–11-16. [Google Scholar]
- Schuler, P.J.; Boehm, F.; Schild, L.R.; Greve, J.; Hoffmann, T.K. Robotik in der Kopf-Hals-Chirurgie. HNO 2021, 69, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Schuler, P.J.; Hoffmann, T.K.; Veit, J.A.; Rotter, N.; Friedrich, D.T.; Greve, J.; Scheithauer, M.O. Hybrid procedure for total laryngectomy with a flexible robot-assisted surgical system. Int. J. Med. Robot. Comput. Assist. Surg. 2016, 13, e1749. [Google Scholar] [CrossRef]
- Krishnan, G.; Krishnan, S. Transoral Robotic Surgery Total Laryngectomy: Evaluation of Functional and Survival Outcomes in a Retrospective Case Series at a Single Institution. ORL 2017, 79, 191–201. [Google Scholar] [CrossRef]
- Lawson, G.; Mendelsohn, A.H.; Van Der Vorst, S.; Bachy, V.; Remacle, M. Transoral robotic surgery total laryngectomy. Laryngoscope 2012, 123, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.V.; Schiff, B.A.; Sarta, C.; Hans, S.; Brasnu, D. Transoral robotic total laryngectomy. Laryngoscope 2013, 123, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Zhang, S.; Song, X.; Chen, L.; Luo, X. Meta-analysis on the risk factors for stomal recurrence after total laryngectomy. J. Clin. Otorhinolaryngol. Head Neck Surg. 2013, 27, 995–999. [Google Scholar]
- Teutsch, S.; Bas, M.; Bier, H.; Knopf, A. Stomarezidive, eine klinisch-pathologische Betrachtung. Laryngo-Rhino-Otologie 2016, 96, 239–243. [Google Scholar] [CrossRef]
- Manfro, G.; Dias, F.L.; De Farias, T.P. Tracheostomy Complications; Springer International Publishing: Heidelbeg, Germany, 2017; pp. 307–319. [Google Scholar]
- Laccourreye, O.; Hans, S.; Borzog-Grayeli, A.; Maulard-Durdux, C.; Brasnu, D.; Housset, M. Complications of postoperative radiation therapy after partial laryngectomy in supraglottic cancer: A long-term evaluation. Otolaryngol. Head Neck Surg. 2000, 122, 752–757. [Google Scholar] [CrossRef]
- Lee, J.H.; Machtay, M.; Unger, L.D.; Weinstein, G.S.; Weber, R.S.; Chalian, A.A.; Rosenthal, D.I. Prophylactic Gastrostomy Tubes in Patients Undergoing Intensive Irradiation for Cancer of the Head and Neck. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Kim, G.; Cho, B.; Park, H.S.; Rho, Y. Pattern of lymph node metastasis in hypopharyngeal squamous cell carcinoma and indications for level VI lymph node dissection. Head Neck 2016, 38, E1969–E1973. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.G.; Laurie, S.A.; Weinstein, G.S.; Mendenhall, W.M.; Adelstein, D.J.; Ang, K.K.; Clayman, G.L.; Fisher, S.G.; Forastiere, A.A.; Harrison, L.B.; et al. American Society of Clinical Oncology Clinical Practice Guideline for the Use of Larynx-Preservation Strategies in the Treatment of Laryngeal Cancer. J. Clin. Oncol. 2006, 24, 3693–3704. [Google Scholar] [CrossRef] [PubMed]
- Robbins, K.T.; Clayman, G.; Levine, P.A.; Medina, J.; Sessions, R.; Shaha, A.; Som, P.; Wolf, G.T. Neck dissection classification update: Revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology–Head and Neck Surgery. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, A.A.; Zhang, Q.; Weber, R.S.; Maor, M.H.; Goepfert, H.; Pajak, T.F.; Morrison, W.H.; Glisson, B.S.; Trotti, A.; Ridge, J.A.; et al. Long-Term Results of RTOG 91-11: A Comparison of Three Nonsurgical Treatment Strategies to Preserve the Larynx in Patients With Locally Advanced Larynx Cancer. J. Clin. Oncol. 2013, 31, 845–852. [Google Scholar] [CrossRef]
