Development of Exhaustion and Acquisition of Regulatory Function by Infiltrating CD8+CD28− T Lymphocytes Dictate Clinical Outcome in Head and Neck Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Monoclonal Antibodies (mAbs) and Immunofluorescence Analyses
2.3. Evaluation of HLA-A2 Positive Patients
2.4. Multidimensional Data Reduction Analysis
2.5. Analysis of G250/CAIX Specific CD8+ T Lymphocytes
2.6. Proliferation Suppression Assay
2.7. Statistical Analyses
3. Results
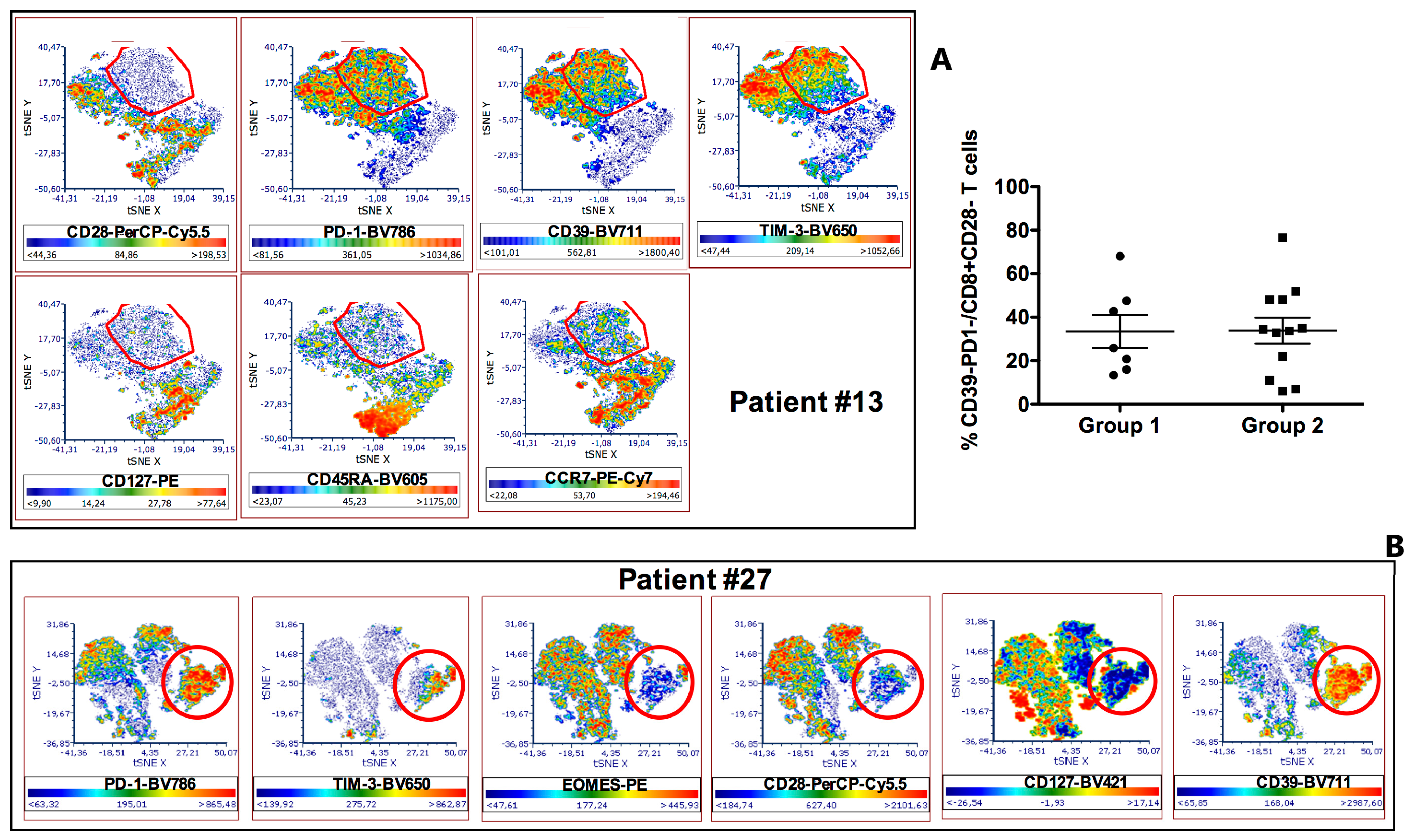
3.1. Comparative Phenotypic and Functional Characterization of Intratumoral T cells between HNSCC Patients with Poor (Group 1) or Good (Group 2) Response to Therapy
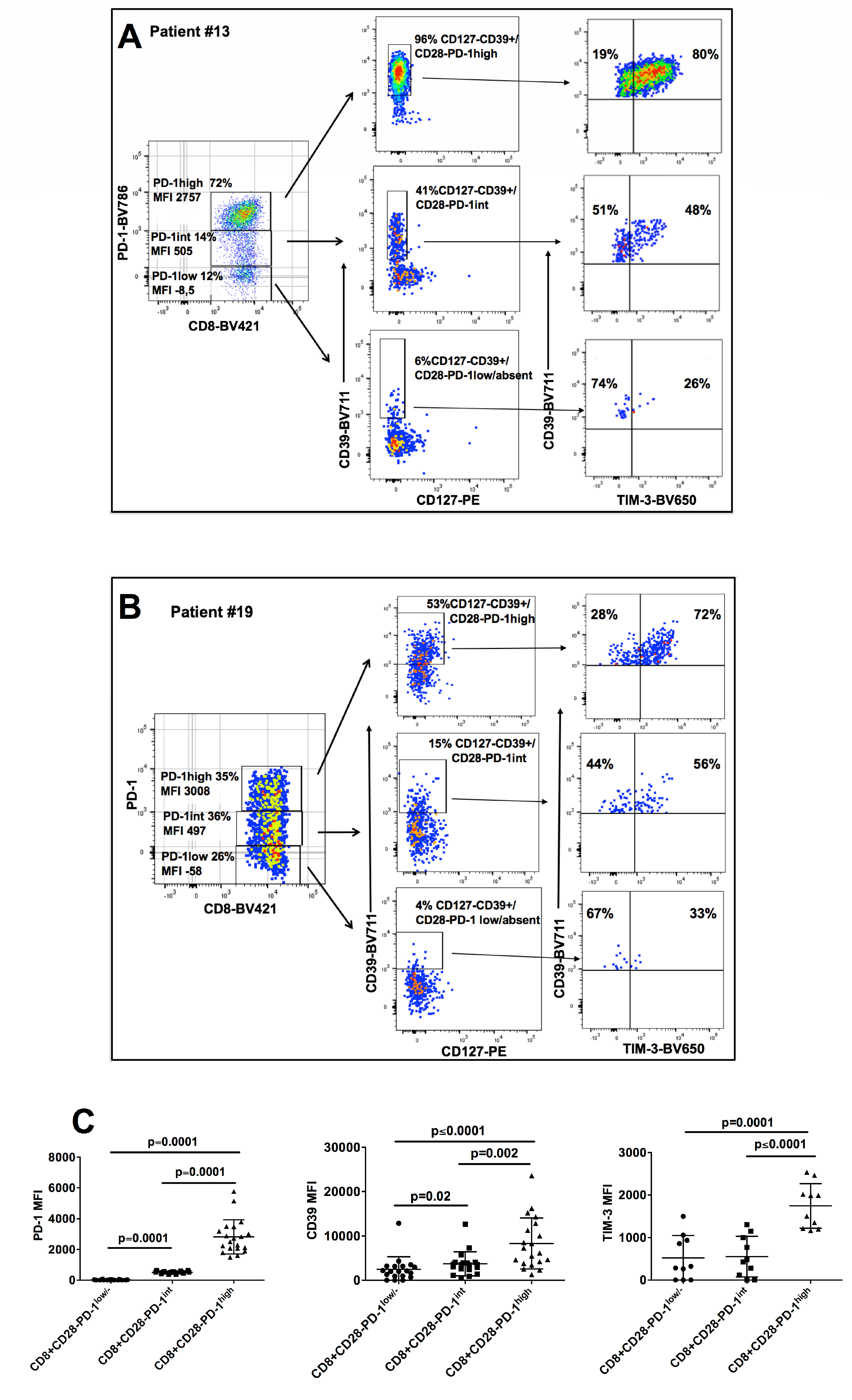
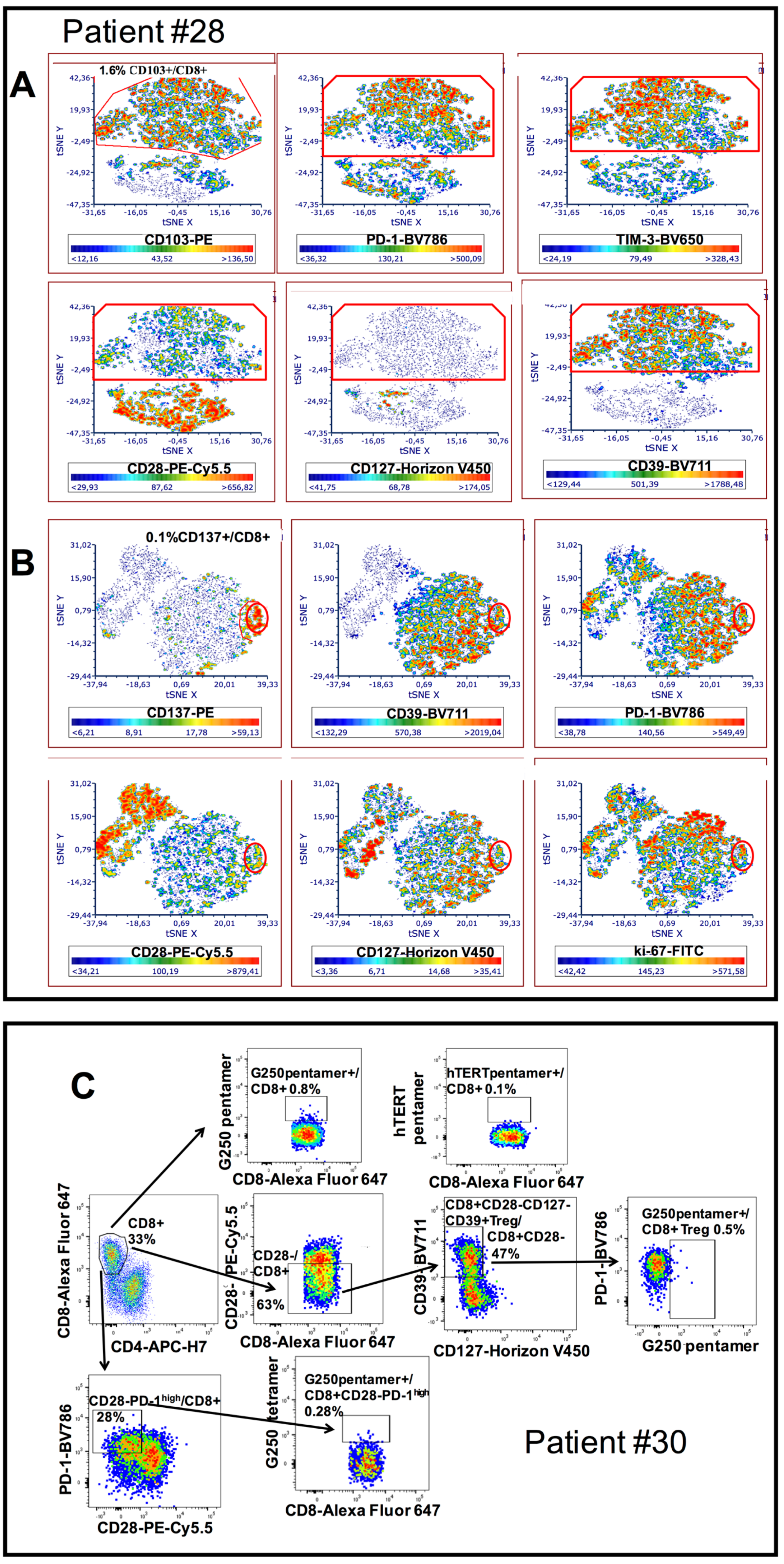
3.2. Phenotypic Characterization of CD8+CD28− HNSCC Infiltrating T Cells
3.3. Characterization of CD8+CD28−PD1hi T cells as CD8+CD28-CD127-CD39+ Treg
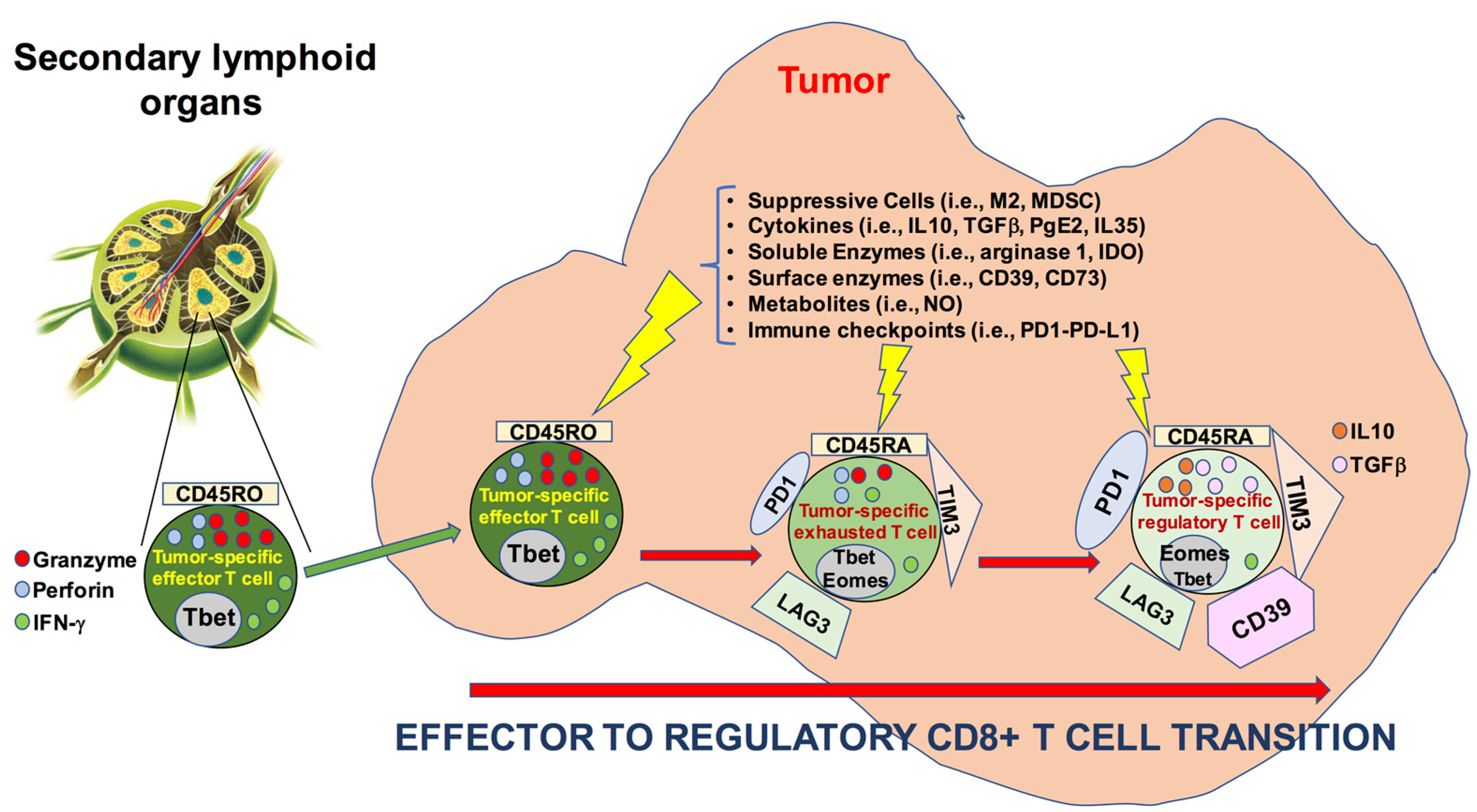
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grégoire, V.; Lefebvre, J.L.; Licitra, L.; Felip, E.; EHNS-ESMO-ESTRO Guidelines Working Group. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21 (Suppl. 5), v184–v186. [Google Scholar] [CrossRef] [PubMed]
- Belgioia, L.; Bacigalupo, A.; Missale, F.; Vecchio, S.; Chiola, I.; Callegari, S.; Verzanini, E.; Peretti, G.; Corvò, R. Individualized treatment of head neck squamous cell carcinoma patients aged 70 or older with radiotherapy alone or associated to cisplatin or cetuximab: Impact of weekly radiation dose on loco-regional control. Med. Oncol. 2019, 36, 42. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.R.; Cupissol, D.; et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [Green Version]
- Maddalo, M.; Borghetti, P.; Tomasini, D.; Corvò, R.; Bonomo, P.; Petrucci, A.; Paiar, F.; Lastrucci, L.; Bonù, M.L.; Greco, D.; et al. Cetuximab and radiotherapy versus cisplatin and radiotherapy for locally advanced head and neck cancer: Long term survival and toxicity outcomes of a randomized phase ii trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 469–477. [Google Scholar] [CrossRef]
- Ling, D.C.; Bakkenist, C.J.; Ferris, R.L.; Clump, D.A. Role of Immunotherapy in Head and Neck Cancer. Semin. Radiat. Oncol. 2018, 28, 12–16. [Google Scholar] [CrossRef]
