Prostate Cancer Mortality Associated with Aggregate Polymorphisms in Androgen-Regulating Genes: The Atherosclerosis Risk in the Communities (ARIC) Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. ARIC Study Design
2.2. SNP Genotyping
2.3. Statistical Analysis
2.3.1. Genetic Risk Score (GRS)
2.3.2. Sensitivity Analyses
3. Results
3.1. Characteristics of Study Sample
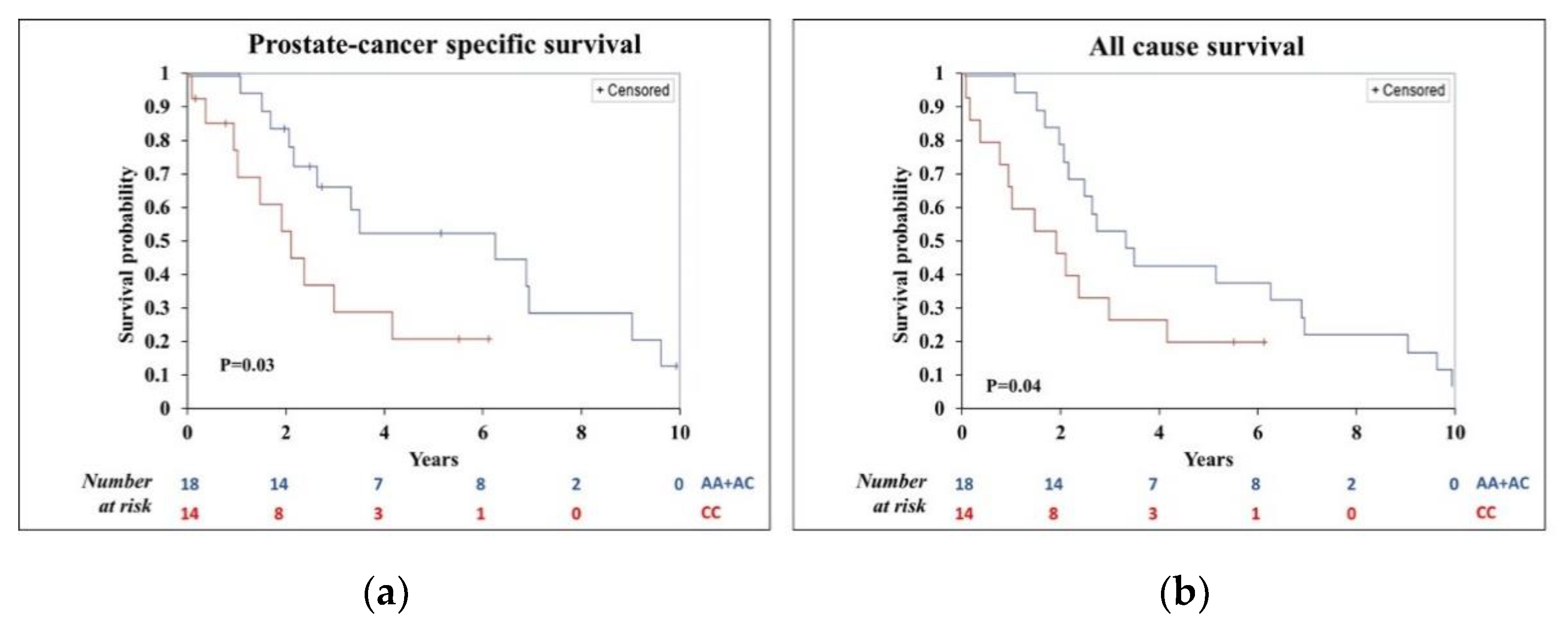
3.2. Univariate Analysis of Cancer-Specific and All-Cause Survival
3.3. Multivariable Analysis of PC-Specific Mortality and All-Cause Mortality for Individual SNPs and a Priori Genetic Risk Score (GRS)
3.3.1. PC-Specific Mortality Associated with Individual SNPs
3.3.2. All-Cause Mortality Associated with Individual SNPs
3.3.3. GRS and PC-Specific and All-Cause Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, C.J.; Smith, M.R.; De Bono, J.S.; Molina, A.; Logothetis, C.J.; De Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Vogelzang, N.J.; et al. Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Hagenbuch, B.; Stieger, B. The SLCO (former SLC21) superfamily of transporters. Mol. Asp. Med. 2013, 34, 396–412. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.; Kanaya, J.; Namiki, M. Adrenal steroids in human prostatic cancer cell lines. Arch. Androl. 2001, 46, 117–125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hearn, J.W.D.; Xie, W.; Nakabayashi, M.; Almassi, N.; Reichard, C.A.; Pomerantz, M.; Kantoff, P.W.; Sharifi, N. Association of HSD3B1 Genotype With Response to Androgen-Deprivation Therapy for Biochemical Recurrence After Radiotherapy for Localized Prostate Cancer. JAMA Oncol. 2018, 4, 558–562. [Google Scholar] [CrossRef]
- Hearn, J.W.D.; AbuAli, G.; Reichard, C.A.; Reddy, C.A.; Magi-Galluzzi, C.; Chang, K.-H.; Carlson, R.; Rangel, L.; Reagan, K.; Davis, B.J.; et al. HSD3B1 and resistance to androgen-deprivation therapy in prostate cancer: A retrospective, multicohort study. Lancet Oncol. 2016, 17, 1435–1444. [Google Scholar] [CrossRef]
- Shiota, M.; Fujimoto, N.; Yokomizo, A.; Takeuchi, A.; Itsumi, M.; Inokuchi, J.; Tatsugami, K.; Uchiumi, T.; Naito, S. SRD5A gene polymorphism in Japanese men predicts prognosis of metastatic prostate cancer with androgen-deprivation therapy. Eur. J. Cancer 2015, 51, 1962–1969. [Google Scholar] [CrossRef]
- Ryan, C.J.; Halabi, S.; Ou, S.-S.; Vogelzang, N.J.; Kantoff, P.; Small, E.J. Adrenal Androgen Levels as Predictors of Outcome in Prostate Cancer Patients Treated with Ketoconazole Plus Antiandrogen Withdrawal: Results from a Cancer and Leukemia Group B Study. Clin. Cancer Res. 2007, 13, 2030–2037. [Google Scholar] [CrossRef]
