Small Extracellular Vesicles in Pre-Therapy Plasma Predict Clinical Outcome in Non-Small-Cell Lung Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Plasma Samples
2.2. Protein Measurement Using the BCA Protein Assay
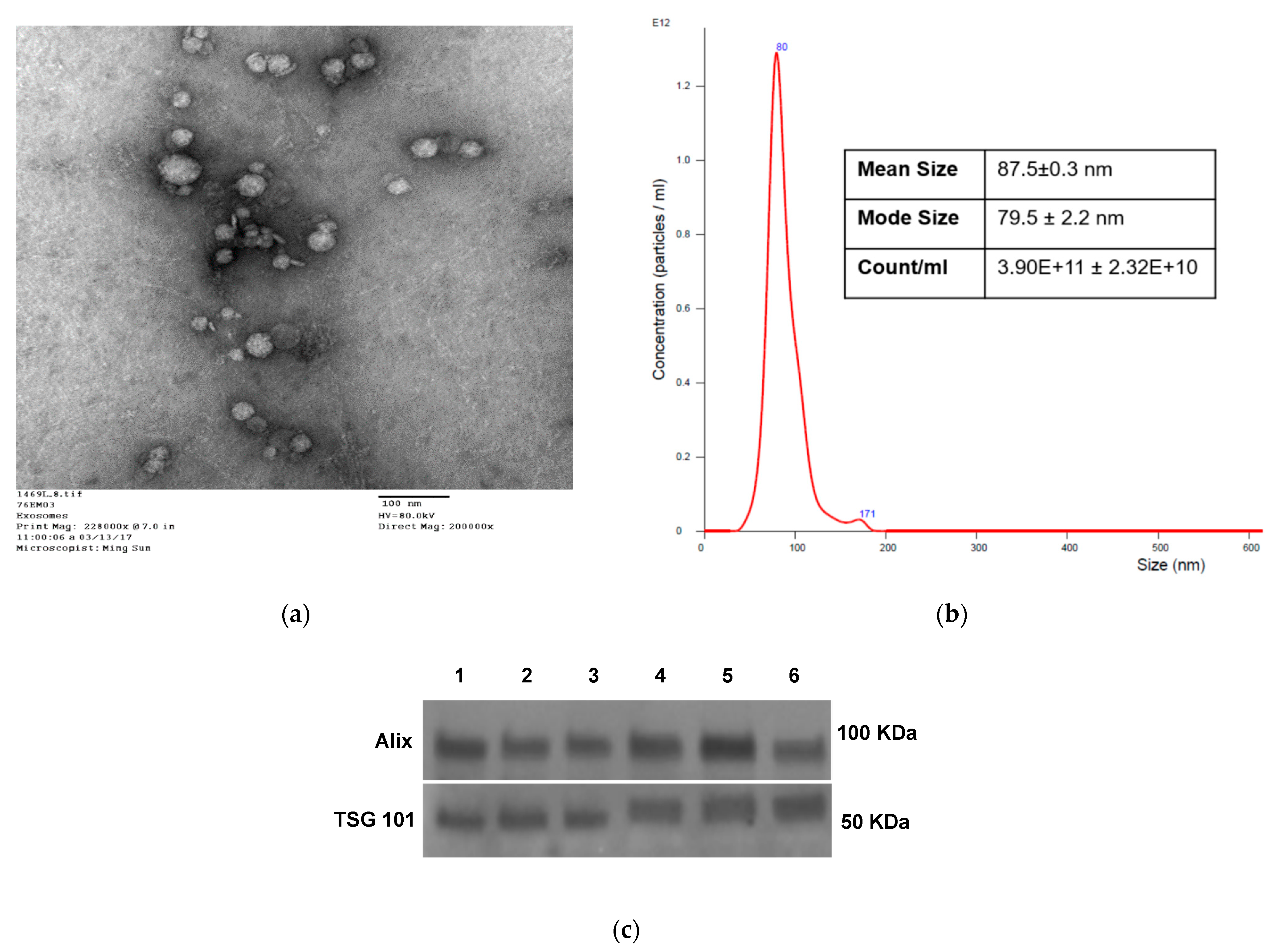
2.3. Exosome Size and Concentration Assessment by Tunable Resistive Pulse Sensing (TRPS)
2.4. Transmission Electron Microscopy (TEM)
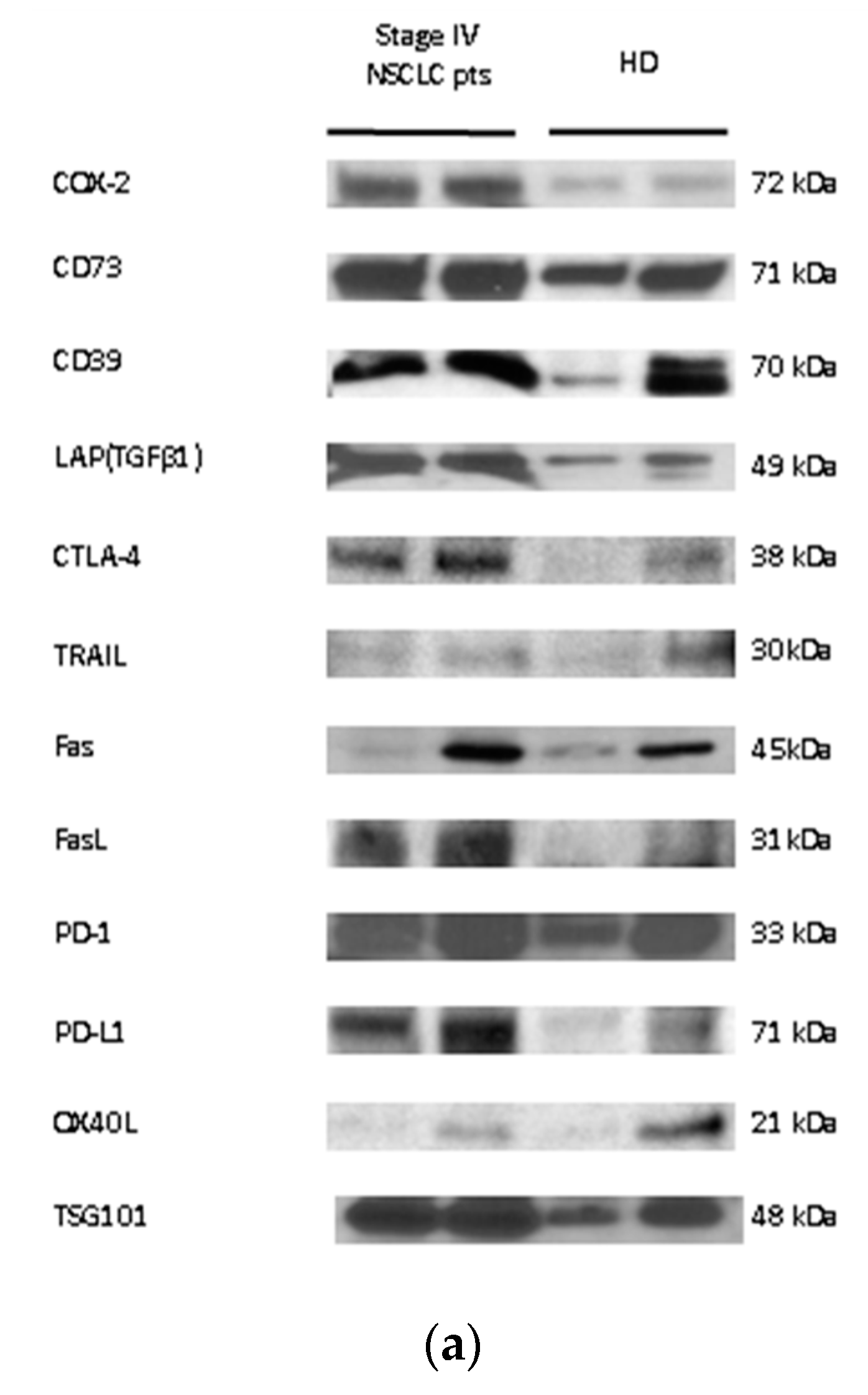
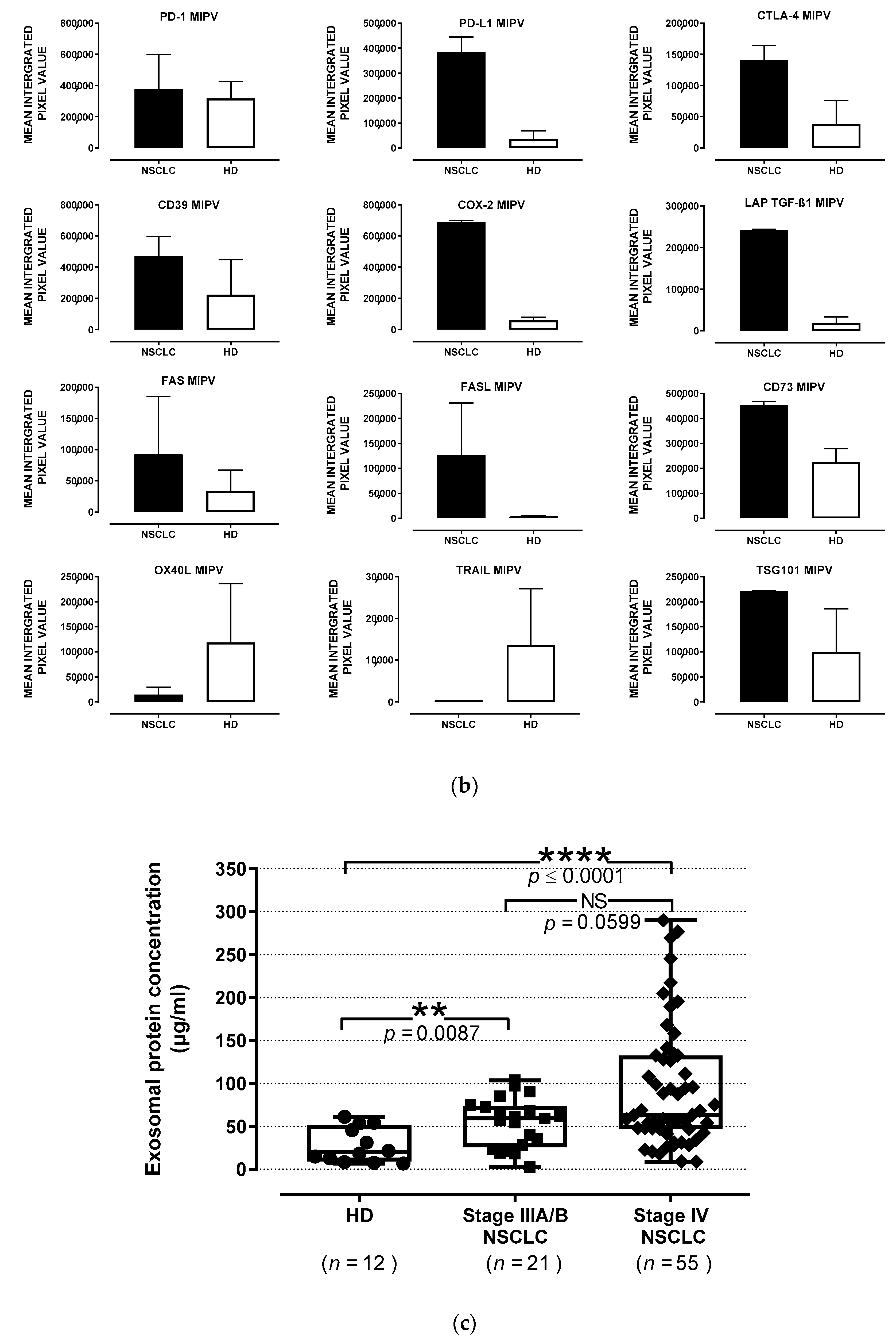
2.5. Detection of the Immune Cargo in Isolated sEV by Western Blots
2.6. Flow Cytometry for Immunophenotypic Analysis and Enumeration of Immune Cells
2.7. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics of NSCLC Patients
3.2. Characterization of Exosomes Isolated from the Plasma of NSCLC Patients
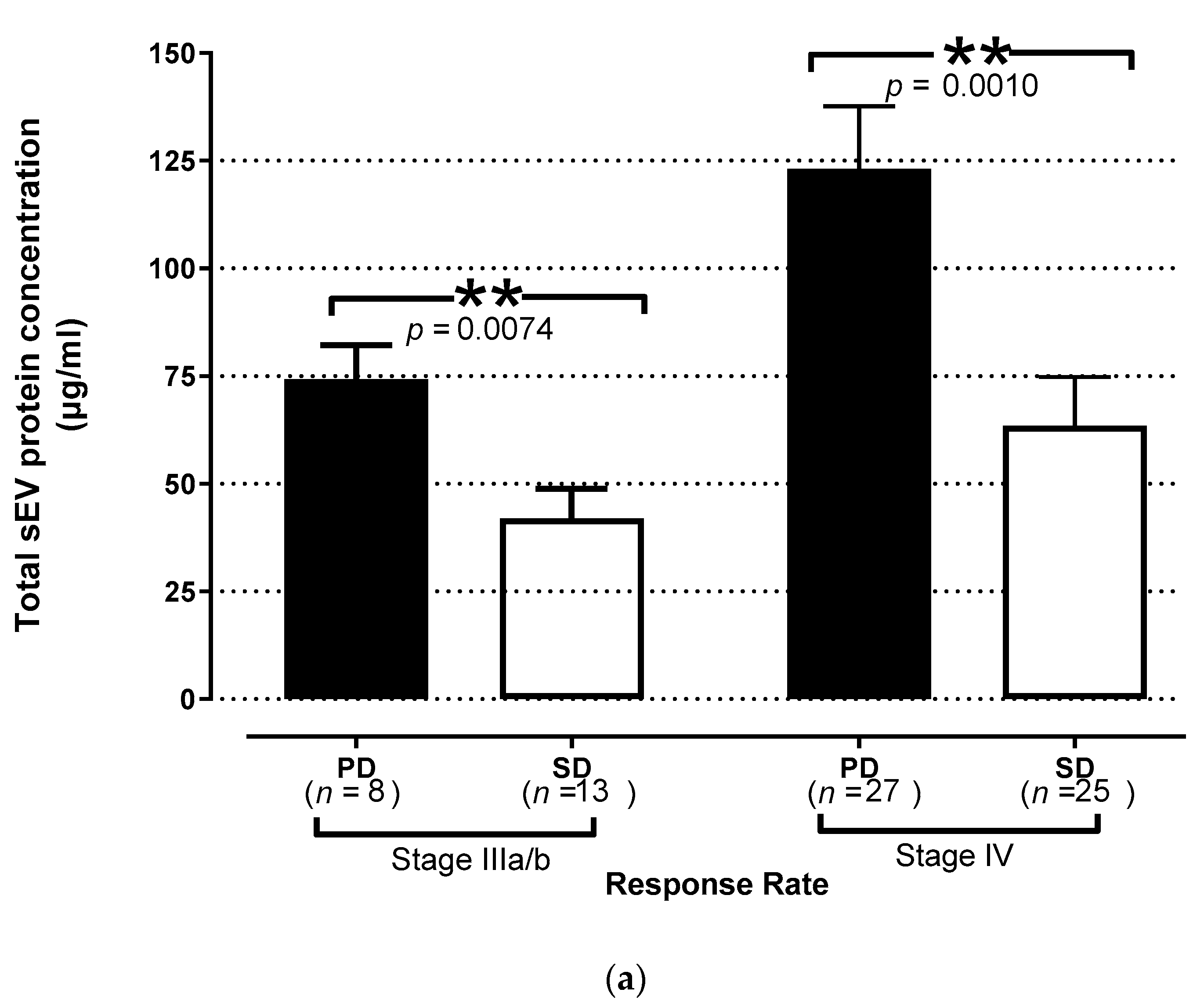
3.3. Association of TEP Levels with the Clinicopathological Characteristics of Chemotherapy-Naïve NSCLC Patients
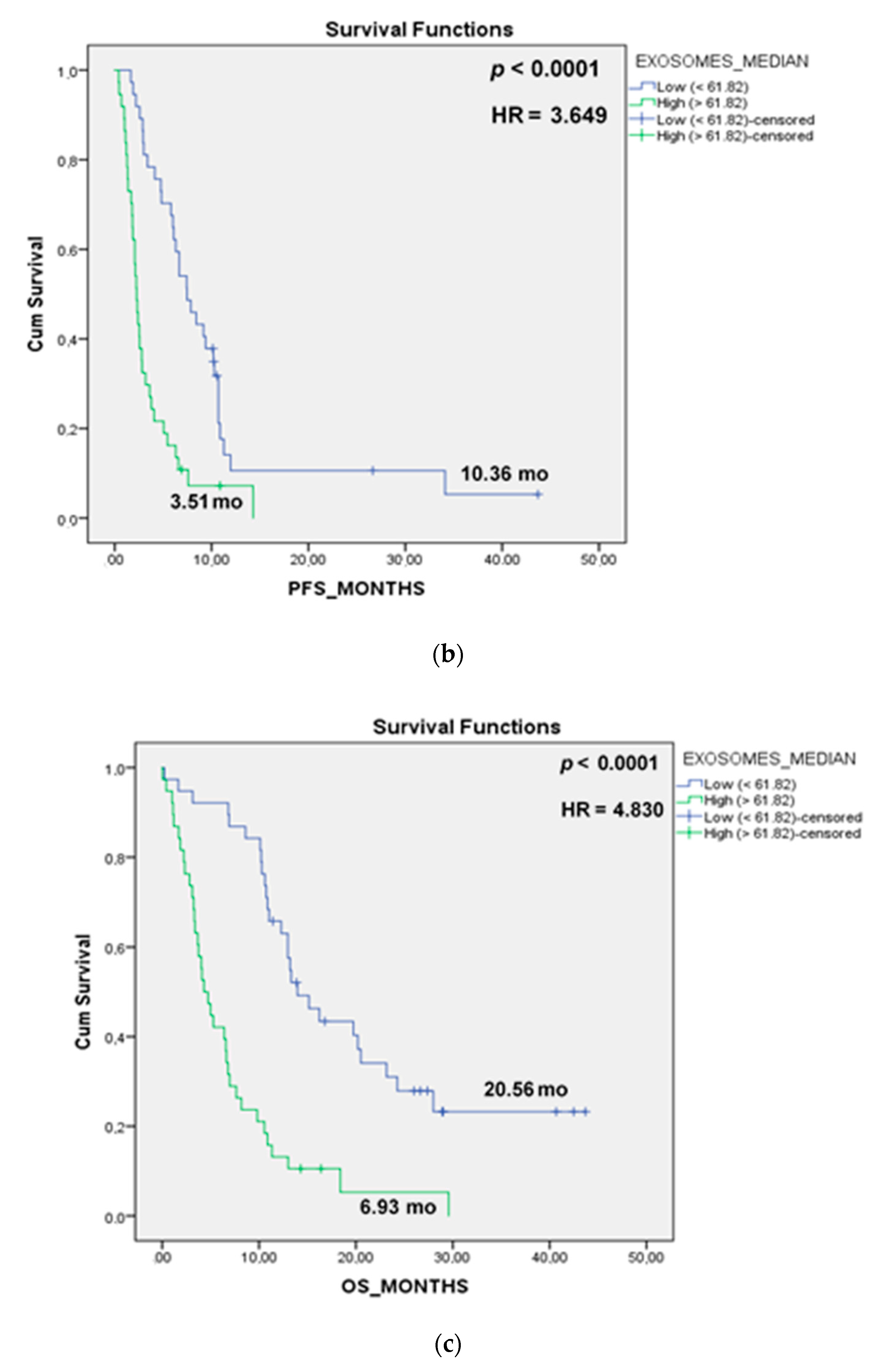
3.4. Prognostic Role of TEP Levels in Patients with NSCLC
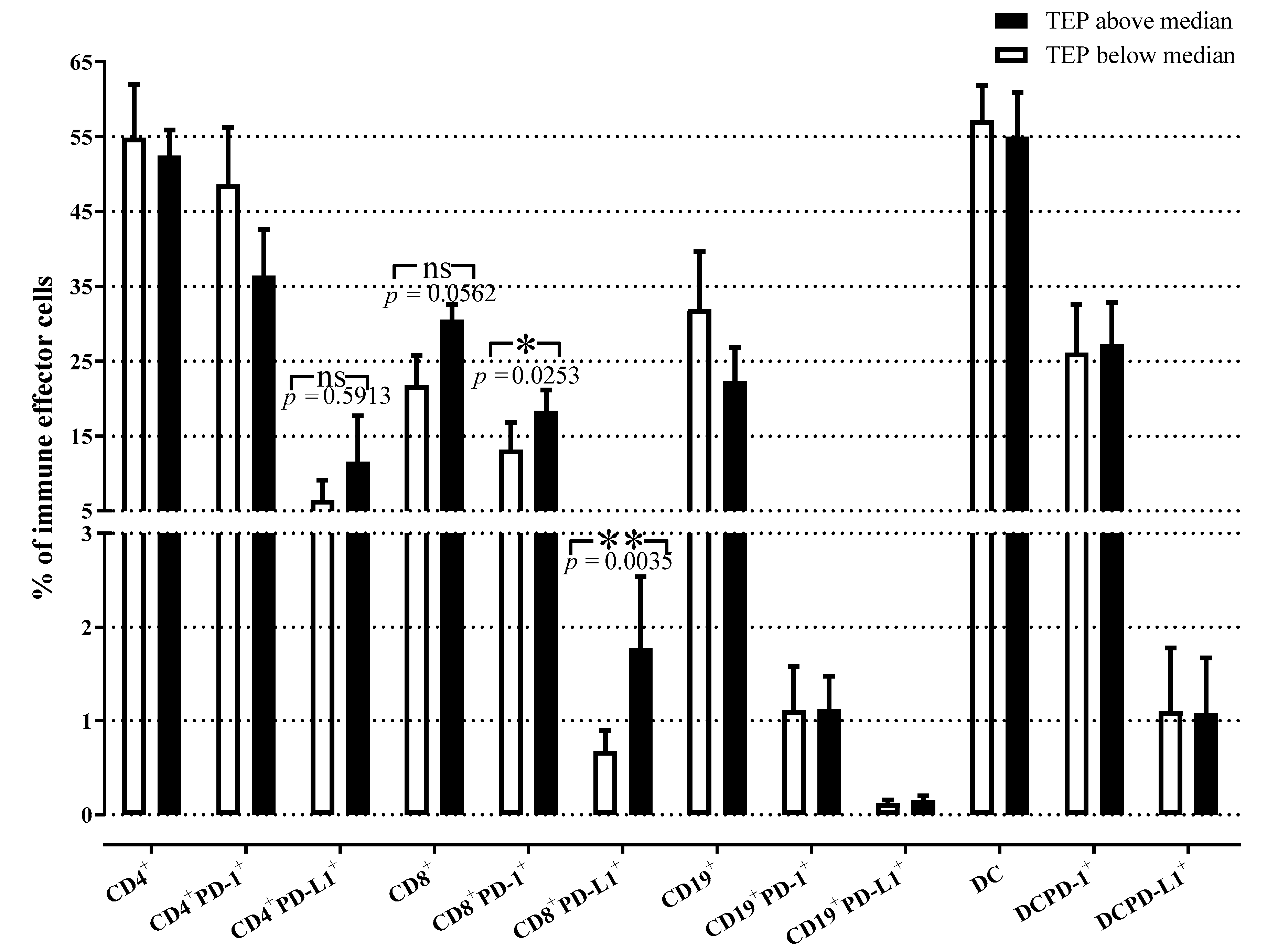
3.5. Quantitative Distribution of Peripheral Immune Effector Cells in NSCLC Patients with High and Low TEP Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Lao, X.Q.; Ho, K.F.; Goggins, W.B.; Tse, S.L.A. Incidence and mortality of lung cancer: Global trends and association with socioeconomic status. Sci. Rep. 2017, 7, 1–9. [Google Scholar]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; Committee, E.G. Early and Locally Advanced Non-Small-Cell Lung Cancer (NSCLC): ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- Hoang, T.; Traynor, A.M.; Schiller, J.H. Novel therapies for lung cancer. Surg. Oncol. 2002, 11, 229–241. [Google Scholar] [CrossRef]
