Towards Targeted Alpha Therapy with Actinium-225: Chelators for Mild Condition Radiolabeling and Targeting PSMA—A Proof of Concept Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
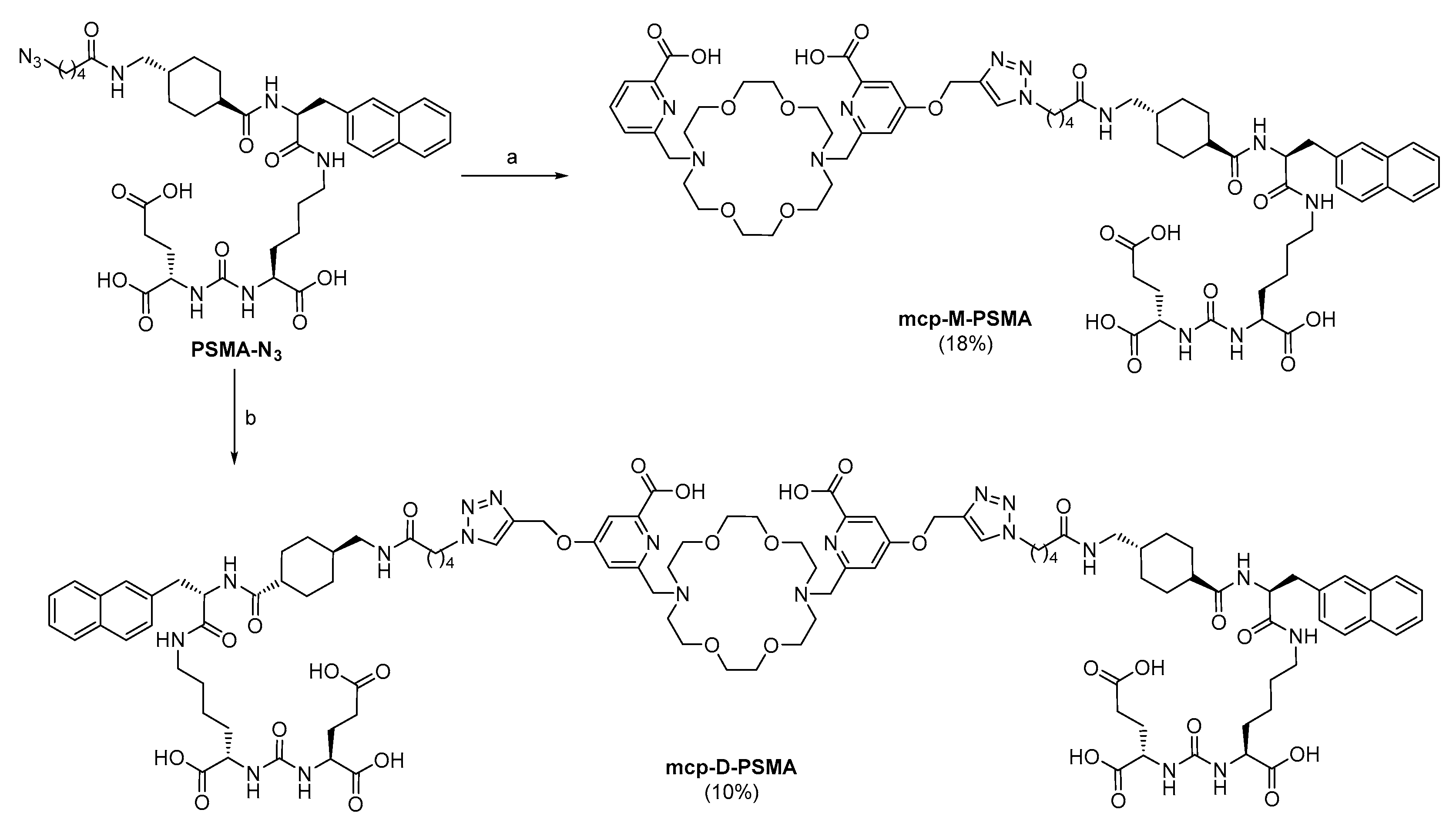
2.1. Chelator Development
2.2. Radiolabeling and Complex Stability
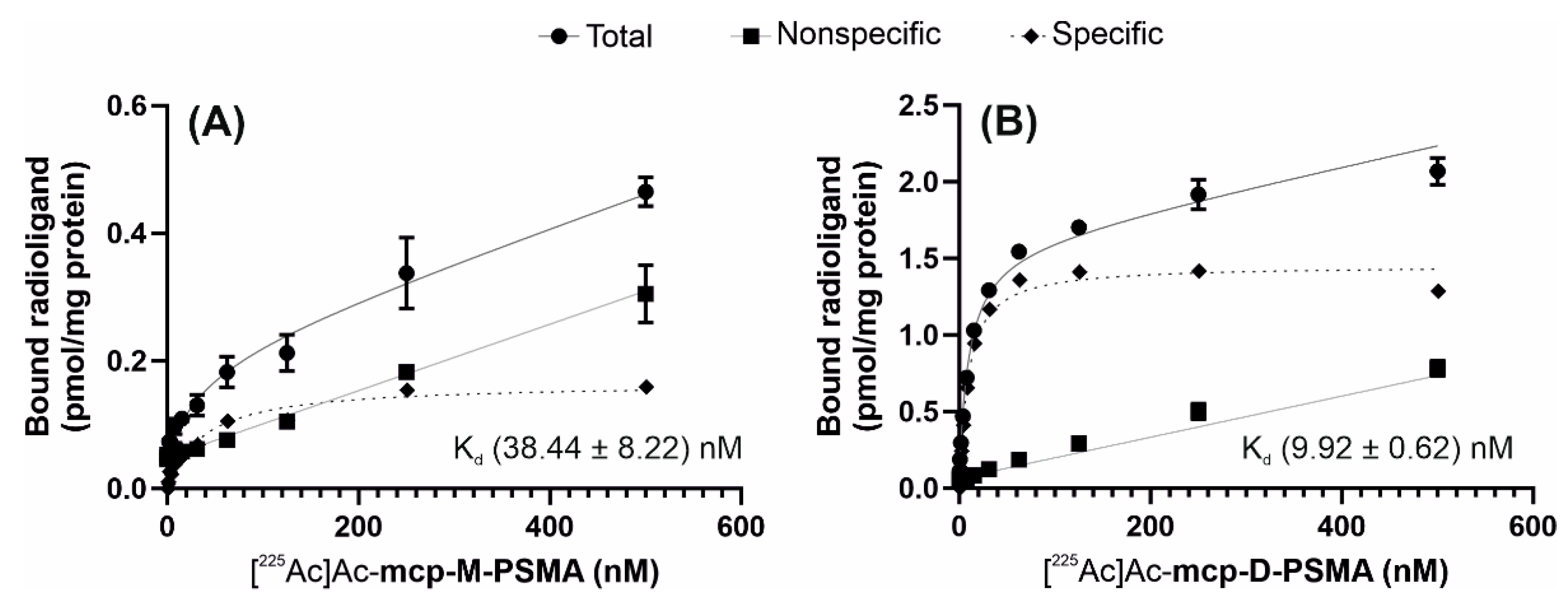
2.3. Characterization of the PSMA-Binding Properties
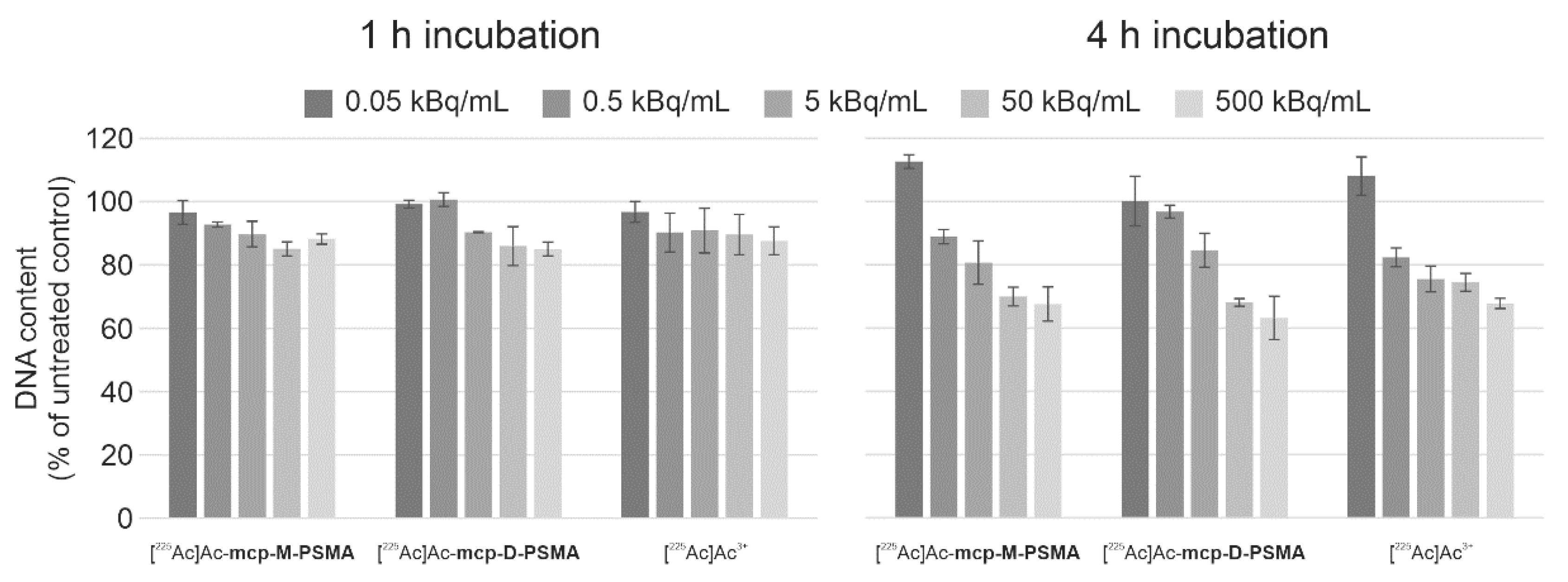
2.4. Cytotoxicity of 225Ac-Labeled PSMA Derivatives
2.5. Clonogenicity in Response to the Incubation with 225Ac-Labeled PSMA Derivatives
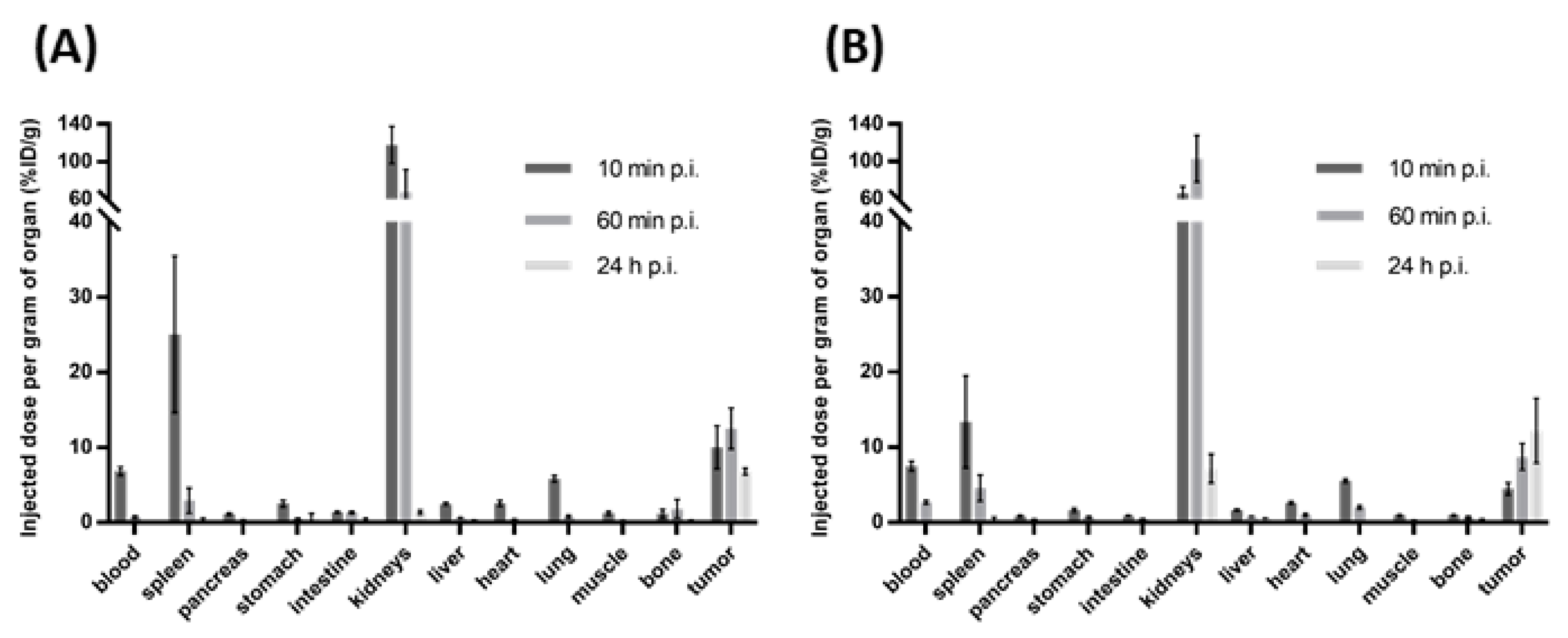
2.6. Small Animal Biodistribution Experiments
3. Materials and Methods
3.1. Chemistry
3.1.1. Methyl 6-((1,4,10,13-tetraoxa-7,16-diazacyclooctadecan-7-yl)methyl)picolinate 1
3.1.2. Methyl 6-((16-((6-(methoxycarbonyl)pyridin-2-yl)methyl)-1,4,10,13-tetraoxa-7,16-diazacyclooctadecan-7-yl)methyl)-4-(prop-2-yn-1-yloxy)picolinate 2
3.1.3. Dimethyl 6,6′-((1,4,10,13-tetraoxa-7,16-diazacyclooctadecane-7,16-diyl)bis(methylene))bis(4-(prop-2-yn-1-yloxy)picolinate) 3
3.1.4. 6-((16-((6-Carboxypyridin-2-yl)methyl)-1,4,10,13-tetraoxa-7,16-diazacyclooctadecan-7-yl)methyl)-4-(prop-2-yn-1-yloxy)picolinic acid mcp-M-click
3.1.5. 6,6′-((1,4,10,13-Tetraoxa-7,16-diazacyclooctadecane-7,16-diyl)bis(methylene))bis(4-(prop-2-yn-1-yloxy)picolinic acid) mcp-D-click
3.1.6. (3RS,10RS,14S)-1-((1s,4S)-4-((5-(4-(((2-Carboxy-6-((16-((6-carboxypyridin-2-yl)methyl)-1,4,10,13-tetraoxa-7,16-diazacyclooctadecan-7-yl)methyl)pyridin-4-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)pentanamido)methyl)cyclohexyl)-3-(naphthalen-2-ylmethyl)-1,4,12-trioxo-2,5,11,13-tetraazahexadecane-10,14,16-tricarboxylic acid mcp-M-PSMA
3.1.7. Bis-Functionalized PSMA Conjugate mcp-D-PSMA
3.2. Radiolabeling
3.3. Cell Culture
3.4. Protein Determination
