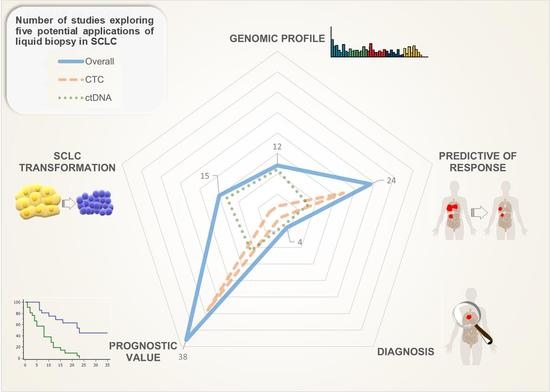
Liquid Biopsy for Small Cell Lung Cancer either De Novo or Transformed: Systematic Review of Different Applications and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Background
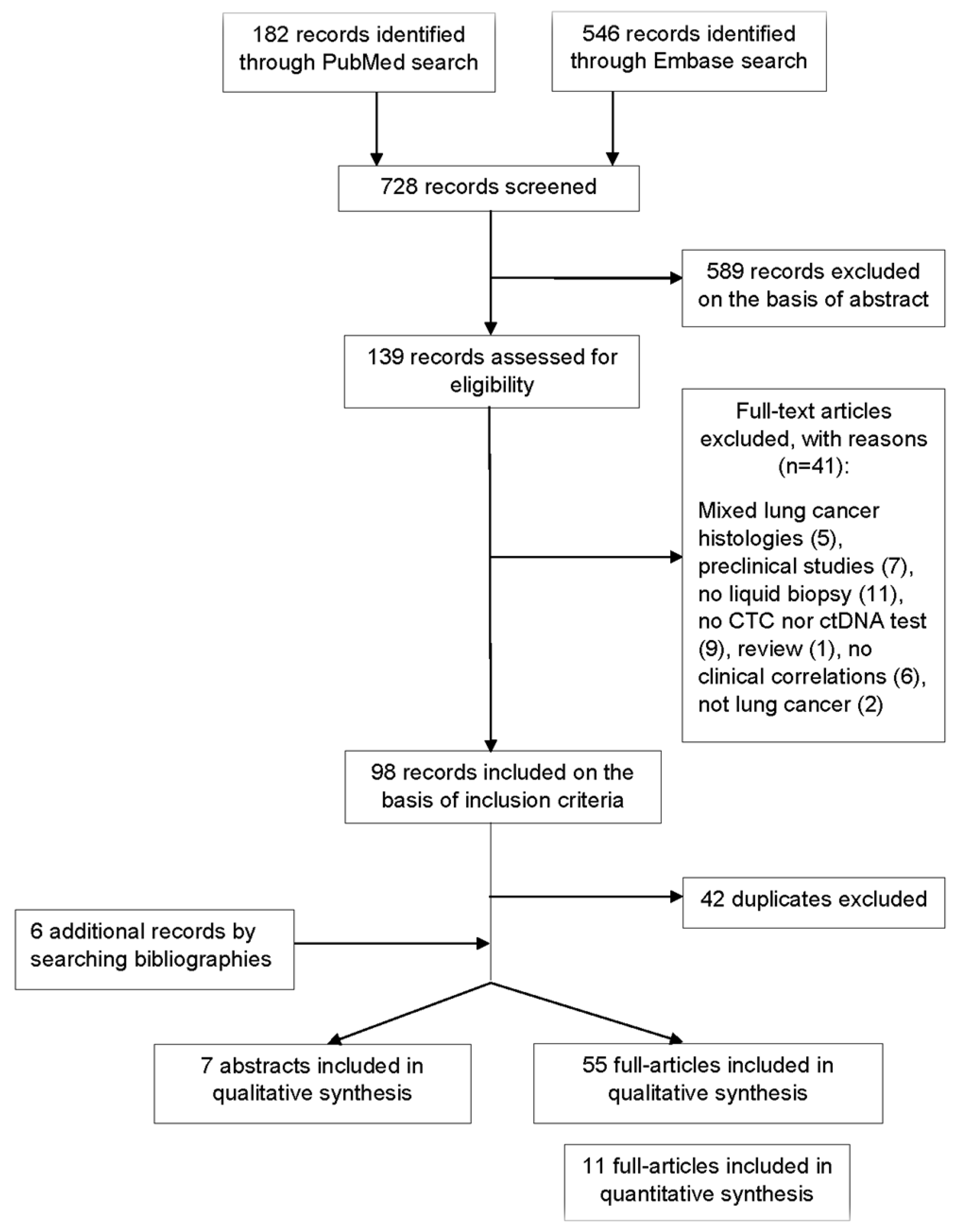
2. Methods
2.1. Definition of the Outcome
2.2. Data Source and Search Strategy
- Analysis of cfDNA/ctDNA or CTCs in plasma/serum of patients with SCLC included histologically transformed SCLC from NSCLC;
- Genomic profiling, diagnosis, treatment response, and/or survival data collected and correlated with cf/ctDNA or CTCs in humans.
- Not specific for SCLC, except in the cases of small cell transformation;
- Analysis of tumoral circulating components different from ctDNA or CTCs.
- -
- Hazard ratios (HR) with 95% confidence intervals (CI);
- -
- Sample size;
- -
- Cut-off of CTC number.