- Stanbrough, M.; Bubley, G.J.; Ross, K.; Golub, T.R.; Rubin, M.A.; Penning, T.M.; Febbo, P.G.; Balk, S.P. Increased Expression of Genes Converting Adrenal Androgens to Testosterone in Androgen-Independent Prostate Cancer. Cancer Res. 2006, 66, 2815–2825. [Google Scholar] [CrossRef]
- Sabharwal, N.; Sharifi, N. HSD3B1 genotypes conferring adrenal-restrictive and adrenal-permissive phenotypes in prostate cancer and beyond. Endocrinology 2019, 160, 2180–2188. [Google Scholar] [CrossRef]
- Agarwal, N.; Hahn, A.W.; Gill, D.M.; Farnham, J.M.; Poole, A.I.; Cannon-Albright, L. Independent validation of effect of HSD3B1 genotype on response to androgen-deprivation therapy in prostate cancer. Am. Med. Assoc. 2017, 3, 856–857. [Google Scholar] [CrossRef]
- Hearn, J.W.D.; Sweeney, C.J.; Almassi, N.; Reichard, C.A.; Reddy, C.A.; Li, H.; Hobbs, B.; Jarrard, D.F.; Chen, Y.-H.; Dreicer, R.; et al. HSD3B1 Genotype and Clinical Outcomes in Metastatic Castration-Sensitive Prostate Cancer. JAMA Oncol. 2020, 6, e196496. [Google Scholar] [CrossRef] [PubMed]
- Mostaghel, E.A.; Cho, E.; Zhang, A.; Alyamani, M.; Kaipainen, A.; Green, S.; Marck, B.T.; Sharifi, N.; Wright, J.L.; Gulati, R.; et al. Association of Tissue Abiraterone Levels and SLCO Genotype with Intraprostatic Steroids and Pathologic Response in Men with High-Risk Localized Prostate Cancer. Clin. Cancer Res. 2017, 23, 4592–4601. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xie, W.; Mostaghel, E.; Nakabayashi, M.; Werner, L.; Sun, T.; Pomerantz, M.; Freedman, M.; Ross, R.; Regan, M.; et al. SLCO2B1 and SLCO1B3 May Determine Time to Progression for Patients Receiving Androgen Deprivation Therapy for Prostate Cancer. J. Clin. Oncol. 2011, 29, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Harshman, L.C.; Xie, W.; Nakabayashi, M.; Qu, F.; Pomerantz, M.M.; Lee, G.-S.M.; Kantoff, P.W. Association of SLCO2B1 Genotypes with Time to Progression and Overall Survival in Patients Receiving Androgen-Deprivation Therapy for Prostate Cancer. J. Clin. Oncol. 2016, 34, 352–359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Audet-Walsh, E.; Bellemare, J.; Nadeau, G.; Lacombe, L.; Fradet, Y.; Fradet, V.; Huang, S.-P.; Bao, B.-Y.; Douville, P.; Girard, H.; et al. SRD5A Polymorphisms and Biochemical Failure After Radical Prostatectomy. Eur. Urol. 2011, 60, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- The Atherosclerosis Risk in Communities (ARIC) Study: Design and Objectives. The ARIC investigators. Am. J. Epidemiol. 1989, 129, 687–702.