3.5. Gel Electrophoresis and Western Blot Analysis
3.6. Saturation Binding Studies
3.7. Cell Viability, Apoptosis and Cell Proliferation Assays
3.8. Colony Formation Assay
3.9. Animal Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nayak, T.; Norenberg, J.; Anderson, T.; Atcher, R. A comparison of high- versus low-linear energy transfer somatostatin receptor targeted radionuclide therapy in vitro. Cancer Biother. Radiopharm. 2005, 20, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Zalutsky, M.R.; Reardon, D.A.; Pozzi, O.R.; Vaidyanathan, G.; Bigner, D.D. Targeted alpha-particle radiotherapy with 211At-labeled monoclonal antibodies. Nucl. Med. Biol. 2007, 34, 779–785. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Pruszynski, M. Astatine-211: Production and availability. Curr. Radiopharm. 2011, 4, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, G.; Zalutsky, M.R. Applications of 211At and 223Ra in targeted alpha-particle radiotherapy. Curr. Radiopharm. 2011, 4, 283–294. [Google Scholar] [CrossRef]
- Choi, J.; Vaidyanathan, G.; Koumarianou, E.; Kang, C.M.; Zalutsky, M.R. Astatine-211 labeled anti-HER2 5F7 single domain antibody fragment conjugates: Radiolabeling and preliminary evaluation. Nucl. Med. Biol. 2018, 56, 10–20. [Google Scholar] [CrossRef]
- Thiele, N.A.; Wilson, J.J. Actinium-225 for Targeted α Therapy: Coordination Chemistry and Current Chelation Approaches. Cancer Biother. Radiopharm. 2018, 33, 336–348. [Google Scholar] [CrossRef]
- Roscher, M.; Bakos, G.; Benešová, M. Atomic Nanogenerators in Targeted Alpha Therapies: Curie’s Legacy in Modern Cancer Management. Pharmaceuticals 2020, 13, 76. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. ²¹³Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef]