2.3. Statistical Analysis
3. Results
3.1. Diagnostic
3.2. Genomic Profiling
3.3. Predictive
3.4. Prognostic
Meta-Analysis
3.5. Small-Cell Transformation of NSCLC
4. Discussion
4.1. Diagnosis
4.2. Genomic Profiling
4.3. Predictive Value
4.4. Prognostic Value
4.5. Value of CTCs and ctDNA Changes in Small-Cell Transformation of NSCLC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pietanza, M.C.; Byers, L.A.; Minna, J.D.; Rudin, C.M. Small Cell Lung Cancer: Will Recent Progress Lead to Improved Outcomes? Clin. Cancer Res. 2015, 21, 2244–2255. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Cancer Facts & Figures 2020; American Cancer Society: Atlanta, GA, USA, 2020. [Google Scholar]
- Oser, M.G.; Niederst, M.J.; Sequist, L.V.; Engelman, J.A. Transformation from Non-Small-Cell Lung Cancer to Small-Cell Lung Cancer: Molecular Drivers and Cells of Origin. Lancet Oncol. 2015, 16, e165–e172. [Google Scholar] [CrossRef] [Green Version]
- Niederst, M.J.; Sequist, L.V.; Poirier, J.T.; Mermel, C.H.; Lockerman, E.L.; Garcia, A.R.; Katayama, R.; Costa, C.; Ross, K.N.; Moran, T.; et al. RB Loss in Resistant EGFR Mutant Lung Adenocarcinomas That Transform to Small-Cell Lung Cancer. Nat. Commun. 2015, 6, 6377. [Google Scholar] [CrossRef]
- Blackhall, F.; Frese, K.K.; Simpson, K.; Kilgour, E.; Brady, G.; Dive, C. Will Liquid Biopsies Improve Outcomes for Patients with Small-Cell Lung Cancer? Lancet Oncol. 2018, 19, e470–e481. [Google Scholar] [CrossRef]
- Siravegna, G.; Mussolin, B.; Buscarino, M.; Corti, G.; Cassingena, A.; Crisafulli, G.; Ponzetti, A.; Cremolini, C.; Amatu, A.; Lauricella, C.; et al. Clonal Evolution and Resistance to EGFR Blockade in the Blood of Colorectal Cancer Patients. Nat. Med. 2015, 21, 795–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolfo, C.; Mack, P.C.; Scagliotti, G.V.; Baas, P.; Barlesi, F.; Bivona, T.G.; Herbst, R.S.; Mok, T.S.; Peled, N.; Pirker, R.; et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J. Thorac. Oncol. 2018, 13, 1248–1268. [Google Scholar] [CrossRef] [Green Version]
- Massihnia, D.; Pizzutilo, E.G.; Amatu, A.; Tosi, F.; Ghezzi, S.; Bencardino, K.; Di Masi, P.; Righetti, E.; Patelli, G.; Scaglione, F.; et al. Liquid Biopsy for Rectal Cancer: A Systematic Review. Cancer Treat. Rev. 2019, 79, 101893. [Google Scholar] [CrossRef] [PubMed]
- Buono, G.; Gerratana, L.; Bulfoni, M.; Provinciali, N.; Basile, D.; Giuliano, M.; Corvaja, C.; Arpino, G.; Mastro, L.D.; Placido, S.D.; et al. Circulating Tumor DNA Analysis in Breast Cancer: Is It Ready for Prime-Time? Cancer Treat. Rev. 2019, 73, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.; Foy, V.; Ayub, M.; Leong, H.S.; Schofield, P.; Sahoo, S.; Descamps, T.; Kilerci, B.; Smith, N.K.; Carter, M.; et al. Profiling of Circulating Free DNA Using Targeted and Genome-Wide Sequencing in Patients with SCLC. J. Thorac. Oncol. 2020, 15, 216–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Cuesta, L.; Perdomo, S.; Avogbe, P.H.; Leblay, N.; Delhomme, T.M.; Gaborieau, V.; Abedi-Ardekani, B.; Chanudet, E.; Olivier, M.; Zaridze, D.; et al. Identification of Circulating Tumor DNA for the Early Detection of Small-Cell Lung Cancer. EBioMedicine 2016, 10, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Almodovar, K.; Iams, W.T.; Meador, C.B.; Zhao, Z.; York, S.; Horn, L.; Yan, Y.; Hernandez, J.; Chen, H.; Shyr, Y.; et al. Longitudinal Cell-Free DNA Analysis in Patients with Small Cell Lung Cancer Reveals Dynamic Insights into Treatment Efficacy and Disease Relapse. J. Thorac. Oncol. 2018, 13, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Wakelee, H.A.; Gadgeel, S.M.; Goldman, J.W.; Reckamp, K.L.; Karlovich, C.A.; Melnikova, V.; Jean-Charles, S.; Yu, H.A.; Solomon, B.J.; Perol, M.; et al. Epidermal Growth Factor Receptor (EGFR) Genotyping of Matched Urine, Plasma and Tumor Tissue from Non-Small Cell Lung Cancer (NSCLC) Patients (Pts) Treated with Rociletinib. J. Clin. Oncol. 2016, 34 (Suppl. 15), 9001. [Google Scholar] [CrossRef]
- CELLSEARCH®. Available online: https://www.cellsearchctc.com/ (accessed on 5 July 2020).
