Clinical, Pathological and Prognostic Features of Rare BRAF Mutations in Metastatic Colorectal Cancer (mCRC): A Bi-Institutional Retrospective Analysis (REBUS Study)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Extraction and Assessment of RAS and BRAF Mutational Status
2.2. Assessment of MMR/MSI Status
2.3. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
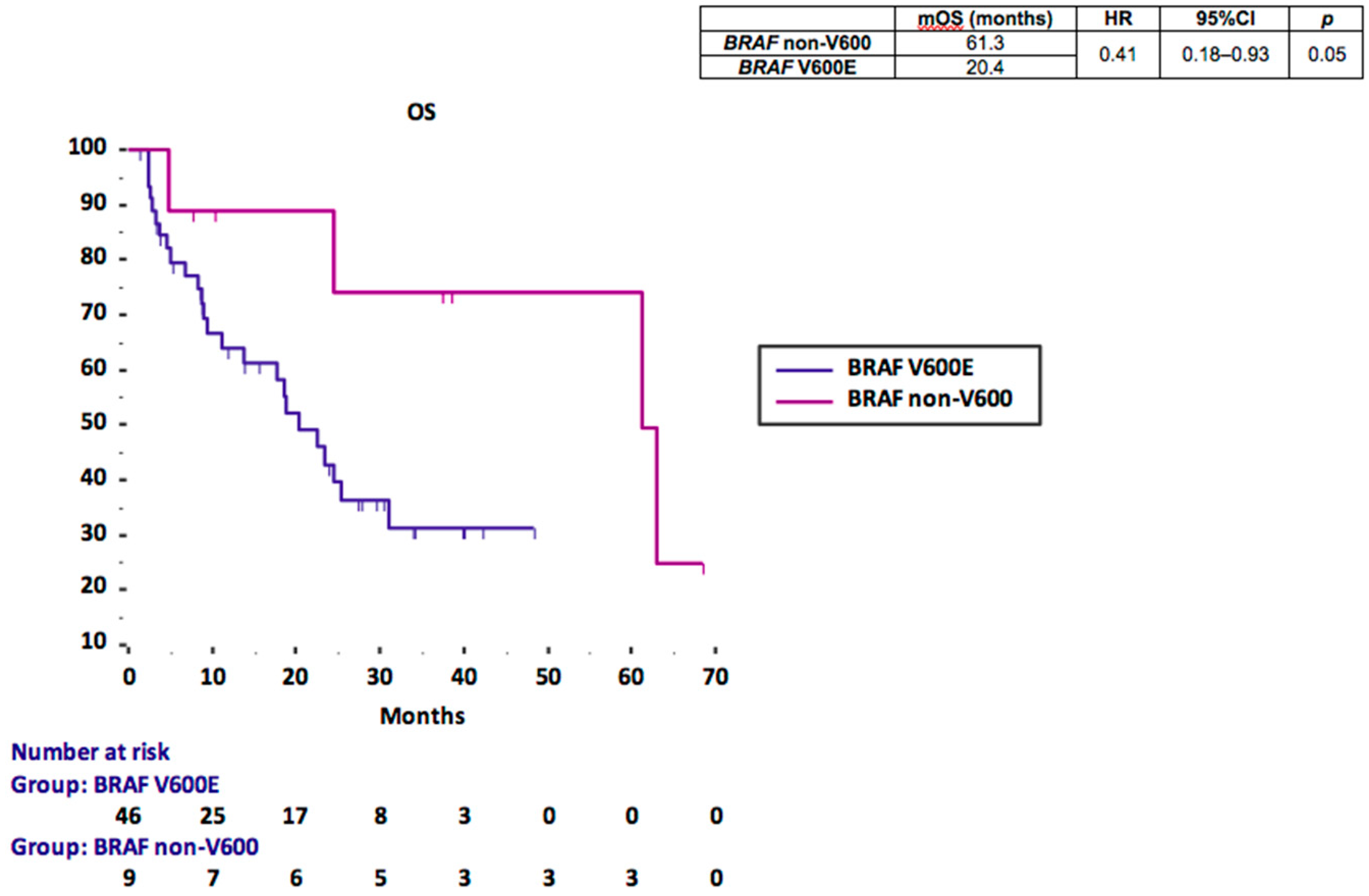
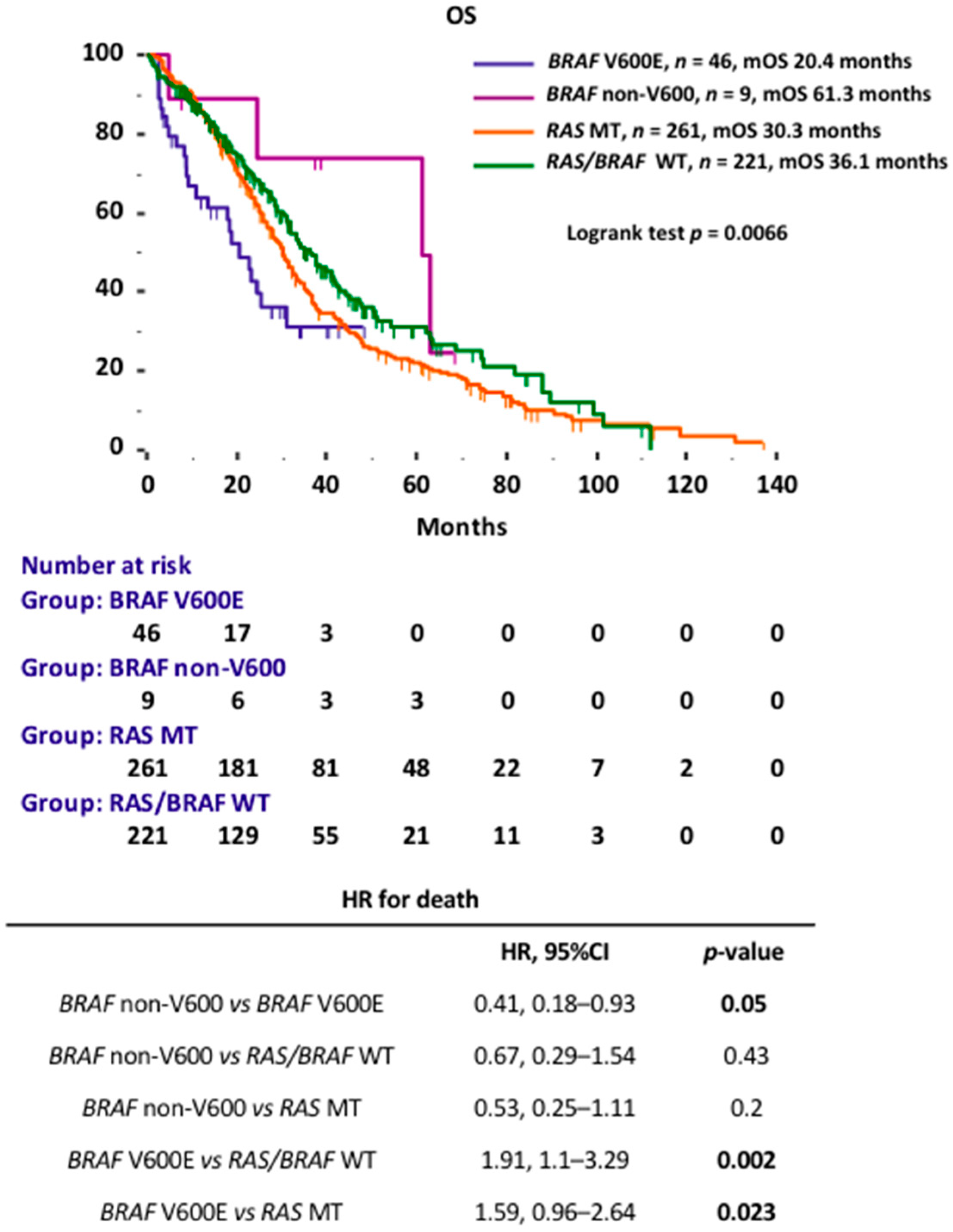
3.2. BRAF Prognostic Performance
4. Discussion
5. Conclusions
- BRAF V600E mutation is associated with poor prognosis in the setting of mCRC.