- Kratochwil, C.; Schmidt, K.; Afshar-Oromieh, A.; Bruchertseifer, F.; Rathke, H.; Morgenstern, A.; Haberkorn, U.; Giesel, F.L. Targeted alpha therapy of mCRPC: Dosimetry estimate of 213Bismuth-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Poeppel, T.D.; Handkiewicz-Junak, D.; Andreeff, M.; Becherer, A.; Bockisch, A.; Fricke, E.; Geworski, L.; Heinzel, A.; Krause, B.J.; Krause, T.; et al. EANM guideline for radionuclide therapy with radium-223 of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 824–845. [Google Scholar] [CrossRef]
- Gott, M.; Steinbach, J.; Mamat, C. The radiochemical and radiopharmaceutical applications of radium. Open Chem. 2016, 14, 118–129. [Google Scholar] [CrossRef]
- Steinberg, J.; Bauer, D.; Reissig, F.; Köckerling, M.; Pietzsch, H.-J.; Mamat, C. Modified Calix[4]crowns as Molecular Receptors for Barium. ChemistryOpen 2018, 7, 432–438. [Google Scholar] [CrossRef]
- Bauer, D.; Gott, M.; Steinbach, J.; Mamat, C. Chelation of heavy group 2 (radio)metals by p-tert-butylcalix[4]arene-1,3-crown-6 and logK determination via NMR. Spectrochim. Acta A 2018, 199, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.; Blumberg, M.; Köckerling, M.; Mamat, C. A Comparative Evaluation of Calix[4]arene-1,3-crown-6 as a Ligand for Selected Divalent Cations of Radiopharmaceutical Interest. RSC Adv. 2019, 9, 32357–32366. [Google Scholar] [CrossRef]
- Abou, D.S.; Thiele, N.A.; Gutsche, N.T.; Villmer, A.; Zhang, H.; Woods, J.J.; Baidoo, K.E.; Escorcia, F.E.; Wilson, J.J.; Thorek, D.L.J. Towards the stable chelation of radium for biomedical applications with an 18-membered macrocyclic ligand. Chem. Sci. 2021, 12, 3733–3742. [Google Scholar] [CrossRef]
- Reissig, F.; Bauer, D.; Pietzsch, H.-J.; Steinbach, J.; Mamat, C. Synthesis and functionalization of radium-doped barium sulfate nanoparticles. J. Med. Imag. Radiat. Sci. 2019, 50, S38. [Google Scholar] [CrossRef]