- Langendijk, J.A.; Ferlito, A.; Takes, R.P.; Rodrigo, J.P.; Suárez, C.; Strojan, P.; Haigentz, M.; Rinaldo, A.; Tapia, J.P.R. Postoperative strategies after primary surgery for squamous cell carcinoma of the head and neck. Oral Oncol. 2010, 46, 577–585. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Jung, G.M.; Borel, C.; Bronner, G.; Flesch, H.; Velten, M. Prognostic factors of survival in head and neck cancer patients treated with surgery and postoperative radiation therapy. Acta Oto-Laryngol. 2008, 128, 706–712. [Google Scholar] [CrossRef]
- Bernier, J.; Cooper, J.S. Chemoradiation after Surgery for High-Risk Head and Neck Cancer Patients: How Strong Is the Evidence? Oncologist 2005, 10, 215–224. [Google Scholar] [CrossRef]
- Group* DOVaLCS. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N. Engl. J. Med. 1991, 324, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, P.; Baujat, B.; Holostenco, V.; Bourredjem, A.; Baey, C.; Bourhis, J.; Pignon, J.-P. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): A comprehensive analysis by tumour site. Radiother. Oncol. 2011, 100, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.S.; Berkey, B.A.; Forastiere, A.; Cooper, J.; Maor, M.; Goepfert, H.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; et al. Outcome of salvage total laryngectomy following organ preservation therapy: The Radiation Therapy Oncology Group trial 91-11. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Budach, V.; Cho, C.-H.; Sedlmaier, B.; Wittlinger, M.; Iro, H.; Engenhart-Cabillic, R.; Hautmann, M.; Strutz, J.; Flentje, M.; Hueltenschmidt, B.; et al. Five years’ results of the German ARO 04-01 trial of concurrent 72 Gy hyperfractionated accelerated radiation therapy (HART) plus once weekly cisplatinum/5-FU versus mitomycin C/5-FU in stage IV head and neck cancer. J. Clin. Oncol. 2012, 30, 5512. [Google Scholar] [CrossRef]
- Budach, V.; Stromberger, C.; Poettgen, C.; Baumann, M.; Budach, W.; Grabenbauer, G.; Marnitz, S.; Olze, H.; Wernecke, K.-D.; Ghadjar, P. Hyperfractionated Accelerated Radiation Therapy (HART) of 70.6 Gy With Concurrent 5-FU/Mitomycin C Is Superior to HART of 77.6 Gy Alone in Locally Advanced Head and Neck Cancer: Long-term Results of the ARO 95-06 Randomized Phase III Trial. Int. J. Radiat. Oncol. 2015, 91, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Bauml, J.M.; Vinnakota, R.; Park, Y.A.; Bates, S.E.; Fojo, T.; Aggarwal, C.; Di Stefano, J.; Knepley, C.; Limaye, S.; Mamtani, R.; et al. Cisplatin versus cetuximab with definitive concurrent radiotherapy for head and neck squamous cell carcinoma: An analysis of Veterans Health Affairs data. Cancer 2019, 125, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Xiang, M.; Holsinger, F.C.; Colevas, A.D.; Chen, M.M.; Beadle, B.M.; Beadle, B.M. Survival of patients with head and neck cancer treated with definitive radiotherapy and concurrent cisplatin or concurrent cetuximab: A Surveillance, Epidemiology, and End Results-Medicare analysis. Cancer 2018, 124, 4486–4494. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef]
- Nuyts, S.; Lambrecht, M.; Duprez, F.; Daisne, J.-F.; Van Gestel, D.; Weyngaert, D.V.D.; Platteaux, N.; Geussens, Y.; Voordeckers, M.; Madani, I.; et al. Reduction of the dose to the elective neck in head and neck squamous cell carcinoma, a randomized clinical trial using intensity modulated radiotherapy (IMRT). Dosimetrical analysis and effect on acute toxicity. Radiother. Oncol. 2013, 109, 323–329. [Google Scholar] [CrossRef]