- Prognosis of BRAF-mutated mCRC significantly varies depending on the specific missense mutation. Namely, rare non-V600 mutations identify a distinct subgroup of patients with favorable prognosis and peculiar phenotype.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orlandi, A.; Calegari, A.; Inno, A.; Berenato, R.; Caporale, M.; Niger, M.; Bossi, I.; Di Bartolomeo, M.; de Braud, F.; Pietrantonio, F. BRAF in metastatic colorectal cancer: The future starts now. Pharmacogenomics 2015, 16, 2069–2081. [Google Scholar] [CrossRef]
- Clancy, C.; Burke, J.P.; Kalady, M.F.; Coffey, J.C. BRAF mutation is associated with distinct clinicopathological characteristics in colorectal cancer: A systematic review and meta-analysis. Colorectal Dis. 2013, 15, e711–e718. [Google Scholar] [CrossRef]
- Jones, J.C.; Renfro, L.A.; Al-Shamsi, H.O.; Schrock, A.B.; Rankin, A.; Zhang, B.Y.; Kasi, P.M.; Voss, J.S.; Leal, A.D.; Sun, J.; et al. (Non-V600) BRAF Mutations Define a Clinically Distinct Molecular Subtype of Metastatic Colorectal Cancer. J. Clin. Oncol. 2017, 35, 2624–2630. [Google Scholar] [CrossRef]
- Loupakis, F.; Moretto, R.; Aprile, G.; Muntoni, M.; Cremolini, C.; Iacono, D.; Casagrande, M.; Ferrari, L.; Salvatore, L.; Schirripa, M.; et al. Clinico-pathological nomogram for predicting BRAF mutational status of metastatic colorectal cancer. Br. J. Cancer 2016, 114, 30–36. [Google Scholar] [CrossRef]
- Tran, B.; Kopetz, S.; Tie, J.; Gibbs, P.; Jiang, Z.Q.; Lieu, C.H.; Agarwal, A.; Maru, D.M.; Sieber, O.; Desai, J. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011, 117, 4623–4632. [Google Scholar] [CrossRef] [Green Version]
- Venderbosch, S.; Nagtegaal, I.D.; Maughan, T.S.; Smith, C.G.; Cheadle, J.P.; Fisher, D.; Kaplan, R.; Quirke, P.; Seymour, M.T.; Richman, S.D.; et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: A pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin. Cancer Res. 2014, 20, 5322–5330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taieb, J.; Jung, A.; Sartore-Bianchi, A.; Peeters, M.; Seligmann, J.; Zaanan, A.; Burdon, P.; Montagut, C.; Laurent-Puig, P. The Evolving Biomarker Landscape for Treatment Selection in Metastatic Colorectal Cancer. Drugs 2019, 79, 1375–1394. [Google Scholar] [CrossRef] [Green Version]
- Clarke, C.N.; Kopetz, E.S. BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: Clinical characteristics, clinical behavior, and response to targeted therapies. J. Gastrointest. Oncol. 2015, 6, 660–667. [Google Scholar] [PubMed]
- Richman, S.D.; Seymour, M.T.; Chambers, P.; Elliott, F.; Daly, C.L.; Meade, A.M.; Taylor, G.; Barrett, J.H.; Quirke, P. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: Results from the MRC FOCUS trial. J. Clin. Oncol. 2009, 27, 5931–5937. [Google Scholar] [CrossRef] [PubMed]