- An, H.J.; Kim, M.H.; Kim, S.H.; Lee, G.; Song, D.H. Prognostic role of extracellular vesicles in squamous cell carcinoma of the lung. Thorac. Cancer 2020, 11, 1989–1995. [Google Scholar] [CrossRef]
- Raimondo, S.; Pucci, M.; Alessandro, R.; Fontana, S. Extracellular vesicles and tumor-immune escape: Biological functions and clinical perspectives. Int. J. Mol. Sci. 2020, 21, 2286. [Google Scholar] [CrossRef]
- Amiri, A.; Pourhanifeh, M.H.; Mirzaei, H.R.; Nahand, J.S.; Moghoofei, M.; Sahebnasagh, R.; Mirzaei, H.; Hamblin, M.R. Exosomes and Lung cancer: Roles in pathophysiology, diagnosis and therapeutic applications. Curr. Med. Chem. 2020, 27, 308–328. [Google Scholar] [CrossRef]
- Pretti, M.A.M.; Bernardes, S.S.; da Cruz, J.G.V.; Boroni, M.; Possik, P.A. Extracellular vesicle-mediated crosstalk between melanoma and the immune system: Impact on tumor progression and therapy response. J. Leukoc. Biol. 2020, 108, 1101–1115. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Tumor-Derived Exosomes and Their Role in Cancer Progression. In Advances in Clinical Chemistry; Academic Press Inc.: Cambridge, MA, USA, 2016; Volume 74, pp. 103–141. ISBN 9780128046890. [Google Scholar]
- Zhang, H.; Lyden, D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat. Protoc. 2019, 14, 1027–1053. [Google Scholar] [CrossRef]
- Whiteside, T.L. Exosomes and tumor-mediated immune suppression. J. Clin. Investig. 2016, 126, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Gao, Y.; Li, N.; Shao, F.; Wang, C.; Wang, P.; Yang, Z.; Li, R.; He, J. Exosomes: New players in cancer. Oncol. Rep. 2017, 38, 665–675. [Google Scholar] [CrossRef]
- Whiteside, T.L. Exosomes in cancer: Another mechanism of tumor-induced immune suppression. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2017; Volume 1036, pp. 81–89. [Google Scholar]