- Garden, A.S.; Harris, J.; Trotti, A.; Jones, C.U.; Carrascosa, L.; Cheng, J.D.; Spencer, S.S.; Forastiere, A.; Weber, R.S.; Ang, K.K. Long-Term Results of Concomitant Boost Radiation Plus Concurrent Cisplatin for Advanced Head and Neck Carcinomas: A Phase II Trial of the Radiation Therapy Oncology Group (RTOG 99-14). Int. J. Radiat. Oncol. 2008, 71, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Machtay, M.; Moughan, J.; Trotti, A.; Garden, A.S.; Weber, R.S.; Cooper, J.S.; Forastiere, A.A.; Ang, K.K. Factors Associated With Severe Late Toxicity After Concurrent Chemoradiation for Locally Advanced Head and Neck Cancer: An RTOG Analysis. J. Clin. Oncol. 2008, 26, 3582–3589. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.; O’Neill, A.; Rabinowits, G.; Tishler, R.; Khuri, F.; Adkins, D.; Clark, J.; Sarlis, N.; Lorch, J.; Beitler, J.J.; et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 257–264. [Google Scholar] [CrossRef]
- Worden, F.P.; Moyer, J.; Lee, J.S.; Taylor, J.M.G.; Urba, S.G.; Eisbruch, A.; Teknos, T.N.; Chepeha, D.B.; Prince, M.E.; Hogikyan, N.; et al. Chemoselection as a strategy for organ preservation in patients with T4 laryngeal squamous cell carcinoma with cartilage invasion. Laryngoscope 2009, 119, 1510–1517. [Google Scholar] [CrossRef]
- Budach, W.; Bölke, E.; Kammers, K.; Gerber, P.A.; Orth, K.; Gripp, S.; Matuschek, C. Induction chemotherapy followed by concurrent radio-chemotherapy versus concurrent radio-chemotherapy alone as treatment of locally advanced squamous cell carcinoma of the head and neck (HNSCC): A meta-analysis of randomized trials. Radiother. Oncol. 2016, 118, 238–243. [Google Scholar] [CrossRef]
- Dietz, A.; Wichmann, G.; Kuhnt, T.; Pfreundner, L.; Hagen, R.; Scheich, M.; Kölbl, O.; Hautmann, M.G.; Strutz, J.; Schreiber, F.; et al. Induction chemotherapy (IC) followed by radiotherapy (RT) versus cetuximab plus IC and RT in advanced laryngeal/hypopharyngeal cancer resectable only by total laryngectomy—final results of the larynx organ preservation trial DeLOS-II. Ann. Oncol. 2018, 29, 2105–2114. [Google Scholar] [CrossRef]
- Lefebvre, J.-L.; Ang, K.K.; Panel, O.B.O.T.L.P.C. Larynx preservation clinical trial design: Key issues and recommendations-A consensus panel summary. Head Neck 2009, 31, 429–441. [Google Scholar] [CrossRef]
- Dziegielewski, P.T.; O’Connell, D.A.; Klein, M.; Fung, C.; Singh, P.; Mlynarek, M.A.; Fung, D.; Harris, J.R.; Seikaly, H. Primary total laryngectomy versus organ preservation for T3/T4a laryngeal cancer: A population-based analysis of survival. J. Otolaryngol. Head Neck Surg. 2012, 41, S56–S64. [Google Scholar]
- Timme, D.W.; Jonnalagadda, S.; Patel, R.; Rao, K.; Robbins, K.T. Treatment selection for T3/T4a laryngeal cancer: Chemoradiation versus primary surgery. Ann. Otol. Rhinol. Laryngol. 2015, 124, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, A.; Chaves, A.L.; Kowalski, L.P.; Wolf, G.T.; Saba, N.F.; Forastiere, A.A.; Beitler, J.J.; Nibu, K.-I.; Bradford, C.R.; Suárez, C.; et al. Organ preservation with chemoradiation in advanced laryngeal cancer: The problem of generalizing results from randomized controlled trials. Auris Nasus Larynx 2017, 44, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Swisher-McClure, S.; Mitra, N.; Li, J.; Cohen, R.B.; Ahn, P.H.; Lukens, J.N.; Chalian, A.A.; Weinstein, G.S.; O’Malley, B.W.; et al. Total Laryngectomy Versus Larynx Preservation for T4a Larynx Cancer: Patterns of Care and Survival Outcomes. Int. J. Radiat. Oncol. 2015, 92, 594–601. [Google Scholar] [CrossRef]