- Hodgkinson, C.L.; Morrow, C.J.; Li, Y.; Metcalf, R.L.; Rothwell, D.G.; Trapani, F.; Polanski, R.; Burt, D.J.; Simpson, K.L.; Morris, K.; et al. Tumorigenicity and Genetic Profiling of Circulating Tumor Cells in Small-Cell Lung Cancer. Nat. Med. 2014, 20, 897–903. [Google Scholar] [CrossRef]
- Hou, J.-M.; Krebs, M.G.; Lancashire, L.; Sloane, R.; Backen, A.; Swain, R.K.; Priest, L.J.C.; Greystoke, A.; Zhou, C.; Morris, K.; et al. Clinical Significance and Molecular Characteristics of Circulating Tumor Cells and Circulating Tumor Microemboli in Patients with Small-Cell Lung Cancer. J. Clin. Oncol. 2012, 30, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Tanaka, F.; Ono, A.; Yoneda, K.; Takahashi, T.; Murakami, H.; Nakamura, Y.; Tsuya, A.; Kenmotsu, H.; Shukuya, T.; et al. Prognostic Impact of Circulating Tumor Cells in Patients with Small Cell Lung Cancer. J. Thorac. Oncol. 2012, 7, 512–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiltermann, T.J.N.; Pore, M.M.; van den Berg, A.; Timens, W.; Boezen, H.M.; Liesker, J.J.W.; Schouwink, J.H.; Wijnands, W.J.A.; Kerner, G.S.M.A.; Kruyt, F.A.E.; et al. Circulating Tumor Cells in Small-Cell Lung Cancer: A Predictive and Prognostic Factor. Ann. Oncol. 2012, 23, 2937–2942. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemio. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical Methods for Incorporating Summary Time-to-Event Data into Meta-Analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-L.; Liu, C.-H.; Li, J.; Ma, X.-P.; Gong, P. Clinical Significance of Circulating Tumor Cells in Patients with Small-Cell Lung Cancer. Tumori 2017, 103, 242–248. [Google Scholar] [CrossRef]
- Board, R.E.; Williams, V.S.; Knight, L.; Shaw, J.; Greystoke, A.; Ranson, M.; Dive, C.; Blackhall, F.H.; Hughes, A. Isolation and Extraction of Circulating Tumor DNA from Patients with Small Cell Lung Cancer. Ann. N. Y. Acad. Sci. 2008, 1137, 98–107. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Niu, H.; Nackaerts, K.; Csoszi, T.; Ostoros, G.; Mark, Z.; Baik, C.; Joy, A.A.; Chouaid, C.; Jaime, J.C.; et al. Randomized Phase II Study of Paclitaxel plus Alisertib versus Paclitaxel plus Placebo as Second-Line Therapy for SCLC: Primary and Correlative Biomarker Analyses. J. Thorac. Oncol. 2020, 15, 274–287. [Google Scholar] [CrossRef] [Green Version]
- Devarakonda, S.; Sankararaman, S.; Herzog, B.H.; Gold, K.A.; Waqar, S.N.; Ward, J.P.; Raymond, V.M.; Lanman, R.B.; Chaudhuri, A.A.; Owonikoko, T.K.; et al. Circulating Tumor DNA Profiling in Small-Cell Lung Cancer Identifies Potentially Targetable Alterations. Clin. Cancer Res. 2019, 25, 6119–6126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, M.; Thompson, J.; Fisher, H.; Zhang, P.; Huang, C.-C.; Wang, L. Genomic Alterations of Plasma Cell-Free DNAs in Small Cell Lung Cancer and Their Clinical Relevance. Lung Cancer 2018, 120, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Nong, J.; Gong, Y.; Guan, Y.; Yi, X.; Yi, Y.; Chang, L.; Yang, L.; Lv, J.; Guo, Z.; Jia, H.; et al. Circulating Tumor DNA Analysis Depicts Subclonal Architecture and Genomic Evolution of Small Cell Lung Cancer. Nat. Commun. 2018, 9, 3114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbreteau, G.; Langlais, A.; Greillier, L.; Audigier-Valette, C.; Uwer, L.; Hureaux, J.; Moro-Sibilot, D.; Guisier, F.; Carmier, D.; Madelaine, J.; et al. Circulating Tumor DNA as a Prognostic Determinant in Small Cell Lung Cancer Patients Receiving Atezolizumab. J. Clin. Med. 2020, 9, 3861. [Google Scholar] [CrossRef]
- Yaung, S.; Woestmann, C.; Xi, L.; Ju, C.; Hinzmann, B.; Thomas, M.; Lasitschka, F.; Meister, M.; Schneider, M.; Herth, F.J.F.; et al. 1744P-Mutational Profiling of Tumour Tissue and Sequential Plasma Illustrates Emergent Clones during Treatment in Late Stage Small Cell Lung Cancer (SCLC). Ann. Oncol. 2019, 30, v713–v714. [Google Scholar] [CrossRef]
- Aggarwal, C.; Badola, S.; Shin, H.; Bedford, L.; Collins, S.; Derk, B.; Fostel, J.; Ecsedy, J.; Evans, T.; Bauml, J.; et al. PUB140 A Pilot Study to Assess Circulating Tumor Cells, Circulating Tumor Cell DNA and Cell Free DNA in Patients with Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, S1527. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Wang, Z.; Ni, X.; Duan, J.; Gao, Y.; Zhuo, M.; Li, R.; Zhao, J.; Ma, Q.; Bai, H.; et al. Inferring the Evolution and Progression of Small-Cell Lung Cancer by Single-Cell Sequencing of Circulating Tumor Cells. Clin. Cancer Res. 2019, 25, 5049–5060. [Google Scholar] [CrossRef]
- Gonzalez, R.; Silva, J.M.; Sanchez, A.; Dominguez, G.; Garcia, J.M.; Chen, X.Q.; Stroun, M.; Provencio, M.; España, P.; Anker, P.; et al. Microsatellite Alterations and TP53 Mutations in Plasma DNA of Small-Cell Lung Cancer Patients: Follow-up Study and Prognostic Significance. Ann. Oncol. 2000, 11, 1097–1104. [Google Scholar] [CrossRef]