- Reissig, F.; Zarschler, K.; Hübner, R.; Pietzsch, H.-J.; Kopka, K.; Mamat, C. Sub-10 nm barium sulfate nanoparticles as universal radionuclide carriers for theranostic applications and targeted alpha therapy. ChemistryOpen 2020, 9, 797–805. [Google Scholar] [CrossRef]
- Bilewicz, A.; Cedrowska, E.; Gaweda, W.; Bruchertseifer, F.; Morgenstern, A. Barium ferrite magnetic nanoparticles labeled with 223Ra: A new potential magnetic radiobioconjugate for targeted alpha therapy. J. Label. Compds. Radiopharm. 2019, 62, 103. [Google Scholar] [CrossRef]
- Vasiliev, A.N.; Severin, A.; Lapshina, E.; Chernykh, E.; Ermolaev, S.; Kalmykov, S. Hydroxyapatite particles as carriers for 223Ra. J. Radioanal. Nucl. Chem. 2017, 311, 1503–1509. [Google Scholar] [CrossRef]
- Suchánková, P.; Kukleva, E.; Štamberg, K.; Nykl, P.; Vlk, M.; Kozempel, J. Study of 223Ra uptake mechanism on hydroxyapatite and titanium dioxide nanoparticles as a function of pH. RSC Adv. 2020, 10, 3659–3666. [Google Scholar] [CrossRef]
- Gott, M.; Yang, P.; Kortz, U.; Stephan, H.; Pietzsch, H.-J.; Mamat, C. Evaluation of Barium and Radium Polyoxopalladates for Radiopharmaceutical Applications. Chem. Commun. 2019, 55, 7631–7634. [Google Scholar] [CrossRef]
- Hagemann, U.B.; Ellingsen, C.; Schuhmacher, J.; Kristian, A.; Mobergslien, A.; Cruciani, V.; Wickstroem, K.; Schatz, C.A.; Kneip, C.; Golfier, S.; et al. Mesothelin-Targeted Thorium-227 Conjugate (MSLN-TTC): Preclinical Evaluation of a New Targeted Alpha Therapy for Mesothelin-Positive Cancers. Clin. Cancer Res. 2019, 25, 4723–4734. [Google Scholar] [CrossRef]