- Atherosclerosis Risk in Communities (ARIC) Study. ARIC Documentation. Available online: https://sites.cscc.unc.edu/aric/desc_pub (accessed on 3 January 2021).
- George, K.M.; Folsom, A.R.; Kucharska-Newton, A.; Mosley, T.H.; Heiss, G. Factors Related to Differences in Retention among African American and White Participants in the Atherosclerosis Risk in Communities Study (ARIC) Prospective Cohort: 1987–2013. Ethn. Dis. 2017, 27, 31–38. [Google Scholar] [CrossRef][Green Version]
- Joshu, C.E.; Barber, J.R.; Coresh, J.; Couper, D.J.; Mosley, T.H.; Vitolins, M.Z.; Butler, K.R.; Nelson, H.H.; Prizment, A.E.; Selvin, E.; et al. Enhancing the infrastructure of the Atherosclerosis Risk in Communities (ARIC) Study for cancer epidemiology research: ARIC cancer. Cancer Epidemiol. Prev. Biomark. 2018, 27, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Pankow, J.S.; Tang, W.; Pankratz, N.; Guan, W.; Weng, L.C.; Cushman, M.; Boerwinkle, E.; Folsom, A.R. Identification of Genetic Variants Linking Protein C and Lipoprotein Metabolism: The ARIC Study (Atherosclerosis Risk in Communities). Arter. Thromb. Vasc. Biol. 2017, 37, 589–597. [Google Scholar] [CrossRef]
- Tang, W.; Saratzis, A.; Pattee, J.; Smith, J.; Pankratz, N.; Leavy, O.C.; Guan, W.; Dudbridge, F.; Pankow, J.S.; Kitas, G.D.; et al. Replication of Newly Identified Genetic Associations Between Abdominal Aortic Aneurysm and SMYD2, LINC00540, PCIF1/MMP9/ZNF335, and ERG. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Marchini, J.; Howie, B. Genotype imputation for genome-wide association studies. Nat. Rev. Genet. 2010, 11, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.P.; Gray, R.J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; Fong, L.; Rosenberg, J.E.; Kantoff, P.; Raynaud, F.; Martins, V.; Lee, G.; Kheoh, T.; Kim, J.; et al. Phase I Clinical Trial of the CYP17 Inhibitor Abiraterone Acetate Demonstrating Clinical Activity in Patients With Castration-Resistant Prostate Cancer Who Received Prior Ketoconazole Therapy. J. Clin. Oncol. 2010, 28, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Tsodikov, A.; Gulati, R.; Ms, T.M.D.C.; Heijnsdijk, E.A.M.; Ma, R.A.H.; Mariotto, A.B.; De Koning, H.J.; Etzioni, R. Is prostate cancer different in black men? Answers from 3 natural history models. Cancer 2017, 123, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Sherry, S.T.; Ward, M.-H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Hearn, J.W.; Sweeney, C.; Almassi, N.; Reichard, C.A.; Reddy, C.A.; Hobbs, B.; Jarrard, D.F.; Chen, Y.-H.; Dreicer, R.; Garcia, J.A.; et al. HSD3B1 and overall survival (OS) in men with low-volume (LV) metastatic prostate cancer (PCa) treated with androgen deprivation therapy (ADT) or chemohormonal therapy in the CHAARTED Randomized trial. J. Clin. Oncol. 2019, 37, 5020. [Google Scholar] [CrossRef]
- Harper, M.E.; Pike, A.; Peeling, W.B.; Griffiths, K. Steroids of adrenal origin metabolized by human prostatic tissue both in vivo and in vitro. J. Endocrinol. 1974, 60, 117–125. [Google Scholar] [CrossRef]
- Weisser, H.; Krieg, M. Kinetic analysis of androstenedione 5α-reductase in epithelium and stroma of human prostate. Steroids 1997, 62, 589–594. [Google Scholar] [CrossRef]
