Ultra-High Dose Rate Transmission Beam Proton Therapy for Conventionally Fractionated Head and Neck Cancer: Treatment Planning and Dose Rate Distributions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment Planning and Quality Comparison
2.2. UHDR Analyses
2.2.1. Dose-Rate
2.2.2. Beam-on Irradiation Time
2.3. Plan Splitting
3. Results
3.1. Quality Comparison
3.2. Dose-Rate
3.3. Total Irradiation Time
4. Discussion
4.1. Plan Quality
4.2. Dose-Rate
4.3. Total Irradiation Time
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mou, B.; Beltran, C.J.; Park, S.S.; Olivier, K.R.; Furutani, K.M. Feasibility of proton transmission-beam stereotactic ablative radiotherapy versus photon stereotactic ablative radiotherapy for lung tumors: A dosimetric and feasibility study. PLoS ONE 2014, 9, e98621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Water, S.; Safai, S.; Schippers, J.M.; Weber, D.C.; Lomax, A.J. Towards FLASH proton therapy: The impact of treatment planning and machine characteristics on achievable dose rates. Acta. Oncol. 2019. [Google Scholar] [CrossRef]
- van Marlen, P.; Dahele, M.; Folkerts, M.; Abel, E.; Slotman, B.J.; Verbakel, W.F.A.R. Bringing FLASH to the clinic: Treatment planning considerations for ultrahigh dose-rate proton beams. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Hornsey, S.; Alper, T. Unexpected dose-rate effect in the killing of mice by radiation. Nature 1966, 210, 212–213. [Google Scholar] [CrossRef]
- Berry, R.J.; Hall, E.J.; Forster, D.W.; Storr, T.H.; Goodman, M.J. Survival of mammalian cells exposed to x rays at ultra-high dose-rates. Br. J. Radiol. 1969, 42, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Field, S.B.; Bewley, D.K. Effects of dose-rate on the radiation response of rat skin. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1974, 26, 259–267. [Google Scholar] [CrossRef]
- Favaudon, V.; Caplier, L.; Monceau, V. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245. [Google Scholar] [CrossRef]