- Levy, M.Y.; Cicic, D.; Bergonio, G.; Berger, M. Trial in Progress: Phase I Study of Actinium-225 (225Ac)-Lintuzumab in Patients with Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2017, 17, S329–S330. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Thiele, N.A.; Brown, V.; Kelly, J.M.; Amor-Coarasa, A.; Jermilova, U.; MacMillan, S.N.; Nikolopoulou, A.; Ponnala, S.; Ramogida, C.F.; Robertson, A.K.H.; et al. An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chem. Int. Ed. Engl. 2017, 56, 14712–14717. [Google Scholar] [CrossRef] [PubMed]
- Roca-Sabio, A.; Mato-Iglesias, M.; Esteban-Gómez, D.; de Blas, A.; Rodríguez-Blas, T.; Platas-Iglesias, C. The effect of ring size variation on the structure and stability of lanthanide(III) complexes with crown ethers containing picolinate pendants. Dalton Trans. 2011, 40, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Amor-Coarasa, A.; Ponnala, S.; Nikolopoulou, A.; Williams, C., Jr.; Thiele, N.A.; Schlyer, D.; Wilson, J.J.; DiMagno, S.G.; Babich, J.W. A Single Dose of 225Ac-RPS-074 Induces a Complete Tumor Response in an LNCaP Xenograft Model. J. Nucl. Med. 2019, 60, 649–655. [Google Scholar] [CrossRef]
- Thirumurugan, P.; Dariusz Matosiuk, D.; Jozwiak, K. Click Chemistry for Drug Development and Diverse Chemical–Biology Applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef]
- Roca-Sabio, A.; Mato-Iglesias, M.; Esteban-Gómez, D.; Tóth, E.; de Blas, A.; Platas-Iglesias, C.; Rodríguez-Blas, T. Macrocyclic receptor exhibiting unprecedented selectivity for light lanthanides. J. Am. Chem. Soc. 2009, 131, 3331–3341. [Google Scholar] [CrossRef]
- Kozempel, J.; Mokhodoeva, O.; Vlk, M. Progress in Targeted Alpha-Particle Therapy. What We Learned about Recoils Release from In Vivo Generators. Molecules 2018, 23, 581. [Google Scholar] [CrossRef]