- Seligmann, J.F.; Fisher, D.; Smith, C.G.; Richman, S.D.; Elliott, F.; Brown, S.; Adams, R.; Maughan, T.; Quirke, P.; Cheadle, J.; et al. Investigating the poor outcomes of BRAF-mutant advanced colorectal cancer: Analysis from 2530 patients in randomised clinical trials. Ann. Oncol. 2017, 28, 562–568. [Google Scholar] [CrossRef]
- Loupakis, F.; Intini, R.; Cremolini, C.; Orlandi, A.; Sartore-Bianchi, A.; Pietrantonio, F.; Pella, N.; Spallanzani, A.; Dell’Aquila, E.; Scartozzi, M.; et al. A validated prognostic classifier for (V600E)BRAF-mutated metastatic colorectal cancer: The ‘BRAF BeCool’ study. Eur. J. Cancer 2019, 118, 121–130. [Google Scholar] [CrossRef]
- Ardekani, G.S.; Jafarnejad, S.M.; Tan, L.; Saeedi, A.; Li, G. The prognostic value of BRAF mutation in colorectal cancer and melanoma: A systematic review and meta-analysis. PLoS ONE 2012, 7, e47054. [Google Scholar]
- Schirripa, M.; Bergamo, F.; Cremolini, C.; Casagrande, M.; Lonardi, S.; Aprile, G.; Yang, D.; Marmorino, F.; Pasquini, G.; Sensi, E.; et al. BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br. J. Cancer 2015, 112, 1921–1928. [Google Scholar] [CrossRef] [Green Version]
- Tosi, F.; Magni, E.; Amatu, A.; Mauri, G.; Bencardino, K.; Truini, M.; Veronese, S.; De Carlis, L.; Ferrari, G.; Nichelatti, M.; et al. Effect of KRAS and BRAF Mutations on Survival of Metastatic Colorectal Cancer After Liver Resection: A Systematic Review and Meta-Analysis. Clin. Colorectal Cancer 2017, 16, e153–e163. [Google Scholar] [CrossRef] [PubMed]
- Margonis, G.A.; Buettner, S.; Andreatos, N.; Kim, Y.; Wagner, D.; Sasaki, K.; Beer, A.; Schwarz, C.; Løes, I.M.; Smolle, M.; et al. Association of BRAF Mutations With Survival and Recurrence in Surgically Treated Patients With Metastatic Colorectal Liver Cancer. JAMA Surg. 2018, 153, e180996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, Y.; Muneoka, Y.; Nagahashi, M.; Ichikawa, H.; Tajima, Y.; Hirose, Y.; Ando, T.; Nakano, M.; Sakata, J.; Kameyama, H.; et al. BRAF V600E and SRC mutations as molecular markers for predicting prognosis and conversion surgery in Stage IV colorectal cancer. Sci. Rep. 2019, 9, 2466. [Google Scholar] [CrossRef] [Green Version]
- Pietrantonio, F.; Petrelli, F.; Coinu, A.; Di Bartolomeo, M.; Borgonovo, K.; Maggi, C.; Cabiddu, M.; Iacovelli, R.; Bossi, I.; Lonati, V.; et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: A meta-analysis. Eur. J. Cancer 2015, 51, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Bokemeyer, C.; Van Cutsem, E.; Rougier, P.; Ciardiello, F.; Heeger, S.; Schlichting, M.; Celik, I.; Köhne, C.H. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: Pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur. J. Cancer 2012, 48, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, R.B. New therapeutic strategies for BRAF mutant colorectal cancers. J. Gastrointest. Oncol. 2015, 6, 650–659. [Google Scholar]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef] [Green Version]