- Girdhani, S.; Abel, E.; Katsis, A.; Rodriquez, A.; Senapati, S.; KuVillanueva, A.; Jackson, I.L.; Eley, J.; Vujaskovic, Z.; Parry, R. Flash: A Novel Paradigm Changing Tumor Irradiation Platform that Enhances Therapeutic Ratio by Reducing Normal Tissue Toxicity And Activating Immune Pathways. In Proceedings of the AACR Annual Meeting 2019, Atlanta, GA, USA, 29 March–3 April 2019; pp. 1538–7445. [Google Scholar]
- Montay-Gruel, P.; Petersson, K.; Jaccard, M.; Boivin, G.; Germond, J.-F.; Petit, B.; Doenlen, R.; Favaudon, V.; Bochud, F.; Bailat, C.; et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose-rates above 100 Gy/s. Radiother. Oncol. 2017, 124, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Bouchet, A.; Jaccard, M.; Patin, D.; Serduc, R.; Aim, W.; Petersson, K.; Petit, B.; Bailat, C.; Bourhis, J.; et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother. Oncol. 2018, 129, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Acharya, M.M.; Petersson, K.; Alikhani, L.; Yakkala, C.; Allen, B.D.; Ollivier, J.; Petit, B.; Jorge, P.G.; Syage, A.R.; et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10943–10951. [Google Scholar] [CrossRef] [Green Version]
- Simmons, D.A.; Lartey, F.M.; Schüler, E.; Rafat, M.; King, G.; Kim, A.; Ko, R.; Semaan, S.; Gonzalez, S.; Jenkins, M.; et al. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother. Oncol. 2019, 139, 4–10. [Google Scholar] [CrossRef]
- Loo, B.W.; Schuler, E.; Lartey, F.M.; Rafat, M.; King, G.J.; Trovati, S.; Koong, A.C.; Maxim, P.G. (P003) Delivery of ultra-rapid FLASH radiation therapy and demonstration of normal tissue sparing after abdominal irradiation of mice. Radiat. Oncol. 2017, 98, E16. [Google Scholar] [CrossRef] [Green Version]
- Diffenderfer, E.S.; Verginadis, I.I.; Kim, M.M.; Shoniyozov, K.; Velalopoulou, A.; Goia, D.; Putt, M.; Hagan, S.; Avery, S.; Teo, K.; et al. Design, implementation, and in vivo validation of a novel proton FLASH radiation therapy system. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 440–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vozenin, M.-C.; De Fornel, P.; Petersson, K.; Favaudon, V.; Jaccard, M.; Germond, J.-F.; Petit, B.; Burki, M.; Ferrand, G.; Patin, D.; et al. The advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients. Clin. Cancer Res. 2019, 25, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourhis, J.; Sozzi, W.J.; Jorge, P.G.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Ozsahin, M.; Bochud, F.; Germond, J.-F.; et al. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol 2019, 139, 18–22. [Google Scholar] [CrossRef]