- Hong, C.S.; Sharma, P.; Yerneni, S.S.; Simms, P.; Jackson, E.K.; Whiteside, T.L.; Boyiadzis, M. Circulating exosomes carrying an immunosuppressive cargo interfere with cellular immunotherapy in acute myeloid leukemia. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Li, C.; Donninger, H.; Eaton, J.; Yaddanapudi, K. Regulatory Role of Immune Cell-Derived Extracellular Vesicles in Cancer: The Message Is in the Envelope. Front. Immunol. 2020, 11, 1525. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, Y.; Song, X.; Xie, L.; Zhao, S.; Song, X. Tumor-Derived Exosomal miRNAs as Diagnostic Biomarkers in Non-Small Cell Lung Cancer. Front. Oncol. 2020, 10, 2236. [Google Scholar] [CrossRef]
- Jin, X.; Chen, Y.; Chen, H.; Fei, S.; Chen, D.; Cai, X.; Liu, L.; Lin, B.; Su, H.; Zhao, L.; et al. Evaluation of tumor-derived exosomal miRNA as potential diagnostic biomarkers for early-stage non-small cell lung cancer using next-generation sequencing. Clin. Cancer Res. 2017, 23, 5311–5319. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yu, L.; Lin, X.; Zheng, Q.; Zhang, S.; Chen, D.; Pan, X.; Huang, Y. Combination of Serum miRNAs with Serum Exosomal miRNAs in Early Diagnosis for Non-Small-Cell Lung Cancer. Cancer Manag. Res. 2020, 12, 485–495. [Google Scholar] [CrossRef]
- Niu, L.; Song, X.; Wang, N.; Xue, L.; Song, X.; Xie, L. Tumor-derived exosomal proteins as diagnostic biomarkers in non-small cell lung cancer. Cancer Sci. 2019, 110, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Sandfeld-Paulsen, B.; Jakobsen, K.R.; Bæk, R.; Folkersen, B.H.; Rasmussen, T.R.; Meldgaard, P.; Varming, K.; Jørgensen, M.M.; Sorensen, B.S. Exosomal proteins as diagnostic biomarkers in lung cancer. J. Thorac. Oncol. 2016, 11, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Rabinowits, G.; Gerçel-Taylor, C.; Day, J.M.; Taylor, D.D.; Kloecker, G.H. Exosomal microRNA: A diagnostic marker for lung cancer. Clin. Lung Cancer 2009, 10, 42–46. [Google Scholar] [CrossRef]
