Feasibility of Proton Beam Therapy as a Rescue Therapy in Heavily Pre-Treated Retinoblastoma Eyes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
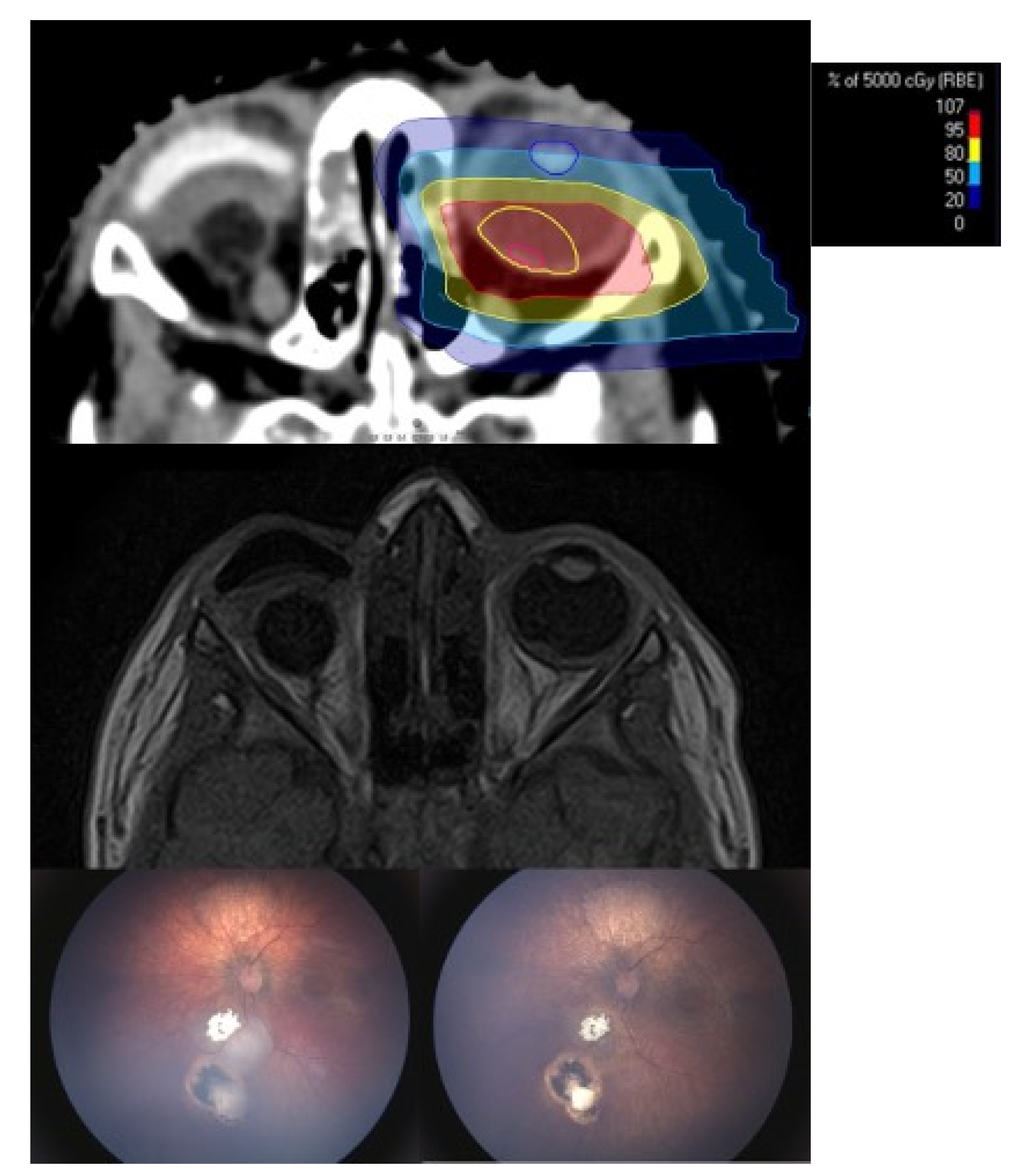
Treatment Technique
3. Results
3.1. Rate of Eye Preservation and Tumor Control
3.2. Radiation Induced Side Effects
3.3. Visual Outcome
3.4. Metastatic Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hilgartner, H.L. Report of case of double glioma treated with x-ray. 1903. Tex. Med. 2005, 101, 10. [Google Scholar]
- Munier, F.L.; Verwey, J.; Pica, A.; Balmer, A.; Zografos, L.; Abouzeid, H.; Timmerman, B.; Goitein, G.; Moeckli, R. New developments in external beam radiotherapy for retinoblastoma: From lens to normal tissue-sparing techniques. Clin. Exp. Ophthalmol. 2008, 36, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Kleinerman, R.A.; Tucker, M.A.; Tarone, R.E.; Abramson, D.H.; Seddon, J.M.; Stovall, M.; Li, F.P.; Fraumeni, J.F., Jr. Risk of New Cancers After Radiotherapy in Long-Term Survivors of Retinoblastoma: An Extended Follow-Up. J. Clin. Oncol. 2005, 23, 2272–2279. [Google Scholar] [CrossRef]
- Marees, T.; Moll, A.C.; Imhof, S.M.; De Boer, M.R.; Ringens, P.J.; Van Leeuwen, F.E. Risk of Second Malignancies in Survivors of Retinoblastoma: More Than 40 Years of Follow-up. J. Natl. Cancer Inst. 2008, 100, 1771–1779. [Google Scholar] [CrossRef]
- Yu, C.-L.; Tucker, M.A.; Abramson, D.H.; Furukawa, K.; Seddon, J.M.; Stovall, M.; Fraumeni, J.F.; Kleinerman, R.A. Cause-Specific Mortality in Long-Term Survivors of Retinoblastoma. J. Natl. Cancer Inst. 2009, 101, 581–591. [Google Scholar] [CrossRef]
- Temming, P.; Arendt, M.; Viehmann, A.; Eisele, L.; Le Guin, C.H.D.; Schündeln, M.M.; Biewald, E.; Astrahantseff, K.; Wieland, R.; Bornfeld, N.; et al. Incidence of second cancers after radiotherapy and systemic chemotherapy in heritable retinoblastoma survivors: A report from the German reference center. Pediatr. Blood Cancer 2017, 64, 71–80. [Google Scholar] [CrossRef]
- Temming, P.; Arendt, M.; Viehmann, A.; Eisele, L.; Le Guin, C.H.; Schündeln, M.M.; Biewald, E.; Mäusert, J.; Wieland, R.; Bornfeld, N.; et al. How Eye-Preserving Therapy Affects Long-Term Overall Survival in Heritable Retinoblastoma Survivors. J. Clin. Oncol. 2016, 34, 3183–3188. [Google Scholar] [CrossRef] [PubMed]