- von der Grün, J.; Rödel, F.; Brandts, C.; Fokas, E.; Guckenberger, M.; Rödel, C.; Balermpas, P. Targeted Therapies and Immune-Checkpoint Inhibition in Head and Neck Squamous Cell Carcinoma: Where Do We Stand Today and Where to Go? Cancers 2019, 11, 472. [Google Scholar] [CrossRef] [Green Version]
- Le, Q.T.; Colevas, A.D.; O’Sullivan, B.; Lee, A.W.M.; Lee, N.; Ma, B.; Siu, L.L.; Waldron, J.; Lim, C.M.; Riaz, N.; et al. Current Treatment Landscape of Nasopharyngeal Carcinoma and Potential Trials Evaluating the Value of Immunotherapy. J. Natl. Cancer Inst. 2019, 111, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.-W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016, 1, e89829. [Google Scholar] [CrossRef] [Green Version]
- McDermott, J.D.; Bowles, D.W. Epidemiology of Head and Neck Squamous Cell Carcinomas: Impact on Staging and Prevention Strategies. Curr. Treat. Options Oncol. 2019, 20, 43. [Google Scholar] [CrossRef]
- Lee, N.; Zakka, L.R.; Mihm, M.C., Jr.; Schatton, T. Tumour-infiltrating lymphocytes in melanoma prognosis and cancer immunotherapy. Pathology 2016, 48, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Erdag, G.; Schaefer, J.T.; Smolkin, M.E.; Deacon, D.H.; Shea, S.M.; Dengel, L.T.; Patterson, J.W.; Slingluff, C.L., Jr. Immunotype and immunohistologic characteristics of tumor infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012, 72, 1070–1080. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Shen, Y.; Wen, S.; Yamada, S.; Jungbluth, A.A.; Gnjatic, S.; Bajorin, G.F.; Reuter, V.E.; Herr, H.; Old, L.J.; et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 3967–3972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sideras, K.; Biermann, K.; Verheij, J.; Takkenberg, B.R.; Mancham, S.; Hansen, B.E.; Schutz, H.M.; de Man, R.A.; Sprengers, D.; Buschow, S.I.; et al. PD-L1, Galectin-9 and CD8+ tumor-infiltrating lymphocytes are associated with survival in hepatocellular carcinoma. Oncoimmunology 2017, 6, e1273309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balermpas, P.; Rödel, F.; Rödel, C.; Krause, M.; Linge, A.; Lohaus, F.; Baumann, M.; Tinhofer, I.; Budach, V.; Gkika, E.; et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG). Int. J. Cancer 2016, 138, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Partlova, S.; Boucek, J.; Kloudova, K.; Lukesova, E.; Zabrodsky, M.; Grega, M.; Fučíková, J.; Truxová, I.; Tachezy, R.; Špíšek, R.; et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology 2015, 4, e965570. [Google Scholar] [CrossRef]