- de Kruijff, R.M.; Wolterbeek, H.T.; Denkova, A.G. A Critical Review of Alpha Radionuclide Therapy-How to Deal with Recoiling Daughters? Pharmaceuticals 2015, 8, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, C.; Yuan, Z.; Rodriguez-Rodriguez, C.; Robertson, A.; Radchenko, V.; Perron, R.; Gendron, D.; Causey, P.; Gao, F.; et al. Synthesis and Evaluation of a Macrocyclic Actinium-225 Chelator, Quality Control and In Vivo Evaluation of 225 Ac-crown-αMSH Peptide. Chem. Eur. J. 2020, 26, 11435–11440. [Google Scholar] [CrossRef]
- Schäfer, M.; Bauder-Wüst, U.; Leotta, K.; Zoller, F.; Mier, W.; Haberkorn, U.; Eisenhut, M.; Eder, M. A dimerized urea-based inhibitor of the prostate-specific membrane antigen for 68Ga-PET imaging of prostate cancer. EJNMMI Res. 2012, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Frei, A.; Fischer, E.; Childs, B.C.; Holland, J.P.; Alberto, R. Two is better than one: Difunctional high-affinity PSMA probes based on a [CpM(CO)3] (M = Re/99mTc) scaffold. Dalton Trans. 2019, 48, 14600–14605. [Google Scholar] [CrossRef] [PubMed]
- Mammen, M.; Choi, S.K.; Whitesides, G.M. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew. Chem. Int. Ed. Engl. 1998, 37, 2754–2794. [Google Scholar] [CrossRef]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- O’Keefe, D.S.; Bacich, D.J.; Heston, W.D. Comparative analysis of prostate-specific membrane antigen (PSMA) versus a prostate-specific membrane antigen-like gene. Prostate 2004, 58, 200–210. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Kuratsukuri, K.; Landas, S.; Imaida, K.; Rovito, P.M., Jr.; Wang, C.Y.; Haas, G.P. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J. Surg. 2006, 30, 628–636. [Google Scholar] [CrossRef]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Haberkorn, U.; Eisenhut, M.; Kopka, K. Preclinical evaluation of a bispecific low-molecular heterodimer targeting both PSMA and GRPR for improved PET imaging and therapy of prostate cancer. Prostate 2014, 74, 659–668. [Google Scholar] [CrossRef]