- Alipoor, S.D.; Mortaz, E.; Varahram, M.; Movassaghi, M.; Kraneveld, A.D.; Garssen, J.; Adcock, I.M. The potential biomarkers and immunological effects of tumor-derived exosomes in lung cancer. Front. Immunol. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Reclusa, P.; Taverna, S.; Pucci, M.; Durendez, E.; Calabuig, S.; Manca, P.; Serrano, M.J.; Sober, L.; Pauwels, P.; Russo, A.; et al. Exosomes as diagnostic and predictive biomarkers in lung cancer. J. Thorac. Dis. 2017, 9, S1373–S1382. [Google Scholar] [CrossRef]
- Yuwen, D.-L.; Sheng, B.-B.; Liu, J.; Wenyu, W.; Shu, Y.-Q. MiR-146a-5p level in serum exosomes predicts therapeutic effect of cisplatin in non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2650–2658. [Google Scholar] [PubMed]
- Sandfeld-Paulsen, B.; Aggerholm-Pedersen, N.; Baek, R.; Jakobsen, K.R.; Meldgaard, P.; Folkersen, B.H.; Rasmussen, T.R.; Varming, K.; Jørgensen, M.M.; Sorensen, B.S. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol. Oncol. 2016, 10, 1595–1602. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.-N.; Yerneni, S.S.; Brunner, C.; Theodorakis, J.; Hoffmann, T.K.; Whiteside, T.L. Plasma-derived Exosomes Reverse Epithelial-to-Mesenchymal Transition after Photodynamic Therapy of Patients with Head and Neck Cancer. Oncoscience 2018, 5, 75–87. [Google Scholar] [CrossRef]