- Thomas, A.; Vilimas, R.; Trindade, C.; Erwin-Cohen, R.; Roper, N.; Xi, L.; Krishnasamy, V.; Levy, E.; Mammen, A.; Nichols, S.; et al. Durvalumab in Combination with Olaparib in Patients with Relapsed SCLC: Results from a Phase II Study. J. Thorac. Oncol. 2019, 14, 1447–1457. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, C.; Lv, F.; Wang, J.; Han, W.; Nie, J.; Dai, L.; Hu, W.; Chen, X.; Ma, X.; et al. Molecular Analysis of Cell-Free DNA Identifies Distinct Molecular Features in Patients with Chemosensitive and Chemorefractory Small Cell Lung Cancer. Cancer Commun. 2019, 39, 20. [Google Scholar] [CrossRef] [Green Version]
- Messaritakis, I.; Stoltidis, D.; Kotsakis, A.; Dermitzaki, E.-K.; Koinis, F.; Lagoudaki, E.; Koutsopoulos, A.; Politaki, E.; Apostolaki, S.; Souglakos, J.; et al. TTF-1- and/or CD56-Positive Circulating Tumor Cells in Patients with Small Cell Lung Cancer (SCLC). Sci. Rep. 2017, 7, 45351. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, C.; Wang, X.; Ranganathan, A.; Torigian, D.; Troxel, A.; Evans, T.; Cohen, R.B.; Vaidya, B.; Rao, C.; Connelly, M.; et al. Circulating Tumor Cells as a Predictive Biomarker in Patients with Small Cell Lung Cancer Undergoing Chemotherapy. Lung Cancer 2017, 112, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Belani, C.P.; Dahlberg, S.E.; Rudin, C.M.; Fleisher, M.; Chen, H.X.; Takebe, N.; Velasco, M.R.; Tester, W.J.; Sturtz, K.; Hann, C.L.; et al. Vismodegib or Cixutumumab in Combination with Standard Chemotherapy for Patients with Extensive-Stage Small Cell Lung Cancer: A Trial of the ECOG-ACRIN Cancer Research Group (E1508). Cancer 2016, 122, 2371–2378. [Google Scholar] [CrossRef]
- Wang, X.; Ma, K.; Wang, Y.; He, H.; Hu, J.-F.; Li, W. Evaluation of Circulating Tumor Cells in Predicting Therapeutic Response in Small Cell Lung Cancer Patients. Arch. Med. Res. 2016, 47, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.-M.; Greystoke, A.; Lancashire, L.; Cummings, J.; Ward, T.; Board, R.; Amir, E.; Hughes, S.; Krebs, M.; Hughes, A.; et al. Evaluation of Circulating Tumor Cells and Serological Cell Death Biomarkers in Small Cell Lung Cancer Patients Undergoing Chemotherapy. Am. J. Pathol. 2009, 175, 808–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevilacqua, S.; Gallo, M.; Franco, R.; Rossi, A.; De Luca, A.; Rocco, G.; Botti, G.; Gridelli, C.; Normanno, N. A “Live” Biopsy in a Small-Cell Lung Cancer Patient by Detection of Circulating Tumor Cells. Lung Cancer 2009, 65, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Salgia, R.; Weaver, R.W.; McCleod, M.; Stille, J.R.; Yan, S.B.; Roberson, S.; Polzer, J.; Flynt, A.; Raddad, E.; Peek, V.L.; et al. Prognostic and Predictive Value of Circulating Tumor Cells and CXCR4 Expression as Biomarkers for a CXCR4 Peptide Antagonist in Combination with Carboplatin-Etoposide in Small Cell Lung Cancer: Exploratory Analysis of a Phase II Study. Investig. New Drugs 2017, 35, 334–344. [Google Scholar] [CrossRef] [Green Version]
- Pietanza, M.C.; Waqar, S.N.; Krug, L.M.; Dowlati, A.; Hann, C.L.; Chiappori, A.; Owonikoko, T.K.; Woo, K.M.; Cardnell, R.J.; Fujimoto, J.; et al. Randomized, Double-Blind, Phase II Study of Temozolomide in Combination With Either Veliparib or Placebo in Patients With Relapsed-Sensitive or Refractory Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2386–2394. [Google Scholar] [CrossRef] [PubMed]
- Messaritakis, I.; Politaki, E.; Koinis, F.; Stoltidis, D.; Apostolaki, S.; Plataki, M.; Dermitzaki, E.-K.; Georgoulias, V.; Kotsakis, A. Dynamic Changes of Phenotypically Different Circulating Tumor Cells Sub-Populations in Patients with Recurrent/Refractory Small Cell Lung Cancer Treated with Pazopanib. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koinis, F.; Agelaki, S.; Karavassilis, V.; Kentepozidis, N.; Samantas, E.; Peroukidis, S.; Katsaounis, P.; Hartabilas, E.; Varthalitis, I.I.; Messaritakis, I.; et al. Second-Line Pazopanib in Patients with Relapsed and Refractory Small-Cell Lung Cancer: A Multicentre Phase II Study of the Hellenic Oncology Research Group. Br. J. Cancer 2017, 117, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Igawa, S.; Gohda, K.; Fukui, T.; Ryuge, S.; Otani, S.; Masago, A.; Sato, J.; Murakami, K.; Maki, S.; Katono, K.; et al. Circulating Tumor Cells as a Prognostic Factor in Patients with Small Cell Lung Cancer. Oncol. Lett. 2014, 7, 1469–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Zhao, J.; Jiang, T.; Li, X.; Zhao, C.; Su, C.; Zhou, C. Predictive and Prognostic Value of Folate Receptor-Positive Circulating Tumor Cells in Small Cell Lung Cancer Patients Treated with First-Line Chemotherapy. Oncotarget 2017, 8, 49044–49052. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.-L.; Li, J.; Du, Y.-J.; Zhu, W.-F.; Wu, Y.; Hu, Y.-M.; Chen, Y.-C. CK-19 MRNA-Positive Cells in Peripheral Blood Predict Treatment Efficacy and Survival in Small-Cell Lung Cancer Patients. Med. Oncol. 2013, 30, 755. [Google Scholar] [CrossRef] [PubMed]
- Messaritakis, I.; Nikolaou, M.; Koinis, F.; Politaki, E.; Koutsopoulos, A.; Lagoudaki, E.; Vetsika, E.-K.; Georgoulias, V.; Kotsakis, A. Characterization of DLL3-Positive Circulating Tumor Cells (CTCs) in Patients with Small Cell Lung Cancer (SCLC) and Evaluation of Their Clinical Relevance during Front-Line Treatment. Lung Cancer 2019, 135, 33–39. [Google Scholar] [CrossRef]