- Balermpas, P.; Michel, Y.; Wagenblast, J.; Seitz, O.; Weiss, C.; Rödel, F.; Rödel, C.; Fokas, E. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br. J. Cancer 2014, 110, 501–509. [Google Scholar] [CrossRef]
- Fridman, W.H.; Zitvogel, L.; Sautès-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef]
- Ono, T.; Azuma, K.; Kawahara, A.; Akiba, J.; Kakuma, T.; Chitose, S.; Umeno, H. Pre-treatment CD8+ tumour-infiltrating lymphocyte density predicts distant metastasis after definitive treatment in patients with stage III/IV hypopharyngeal squamous cell carcinoma. Clin. Otolaryngol. 2018, 43, 1312–1320. [Google Scholar] [CrossRef]
- De Meulenaere, A.; Vermassen, T.; Aspeslagh, S.; Zwaenepoel, K.; Deron, P.; Duprez, F.; Rottey, S.; Ferdinande, L. Prognostic markers in oropharyngeal squamous cell carcinoma: Focus on CD70 and tumour infiltrating lymphocytes. Pathology 2017, 49, 397–404. [Google Scholar] [CrossRef]
- van der Heijden, M.; Essers, P.B.M.; de Jong, M.C.; de Roestm, R.H.; Sanduleanu, S.; Verhagen, C.V.M.; Hamming-Vrieze, O.; Hoebers, F.; Lambin, P.; Bartelink, H.; et al. Biological Determinants of Chemo-Radiotherapy Response in HPV-Negative Head and Neck Cancer: A Multicentric External Validation. Front. Oncol. 2020, 9, 1470. [Google Scholar] [CrossRef]
- Reading, J.L.; Gálvez-Cancino, F.; Swanton, C.; Lladser, A.; Peggs, K.S.; Quezada, S.A. The function and dysfunction of memory CD8+ T cells in tumor immunity. Immunol. Rev. 2018, 283, 194–212. [Google Scholar] [CrossRef]
- Crespo, J.; Haoyu, S.; Welling, T.H.; Tian, Z.; Zou, W. T cell anergy, exhaustion, senescence and stemness in the tumour microenvironment. Curr. Opin. Immunol. 2013, 25, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Filaci, G.; Fenoglio, D.; Fravega, M.; Ansaldo, G.; Borgonovo, G.; Traverso, P.; Villaggio, B.; Ferrera, A.; Kunkl, A.; Rizzi, M.; et al. CD8+ CD28- T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J. Immunol. 2007, 179, 4323–4334. [Google Scholar] [CrossRef] [Green Version]
- Parodi, A.; Battaglia, F.; Kalli, F.; Ferrera, F.; Conteduca, G.; Tardito, S.; Stringara, S.; Ivaldi, F.; Negrini, S.; Borgonovo, G.; et al. CD39 is highly involved in mediating the suppression activity of tumor-infiltrating CD8+ T regulatory lymphocytes. Cancer Immunol. Immunother. 