- Eschmann, S.-M.; Paulsen, F.; Reimold, M.; Dittmann, H.; Welz, S.; Reischl, G.; Machulla, H.-J.; Bares, R. Prognostic impact of hypoxia imaging with 18F-misonidazole PET in non-small cell lung cancer and head and neck cancer before radiotherapy. J. Nucl. Med. 2005, 46, 253–260. [Google Scholar]
- Mortensen, L.S.; Johansen, J.; Kallehauge, J.; Primdahl, H.; Busk, M.; Lassen, P.; Alsner, J.; Sørensen, B.S.; Toustrup, K.; Jakobsen, S.; et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: Results from the DAHANCA 24 trial. Radiother. Oncol. 2012, 105, 14–20. [Google Scholar] [CrossRef]
- Hoffmann, T.K. Systemic therapy strategies for head-neck carcinomas: Current status. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2012, 11. [Google Scholar] [CrossRef]
- Silverman, D.A.; Puram, S.V.; Rocco, J.W.; Old, M.O.; Kang, S.Y. Salvage laryngectomy following organ-preservation therapy—An evidence-based review. Oral Oncol. 2019, 88, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Birkeland, A.C.; Rosko, A.J.; Issa, M.R.; Shuman, A.G.; Prince, M.E.; Wolf, G.T.; Bradford, C.R.; McHugh, J.B.; Brenner, J.C.; Spector, M.E. Occult Nodal Disease Prevalence and Distribution in Recurrent Laryngeal Cancer Requiring Salvage Laryngectomy. Otolaryngol. Neck Surg. 2016, 154, 473–479. [Google Scholar] [CrossRef]
- Hussain, T.; Kanaan, O.; Höing, B.; Dominas, N.; Lang, S.; Mattheis, S. Die Rolle der elektiven Neck dissection bei Salvage Laryngektomie—Eine retrospektive Analyse. Laryngo-Rhino-Otologie 2018, 97, 694–701. [Google Scholar] [CrossRef]
- Rosko, A.; Birkeland, A.; Shuman, A.; Prince, M.; Bradford, C.; Wolf, G.; Worden, F.; Eisbruch, A.; Srinivasan, A.; Wong, K.K.; et al. Positron emission tomography-CT prediction of occult nodal metastasis in recurrent laryngeal cancer. Head Neck 2017, 39, 980–987. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Mamgani, A.; Tans, L.; Van Rooij, P.; Levendag, P.C. A Single-Institutional Experience of 15 Years of Treating T3 Laryngeal Cancer With Primary Radiotherapy, With or Without Chemotherapy. Int. J. Radiat. Oncol. 2012, 83, 1000–1006. [Google Scholar] [CrossRef]
- Rothmeier, N.; Hoffmann, T.K.; Lehnerdt, G.; Lang, S.; Mattheis, S. Chirurgisches Management der persistierenden Speichelfistel nach Salvage-Laryngektomie. Laryngo-Rhino-Otologie 2013, 92, 236–243. [Google Scholar] [PubMed]
- Goepfert, R.P.; Hutcheson, K.A.; Lewin, J.S.; Desai, N.G.; Zafereo, M.E.; Hessel, A.C.; Lewis, C.M.; Weber, R.S.; Gross, N.D. Complications, hospital length of stay, and readmission after total laryngectomy. Cancer 2017, 123, 1760–1767. [Google Scholar] [CrossRef] [PubMed]
- Karsten, R.T.; Timmermans, A.J.; Cate, J.T.; Stuiver, M.M.; Brekel, M.W.M.V.D. Direct complications and routine ICU admission after total laryngectomy. Acta Oto-Laryngologica 2018, 138, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Helman, S.N.; Brant, J.A.; Kadakia, S.K.; Newman, J.G.; Cannady, S.B.; Chai, R.L. Factors associated with complications in total laryngectomy without microvascular reconstruction. Head Neck 2018, 40, 2409–2415. [Google Scholar] [CrossRef]
- Hasan, Z.; Dwivedi, R.; Gunaratne, D.; Virk, S.; Palme, C.; Riffat, F. Systematic review and meta-analysis of the complications of salvage total laryngectomy. Eur. J. Surg. Oncol. (EJSO) 2017, 43, 42–51. [Google Scholar] [CrossRef]