- Carter, L.; Rothwell, D.G.; Mesquita, B.; Smowton, C.; Leong, H.S.; Fernandez-Gutierrez, F.; Li, Y.; Burt, D.J.; Antonello, J.; Morrow, C.J.; et al. Molecular Analysis of Circulating Tumor Cells Identifies Distinct Copy-Number Profiles in Patients with Chemosensitive and Chemorefractory Small-Cell Lung Cancer. Nat. Med. 2017, 23, 114–119. [Google Scholar] [CrossRef]
- Palma, J.F.; Woestmann, C.; McNamara, S.; Hinzmann, B.; Fröhler, S.; Adams, H.-P.; Feldkamp, M.; Siemann, S.; Lange, M.; Blüher, A.; et al. Early Assessment of Therapy Response in Small Cell Lung Cancer via Longitudinal CtDNA Analysis. JCO 2018, 36, 8577. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Y.M.; Tang, H.R.; Li, Q.; Li, P.S.; Hu, X.; Guan, Y.F.; Xia, X.F.; Yi, X.; Zhang, J.J.; et al. Clinical Potential of CtDNA-Based TMB in Small Cell Lung Cancer Recieving Chemoradiotherapy. J. Clin. Oncol. 2020, 38 (Suppl. 15), 3536. [Google Scholar] [CrossRef]
- Yaung, S.; Xi, L.; Woestmann, C.; McNamara, S.; Hinzmann, B.; Froehler, S.; Tikoo, N.; Ju, C.; Balasubramanyam, A.; Adams, H.-P.; et al. Ecological Diversity Indices as Measurements of Tumor Heterogeneity Correlates with Clinical Outcomes in Late Stage Small Cell Lung Cancer (SCLC). Annal Oncol. 2018, 29, viii54. [Google Scholar] [CrossRef]
- Kularatne, B.Y.; Lorigan, P.; Browne, S.; Suvarna, S.K.; Smith, M.O.; Lawry, J. Monitoring Tumour Cells in the Peripheral Blood of Small Cell Lung Cancer Patients. Cytometry 2002, 50, 160–167. [Google Scholar] [CrossRef]
- Tay, R.Y.; Fernández-Gutiérrez, F.; Foy, V.; Burns, K.; Pierce, J.; Morris, K.; Priest, L.; Tugwood, J.; Ashcroft, L.; Lindsay, C.R.; et al. Prognostic Value of Circulating Tumour Cells in Limited-Stage Small-Cell Lung Cancer: Analysis of the Concurrent Once-Daily versus Twice-Daily Radiotherapy (CONVERT) Randomised Controlled Trial. Ann. Oncol. 2019, 30, 1114–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Zhu, Y.; Jing, W.; Guo, D.; Kong, L.; Yu, J. Incorporation of Circulating Tumor Cells and Whole-Body Metabolic Tumor Volume of 18F-FDG PET/CT Improves Prediction of Outcome in IIIB Stage Small-Cell Lung Cancer. Chin. J. Cancer Res. 2018, 30, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Messaritakis, I.; Nikolaou, M.; Politaki, E.; Koinis, F.; Lagoudaki, E.; Koutsopoulos, A.; Georgoulia, N.; Georgoulias, V.; Kotsakis, A. Bcl-2 Expression in Circulating Tumor Cells (CTCs) of Patients with Small Cell Lung Cancer (SCLC) Receiving Front-Line Treatment. Lung Cancer 2018, 124, 270–278. [Google Scholar] [CrossRef]
- Pietanza, M.C.; Litvak, A.M.; Varghese, A.M.; Krug, L.M.; Fleisher, M.; Teitcher, J.B.; Holodny, A.I.; Sima, C.S.; Woo, K.M.; Ng, K.K.; et al. A Phase I Trial of the Hedgehog Inhibitor, Sonidegib (LDE225), in Combination with Etoposide and Cisplatin for the Initial Treatment of Extensive Stage Small Cell Lung Cancer. Lung Cancer 2016, 99, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Liu, X.-Q.; Fan, Y.; Liu, Y.-P.; Liu, Y.; Liu, Y.; Ma, L.-X.; Liu, X.-H.; Li, H.; Bao, H.-Z.; et al. Circulating Tumor Cell Counts/Change for Outcome Prediction in Patients with Extensive-Stage Small-Cell Lung Cancer. Future Oncol. 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Normanno, N.; Rossi, A.; Morabito, A.; Signoriello, S.; Bevilacqua, S.; Di Maio, M.; Costanzo, R.; De Luca, A.; Montanino, A.; Gridelli, C.; et al. Prognostic Value of Circulating Tumor Cells’ Reduction in Patients with Extensive Small-Cell Lung Cancer. Lung Cancer 2014, 85, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Wick, J.A.; Sittampalam, G.S.; Nirmalanandhan, V.S.; Ganti, A.K.; Neupane, P.C.; Williamson, S.K.; Godwin, A.K.; Schmitt, S.; Smart, N.J.; et al. A Multicenter Pilot Study Examining the Role of Circulating Tumor Cells as a Blood-Based Tumor Marker in Patients with Extensive Small-Cell Lung Cancer. Front Oncol. 2014, 4, 271. [Google Scholar] [CrossRef] [Green Version]
- Gadgeel, S.M.; Pennell, N.A.; Fidler, M.J.; Halmos, B.; Bonomi, P.; Stevenson, J.; Schneider, B.; Sukari, A.; Ventimiglia, J.; Chen, W.; et al. Phase II Study of Maintenance Pembrolizumab in Patients with Extensive-Stage Small Cell Lung Cancer (SCLC). J. Thorac. Oncol. 2018, 13, 1393–1399. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.-P.; Liu, S.-H.; Chen, C.-T.; Lv, L.; Li, D.; Liu, Q.-Y.; Liu, G.-L.; Wu, Y. Circulating Tumor Cells as a New Predictive and Prognostic Factor in Patients with Small Cell Lung Cancer. J. Cancer 2020, 11, 2113–2122. [Google Scholar] [CrossRef] [PubMed]
- Pizzutilo, E.G.; Lauricella, C.; Cerea, G.; Giannetta, L.G.; Tomasello, G.; Stabile, S.; Motta, V.; Alexiadis, S.; Scaglione, F.; Vanzulli, A.; et al. Concurrent Small-Cell Transformation and Emergence of Trans-C797S and T790M Mutations Under Sequential Treatment With EGFR Inhibitors in Lung Adenocarcinoma. JCO Precis. Oncol. 2019, 1–5. [Google Scholar] [CrossRef]
- Piotrowska, Z.; Niederst, M.J.; Karlovich, C.A.; Wakelee, H.A.; Neal, J.W.; Mino-Kenudson, M.; Fulton, L.; Hata, A.N.; Lockerman, E.L.; Kalsy, A.; et al. Heterogeneity Underlies the Emergence of EGFRT790 Wild-Type Clones Following Treatment of T790M-Positive Cancers with a Third-Generation EGFR Inhibitor. Cancer Discov. 2015, 5, 713–722. [Google Scholar] [CrossRef] [Green Version]