2013, 62, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Huff, W.X.; Kwon, J.H.; Henriquez, M.; Fetcko, K.; Dey, M. The Evolving Role of CD8+CD28- Immunosenescent T Cells in Cancer Immunology. Int. J. Mol. Sci. 2019, 20, 2810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thommen, D.S.; Koelzer, V.H.; Herzig, P.; Roller, A.; Trefny, M.; Dimeloe, S.; Kiialainen, A.; Hanhart, J.; Schill, C.; Hess, C.; et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 2018, 24, 994–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paley, M.A.; Kroy, D.C.; Odorizzi, P.M.; Johnnidis, J.B.; Dolfi, D.V.; Barnett, B.E.; Bikoff, E.K.; Robertson, E.J.; Lauer, G.M.; Reiner, S.L.; et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 2012, 338, 1220–1225. [Google Scholar] [CrossRef] [Green Version]
- Reiser, J.; Banerjee, A. Effector, Memory, and Dysfunctional CD8(+) T Cell Fates in the Antitumor Immune Response. J. Immunol. Res. 2016, 2016, 8941260. [Google Scholar] [CrossRef] [Green Version]
- Canale, F.P.; Ramello, M.C.; Núñez, N.; Araujo Furlan, C.L.; Bossio, S.N.; Gorosito Serrán, M.; Tosello Boari, J.; Del Castillo, A.; Ledesma, M.; Sedlik, C.; et al. CD39 Expression Defines Cell Exhaustion in Tumor-Infiltrating CD8+ T Cells. Cancer Res. 2018, 78, 115–128. [Google Scholar] [CrossRef] [Green Version]
- Wherry, E.J.; Ha, S.J.; Kaech, S.M.; Haining, W.N.; Sarkar, S.; Kalia, V.; Subramaniam, S.; Blattman, J.N.; Barber, D.L.; Ahmed, R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 2007, 27, 670–684. [Google Scholar] [CrossRef] [Green Version]
- Fenoglio, D.; Ferrera, F.; Fravega, M.; Balestra, P.; Battaglia, F.; Proietti, M.; Andrei, C.; Olive, D.; La Cava, A.; Indiveri, F.; et al. Advancements on phenotypic and functional characterization of non-antigen-specific CD8+CD28- regulatory T cells. Hum. Immunol. 2008, 69, 745–750. [Google Scholar] [CrossRef]
- Filaci, G.; Fravega, M.; Negrini, S.; Procopio, F.; Fenoglio, D.; Rizzi, M.; Brenci, S.; Contini, P.; Olive, D.; Ghio, M.; et al. Nonantigen specific CD8+ T suppressor lymphocytes originate from CD8+CD28- T cells and inhibit both T-cell proliferation and CTL function. Hum. Immunol. 2004, 65, 142–156. [Google Scholar] [CrossRef]
- Corgnac, S.; Boutet, M.; Kfoury, M.; Naltet, C.; Mami-Chouaib, F. The Emerging Role of CD8+ Tissue Resident Memory T (TRM) Cells in Antitumor Immunity: A Unique Functional Contribution of the CD103 Integrin. Front. Immunol. 2018, 9, 904. [Google Scholar] [CrossRef] [Green Version]