- Corcoran, R.B.; André, T.; Atreya, C.E.; Schellens, J.H.M.; Yoshino, T.; Bendell, J.C.; Hollebecque, A.; McRee, A.J.; Siena, S.; Middleton, G.; et al. Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAF(V600E)-Mutant Colorectal Cancer. Cancer Discov. 2018, 8, 428–443. [Google Scholar] [CrossRef] [Green Version]
- Grothey, A.; Tabernero, J.; Taieb, J.; Yaeger, R.; Yoshino, T.; Maiello, E.; Fernandez, E.E.; Casado, A.R.; Ross, P.; André, T.; et al. ANCHOR CRC: A single-arm, phase 2 study of encorafenib, binimetinib plus cetuximab in previously untreated BRAF V600E-mutant metastatic colorectal cancer. Ann. Oncol. 2020, 31, S242–S243. [Google Scholar] [CrossRef]
- Cremolini, C.; Di Bartolomeo, M.; Amatu, A.; Antoniotti, C.; Moretto, R.; Berenato, R.; Perrone, F.; Tamborini, E.; Aprile, G.; Lonardi, S.; et al. BRAF codons 594 and 596 mutations identify a new molecular subtype of metastatic colorectal cancer at favorable prognosis. Ann. Oncol. 2015, 26, 2092–2097. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Yaeger, R.; Rodrik-Outmezguine, V.S.; Tao, A.; Torres, N.M.; Chang, M.T.; Drosten, M.; Zhao, H.; Cecchi, F.; Hembrough, T.; et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature 2017, 548, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Schirripa, M.; Biason, P.; Lonardi, S.; Pella, N.; Pino, M.S.; Urbano, F.; Antoniotti, C.; Cremolini, C.; Corallo, S.; Pietrantonio, F.; et al. Class 1, 2, and 3 BRAF-Mutated Metastatic Colorectal Cancer: A Detailed Clinical, Pathologic, and Molecular Characterization. Clin. Cancer Res. 2019, 25, 3954–3961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natalicchio, M.I.; Improta, G.; Zupa, A.; Cursio, O.E.; Stampone, E.; Possidente, L.; Teresa Gerardi, A.M.; Vita, G.; Martini, M.; Cassano, A.; et al. Pyrosequencing evaluation of low-frequency KRAS mutant alleles for EGF receptor therapy selection in metastatic colorectal carcinoma. Future Oncol. 2014, 10, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Calegari, M.A.; Inno, A.; Monterisi, S.; Orlandi, A.; Santini, D.; Basso, M.; Cassano, A.; Martini, M.; Cenci, T.; de Pascalis, I.; et al. A phase 2 study of temozolomide in pretreated metastatic colorectal cancer with MGMT promoter methylation. Br. J. Cancer 2017, 116, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Chan, A.P. PROVEAN web server: A tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 2015, 31, 2745–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaeger, R.; Chatila, W.K.; Lipsyc, M.D.; Hechtman, J.F.; Cercek, A.; Sanchez-Vega, F.; Jayakumaran, G.; Middha, S.; Zehir, A.; Donoghue, M.T.A.; et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell 2018, 33, 125–136.e123. [Google Scholar] [CrossRef] [Green Version]
- Orlandi, A.; Basso, M.; Calegari, M.A.; Barone, C. New life for retrospective study in the precision oncology era. Ann. Oncol. 2015, 26, 2352–2353. [Google Scholar] [CrossRef] [PubMed]