- Shiota, M.; Narita, S.; Akamatsu, S.; Fujimoto, N.; Sumiyoshi, T.; Fujiwara, M.; Uchiumi, T.; Habuchi, T.; Ogawa, O.; Eto, M. Association of Missense Polymorphism inHSD3B1With Outcomes Among Men with Prostate Cancer Treated with Androgen-Deprivation Therapy or Abiraterone. JAMA Netw. Open 2019, 2, e190115. [Google Scholar] [CrossRef]
- Almassi, N.; Reichard, C.; Li, J.; Russell, C.; Perry, J.; Ryan, C.J.; Friedlander, T.; Sharifi, N. HSD3B1 and Response to a Nonsteroidal CYP17A1 Inhibitor in Castration-Resistant Prostate Cancer. JAMA Oncol. 2018, 4, 554. [Google Scholar] [CrossRef]
- Alyamani, M.; Emamekhoo, H.; Park, S.; Taylor, J.; Almassi, N.; Upadhyay, S.; Tyler, A.; Berk, M.P.; Hu, B.; Hwang, T.H.; et al. HSD3B1(1245A>C) variant regulates dueling abiraterone metabolite effects in prostate cancer. J. Clin. Investig. 2018, 128, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Terbuch, A.; Dolling, D.; Yu, J.; Wang, H.; Chen, Y.; Fountain, J.; Bertan, C.; Sharp, A.; Carreira, S.; et al. Treatment with abiraterone and enzalutamide does not overcome poor outcome from metastatic castration-resistant prostate cancer in men with the germline homozygous HSD3B1 c.1245C genotype. Ann. Oncol. 2020, 31, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, N.; Kubo, T.; Inatomi, H.; Bui, H.T.; Shiota, M.; Sho, T.; Matsumoto, T. Polymorphisms of the androgen transporting gene SLCO2B1 may influence the castration resistance of prostate cancer and the racial differences in response to androgen deprivation. Prostate Cancer Prostatic Dis. 2013, 16, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Mahal, B.A.; Alshalalfa, M.; Kensler, K.H.; Chowdhury-Paulino, I.; Kantoff, P.; Mucci, L.A.; Schaeffer, E.M.; Spratt, D.; Yamoah, K.; Nguyen, P.L.; et al. Racial Differences in Genomic Profiling of Prostate Cancer. N. Engl. J. Med. 2020, 383, 1083–1085. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All Men | White Men | Black Men | p-Value 1 |
|---|---|---|---|---|
| Number of men | 622 | 489 | 133 | |
| Mean age at diagnosis (SD), y | 69.7 (6.3) | 69.9 (6.3) | 69.8 (6.3) | <0.12 2 |
| Median (range) | 70 (53–86) | 70 (53–86) | 68 (54–84) | |
| Stage at diagnosis 3 (%) | 0.14 | |||
| 1 and 2 combined 3 4 | 82.0 12.2 5.8 | 82.8 12.3 4.9 | 79.2 11.2 9.6 | |
| Grade at diagnosis 3 (%) | 0.0005 | |||
| 1 2 3 Unknown | 20.6 46.1 27.7 5.6 | 21.9 48.9 24.1 5.1 | 15.8 36.1 40.6 7.5 | |
| First course of treatment 4 | ||||
| Surgery (yes, %) Radiation (yes, %) Hormonal therapy (yes, %) | 51.3 17.6 25.4 | 51.3 20.0 25.0 | 51.1 16.9 26.7 | 0.98 0.45 0.75 |
| Co-morbidities at baseline | ||||
| Diabetes (yes, %) Cardiovascular disease (yes, %) | 8.1 6.4 | 8.1 7.0 | 8.0 4.0 | 0.96 0.14 |
| Co-morbidities before diagnosis | ||||
| Diabetes (yes, %) Cardiovascular disease (yes, %) | 14.0 12.1 | 13.7 12.5 | 15.3 10.4 | 0.65 0.15 |
| Number of deaths | 350 | 289 | 61 | |
| Incidence rate of death from any cause (per 1000 men) | 47.9 | 49.7 | 41.1 | |
| Incidence rate of PC-specific death (per 1000 men) | 10.2 | 10.0 | 11.0 | |