- Dam, J.H.; Olsen, B.B.; Baun, C.; Høilund-Carlsen, P.F.; Thisgaard, H. A PSMA Ligand Labeled with Cobalt-55 for PET Imaging of Prostate Cancer. Mol. Imaging Biol. 2017, 19, 915–922. [Google Scholar] [CrossRef]
- Benešová, M.; Schäfer, M.; Bauder-Wüst, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Wurzer, A.; Parzinger, M.; Konrad, M.; Beck, R.; Günther, T.; Felber, V.; Färber, S.; Di Carlo, D.; Wester, H.J. Preclinical comparison of four [18F, natGa]rhPSMA-7 isomers: Influence of the stereoconfiguration on pharmacokinetics. EJNMMI Res. 2020, 10, 149. [Google Scholar] [CrossRef]
- Hanna, J.R.; Allan, C.; Lawrence, C.; Meyer, O.; Wilson, N.D.; Hulme, A.N. Optimizing the Readout of Lanthanide-DOTA Complexes for the Detection of Ligand-Bound Copper(I). Molecules 2017, 22, 802. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.M.; Severns, V.; Brown, D.C.; Bisoffi, M.; Sillerud, L.O. Prostate cancer targeting motifs: Expression of ανβ3, neurotensin receptor 1, prostate specific membrane antigen, and prostate stem cell antigen in human prostate cancer cell lines and xenografts. Prostate 2012, 72, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, F.; Debarbieux, F.; Kronland-Martinet, C.; Cojocaru, G.R.; Popa-Wagner, A. Combined two-photon laser-scanning microscopy and spectral microCT X-ray imaging to characterize the cellular signature and evolution of microstroke foci. Rom. J. Morphol. Embryol. 2012, 53, 671–675. [Google Scholar]






| RCY (%) | [225Ac]Ac-macropa | [225Ac]Ac-mcp-M-click | [225Ac]Ac-mcp-D-click | [225Ac]Ac-mcp-M-COOH | [225Ac]Ac-mcp-M-PSMA | [225Ac]Ac-mcp-D-PSMA | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| c [M] | 10−5 | 10−6 | 10−7 | 10−5 | 10−6 | 10−7 | 10−5 | 10−6 | 10−7 | 10−5 | 10−6 | 10−7 | 10−5 | 10−6 | 10−7 | 10−5 | 10−6 | 10−7 |