- Schüler, E.; Trovati, S.; King, G.; Lartey, F.; Rafat, M.; Villegas, M.; Praxel, A.J.; Loo, B.W., Jr.; Maxim, P.G. Experimental platform for ultra-high dose rate FLASH irradiation of small animals using a clinical linear accelarator. Int. J. Radiat. Oncol. Biol. Phys. 2017, 1, 195–203. [Google Scholar] [CrossRef]
- Patriarca, A.; Fouillade, C.; Auger, M.; Martin, F.; Pouzoulet, F.; Nauraye, C.; Heinrich, S.; Favaudon, V.; Meyroneinc, S.; Dendale, R.; et al. Experimental set-up for FLASH proton irradiation of small animals using a clinical system. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 619–626. [Google Scholar] [CrossRef]
- Perez, J.; Magliari, A.; Folkerts, M.; Kopone, T.; Vanderstraeten, R.; Abel, E.; Smith, C. FLASH Radiotherapy: A Look at Ultra-High Dose Rate Research and Treatment Plans. In Proceedings of the 2019 AAMD National Meeting, Garden Grove, CA, USA, 10–15 June 2019; 2019. [Google Scholar]
- Darafsheh, A.; Hao, Y.; Zwart, T.; Wagner, M.; Catanzano, D.; Williamson, J.F.; Knutson, N.; Sun, B.; Mutic, S.; Zhao, T. Feasibility of proton FLASH irradiation using a synchrocyclotron for preclinical studies. Med. Phys. 2020, 47, 4348–4355. [Google Scholar] [CrossRef]
- Zhang, Q.; Cascio, E.; Li, C.; Yang, Q.; Gerweck, L.E.; Huang, P.; Gottschalk, B.; Flanz, J.; Schuemann, J. FLASH investigations using protons: Design of delivery system, preclinical setup and confirmation of FLASH effect with protons in animal systems. Radiat. Res. 2020, 194, 656–664. [Google Scholar] [CrossRef]
- Durante, M.; Bräuer-Krisch, E.; Hill, M. Faster and safer? FLASH ultra-high dose rate in radiotherapy. Br. J. Radiol. 2018, 91, 20170628. [Google Scholar] [CrossRef]
- Petersson, K.; Adrian, G.; Butterworth, K.; McMahon, S.J. A quantitative analysis of the role of oxygen tension in FLASH radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2020. [Google Scholar] [CrossRef] [PubMed]