- Hall, F.T.; O’Brien, C.J.; Clifford, A.R.; McNeil, E.B.; Bron, L.; Jackson, M.A. Clinical outcome following total laryngectomy for cancer. ANZ J. Surg. 2003, 73, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Homma, A.; Oridate, N.; Suzuki, F.; Hatakeyama, H.; Suzuki, K.; Nishioka, T.; Shirato, H.; Fukuda, S. Surgical complications of salvage total laryngectomy following concurrent chemoradiotherapy. Int. J. Clin. Oncol. 2008, 13, 521–527. [Google Scholar] [CrossRef]
- Upile, T.; Triaridis, S.; Kirkland, P.; Archer, D.; Searle, A.; Irving, C.; Evans, P.R. The management of carotid artery rupture. Eur. Arch. Oto-Rhino-Laryngol. 2005, 262, 555–560. [Google Scholar] [CrossRef]
- Grau, C.; Johansen, L.V.; Hansen, H.S.; Andersen, E.; Godballe, C.; Andersen, L.J.; Hald, J.; Møller, H.; Overgaard, M.; Bastholt, L.; et al. Salvage laryngectomy and pharyngocutaneous fistulae after primary radiotherapy for head and neck cancer: A national survey from DAHANCA. Head Neck 2003, 25, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, M.; Milisavljevic, D.; Zivic, M.; Stojanov, D.; Stankovic, P. Primary and salvage total laryngectomy. Influential factors, complications, and survival. J. BUON 2015, 20, 527–539. [Google Scholar] [PubMed]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery. Surg. Infect. 2013, 14, 73–156. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.N.; Jayawardena, A.D.L.; Walden, R.L.; Penn, E.B.; Francis, D.O. Evidence-Based Use of Perioperative Antibiotics in Otolaryngology. Otolaryngol. Neck Surg. 2018, 158, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Dedivitis, R.A.; Aires, F.T.; Cernea, C.R.; Brandão, L.G. Pharyngocutaneous fistula after total laryngectomy: Systematic review of risk factors. Head Neck 2015, 37, 1691–1697. [Google Scholar] [CrossRef]
- Formeister, E.J.; Alemi, A.S.; El-Sayed, I.; George, J.R.; Ha, P.; Knott, P.D.; Ryan, W.R.; Seth, R.; Tamplen, M.L.; Heaton, C.M. Shorter interval between radiation therapy and salvage laryngopharyngeal surgery increases complication rates following microvascular free tissue transfer. Am. J. Otolaryngol. 2018, 39, 548–552. [Google Scholar] [CrossRef]
- Miles, B.A. Moving Toward Improved Outcomes in Salvage Laryngectomy. Ann. Surg. Oncol. 2017, 25, 1110–1111. [Google Scholar] [CrossRef]
- Achim, V.; Bash, J.; Mowery, A.; Guimaraes, A.R.; Li, R.; Schindler, J.; Wax, M.; Andersen, P.; Clayburgh, D. Prognostic Indication of Sarcopenia for Wound Complication After Total Laryngectomy. JAMA Otolaryngol. Neck Surg. 2017, 143, 1159–1165. [Google Scholar] [CrossRef]
- Filimonov, A.; Brady, J.S.; Govindan, A.; Merchant, A.; Eloy, J.A.; Baredes, S.; Park, R.C.W. Postoperative complications of total laryngectomy in diabetic patients. Laryngoscope 2017, 127, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Gourin, C.G.; Stewart, C.M.; Frick, K.D.; Fakhry, C.; Pitman, K.T.; Eisele, D.W.; Austin, J.M. Association of Hospital Volume With Laryngectomy Outcomes in Patients With Larynx Cancer. JAMA Otolaryngol. Neck Surg. 2019, 145, 62–70. [Google Scholar] [CrossRef]
- Boenninghaus, H.G.; Lenarz, T. Hals-Nasen-Ohren-Heilkunde; Chapter: MdE-Werte (GdB-Werte); Springer: Heidelberg, Germany, 2012. [Google Scholar]