| BRAF Mutation | Class | Kinase Activity | Number of Patients, N (%) |
|---|---|---|---|
| V600E | 1 | High | 46 (84) |
| K601E | 2 | High | 1 (1.8) |
| G469A | 2 | High | 1 (1.8) |
| G469R | 2 | Intermediate | 1 (1.8) |
| G466E | 3 | Low | 1 (1.8) |
| G466A | 3 | Low | 1 (1.8) |
| D594N | 3 | None | 1 (1.8) |
| D594G | 3 | None | 2 (3.4) |
| T599I | / | Unknown | 1 (1.8) |
| Characteristics | BRAF V600E Total = 46, N (%) | BRAF Non-V600 Total = 9, N (%) | p |
|---|---|---|---|
| Gender | |||
| Female | 24 (52.2) | 3 (33.4) | 0.3 |
| Male | 22 (47.8) | 6 (66.6) | |
| Age | |||
| median, years | 66 | 61 | |
| range, years | 42–85 | 45–79 | |
| ≤65 years | 22 (47.8) | 5 (55.5) | 0.6 |
| >65 years | 24 (52.2) | 4 (44.5) | |
| ECOG PS | |||
| 0 | 25 (54.3) | 5 (55.5) | 0.9 |
| 1–2 | 21 (45.7) | 4 (44.5) | |
| Metastases onset | |||
| Synchronous | 27 (58.7) | 6 (66.6) | 0.6 |
| Metachronous | 19 (41.3) | 3 (33.4) | |
| Mucinous histology | |||
| Y | 11 (23.9) | 1 (11.1) | 0.39 |
| N | 35 (76.1) | 8 (88.9) | |
| Primary tumor location | |||
| Right | 25 (54.3) | 1 (11.1) | 0.017 |
| Left | 21 (45.7) | 8 (88.9) | |
| Number of metastatic sites | |||
| 1 | 13 (28.3) | 6 (66.6) | 0.026 |
| >1 | 33 (71.7) | 3 (33.4) | |
| Metastatic sites | |||
| Liver metastases | 30 (65.2) | 7 (77.8) | 0.46 |
| Lymph node metastases | 28 (60.8) | 3 (33.4) | 0.12 |
| Lung metastases | 18 (39.1) | 1 (11.1) | 0.1 |
| Peritoneal metastases | 14 (30.4) | 2 (22.2) | 0.61 |
| Grading | |||
| 2 | 19 (41.3) | 7 (77.8) | 0.045 |
| 3 | 27 (58.7) | 2 (22.3) | |
| MSI/MMR status | |||
| MSI-H/MMR-deficient | 8 (17.4) | / | <0.001 |
| MSS/MMR-proficient | 25 (54.3) | 7 (77.7) | |
| NE | 13 (28.3) | 2 (22.2) | |
| Resection of metastases | |||
| Y | 8 (17.4) | 6 (66.6) | 0.002 |
| N | 38 (82.6) | 3 (33.4) | |
| BRAF Be Cool score | |||
| Low risk | 26 (56.5) | NA | |
| Intermediate risk | 19 (41.3) | NA | |
| High risk | 1 (2.2) | NA | |
| Lines of treatment | |||
| 1 | 27 (58.7) | 3 (33.4) | 0.16 |
| 2 | 11 (23.9) | 1 (11.1) | 0.39 |
| ≥3 | 8 (17.4) | 5 (55.5) | 0.01 |
| First line regimen | |||
| Triplet-based | 7 (15.2) | 1 (11.1) | 0.74 |
| Doublet-based | 39 (84.8) | 8 (88.9) | |
| Administered drugs | |||
| Fluoropyrimidines | 45 (97.8) | 9 (100) | 0.65 |
| Oxaliplatin | 42 (91.3) | 7 (77.8) | 0.23 |
| Irinotecan | 22 (47.8) | 4 (44.5) | 0.85 |
| Raltitrexed | 1 (2.2) | / | 0.65 |
| Bevacizumab | 29 (63) | 6 (66.6) | 0.83 |
| Aflibercept | 1 (2.2) | 1 (11.1) | 0.19 |
| Cetuximab | 4 (8.7) | 1 (11.1) | 0.81 |
| Panitumumab | 1 (2.2) | 1 (11.1) | 0.19 |
| Anti-BRAF | 4 (8.7) | / | 0.35 |
| Regorafenib | 3 (6.5) | / | 0.43 |
| TAS-102 | 1 (2.2) | 2 (22.2) | 0.01 |
| Variable | Overall Survival (OS) | |||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||||
| HR | 95%CI | p | HR | 95%CI | p | |
| Gender | ||||||
| Male vs. Female | 0.55 | 0.25–1.21 | 0.09 | |||