- Mami-Chouaib, F.; Blanc, C.; Corgnac, S.; Hans, S.; Malenica, I.; Granier, C.; Tihy, I.; Tartour, E. Resident memory T cells, critical components in tumor immunology. J. Immunother. Cancer 2018, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Carr, A.; Ito, F.; Teitz-Tennenbaum, S.; Chang, A.E. Polarization effects of 4-1BB during CD28 costimulation in generating tumor-reactive T cells for cancer immunotherapy. Cancer Res. 2003, 63, 2546–2552. [Google Scholar] [PubMed]
- Wolfl, M.; Kuball, J.; Ho, W.Y.; Nguyen, H.; Manley, T.J.; Bleakley, M.; Greenberg, P.D. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood 2007, 110, 201–210. [Google Scholar] [CrossRef]
- Schmitt, A.; Barth, T.F.; Beyer, E.; Borchert, F.; Rojewski, M.; Chen, J.; Guillaume, P.; Gronau, S.; Greiner, J.; Möller, P.; et al. The tumor antigens RHAMM and G250/CAIX are expressed in head and neck squamous cell carcinomas and elicit specific CD8+ T cell responses. Int. J. Oncol. 2009, 34, 629–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, J.T.; Hamilton, R.L.; Ohnishi, K.; Ikeura, M.; Potter, D.M.; Nikiforova, M.N.; Ferrone, S.; Jakacki, R.I.; Pollack, I.F.; Okada, H. LOH in the HLA class I region at 6p21 is associated with shorter survival in newly diagnosed adult glioblastoma. Clin. Cancer Res. 2013, 19, 1816–1826. [Google Scholar] [CrossRef] [Green Version]
- Mittelbronn, M.; Simon, P.; Löffler, C.; Capper, D.; Bunz, B.; Harter, P.; Schlaszus, H.; Schleich, A.; Tabatabai, G.; Goeppert, B.; et al. Elevated HLA-E levels in human glioblastomas but not in grade I to III astrocytomas correlate with infiltrating CD8+ cells. J. Neuroimmunol. 2007, 189, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, B.G.; Charlebois, R.; Chouinard, G.; Allard, B.; Pommey, S.; Saad, F.; Stagg, J. CD73 Expression Is an Independent Prognostic Factor in Prostate Cancer. Clin. Cancer Res. 2016, 22, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Ness, N.; Andersen, S.; Valkov, A.; Nordby, Y.; Donnem, T.; Al-Saad, S.; Busund, L.T.; Bremnes, R.M.; Richardsen, E. Infiltration of CD8+ lymphocytes is an independent prognostic factor of biochemical failure-free survival in prostate cancer. Prostate 2014, 74, 1452–1461. [Google Scholar] [CrossRef]
- Kärjä, V.; Aaltomaa, S.; Lipponen, P.; Isotalo, T.; Talja, M.; Mokka, R. Tumour-infiltrating lymphocytes: A prognostic factor of PSA-free survival in patients with local prostate carcinoma treated by radical prostatectomy. Anticancer Res. 2005, 25, 4435–4438. [Google Scholar] [PubMed]