- Minari, R.; Bordi, P.; Del Re, M.; Facchinetti, F.; Mazzoni, F.; Barbieri, F.; Camerini, A.; Comin, C.E.; Gnetti, L.; Azzoni, C.; et al. Primary Resistance to Osimertinib Due to SCLC Transformation: Issue of T790M Determination on Liquid Re-Biopsy. Lung Cancer 2018, 115, 21–27. [Google Scholar] [CrossRef]
- Iijima, Y.; Hirotsu, Y.; Mochizuki, H.; Amemiya, K.; Oyama, T.; Uchida, Y.; Kobayashi, Y.; Tsutsui, T.; Kakizaki, Y.; Miyashita, Y.; et al. Dynamic Changes and Drug-Induced Selection of Resistant Clones in a Patient With EGFR-Mutated Adenocarcinoma That Acquired T790M Mutation and Transformed to Small-Cell Lung Cancer. Clin. Lung Cancer 2018, 19, e843–e847. [Google Scholar] [CrossRef]
- Mooradian, M.J.; Piotrowska, Z.; Drapkin, B.J.; Dias-Santagata, D.; Marcoux, N.; Arnaoutakis, K.; Nagy, R.J.; Lanman, R.; Iafrate, A.J.; Farago, A.F.; et al. Clonal Evolution and the Role of Serial Liquid Biopsies in a Case of Small-Cell Lung Cancer–Transformed EGFR Mutant Non–Small-Cell Lung Cancer. JCO Precis. Oncol. 2017, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.; Stewart, E.L.; Martins-Filho, S.N.; Cabanero, M.; Wang, A.; Bao, H.; Wu, X.; Patel, D.; Chen, Z.; Law, J.H.; et al. Early Detection of Multiple Resistance Mechanisms by CtDNA Profiling in a Patient With EGFR-Mutant Lung Adenocarcinoma Treated With Osimertinib. Clin. Lung Cancer 2020. [Google Scholar] [CrossRef]
- Tsui, D.W.Y.; Murtaza, M.; Wong, A.S.C.; Rueda, O.M.; Smith, C.G.; Chandrananda, D.; Soo, R.A.; Lim, H.L.; Goh, B.C.; Caldas, C.; et al. Dynamics of Multiple Resistance Mechanisms in Plasma DNA during EGFR-targeted Therapies in Non-small Cell Lung Cancer. EMBO Mol. Med. 2018, 10. [Google Scholar] [CrossRef]
- Vendrell, J.A.; Quantin, X.; Serre, I.; Solassol, J. Combination of Tissue and Liquid Biopsy Molecular Profiling to Detect Transformation to Small Cell Lung Carcinoma during Osimertinib Treatment. Ther. Adv. Med. Oncol. 2020, 12, 1758835920974192. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Q.; Wang, B.; Zhou, Q.; Yan, L.; Zhang, Z.; Chen, H.; Su, J.; Xie, Z.; Niu, F.; et al. JCES01.24 Molecular Mechanism of Transformation from Adenocarcinoma to Small-Cell Lung Cancer after EGFR-TKI. J. Thorac. Oncol. 2017, 12, S240–S241. [Google Scholar] [CrossRef] [Green Version]
- Nishioka, N.; Yamada, T.; Harita, S.; Hirai, S.; Katayama, Y.; Nakano, T.; Okura, N.; Tamiya, N.; Kaneko, Y.; Uchino, J.; et al. Successful Sequential Treatment of Refractory Tumors Caused by Small Cell Carcinoma Transformation and EGFR-T790M Mutation Diagnosed by Repeated Genetic Testing in a Patient with Lung Adenocarcinoma Harboring Epidermal Growth Factor Receptor Mutations: A Case Report. Respir. Med. Case Rep. 2018, 25, 261–263. [Google Scholar] [CrossRef]
- Ou, S.-H.I.; Lee, T.K.; Young, L.; Fernandez-Rocha, M.Y.; Pavlick, D.; Schrock, A.B.; Zhu, V.W.; Milliken, J.; Ali, S.M.; Gitlitz, B.J. Dual Occurrence of ALK G1202R Solvent Front Mutation and Small Cell Lung Cancer Transformation as Resistance Mechanisms to Second Generation ALK Inhibitors without Prior Exposure to Crizotinib. Pitfall of Solely Relying on Liquid Re-Biopsy? Lung Cancer 2017, 106, 110–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, X.; Zhuo, M.; Su, Z.; Duan, J.; Gao, Y.; Wang, Z.; Zong, C.; Bai, H.; Chapman, A.R.; Zhao, J.; et al. Reproducible Copy Number Variation Patterns among Single Circulating Tumor Cells of Lung Cancer Patients. Proc. Natl. Acad. Sci. USA 2013, 110, 21083–21088. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zou, C.; Zhang, J.; Jiang, W.; Guan, F.; Tang, K.; Li, S.; Li, G.; Wang, J.; Ke, Z. Dynamically Monitoring the Clonal Evolution of Lung Cancer Based on the Molecular Characterization of Circulating Tumor Cells Using Aptamer Cocktail-Modified Nanosubstrates. ACS Appl. Mater. Interfaces 2020, 12, 5671–5679. [Google Scholar] [CrossRef]
- Alì, G.; Bruno, R.; Giordano, M.; Prediletto, I.; Marconi, L.; Zupo, S.; Fedeli, F.; Ribechini, A.; Chella, A.; Fontanini, G. Small Cell Lung Cancer Transformation and the T790M Mutation: A Case Report of Two Acquired Mechanisms of TKI Resistance Detected in a Tumor Rebiopsy and Plasma Sample of EGFR-Mutant Lung Adenocarcinoma. Oncol. Lett. 2016, 12, 4009–4012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennon, A.M.; Buchanan, A.H.; Kinde, I.; Warren, A.; Honushefsky, A.; Cohain, A.T.; Ledbetter, D.H.; Sanfilippo, F.; Sheridan, K.; Rosica, D.; et al. Feasibility of Blood Testing Combined with PET-CT to Screen for Cancer and Guide Intervention. Science 2020, 369. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Swanton, C.; Seiden, M.V.; Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Smith, D.; Richards, D.; et al. Sensitive and Specific Multi-Cancer Detection and Localization Using Methylation Signatures in Cell-Free DNA. Ann. Oncol. 2020, 31, 745–759. [Google Scholar] [CrossRef]
- Menarini Silicon Biosystems. CELLSEARCH® Circulating Tumor Cell Kit (Epithelial) Instructions for Use. Available online: https://documents.cellsearchctc.com/ (accessed on 25 July 2020).