- Theodoraki, M.N.; Yerneni, S.; Gooding, W.E.; Ohr, J.; Clump, D.A.; Bauman, J.E.; Ferris, R.L.; Whiteside, T.L. Circulating exosomes measure responses to therapy in head and neck cancer patients treated with cetuximab, ipilimumab, and IMRT. Oncoimmunology 2019, 8, e1593805. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for lung cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Hong, C.S.; Funk, S.; Muller, L.; Boyiadzis, M.; Whiteside, T.L. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J. Extracell. Vesicles 2016, 5, 29289. [Google Scholar] [CrossRef]
- Ludwig, S.; Floros, T.; Theodoraki, M.N.; Hong, C.S.; Jackson, E.K.; Lang, S.; Whiteside, T.L. Suppression of Lymphocyte Functions by Plasma Exosomes Correlates with Disease Activity in Patients with Head and Neck Cancer. Clin. Cancer Res. 2017, 23, 4843–4854. [Google Scholar] [CrossRef]
- Theodoraki, M.-N.; Hoffmann, T.K.; Jackson, E.K.; Whiteside, T.L. Exosomes in HNSCC plasma as surrogate markers of tumour progression and immune competence. Clin. Exp. Immunol. 2018, 194, 67–78. [Google Scholar] [CrossRef]
- Ludwig, N.; Hong, C.; Ludwig, S.; Azambuja, J.H.; Sharma, P.; Theodoraki, M.; Whiteside, T.L. Isolation and Analysis of Tumor-Derived Exosomes. Curr. Protoc. Immunol. 2019, 127, e91. [Google Scholar] [CrossRef]
- Sharma, P.; Ludwig, S.; Muller, L.; Hong, C.S.; Kirkwood, J.M.; Ferrone, S.; Whiteside, T.L. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J. Extracell. Vesicles 2018, 7, 1435138. [Google Scholar] [CrossRef]
- Sharma, P.; Diergaarde, B.; Ferrone, S.; Kirkwood, J.M.; Whiteside, T.L. Melanoma cell-derived exosomes in plasma of melanoma patients suppress functions of immune effector cells. Sci. Rep. 2020, 10, 92. [Google Scholar] [CrossRef]
- Ludwig, N.; Whiteside, T.L.; Reichert, T.E. Challenges in Exosome Isolation and Analysis in Health and Disease. Int. J. Mol. Sci. 2019, 20, 4684. [Google Scholar] [CrossRef]