- Eadie, T.L.; Bowker, B.C. Coping and Quality of Life after Total Laryngectomy. Otolaryngol. Neck Surg. 2012, 146, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Slesina, W.; Rennert, D.; Weber, A. Patientenbesuche im Krankenhaus durch Besuchsdienste von Krebs-Selbsthilfegruppen—Zur Prozess- und Ergebnisqualität. Das Gesundh. 2014, 76, 847–855. [Google Scholar] [CrossRef]
- Lorenz, K.J. Stimmrehabilitation nach Laryngektomie. HNO 2014, 93, 44–66. [Google Scholar] [CrossRef]
- Doescher, J.; Scheckenbach, K.; Angerstein, W.; Veit, J.A.; Schuler, P.J.; Laban, S.; Thierauf, J.; Theodoraki, M.-N.; Greve, J.; Hoffmann, T.K. Evaluation of Customized Prosthesis for Irregularly Formed Tracheostoma After Laryngectomy. Ann. Otol. Rhinol. Laryngol. 2015, 125, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, S.; Kleinsasser, N.; Scherzad, A.; Kraus, F.; Hagen, R. Microvascular reconstruction of the larynx following total laryngectomy. Laryngorhinootologie 2018, 97, 100–109. [Google Scholar] [PubMed]
- Rosa, V.M.; Fores, J.M.L.; Da Silva, E.P.F.; Guterres, E.O.; Marcelino, A.; Nogueira, P.C.; Baia, W.R.M.; Kulcsar, M.A.V. Interdisciplinary interventions in the perioperative rehabilitation of total laryngectomy: An integrative review. Clinics 2018, 73. [Google Scholar] [CrossRef]
- Landis, B.N.; Giger, R.; Lacroix, J.-S.; Dulguerov, P. Swimming, snorkeling, breathing, smelling, and motorcycling after total laryngectomy. Am. J. Med. 2003, 114, 341–342. [Google Scholar] [CrossRef]
- Mallis, A.; Goumas, P.D.; Mastronikolis, N.S.; Panogeorgou, T.; Stathas, T.; Prodromaki, K.; Papadas, T.A. Factors influencing quality of life after total laryngectomy: A study of 92 patients. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 937–942. [Google Scholar] [PubMed]
- Minovi, A.; Nowak, C.; Marek, A. Quality of life bei Langzeitüberlebenden nach Laryngektomie. Laryngo-Rhino-Otologie 2009, 88, 18–22. [Google Scholar] [CrossRef]
- Patel, R.S.; Mohr, T.; Hartman, C.; Stach, C.; Sikora, A.G.; Zevallos, J.P.; Sandulache, V.C. Tracheoesophageal Prosthesis Use Is Associated With Improved Overall Quality of Life in Veterans With Laryngeal Cancer. Ann. Otol. Rhinol. Laryngol. 2018, 127, 421–428. [Google Scholar] [CrossRef]
- Hanna, E.; Sherman, A.; Cash, D.; Adams, D.; Vural, E.; Fan, C.-Y.; Suen, J.Y. Quality of Life for Patients Following Total Laryngectomy vs Chemoradiation for Laryngeal Preservation. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 875–879. [Google Scholar] [CrossRef]
- Trivedi, N.P.; Swaminathan, D.K.; Thankappan, K.; Chatni, S.; Kuriakose, M.A.; Iyer, S. Comparison of Quality of Life in Advanced Laryngeal Cancer Patients after Concurrent Chemoradiotherapy vs Total Laryngectomy. Otolaryngol. Neck Surg. 2008, 139, 702–707. [Google Scholar] [CrossRef] [PubMed]
- LoTempio, M.M.; Wang, K.H.; Sadeghi, A.; DeLacure, M.D.; Juillard, G.F.; Wang, M.B. Comparison of quality of life outcomes in laryngeal cancer patients following chemoradiation vs. total laryngectomy. Otolaryngol. Neck Surg. 2005, 132, 948–953. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, T.K. Total Laryngectomy—Still Cutting-Edge? Cancers 2021, 13, 1405. https://doi.org/10.3390/cancers13061405
Hoffmann TK. Total Laryngectomy—Still Cutting-Edge? Cancers. 2021; 13(6):1405. https://doi.org/10.3390/cancers13061405
Chicago/Turabian StyleHoffmann, Thomas K. 2021. "Total Laryngectomy—Still Cutting-Edge?" Cancers 13, no. 6: 1405. https://doi.org/10.3390/cancers13061405
APA StyleHoffmann, T. K. (2021). Total Laryngectomy—Still Cutting-Edge? Cancers, 13(6), 1405. https://doi.org/10.3390/cancers13061405