- Yu, N.; Zhou, J.; Cui, F.; Tang, X. Circulating Tumor Cells in Lung Cancer: Detection Methods and Clinical Applications. Lung 2015, 193, 157–171. [Google Scholar] [CrossRef]
- Wang, J.; Gong, Y.; Nong, J.; Yi, Y.; Guan, Y.; Yang, L.; Jia, H.; Zhang, S.; Yi, X.; Liao, Z.; et al. MA 01.03 The Potential of CtDNA Sequencing in Disease Monitoring and Depicting Genomic Evolution of Small-Cell Lung Cancer Under Therapy. J. Thorac. Oncol. 2017, 12, S1800. [Google Scholar] [CrossRef]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G.; et al. Comprehensive Genomic Profiles of Small Cell Lung Cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef]
- Rudin, C.M.; Durinck, S.; Stawiski, E.W.; Poirier, J.T.; Modrusan, Z.; Shames, D.S.; Bergbower, E.A.; Guan, Y.; Shin, J.; Guillory, J.; et al. Comprehensive Genomic Analysis Identifies SOX2 as a Frequently Amplified Gene in Small-Cell Lung Cancer. Nat. Genet. 2012, 44, 1111–1116. [Google Scholar] [CrossRef]
- Swanton, C.; Venn, O.; Aravanis, A.; Hubbell, E.; Maddala, T.; Beausang, J.F.; Filippova, D.; Gross, S.; Jamshidi, A.; Shen, L.; et al. Prevalence of Clonal Hematopoiesis of Indeterminate Potential (CHIP) Measured by an Ultra-Sensitive Sequencing Assay: Exploratory Analysis of the Circulating Cancer Genome Atlas (CCGA) Study. JCO 2018, 36, 12003. [Google Scholar] [CrossRef]
- Gay, C.M.; Stewart, C.A.; Park, E.M.; Diao, L.; Groves, S.M.; Heeke, S.; Nabet, B.Y.; Fujimoto, J.; Solis, L.M.; Lu, W.; et al. Patterns of Transcription Factor Programs and Immune Pathway Activation Define Four Major Subtypes of SCLC with Distinct Therapeutic Vulnerabilities. Cancer Cell 2021. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.; Gay, C.M.; Xi, Y.; Sivajothi, S.; Sivakamasundari, V.; Fujimoto, J.; Bolisetty, M.; Hartsfield, P.M.; Balasubramaniyan, V.; Chalishazar, M.D.; et al. Single-Cell Analyses Reveal Increased Intratumoral Heterogeneity after the Onset of Therapy Resistance in Small-Cell Lung Cancer. Nat. Cancer 2020, 1, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Farago, A.F.; Yeap, B.Y.; Stanzione, M.; Hung, Y.P.; Heist, R.S.; Marcoux, J.P.; Zhong, J.; Rangachari, D.; Barbie, D.A.; Phat, S.; et al. Combination Olaparib and Temozolomide in Relapsed Small Cell Lung Cancer. Cancer Discov. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, H.; Sen, T.; Rudin, C.M. Targeted Therapies and Biomarkers in Small Cell Lung Cancer. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Sabari, J.K.; Lok, B.H.; Laird, J.H.; Poirier, J.T.; Rudin, C.M. Unravelling the Biology of SCLC: Implications for Therapy. Nat. Rev. Clin. Oncol. 2017, 14, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Katsurada, M.; Nagano, T.; Tachihara, M.; Kiriu, T.; Furukawa, K.; Koyama, K.; Otoshi, T.; Sekiya, R.; Hazama, D.; Tamura, D.; et al. Baseline Tumor Size as a Predictive and Prognostic Factor of Immune Checkpoint Inhibitor Therapy for Non-Small Cell Lung Cancer. Anticancer Res. 2019, 39, 815–825. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.-T.; Cui, X.; Chen, Q.; Li, Y.-F.; Cui, Y.-H.; Wang, Y.; Jiang, J. Circulating Tumor Cell Status Monitors the Treatment Responses in Breast Cancer Patients: A Meta-Analysis. Sci. Rep. 2017, 7, 43464. [Google Scholar] [CrossRef] [Green Version]
- Dowlati, A.; Lipka, M.B.; McColl, K.; Dabir, S.; Behtaj, M.; Kresak, A.; Miron, A.; Yang, M.; Sharma, N.; Fu, P.; et al. Clinical Correlation of Extensive-Stage Small-Cell Lung Cancer Genomics. Ann. Oncol. 2016, 27, 642–647. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.-T.; Li, B.-G. Prognostic Significance of Circulating Tumor Cells in Small--Cell Lung Cancer Patients: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2014, 15, 8429–8433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, L.; Cui, S.; Ding, J.; Sun, Y.; Zhang, L.; Zhao, Y.; Gu, A.; Chu, T.; Wang, H.; Zhong, H.; et al. Dynamics of EGFR Mutations in Plasma Recapitulates the Clinical Response to EGFR-TKIs in NSCLC Patients. Oncotarget 2017, 8, 63846–63856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Zhuo, M.; Ye, X.; Bai, H.; Wang, Z.; Sun, Y.; Zhao, J.; An, T.; Duan, J.; Wu, M.; et al. Quantification of Mutant Alleles in Circulating Tumor DNA Can Predict Survival in Lung Cancer. Oncotarget 2016, 7, 20810–20824. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Ref. | Herbreteau 2020 [29] | Mohan 2020 [10] | Owonikoko 2020 [25] | Devarakonda 2019 [26] | Du 2018 [27] | Almodovar 2018 [12] | Nong 2018 [28] |
|---|---|---|---|---|---|---|---|