- Ludwig, N.; Razzo, B.M.; Yerneni, S.S.; Whiteside, T.L. Optimization of cell culture conditions for exosome isolation using mini-size exclusion chromatography (mini-SEC). Exp. Cell Res. 2019, 378, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Kano, M.; Akutsu, Y.; Hanari, N.; Hoshino, I.; Murakami, K.; Usui, A.; Suito, H.; Takahashi, M.; Otsuka, R.; et al. Quantification of plasma exosome is a potential prognostic marker for esophageal squamous cell carcinoma. Oncol. Rep. 2016, 36, 2535–2543. [Google Scholar] [CrossRef]
- Rijavec, E.; Coco, S.; Genova, C.; Rossi, G.; Longo, L.; Grossi, F. Liquid Biopsy in Non-Small Cell Lung Cancer: Highlights and Challenges. Cancers 2019, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhan, Y.; Luo, J.; Feng, J.; Lu, J.; Zheng, H.; Wen, Q.; Fan, S. Roles of exosomes in the carcinogenesis and clinical therapy of non-small cell lung cancer. Biomed. Pharmacother. 2019, 111, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, Z.; Yuan, S.; Xie, W.; Li, C.; Hu, Z.; Xiang, Y.; Wu, N.; Wu, L.; Bai, L.; et al. Circulating exosomal microRNAs as prognostic biomarkers for non-small-cell lung cancer. Oncotarget 2017, 8, 13048–13058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, H. Serum exosomal miR-378 upregulation is associated with poor prognosis in non–small-cell lung cancer patients. J. Clin. Lab. Anal. 2020, 34, e23237. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, C.; Zhi, C.; Liang, W.; Wang, X.; Chen, X.; Lv, T.; Shen, Q.; Song, Y.; Lin, D.; et al. Clinical significance of PD-L1 expression in serum-derived exosomes in NSCLC patients. J. Transl. Med. 2019, 17, 355. [Google Scholar] [CrossRef]