| Median follow-up time (range, y) | 11.6 (0–29.1) | 11.0 (0.11–29.1) | 10.5 (0–27.7) |
| SNP | Gene (Chromosome) Function | Major Allele | Minor Allele | Frequency of Major Allele | Risk Allele 1 | ||
|---|---|---|---|---|---|---|---|
| All | White Men | Black Men | |||||
| rs1047303 (1245C) Missense C–gain of function | HSD3B1 (1) Androgen synthesis and production | A | C | 0.72 | 0.68 | 0.88 | C |
| rs1789693 Intron | SLCO2B1(11) Androgen transport and uptake | A | T | 0.59 | 0.66 | 0.34 | A 2 |
| rs12422149 Missense | SLCO2B1 (11) Androgen transport and uptake | G | A | 0.90 | 0.90 | 0.90 | G |
| rs523349 Missense | SRD5A2 (2) Androgen conversion | C | G | 0.73 | 0.70 | 0.76 | G 2 |
| Polymorphisms | Hazard Ratio and 95% Confidence Intervals for PC-Specific Mortality 1 | ||
|---|---|---|---|
| All | White Men | Black Men | |
| No. of men with PC | 622 | 489 | 133 |
| No. of PC-specific deaths | 74 | 58 | 16 |
| Person-years (per 1000 person-years) | 7273 | 5815 | 1459 |
| SNP (risk allele) 2 | |||
| rs1047303 (C) | 1.40 (0.96–2.06) p = 0.08 | 1.39 (0.93–2.08) p = 0.11 | 1.89 (0.55–6.48) p = 0.76 |
| rs1789693 (A) | 1.46 (0.93–2.27) p = 0.10 | 1.70 (1.01–2.85) p = 0.04 | 0.45 (0.15–1.31) p = 0.14 |
| rs12422149 (G) | 1.22 (0.69–2.18) p = 0.49 | 1.15 (0.62–2.12) p = 0.66 | 1.98 (0.28–13.85) p = 0.50 |
| rs523349 (G) | 1.05 (0.73–1.51) p = 0.76 | 1.26 (0.83–1.91) p = 0.27 | 0.95 (0.43–2.11) p = 0.88 |
| Genetic Risk Score, GRS 3 | |||
| Tertiles | |||
| 0.12–3.76 3.77–4.82 4.83–7.68 | 1 (Reference) 1.39 (0.76–2.55) 1.73 (0.95–3.16) | 1 (Reference) 2.71 (1.15–6.40) 3.21 (1.46–7.07) | 1 (Reference) 0.80 (0.20–3.16) 0.47 (0.06–3.82) |
| Continuous (per risk allele) | 1.26 (1.02–1.56) | 1.39 (1.08–1.77) | 0.85 (0.51–1.42) |
| P-trend 4 | 0.03 | 0.01 | 0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prizment, A.E.; McSweeney, S.; Pankratz, N.; Joshu, C.E.; Hwang, J.H.; Platz, E.A.; Ryan, C.J. Prostate Cancer Mortality Associated with Aggregate Polymorphisms in Androgen-Regulating Genes: The Atherosclerosis Risk in the Communities (ARIC) Study. Cancers 2021, 13, 1958. https://doi.org/10.3390/cancers13081958
Prizment AE, McSweeney S, Pankratz N, Joshu CE, Hwang JH, Platz EA, Ryan CJ. Prostate Cancer Mortality Associated with Aggregate Polymorphisms in Androgen-Regulating Genes: The Atherosclerosis Risk in the Communities (ARIC) Study. Cancers. 2021; 13(8):1958. https://doi.org/10.3390/cancers13081958
Chicago/Turabian StylePrizment, Anna E., Sean McSweeney, Nathan Pankratz, Corinne E. Joshu, Justin H. Hwang, Elizabeth A. Platz, and Charles J. Ryan. 2021. "Prostate Cancer Mortality Associated with Aggregate Polymorphisms in Androgen-Regulating Genes: The Atherosclerosis Risk in the Communities (ARIC) Study" Cancers 13, no. 8: 1958. https://doi.org/10.3390/cancers13081958
APA StylePrizment, A. E., McSweeney, S., Pankratz, N., Joshu, C. E., Hwang, J. H., Platz, E. A., & Ryan, C. J. (2021). Prostate Cancer Mortality Associated with Aggregate Polymorphisms in Androgen-Regulating Genes: The Atherosclerosis Risk in the Communities (ARIC) Study. Cancers, 13(8), 1958. https://doi.org/10.3390/cancers13081958