- Dimaras, H.; Corson, T.W.; Cobrinik, D.; White, A.; Zhao, J.; Munier, F.L.; Abramson, D.H.; Shields, C.L.; Chantada, G.L.; Njuguna, F.; et al. Retinoblastoma. Nat. Rev. Dis. Prim. 2015, 1, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Munier, F.L.; Beck-Popovic, M.; Chantada, G.L.; Cobrinik, D.; Kivelä, T.T.; Lohmann, D.; Maeder, P.; Moll, A.C.; Carcaboso, A.M.; Moulin, A.; et al. Conservative management of retinoblastoma: Challenging orthodoxy without compromising the state of metastatic grace. “Alive, with good vision and no comorbidity”. Prog. Retin. Eye Res. 2019, 73, 100764. [Google Scholar] [CrossRef]
- Mendoza, P.R.; Grossniklaus, H.E. Therapeutic Options for Retinoblastoma. Cancer Control. 2016, 23, 99–109. [Google Scholar] [CrossRef]
- Shields, C.L.; Honavar, S.G.; Meadows, A.T.; Shields, J.A.; Demirci, H.; Singh, A.D.; Friedman, D.L.; Naduvilath, T.J. Chemoreduction plus focal therapy for retinoblastoma: Factors predictive of need for treatment with external beam radiotherapy or enucleation. Am. J. Ophthalmol. 2002, 133, 657–664. [Google Scholar] [CrossRef]
- Abramson, D.H.; Shields, C.L.; Munier, F.L.; Chantada, G.L. Treatment of Retinoblastoma in 2015. JAMA Ophthalmol. 2015, 133, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.P.Y.; Hungerford, J.L.; Kingston, J.E.; Plowman, P.N. Salvage external beam radiotherapy after failed primary chemotherapy for bilateral retinoblastoma: Rate of eye and vision preservation. Br. J. Ophthalmol. 2009, 93, 891–894. [Google Scholar] [CrossRef]
- Timmermann, B. Proton Beam Therapy for Childhood Malignancies: Status Report. Klinische Pädiatrie 2010, 222, 127–133. [Google Scholar] [CrossRef]
- Jairam, V.; Roberts, K.B.; Yu, J.B. Historical Trends in the Use of Radiation Therapy for Pediatric Cancers: 1973-2008. Int. J. Radiat. Oncol. 2013, 85, e151–e155. [Google Scholar] [CrossRef]
- Jung, E.H.; Kim, J.H.; Kim, J.Y.; Jo, D.H.; Yu, Y.S. Outcomes of Proton Beam Radiation Therapy for Retinoblastoma with Vitreous Seeds. J. Pediatr. Hematol. 2018, 40, 569–573. [Google Scholar] [CrossRef]
- Thomas, H.; Timmermann, B. Paediatric proton therapy. Br. J. Radiol. 2020, 93, 20190601. [Google Scholar] [CrossRef]
- Mouw, K.W.; Yeap, S.B.Y.; Caruso, P.; Fay, A.; Misra, M.M.; Sethi, R.V.; Macdonald, S.M.; Chen, Y.-L.; Tarbell, N.J.; Yock, M.T.I.; et al. Analysis of patient outcomes following proton radiation therapy for retinoblastoma. Adv. Radiat. Oncol. 2017, 2, 44–52. [Google Scholar] [CrossRef]
- Bs, R.V.S.; Shih, H.A.; Yeap, B.Y.; Mouw, K.W.; Petersen, R.; Kim, D.Y.; Munzenrider, J.E.; Grabowski, E.; Rodriguez-Galindo, C.; Yock, T.I.; et al. Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer 2014, 120, 126–133. [Google Scholar] [CrossRef]