- Kotsakis, A.; Kallergi, G.; Aggouraki, D.; Lyristi, Z.; Koinis, F.; Lagoudaki, E.; Koutsopoulos, A.; Georgoulias, V.; Vetsika, E.-K. CD8+ PD-1+ T-cells and PD-L1+ circulating tumor cells in chemotherapy-naïve non-small cell lung cancer: Towards their clinical relevance? Ther. Adv. Med. Oncol. 2019, 11, 1758835919853193. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical significance of PD-L1+ exosomes in plasma of head and neck cancer patients. Clin. Cancer Res. 2018, 24, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
| Patients (n = 76) Levels of Exosomes | ||||
|---|---|---|---|---|
| Parameters | n (%) | Low | High | p Value |
| Age (years) | Median 65 (range, 44–84) | 0.037 | ||
| <65 | 38 (50) | 12 | 22 | |
| ≥65 | 38 (50) | 26 | 16 | |
| Gender | 0.544 | |||
| Male | 63 (82.9) | 30 | 33 | |
| Female | 13 (17.1) | 8 | 5 | |
| Stage | 0.318 | |||
| Stage IIIA/B | 23 (30.3) | 14 | 9 | |
| Stage IV | 53 (69.7) | 24 | 29 | |
| Histology | 0.100 | |||
| Squamous | 37 (48.7) | 19 | 18 | |
| Non-squamous | 39 (51.3) | 19 | 20 | |
| EGFR status | 0.484 | |||
| Wild-type | 30 (39.5) | 16 | 14 | |
| Mutant | 2 (2.6) | 0 | 2 | |
| NE | 44 (57.9) | 22 | 22 | |
| Platinum-based chemotherapy regimen | 0.725 | |||
| Taxanes | 35 (47.9) | 18 | 17 | |
| Pemetrexed | 18 (24.7) | 8 | 10 | |
| Gemcitabine | 18 (24.7) | 10 | 8 | |
| Vinorelbine | 2 (2.7) | 1 | 1 | |
| Response to therapy (after 3 cycles) | 0.001 | |||
| PR | 19 (25) | 14 | 5 | |
| SD | 20 (26.3) | 15 | 5 | |
| PD | 35 (46) | 8 | 27 | |
| NE | 2 (2.7) | 1 | 1 | |
| Univariate Analysis | PROGRESSION-FREE SURVIVAL | OVERALL SURVIVAL | ||
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Age (≥65 vs. <65) | 1.351 (0.835–2.187) | 0.220 | 1.028 (0.624–1.696) | 0.912 |
| Gender (Male vs. Female) | 1.190 (0.633–2.237) | 0.386 | 1.320 (0.669–2.607) | 0.424 |
| Histology (SQ vs. Non-SQ) | 1.345 (0.828–2.183) | 0.231 | 1.286 (0.782–2.114) | 0.322 |