- Spitz, D.R.; Buettner, G.R.; Petronek, M.S.; St-Aubin, J.J.; Flynn, R.T.; Waldron, T.J.; Limoli, C.L. An integrated physico-chemical approach for explaining the differential impact of FLASH versus conventional dose rate irradiation on cancer and normal tissue respones. Radiother. Oncol. 2019. [CrossRef]
- Pratx, G.; Kapp, D.S. Ultra-high dose rate FLASH irradiation may spare hypoxic stem cell niches in normal tissues. Int. J. Radiat. Oncol. Biol. Phys. 2019. [Google Scholar] [CrossRef] [PubMed]
- Adrian, G.; Konradsson, E.; Lempart, M.; Bäck, S.; Ceberg, C.; Petersson, K. The FLASH effect depends on oxygen concentration. Br. J. Radiol. 2019, 92, 20190702. [Google Scholar] [CrossRef] [PubMed]
- Vozenin, M.C.; Hendry JHLimoli, C.L. Biological benefits of ultra-high dose rate FLASH radiotherapy: Sleeping beauty awoken. Clin. Oncol. 2019, 31, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.; Montay-Gruel, P.; Gonçalves Jorge, P.; Bailat, C.; Petit, B.; Ollivier, J.; Jeanneret-Sozzi, W.; Ozsahin, M.; Bochud, F.; Moeckli, R.; et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother. Oncol. 2019, 139, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Buchsbaum, J.C.; Coleman, C.N.; Capala, J.; Obcemea, C. FLASH Radiotherapy: New Technology Plus Biology Required. Int. J. Radiat. Oncol. Biol. Phys. 2021. [Google Scholar] [CrossRef]
- Tol, J.P.; Delaney, A.R.; Dahele, M.; Slotman, B.J.; Verbakel, W.F.A.R. Evaluation of a knowledge-based planning solution for head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 612–620. [Google Scholar] [CrossRef]
- Delaney, A.R.; Verbakel, W.F.A.R.; Lindberg, J.; Koponen, T.K.; Slotman, B.J.; Dahele, M. Evaluation of an automated proton planning solution. Cureus 2018, 10, e3696. [Google Scholar] [CrossRef] [Green Version]
- Barten, D.L.J.; Tol, J.P.; Dahele, M.; Slotman, B.J.; Verbakel, W.F.A.R. Comparison of organ-at-risk sparing and plan robustness for spot-scanning proton therapy and volumetric modulated arc photon therapy in head-and-neck cancer. Med. Phys. 2015, 42, 6589–6598. [Google Scholar] [CrossRef]