- Gu, F.M.; Gao, Q.; Shi, G.M.; Zhang, X.; Wang, J.; Jiang, J.H.; Wang, X.-Y.; Shi, Y.-H.; Ding, Z.-B.; Fan, J.; et al. Intratumoral IL-17⁺ cells and neutrophils show strong prognostic significance in intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2012, 19, 2506–2514. [Google Scholar] [CrossRef]
- Näsman, A.; Romanitan, M.; Nordfors, C.; Grün, N.; Johansson, H.; Hammarstedt, L.; Marklund, L.; Munck-Wikland, E.; Dalianis, T.; Ramqvist, T. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS ONE 2012, 7, e38711. [Google Scholar] [CrossRef]
- Krupar, R.; Robold, K.; Gaag, D.; Spanier, G.; Kreutz, M.; Renner, K.; Hellerbrand, C.; Hofstaedter, F.; Bosserhoff, A.K. Immunologic and metabolic characteristics of HPV-negative and HPV-positive head and neck squamous cell carcinomas are strikingly different. Virchows Arch. 2014, 465, 212–299. [Google Scholar] [CrossRef]
- Ward, M.J.; Thirdborough, S.M.; Mellows, T.; Riley, C.; Harris, S.; Suchak, K.; Webb, A.; Hampton, C.; Patel, N.N.; Randall, C.J.; et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br. J. Cancer 2014, 110, 489–500. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.M.; Song, L.J.; Shen, J.; Yue, H.; Han, Y.Q.; Yang, C.L.; Liu, S.-Y.; Deng, J.-W.; Jiang, Y.; Fu, G.-H.; et al. Prognostic and predictive values of immune infiltrate in patients with head and neck squamous cell carcinoma. Hum. Pathol. 2018, 82, 104–112. [Google Scholar] [CrossRef]
- Economopoulou, P.; de Bree, R.; Kotsantis, I.; Psyrri, A. Diagnostic Tumor Markers in Head and Neck Squamous Cell Carcinoma (HNSCC) in the Clinical Setting. Front. Oncol. 2019, 9, 827. [Google Scholar] [CrossRef]
- Linette, G.P.; Carreno, B.M. Tumor-Infiltrating Lymphocytes in the Checkpoint Inhibitor Era. Curr. Hematol. Malig. Rep. 2019, 14, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Hecht, M.; Gostian, A.O.; Eckstein, M.; Rutzner, S.; Von der Grün, J.; Illmer, T.; Hautmann, M.G.; Klautke, G.; Laban, S.; Brunner, T.; et al. Safety and efficacy of single cycle induction treatment with cisplatin/docetaxel/durvalumab/tremelimumab in locally advanced HNSCC: First results of CheckRad-CD8. J. Immunother. Cancer 2020, 8, e001378. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-G.; Donaubauer, A.-J.; Frey, B.; Becker, I.; Rutzner, S.; Eckstein, M.; Sun, R.; Ma, H.; Schubert, P.; Schweizer, C.; et al. Prospective development and validation of a liquid immune profile-based signature (LIPS) to predict response of patients with recurrent/metastatic cancer to immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e001845. [Google Scholar] [CrossRef] [PubMed]