- Mouw, K.W.; Sethi, R.V.; Yeap, B.Y.; MacDonald, S.M.; Chen, Y.-L.E.; Tarbell, N.J.; Yock, T.I.; Munzenrider, J.E.; Adams, J.; Grabowski, E.; et al. Proton Radiation Therapy for the Treatment of Retinoblastoma. Int. J. Radiat. Oncol. 2014, 90, 863–869. [Google Scholar] [CrossRef]
- Chang, J.W.; Yu, Y.S.; Kim, J.Y.; Shin, N.H.; Choi, J.; Kim, J.H.; Kim, S.-J. The Clinical Outcomes of Proton Beam Radiation Therapy for Retinoblastomas That Were Resistant to Chemotherapy and Focal Treatment. Korean J. Ophthalmol. 2011, 25, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Krengli, M.; Hug, E.B.; Adams, J.A.; Smith, A.R.; Tarbell, N.J.; Munzenrider, J.E. Proton radiation therapy for retinoblastoma: Comparison of various intraocular tumor locations and beam arrangements. Int. J. Radiat. Oncol. 2005, 61, 583–593. [Google Scholar] [CrossRef]
- Bäumer, C.; Geismar, D.; Koska, B.; Kramer, P.; Lambert, J.; Lemke, M.; Plaude, S.; Pschichholz, L.; Qamhiyeh, S.; Schiemann, A.; et al. Comprehensive clinical commissioning and validation of the RayStation treatment planning system for proton therapy with active scanning and passive treatment techniques. Phys. Medica 2017, 43, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Mashayekhi, A.; Au, A.K.; Czyz, C.; Leahey, A.; Meadows, A.T.; Shields, J.A. The International Classification of Retinoblastoma Predicts Chemoreduction Success. Ophthalmology 2006, 113, 2276–2280. [Google Scholar] [CrossRef] [PubMed]
- Schipper, J.; Imhoff, S.M.; Tan, K.E.W.P. Precision Megavoltage External Beam Radiation Therapy for Retinoblastoma. In IMRT, IGRT, SBRT—Advances in the Treatment Planning and Delivery of Radiotherapy; Karger Publishers: Berlin, Germany, 2015; Volume 30, pp. 65–80. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Park, Y. Treatment of Retinoblastoma: The Role of External Beam Radiotherapy. Yonsei Med. J. 2015, 56, 1478–1491. [Google Scholar] [CrossRef]
- Baker, M.S.; McConnell, L.K.; Kleinberg, T.T.; Shriver, E.M.; Bilyk, J.R.; Allen, R.C. Orbital sarcomas in retinoblastoma patients. Curr. Opin. Ophthalmol. 2016, 27, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Choi, S.Y.; Kang, H.J.; Lee, J.W.; Kim, H.; Kim, J.H.; Sung, K.W.; Shin, H.Y.; Ahn, H.S.; Park, K.D. Treatment outcome of osteosarcoma after bilateral retinoblastoma: A retrospective study of eight cases. Br. J. Ophthalmol. 2014, 98, 1355–1359. [Google Scholar] [CrossRef]