| EGFR status (WT vs. Mutant) | 1.657 (0.383–7.164) | 0.499 | 2.707 (0.591–12.404) | 0.200 |
| Stage (IV vs. III) | 2.223 (1.265–3.908) | 0.005 | 1.176 (0.689–2.007) | 0.552 |
| Exosomes (high vs. low) | 3.074 (1.856–5.089) | <0.0001 | 3.755 (2.215–6.365) | <0.0001 |
| Multivariate Analysis | PROGRESSION-FREE SURVIVAL | OVERALL SURVIVAL | ||
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Stage (IV vs. III) | 1.955 (1.113–3.435) | 0.020 | N/A | N/A |
| Exosomes (high vs. low) | 2.852 (1.714–4.744) | <0.0001 | 3.755 (2.215–6.365) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vetsika, E.-K.; Sharma, P.; Samaras, I.; Markou, A.; Georgoulias, V.; Whiteside, T.L.; Kotsakis, A. Small Extracellular Vesicles in Pre-Therapy Plasma Predict Clinical Outcome in Non-Small-Cell Lung Cancer Patients. Cancers 2021, 13, 2041. https://doi.org/10.3390/cancers13092041
Vetsika E-K, Sharma P, Samaras I, Markou A, Georgoulias V, Whiteside TL, Kotsakis A. Small Extracellular Vesicles in Pre-Therapy Plasma Predict Clinical Outcome in Non-Small-Cell Lung Cancer Patients. Cancers. 2021; 13(9):2041. https://doi.org/10.3390/cancers13092041
Chicago/Turabian StyleVetsika, Eleni-Kyriaki, Priyanka Sharma, Ioannis Samaras, Alexandra Markou, Vassilis Georgoulias, Theresa L. Whiteside, and Athanasios Kotsakis. 2021. "Small Extracellular Vesicles in Pre-Therapy Plasma Predict Clinical Outcome in Non-Small-Cell Lung Cancer Patients" Cancers 13, no. 9: 2041. https://doi.org/10.3390/cancers13092041
APA StyleVetsika, E.-K., Sharma, P., Samaras, I., Markou, A., Georgoulias, V., Whiteside, T. L., & Kotsakis, A. (2021). Small Extracellular Vesicles in Pre-Therapy Plasma Predict Clinical Outcome in Non-Small-Cell Lung Cancer Patients. Cancers, 13(9), 2041. https://doi.org/10.3390/cancers13092041