| Age (years) | ||||||
| <65 vs. >65 | 0.35 | 0.16–0.74 | 0.004 | 0.38 | 0.16–0.92 | 0.034 |
| ECOG PS | ||||||
| 0 vs. 1–2 | 0.31 | 0.13–0.7 | <0.001 | 0.23 | 0.09–0.59 | 0.002 |
| Metastases onset | ||||||
| Metachronous vs. synchronous | 0.86 | 0.41–1.78 | 0.69 | |||
| Number of metastatic sites | ||||||
| 1 vs. >1 | 0.29 | 0.14–0.61 | 0.003 | 0.28 | 0.08–1.01 | 0.052 |
| Primary tumor location | ||||||
| Left vs. right | 0.51 | 0.23–1.11 | 0.06 | |||
| Liver Metastases | ||||||
| No vs. Yes | 0.74 | 0.34–1.59 | 0.46 | |||
| Lymph node metastases | ||||||
| No vs. Yes | 0.53 | 0.25–1.12 | 0.08 | |||
| Peritoneal metastases | ||||||
| No vs. Yes | 0.87 | 0.37–2.02 | 0.73 | |||
| Lung metastases | ||||||
| No vs. Yes | 0.45 | 0.19–1.03 | 0.02 | 0.57 | 0.24–1.3 | 0.18 |
| MSI/MMR status | ||||||
| MSI-H/MMRdef vs. MSS/MMRpro | 0.74 | 0.24–2.29 | 0.63 | |||
| BRAF | ||||||
| non-V600 vs. V600E | 0.41 | 0.18–0.93 | 0.05 | 0.6 | 0.12–2.88 | 0.52 |
| Mucinous histology | ||||||
| Yes vs. No | 0.49 | 0.21–1.16 | 0.18 | |||
| Grading | ||||||
| 2 vs. 3 | 0.26 | 0.12–0.54 | <0.001 | 0.5 | 0.2–1.28 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calegari, M.A.; Salvatore, L.; Di Stefano, B.; Basso, M.; Orlandi, A.; Boccaccino, A.; Lombardo, F.; Auriemma, A.; Zurlo, I.V.; Bensi, M.; et al. Clinical, Pathological and Prognostic Features of Rare BRAF Mutations in Metastatic Colorectal Cancer (mCRC): A Bi-Institutional Retrospective Analysis (REBUS Study). Cancers 2021, 13, 2098. https://doi.org/10.3390/cancers13092098
Calegari MA, Salvatore L, Di Stefano B, Basso M, Orlandi A, Boccaccino A, Lombardo F, Auriemma A, Zurlo IV, Bensi M, et al. Clinical, Pathological and Prognostic Features of Rare BRAF Mutations in Metastatic Colorectal Cancer (mCRC): A Bi-Institutional Retrospective Analysis (REBUS Study). Cancers. 2021; 13(9):2098. https://doi.org/10.3390/cancers13092098
Chicago/Turabian StyleCalegari, Maria Alessandra, Lisa Salvatore, Brunella Di Stefano, Michele Basso, Armando Orlandi, Alessandra Boccaccino, Fiorella Lombardo, Alessandra Auriemma, Ina Valeria Zurlo, Maria Bensi, and et al. 2021. "Clinical, Pathological and Prognostic Features of Rare BRAF Mutations in Metastatic Colorectal Cancer (mCRC): A Bi-Institutional Retrospective Analysis (REBUS Study)" Cancers 13, no. 9: 2098. https://doi.org/10.3390/cancers13092098
APA StyleCalegari, M. A., Salvatore, L., Di Stefano, B., Basso, M., Orlandi, A., Boccaccino, A., Lombardo, F., Auriemma, A., Zurlo, I. V., Bensi, M., Camarda, F., Ribelli, M., Vivolo, R., Cocomazzi, A., Pozzo, C., Milella, M., Martini, M., Bria, E., & Tortora, G. (2021). Clinical, Pathological and Prognostic Features of Rare BRAF Mutations in Metastatic Colorectal Cancer (mCRC): A Bi-Institutional Retrospective Analysis (REBUS Study). Cancers, 13(9), 2098. https://doi.org/10.3390/cancers13092098