| Demographic and Clinical Variables | Overall (n a = 20) |
|---|---|
| Age | |
| Mean (SD) | 65.1 (11.6) |
| Median (Min, Max) | 65.0 (35.0, 83.0) |
| Gender n (%) | |
| F | 4 (20.0%) |
| M | 16 (80.0%) |
| Smoke n (%) | |
| No | 5 (25.0%) |
| Yes | 15 (75.0%) |
| Alcohol drinker n (%) | |
| No | 13 (65.0%) |
| Yes | 7 (35.0%) |
| HPV (p16) n (%) | |
| Negative | 10 (50.0%) |
| Positive | 10 (50.0%) |
| Site | |
| Oropharynx | 20 (100.0%) |
| cT category (7th ed.) n (%) | |
| T1 | 2 (10.0%) |
| T2 | 6 (30.0%) |
| T4 | 12 (60.0%) |
| cN category (7th ed.) n (%) | |
| N0 | 2 (10.0%) |
| N1 | 3 (15.0%) |
| N2 | 14 (70.0%) |
| N3 | 1 (5.0%) |
| cStage (7th ed.) n (%) | |
| II | 2 (10.0%) |
| III | 2 (10.0%) |
| IV | 16 (80.0%) |
| Therapy | |
| RT b alone | 3 (15.0%) |
| RT + CHT c | 16 (80.0%) |
| Surgery | 1 (5.0%) |
| Response n (%) | |
| Not responder (Group 1) | 7 (35.0%) |
| Responder (Group 2) | 13 (65.0%) |
| T Cell Subsets | Group 1 (n = 7) | Group 2 (n = 13) | p-Value | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| CD3+/total cells | 8 | 7–48 | 27 | 9–36.1 | 0.38 |
| CD4+/CD3+ | 61 | 56–71 | 70 | 54–80 | 0.94 |
| Naïve CD4+ | 3.3 | 0.5–5.2 | 0.8 | 0.3–1.1 | 0.19 |
| CM CD4+ | 8.5 | 1.9–17 | 4.4 | 2–7.6 | 0.69 |
| EM CD4+ | 82 | 72–83 | 83 | 78–89 | 0.50 |
| TEM CD4+ | 7 | 1.3–13 | 8 | 4–13 | 0.63 |
| Naïve CD4+PD1+ | 4.5 | 1.8–10 | 1.9 | 0.8–4 | 0.25 |
| CM CD4+PD1+ | 9 | 5.2–20 | 5 | 3.8–12 | 0.41 |
| EM CD4+PD1+ | 64 | 58–75 | 74 | 56–81 | 0.75 |
| TEM CD4+PD1+ | 8.5 | 3.6–24 | 14 | 6–18 | 0.69 |
| CD4+CD25hiFoxP3+ (CD4+ Treg) | 22 | 11.5–25 | 16.4 | 11.7–20.4 | 0.69 |
| CD4+CD25hiFoxP3+/CD3+ | 14.5 | 9.9–15.6 | 8.8 | 7.9–15.4 | 0.46 |
| CD4+PD1+ Treg | 47 | 23–53 | 43 | 21–48 | 0.59 |
| CD4+CD152+ Treg | 59 | 34–72 | 62 | 45–72 | 0.84 |
| CD4+CD39+ Treg | 48 | 46–94 | 74 | 54–87 | 0.96 |
| CD4+PD-1+/ | 50 | 37–67 | 50 | 44–63 | 0.81 |
| CD4+CD152+ | 27 | 7.2–40 | 29 | 13.2–50 | 0.63 |
| CD4+CD39+ | 48 | 12–61 | 33 | 19–55 | 0.99 |
| CD4+ CD39+PD1+ | 35 | 6–59 | 20 | 7–32 | 0.50 |
| CD4+CD152+PD1+ | 15 | 4.8–20 | 13 | 8–32 | 0.94 |
| T Cell Subsets | Group 1 (n = 7) | Group 2 (n = 13) | p-Value | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| CD3+CD8+/CD3+ | 40.7 | 29–44.1 | 20 | 17–45 | 0.63 |
| CD8+CD28+/CD8+ | 23 | 15–34 | 74 | 67–82 | 0.0006 |