- Delaney, A.R.; Dong, L.; Mascia, A.; Zou, W.; Zhang, Y.; Yin, L.; Rosas, S.; Hrbacek, J.; Lomax, A.J.; Slotman, B.J.; et al. Automated knowledge-based intensity-modulated proton planning: An international multicenter benchmarking study. Cancers (Basel) 2018, 10, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-high dose rate (FLASH) radiotherapy: Silver bullet or fool’s gold? Front. Oncol. 2020, 9, 1563. [Google Scholar] [CrossRef] [Green Version]
- Pedroni, E. Proton beam delivery technique and commissioning issues: Scanned protons. In Proceedings of the Educational meeting PTCOG, Jacksonville, FL, USA, 19 May 2008. [Google Scholar]
- Peeler, C.R.; Mirkovic, D.; Titt, U.; Blanchard, P.; Gunther, J.R.; Mahajan, A.; Mohan, R.; Grosshans, D.R. Clinical evidence of variable proton biological effectiveness in pediatric patients treated for ependymoma. Radiother. Oncol. 2016, 121, 395–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fjæra, L.F.; Li, Z.; Ytre-Hauge, K.S.; Muren, L.P.; Indelicato, D.J.; Lassen-Ramshad, Y.; Engeseth, G.M.; Brydøy, M.; Mairani, A.; Flampouri, S.; et al. Linear energy transfer distributions in the brainstem depending on tumour location in intensity-modulated proton therapy of paediatric cancer. Acta Oncol. 2017, 56, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.Y.; Gu, A.; Wang, W.; Oleinick, N.L.; Machtay, M.; Kong, F.M.S. Ultra-high dose rate effect on circulating immune cells: A potential mechanism for FLASH effect? Radiother. Oncol. 2020, 149, 55–62. [Google Scholar] [CrossRef]
- Bula, C.; Belosi, M.F.; Eichin, M.; Hrbacek, J.; Meer, D. Dynamic beam current control for improved dose accuracy in PBS proton therapy. Phys. Med. Biol. 2019, 64, 175003. [Google Scholar] [CrossRef]
- Gao, H.; Lin, B.; Lin, Y.; Fu, S.; Langen, K.; Liu, T.; Bradley, J. Simultaneous dose and dose rate optimization (SDDRO) for FLASH proton therapy. Med. Phys. 2020. [Google Scholar] [CrossRef]
- Frank, S.J.; Cox, J.D.; Gillin, M.; Mohan, R.; Garden, A.S.; Rosenthal, D.I.; Gunn, G.B.; Weber, R.S.; Kies, M.S.; Lewin, J.S.; et al. Multi-field optimization intensity-modulated proton therapy for head and neck tumors—A translation to practice. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 846–853. [Google Scholar] [CrossRef] [Green Version]