- Mourits, D.L.; Hartong, D.T.; Lissenberg-Witte, B.I.; Bosscha, M.I.; Tan, H.S.; Moll, A.C. Cosmetic results of enucleation and/or external beam radiation therapy in 195 retinoblastoma survivors. Acta Ophthalmol. 2018, 96, 631–640. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, B.J.; Kim, J.H.; Yu, Y.S. Outcomes of Cataract Surgery Following Treatment for Retinoblastoma. Korean J. Ophthalmol. 2017, 31, 52–57. [Google Scholar] [CrossRef][Green Version]
- Miller, D.M.; Murray, T.G.; Cicciarelli, N.L.; Capo, H.; Markoe, A.M. Pars Plana Lensectomy and Intraocular Lens Implantation in Pediatric Radiation-Induced Cataracts in Retinoblastoma. Ophthalmology 2005, 112, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Abramson, D.H. Retinoblastoma: Saving Life with Vision. Annu. Rev. Med. 2014, 65, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.L.; Himelstein, B.; Shields, C.L.; Shields, J.A.; Needle, M.; Miller, D.; Bunin, G.R.; Meadows, A.T. Chemoreduction and Local Ophthalmic Therapy for Intraocular Retinoblastoma. J. Clin. Oncol. 2000, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Kaliki, S.; Rojanaporn, D.; Al-Dahmash, S.; Bianciotto, C.G.; Shields, J.A. Intravenous and intra-arterial chemotherapy for retinoblastoma. Curr. Opin. Ophthalmol. 2012, 23, 202–209. [Google Scholar] [CrossRef]
- Smith, S.J.; Smith, B.D.; Mohney, B.G. Ocular side effects following intravitreal injection therapy for retinoblastoma: A systematic review. Br. J. Ophthalmol. 2014, 98, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.H.; Schaiquevich, P.; Buitrago, E.; Del Sole, M.J.; Zapata, G.; Croxatto, J.O.; Marr, B.P.; Brodie, S.E.; Berra, A.; Chantada, G.L.; et al. Local and Systemic Toxicity of Intravitreal Melphalan for Vitreous Seeding in Retinoblastoma. Ophthalmology 2014, 121, 1810–1817. [Google Scholar] [CrossRef]
- Ravindran, K.; Dalvin, L.A.; Pulido, J.S.; Brinjikji, W. Intra-arterial chemotherapy for retinoblastoma: An updated systematic review and meta-analysis. J. NeuroInterventional Surg. 2019, 11, 1266–1272. [Google Scholar] [CrossRef] [PubMed]


| Patient No. | ICRB | Eye Treated | Age at PBT (Months) | Initial Treatment | Additional Treatment |
|---|---|---|---|---|---|
| 1 | unknown | left | 56 | IVC | IAC, IVitC, Lc, Cc |
| 2 | B | left | 21 | IVC + TCT | IVitC, Lc, Cc |
| 3 | unknown | right | 29 | IVC + IVitC | IAC, Brachy |
| 4 | D | left | 25 | IVC + TCT | IVitC, Lc |
| 5 | D | left | 18 | IVC | none |
| 6 | D | left | 50 | IVC + IVitC + TCT | Brachy, Lc, Cc |
| 7 | A | left | 97 | IVC | IAC, IVitC, Brachy, Lc, Cc |
| 8 | unknown | left | 91 | IVC | IAC |
| 9 | A/B | bilateral | 19 | IVC + TCT | IAC, Lc, Cc |
| 10 | B | left | 14 | IVC + TCT | IAC, IVitC, Brachy, Lc, Cc |