| NP (1 h) | 99 | 99 | 56 | 99 | 99 | 20 | 99 | 99 | 52 | 99 | 99 | 98 | 99 | 99 | 89 | 99 | 99 | 93 |
| NP (7 d) | 99 | 99 | 99 | 99 | 99 | 58 | 99 | 99 | 50 | 99 | 99 | 99 | 99 | 99 | 99 | 99 | 99 | 95 |
| RP (1 h) | 99 | 90 | 37 | 96 | 97 | 23 | 99 | 99 | 41 | 99 | 98 | 98 | / | / | / | / | / | / |
| RP (7 d) | 99 | 99 | 99 | 99 | 99 | 55 | 99 | 94 | 40 | 99 | 99 | 96 | / | / | / | / | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reissig, F.; Bauer, D.; Zarschler, K.; Novy, Z.; Bendova, K.; Ludik, M.-C.; Kopka, K.; Pietzsch, H.-J.; Petrik, M.; Mamat, C. Towards Targeted Alpha Therapy with Actinium-225: Chelators for Mild Condition Radiolabeling and Targeting PSMA—A Proof of Concept Study. Cancers 2021, 13, 1974. https://doi.org/10.3390/cancers13081974
Reissig F, Bauer D, Zarschler K, Novy Z, Bendova K, Ludik M-C, Kopka K, Pietzsch H-J, Petrik M, Mamat C. Towards Targeted Alpha Therapy with Actinium-225: Chelators for Mild Condition Radiolabeling and Targeting PSMA—A Proof of Concept Study. Cancers. 2021; 13(8):1974. https://doi.org/10.3390/cancers13081974
Chicago/Turabian StyleReissig, Falco, David Bauer, Kristof Zarschler, Zbynek Novy, Katerina Bendova, Marie-Charlotte Ludik, Klaus Kopka, Hans-Jürgen Pietzsch, Milos Petrik, and Constantin Mamat. 2021. "Towards Targeted Alpha Therapy with Actinium-225: Chelators for Mild Condition Radiolabeling and Targeting PSMA—A Proof of Concept Study" Cancers 13, no. 8: 1974. https://doi.org/10.3390/cancers13081974
APA StyleReissig, F., Bauer, D., Zarschler, K., Novy, Z., Bendova, K., Ludik, M.-C., Kopka, K., Pietzsch, H.-J., Petrik, M., & Mamat, C. (2021). Towards Targeted Alpha Therapy with Actinium-225: Chelators for Mild Condition Radiolabeling and Targeting PSMA—A Proof of Concept Study. Cancers, 13(8), 1974. https://doi.org/10.3390/cancers13081974