- Mohan, R.; Grosshans, D. Proton therapy—Present and future. Adv. Drug Deliv. Rev. 2017, 109, 26–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxima, P.G.; Tantawib, S.G.; Loo, B.W. PHASER: A platform for clinical translation of FLASH cancer radiotherapy. Radiother. Oncol. 2019, 139, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.R.; Parsons, J.L. FLASH Radiotherapy: Current Knowledge and Future Insights Using Proton-Beam Therapy. Int. J. Mol. Sci. 2020, 21, 6492. [Google Scholar] [CrossRef] [PubMed]
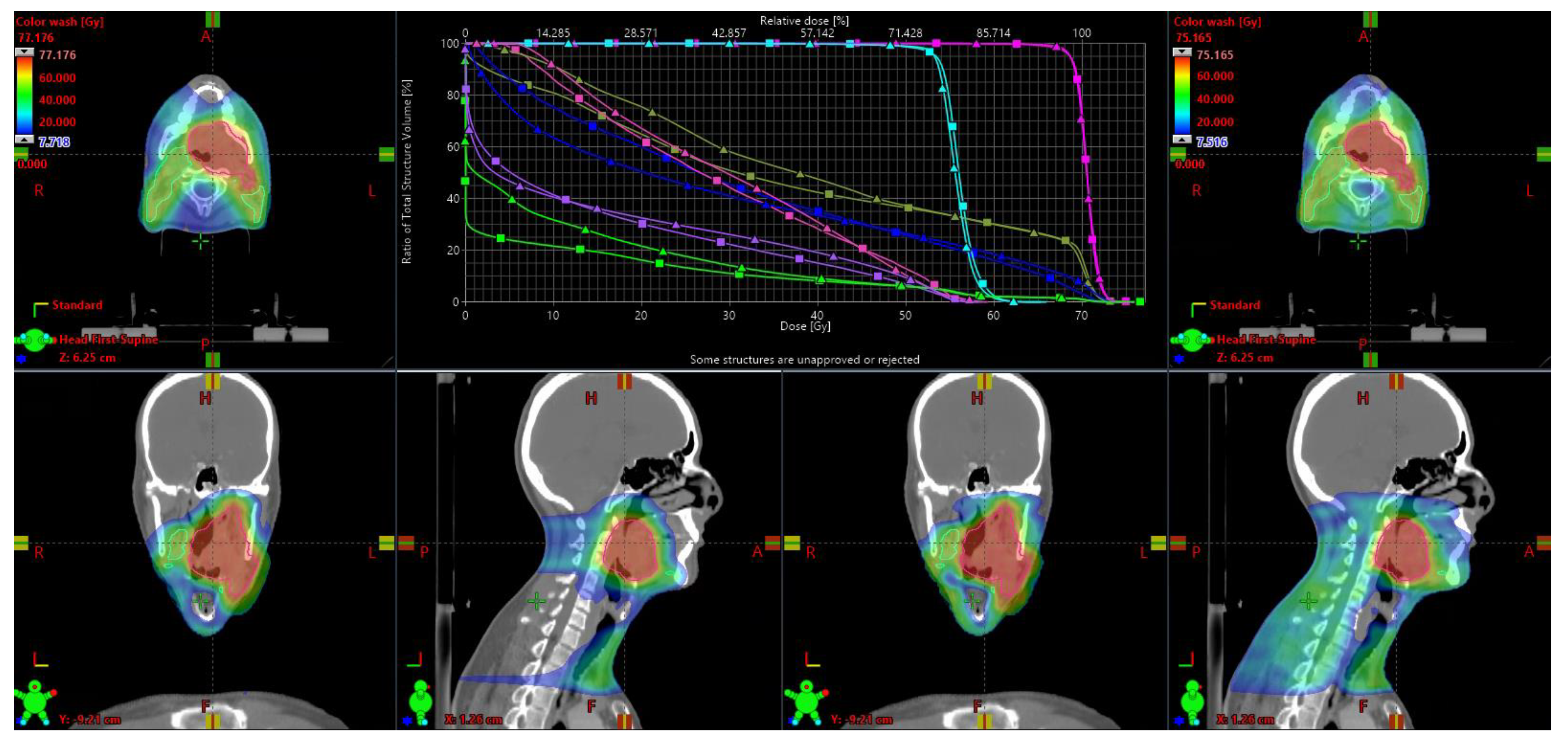


| Structure | Metric | Pavg VMAT | Pavg IMPT | Pavg TB | ∆Pavg (TB-IMPT) | ∆Pavg (TB-VMAT) |
|---|---|---|---|---|---|---|
| PTVB | Dmean V95% | 102.4 99.3 | 101.2 98.7 | 100.8 99.2 | −0.3 † 0.5 | −1.5 −0.1 |
| V107% | 2.2 | 0.7 | 0.0 | −0.7 | −2.2 | |
| PTVE | Dmean | 104.4 | 103.9 | 102.7 | −1.2 † | −1.7 † |
| V95% | 98.8 | 97.4 | 98.1 | 0.6 † | −0.9 † | |
| Davg VMAT | Davg IMPT | Davg TB | ∆Davg (TB-IMPT) | ∆Davg (TB-VMAT) | ||
| Spinal cord | Dmax | 42.7 | 36 | 40.6 | 4.6 | −2.0 † |
| Brainstem * | Dmax | 38.4 | 23.8 | 35.3 | 11.5 | −3.1 |
| Oral cavity | Dmean | 38.6 | 25.2 | 29.3 | 4.1 † | −9.3 † |
| Cricopharynx | Dmean | 23.2 | 13.7 | 12.5 | −1.3 † | −10.8 † |
| Esophagus | Dmean | 16.7 | 11.5 | 10.2 | −1.3 † | −6.5 † |
| SMG (c) ** | Dmean | 35.1 | 25.2 | 26.6 | 1.4 † | −8.5 † |
| SMG (i) | Dmean | 62.3 | 59.7 | 60.2 | 0.5 | −2.1 |
| Lower larynx | Dmean | 20.2 | 10.2 | 9.4 | −0.8 † | −10.8 † |
| Upper larynx | Dmean | 37.8 | 29 | 27.8 | −1.2 | −10.1 † |
| Parotid (c) | Dmean | 21.0 | 12.8 | 13.4 | 0.5 | −7.6 † |
| Parotid (i) | Dmean | 25.3 | 18.4 | 18.4 | 0.1 | −6.9 † |
| Pharynx Inf | Dmean | 27.6 | 16.6 | 15.4 | −1.1 † | −12.1 † |
| Pharynx Med | Dmean | 50.4 | 39.8 | 39.3 | −0.5 | −11.1 † |
| Pharynx Sup | Dmean | 57.4 | 52.3 | 52.4 | 0.1 | −5.0 † |
| UES | Dmean | 15.5 | 9.3 | 7.4 | −1.9 † | −8.1 † |
| Body | Dmean | 11.4 | 6.9 | 9.4 | 2.5 † | −2.0 † |
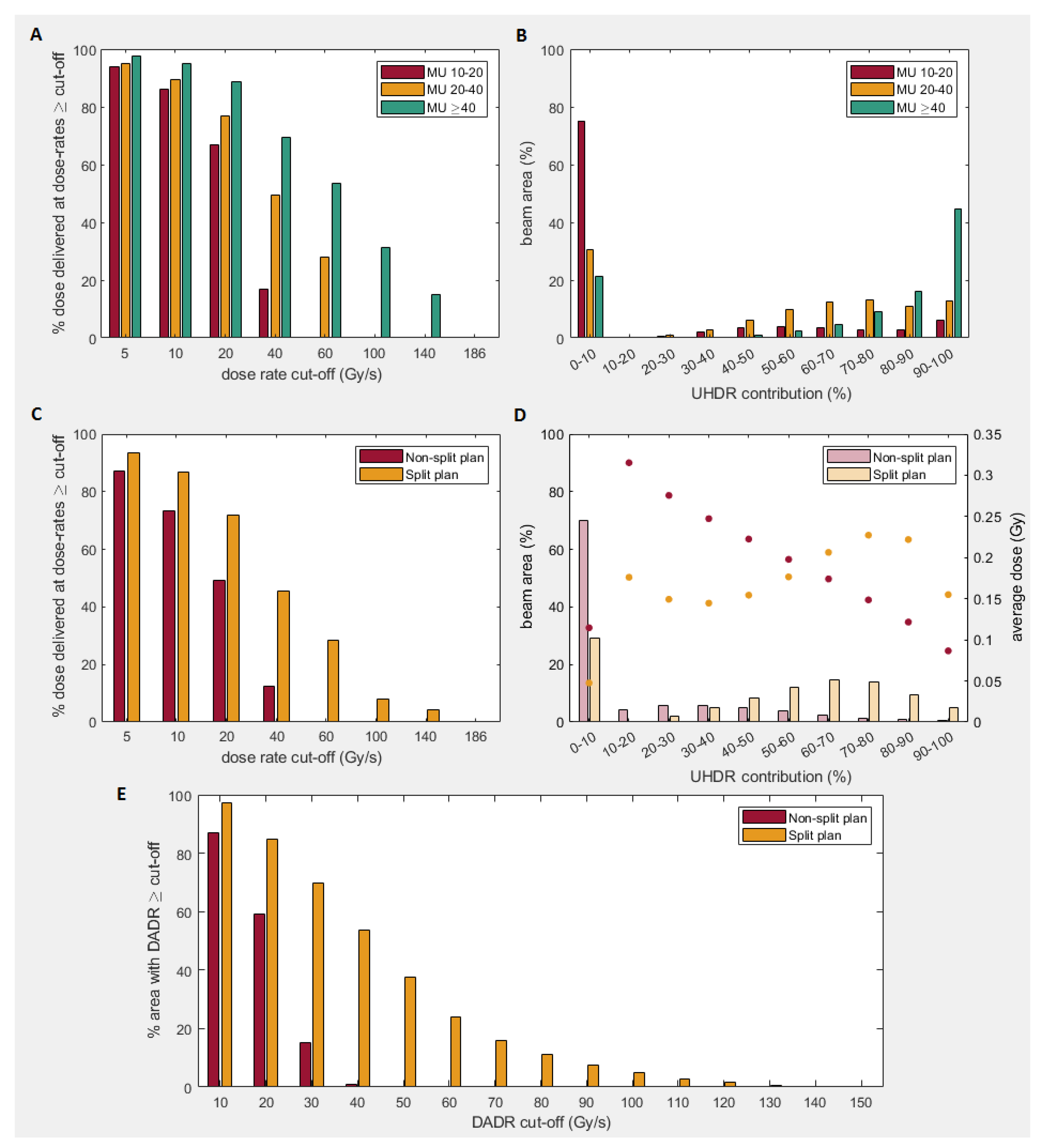
| Threshold (cGy) | DADR (Gy/s) Mean(std) | % Dose Delivered at UHDR Mean(std) | UHDR-Contribution ≥80%/≥90% (%) Mean(std) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NS | S | MU 10–20 | MU 20–40 | MU ≥40 | NS | S | MU 10–20 | MU 20–40 | MU ≥40 | NS | S | |