| 11 | E | left | 26 | IVC | IAC, IVitC |
| 12 | B/A | bilateral | 23 | IVC + TCT | IAC, IVitC, Lc, Cc |
| 13 | B | right | 15 | IVC | none |
| Patient No. | Follow-Up in Months | Additional Treatment After PBT | Complications | Globe Salvage | Histopathologic Viable Tumor Cells | Metastatic Disease | Deaths |
|---|---|---|---|---|---|---|---|
| 1 | 2 * | - | Ciliary body failure | no | no | unknown | unknown |
| 2 | 16 | - | - | yes | - | - | - |
| 3 | 4 * | - | - | no | yes | yes | yes |
| 4 | 6 * | IVitC, Brachy, IAC (ex domo) | - | no | unknown | unknown | unknown |
| 5 | 25 | IVitC | Dry eye disease | no | yes | no | no |
| 6 | 46 | Lensectomy + IOL | Cataract, radiation retinopathy | yes | - | - | - |
| 7 | 39 | pKPL AMT | Cataract, radiation retinopathy | yes | - | yes | no |
| 8 | 36 | - | - | no | no | no | no |
| 9 | 39 | Lensectomy + IOL | Cataract (bilateral) | yes | - | - | - |
| 10 | 21 | - | Cataract, radiation retinopathy | yes | - | - | - |
| 11 | 8 * | Triamcinolonesub-tenon | Exudative retinal detachment | no | yes | no | no |
| 12 | 15 | - | - | yes | - | - | - |
| 13 | 12 | - | - | yes | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biewald, E.; Kiefer, T.; Geismar, D.; Schlüter, S.; Manthey, A.; Westekemper, H.; Wulff, J.; Timmermann, B.; Ketteler, P.; Schönberger, S.; et al. Feasibility of Proton Beam Therapy as a Rescue Therapy in Heavily Pre-Treated Retinoblastoma Eyes. Cancers 2021, 13, 1862. https://doi.org/10.3390/cancers13081862
Biewald E, Kiefer T, Geismar D, Schlüter S, Manthey A, Westekemper H, Wulff J, Timmermann B, Ketteler P, Schönberger S, et al. Feasibility of Proton Beam Therapy as a Rescue Therapy in Heavily Pre-Treated Retinoblastoma Eyes. Cancers. 2021; 13(8):1862. https://doi.org/10.3390/cancers13081862
Chicago/Turabian StyleBiewald, Eva, Tobias Kiefer, Dirk Geismar, Sabrina Schlüter, Anke Manthey, Henrike Westekemper, Jörg Wulff, Beate Timmermann, Petra Ketteler, Stefan Schönberger, and et al. 2021. "Feasibility of Proton Beam Therapy as a Rescue Therapy in Heavily Pre-Treated Retinoblastoma Eyes" Cancers 13, no. 8: 1862. https://doi.org/10.3390/cancers13081862
APA StyleBiewald, E., Kiefer, T., Geismar, D., Schlüter, S., Manthey, A., Westekemper, H., Wulff, J., Timmermann, B., Ketteler, P., Schönberger, S., Metz, K. A., Ting, S., Göricke, S., Bechrakis, N. E., & Bornfeld, N. (2021). Feasibility of Proton Beam Therapy as a Rescue Therapy in Heavily Pre-Treated Retinoblastoma Eyes. Cancers, 13(8), 1862. https://doi.org/10.3390/cancers13081862