| CD8+CD28−/CD8+ | 77 | 66–85 | 26 | 18–33 | 0.0006 |
| Naïve CD8+/CD8+ | 1 | 0.2–4.1 | 0.6 | 0.3–1.7 | 0.66 |
| CM CD8+/CD8+ | 1.8 | 0.1–3.3 | 1.2 | 0.4–2.5 | 0.72 |
| EM CD8+/CD8+ | 62 | 49–74 | 79 | 63–84 | 0.07 |
| TEM CD8+/CD8+ | 31 | 24–50 | 17 | 14–32 | 0.14 |
| CD8+CD28−CD127−CD39+ (CD8+ Treg) | 28.9 | 16–51 | 6.2 | 4.4–15.9 | 0.03 |
| CD8+CD28-CD127−CD39+PD-1+ | 10.6 | 2.1–27.9 | 2.1 | 0.4–2.8 | 0.18 |
| CD8+PD-1+ | 60 | 36.4–81 | 61 | 52–67 | 0.99 |
| CD8+PD-1- | 37 | 18–51 | 34 | 23–38 | 0.84 |
| CD8+CD152+ | 5 | 2–8.7 | 2.3 | 1.5–8 | 0.69 |
| CD8+CD39+ | 50 | 25–54 | 20.6 | 12–47.5 | 0.25 |
| CD8+PD1+CD152+ | 4 | 0.7–7 | 1.7 | 0.5–4.5 | 0.51 |
| CD8+PD1+CD39+ | 38 | 4.3–48 | 18 | 10–39 | 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fenoglio, D.; Belgioia, L.; Parodi, A.; Missale, F.; Bacigalupo, A.; Tarke, A.; Incandela, F.; Negrini, S.; Vecchio, S.; Altosole, T.; et al. Development of Exhaustion and Acquisition of Regulatory Function by Infiltrating CD8+CD28− T Lymphocytes Dictate Clinical Outcome in Head and Neck Cancer. Cancers 2021, 13, 2234. https://doi.org/10.3390/cancers13092234
Fenoglio D, Belgioia L, Parodi A, Missale F, Bacigalupo A, Tarke A, Incandela F, Negrini S, Vecchio S, Altosole T, et al. Development of Exhaustion and Acquisition of Regulatory Function by Infiltrating CD8+CD28− T Lymphocytes Dictate Clinical Outcome in Head and Neck Cancer. Cancers. 2021; 13(9):2234. https://doi.org/10.3390/cancers13092234
Chicago/Turabian StyleFenoglio, Daniela, Liliana Belgioia, Alessia Parodi, Francesco Missale, Almalina Bacigalupo, Alison Tarke, Fabiola Incandela, Simone Negrini, Stefania Vecchio, Tiziana Altosole, and et al. 2021. "Development of Exhaustion and Acquisition of Regulatory Function by Infiltrating CD8+CD28− T Lymphocytes Dictate Clinical Outcome in Head and Neck Cancer" Cancers 13, no. 9: 2234. https://doi.org/10.3390/cancers13092234
APA StyleFenoglio, D., Belgioia, L., Parodi, A., Missale, F., Bacigalupo, A., Tarke, A., Incandela, F., Negrini, S., Vecchio, S., Altosole, T., Vlah, S., Astone, G., Costabile, F., Ascoli, A., Ferrera, F., Schenone, G., De Palma, R., Signori, A., Peretti, G., ... Filaci, G. (2021). Development of Exhaustion and Acquisition of Regulatory Function by Infiltrating CD8+CD28− T Lymphocytes Dictate Clinical Outcome in Head and Neck Cancer. Cancers, 13(9), 2234. https://doi.org/10.3390/cancers13092234