| 0.5 | 18.3 (0.6) | 39.4 (4.2) | 10 (0.4) | 38 (2.1) | 50 (2.1) | 10 (0.8) | 38 (4.2) | 5 (1.0)/ 3(1.1) | 18 (2.6)/ 10 (2.8) | 44 (1.6)/ 32 (4.9) | 1 (0.6)/ 1 (0.4) | 12 (2.6)/ 4 (1.6) |
| 1 | 19.6 (0.4) | 42.3 (3.9) | 12 (0.4) | 42 (1.4) | 58 (1.3) | 11 (0.6) | 41 (3.7) | 6 (1.5)/ 4(1.5) | 20 (3.4)/ 11 (3.4) | 51 (2.9)/ 37 (6.7) | 1 (0.6)/ 1 (0.4) | 13 (2.9)/ 5 (1.9) |
| 2 | 21.3 (0.4) | 46.2 (3.6) | 17 (1.2) | 49 (1.1) | 69 (2.5) | 12 (0.4) | 46 (3.1) | 9 (2.6)/ 6(2.5) | 24 (4.9)/ 13 (4.5) | 61 (5.6)/ 45 (9.8) | 1 (0.8)/ 1 (0.5) | 15 (3.5)/ 5 (2.2) |
| 3 | 22.4 (0.5) | 49.1 (3.5) | 23 (2.8) | 55 (2.3) | 79 (4.5) | 14 (0.5) | 50 (2.7) | 11 (4.1)/ 7 (3.7) | 27 (6.2)/ 14 (5.4) | 69 (8.1)/ 51 (12.5) | 2 (0.8)/ 1 (0.5) | 16 (4.0)/ 6 (2.5) |
| 4 | 23.2 (0.6) | 51.4 (3.6) | 28 (4.6) | 60 (3.0) | 83 (4.7) | 15 (0.6) | 53 (2.7) | 11 (5.2)/ 7 (4.5) | 28 (6.6)/ 14 (5.5) | 72 (8.6)/ 52 (13.2) | 2 (0.9)/ 1 (0.5) | 17 (4.3)/ 6 (2.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Marlen, P.; Dahele, M.; Folkerts, M.; Abel, E.; Slotman, B.J.; Verbakel, W. Ultra-High Dose Rate Transmission Beam Proton Therapy for Conventionally Fractionated Head and Neck Cancer: Treatment Planning and Dose Rate Distributions. Cancers 2021, 13, 1859. https://doi.org/10.3390/cancers13081859
van Marlen P, Dahele M, Folkerts M, Abel E, Slotman BJ, Verbakel W. Ultra-High Dose Rate Transmission Beam Proton Therapy for Conventionally Fractionated Head and Neck Cancer: Treatment Planning and Dose Rate Distributions. Cancers. 2021; 13(8):1859. https://doi.org/10.3390/cancers13081859
Chicago/Turabian Stylevan Marlen, Patricia, Max Dahele, Michael Folkerts, Eric Abel, Ben J. Slotman, and Wilko Verbakel. 2021. "Ultra-High Dose Rate Transmission Beam Proton Therapy for Conventionally Fractionated Head and Neck Cancer: Treatment Planning and Dose Rate Distributions" Cancers 13, no. 8: 1859. https://doi.org/10.3390/cancers13081859
APA Stylevan Marlen, P., Dahele, M., Folkerts, M., Abel, E., Slotman, B. J., & Verbakel, W. (2021). Ultra-High Dose Rate Transmission Beam Proton Therapy for Conventionally Fractionated Head and Neck Cancer: Treatment Planning and Dose Rate Distributions. Cancers, 13(8), 1859. https://doi.org/10.3390/cancers13081859