| Assay | 5 genes, QIAseq Targeted DNA custom panel | 110 genes, custom panel | 80 genes, custom panel with PlasmaSelect-R | 54–73 genes, Guardant 360 | 127 genes, xGen Pan-Cancer Panel (AF >5%) | 14 genes, custom panel with Resolution Bioscience targeted hybrid capture | 430 genes, targeted deep sequencing, custom panel |
| N. | 68 | 62 | 140 | 594 | 17 | 27 | 22 |
| Time of sample collection | At relapse | At diagnosis | At relapse | Any | At diagnosis | Any | At diagnosis |
| GENE | % mut | % mut | % mut | % mut | % mut | % mut | % mut |
| TP53 | 65 | 79 | 86 | 72 | 24 | 67 | 91 |
| KMT2D | - | 13 | - | - | 76 | - | - |
| RB1 | 51 | 32 | 58 | 18 | 24 | 37 | 64 |
| SLIT2 | - | 8 | - | - | - | - | 27 |
| MTOR | - | - | - | 2 | 47 | - | 14 |
| NOTCH1 | 6 | 13 | 15 | 6 | 53 | 15 | 9 |
| ATRX | - | - | 11 | - | 30 | - | 9 |
| NF1 | - | 2 | - | 13 | 24 | - | 9 |
| COLL22A1 | - | 13 | 15 | - | - | - | - |
| CREBBP | - | 5 | 13 | - | - | - | 18 |
| BRCA2 | - | 2 | - | 6 | 24 | - | 18 |
| TP73 | - | 10 | 14 | - | - | - | - |
| EP300 | - | 8 | 8 | - | 29 | - | 14 |
| APC | - | 3 | 6 | 10 | 41 | - | 14 |
| NOTCH3 | 8 | 5 | 9 | - | - | 11 | 14 |
| ATM | - | - | - | 3 | 35 | - | 9 |
| ARID1A | - | 0 | - | 12 | 53 | - | 5 |
| AR | - | 2 | 8 | 8 | 18 | - | 9 |
| PIK3CA | - | 5 | 4 | 8 | - | 11 | 14 |
| PTEN | - | 3 | 5 | 5 | 6 | 7 | 5 |
| EGFR | - | 2 | 2 | 14 | 18 | - | 5 |
| PDGFRA | - | 3 | - | 5 | 12 | - | 5 |
| BRCA1 | - | 2 | - | 8 | 12 | - | 0 |
| Ref. | Mohan 2020 [10] | Devarakonda 2019 [26] | Du 2018 [27] | Almodovar 2018 [12] | Nong 2018 [28] |
|---|---|---|---|---|---|
| Assay | Whole genome sequencing | 54–73 genes, Guardant 360 | Whole genome sequencing | 14 genes, custom panel with Resolution Bioscience targeted hybrid capture | 430 genes, targeted deep sequencing, custom panel |
| N. | 62 | 594 | 24 | 27 | 22 |
| Time of sample collection | At diagnosis | Any | At diagnosis | Any | At diagnosis |
| GENE | % CNV | % CNV | % CNV | % CNV | % CNV |
| RASSF1 | 55 | - | 58 | - | - |
| SOX2 | 52 | - | 38 | - | - |
| FHIT | 58 | - | 29 | - | - |
| FGF10 | - | - | 38 | - | - |
| RB1 | 35 | 0 | 38 | 44 | 23 |
| CNTN3 | 59 | - | 0 | - | - |
| CCNE1 | - | 13 | 33 | - | - |
| PIK3CA | - | 23 | 0 | 30 | - |
| CD274 | 20 | - | 25 | - | - |
| MYCL | 22 | - | 41 | - | 9 |
| TP53 | - | 0 | 67 | 22 | 5 |
| MYC | 30 | 12 | 71 | - | 5 |
| KIF2A | 29 | - | 0 | - | - |
| FGFR1 | 17 | 9 | 25 | 0 | - |
| NFIB | 23 | - | 0 | - | - |
| MYCN | 10 | - | 21 | 0 | 5 |
| KIT | 3 | 3 | 0 | 15 | - |
| REF. | N. | Assay | Results |
|---|---|---|---|
| ctDNA | |||
| Vendrell 2020 [71] | 3 | ddPCR and NGS | In 2 patients, elevation of AF in ctDNA of EGFRdel19 (from 6% to 17%) and TP53 M246K (from 6% to 24%), and of EGFRL858R (from 4% to 6%) and TP53 L194R (from 3% to 6%), respectively, concurrent with evidence of tSCLC. Not available AF at the moment of transformation for the third patient. In all patients, the levels of the EGFR mutations in terms of copies/mL of plasma raised with SCLC progression. |
| Schmid 2020 [69] | 1 | NGS (Geneseeq Prime 425-gene) | Elevation of AF in ctDNA of EGFRdel19 (from 0% to 23%), T790M (from 2% to 18%), RB1Q850X (from 0% to 5%), and TP53M237I (from 0% to 4%) concurrent with evidence of t SCLC. Subsequently, a reduction in AFs of these mutations was achieved with cisplatin-etoposide+RT. |
| Pizzutilo 2019 [64] | 1 | ddPCR (EGFR) | Elevation of AF in ctDNA of EGFRdel19 (from 25% to 60%) with reduction in T790M/del19 Ratio (from 0.24 to 0.02) and detection of C797S concurrent with evidence of tSCLC. |
| Minari 2018 [66] | 2 | ddPCR (EGFR) | Elevation of AF in ctDNA of EGFRdel19 (from 10% to 22%) and of EGFRL858R (from 20% to 81%), respectively, concurrent with evidence of tSCLC in 2 patients. |
| Iijima 2018 [67] | 1 | NGS (43 genes) | Elevation of AF in ctDNA of EGFRdel19 (from 12% to 72%) and TP53F134fs (similar AF) concurrent with evidence of tSCLC. Subsequent carboplatin-etoposide treatment led to a drop in AFs. |
| Tsui 2018 [70] | 3 | Targeted NGS and WGS | 2/3 retained EGFR activating mutation after transformation in ctDNA and tissue, 0/3 presented T790M. Elevation in AFs of EGFR concurrent with evidence of PD of SCLC. TP53 mutation was present before transformation and increased in 3/3 patients with PD of SCLC, together with the emergence of CNAs of genes such as MYCL1, SOX2, SOX4, and EGFR. |
| Nishioka 2018 [73] | 1 | NA | Evidence of EGFR T790M mutation in ctDNA after treatment for tSCLC, leading to successful therapy with osimertinib. |
| Mooradian 2017 [68] | 1 | NGS (Guardant360) | Elevation of AF in ctDNA of EGFRdel19 (from 11% to 46%), TP53V173L (from 11% to 55%), PIK3CAE726K (from 3% to 51%), and PIK3CAE545K (from 3% to 54%), concurrent with evidence of PD of tSCLC. |
| Ou 2017 [74] | 1 | NGS (FoundationACT) | After PD to 2° line lorlatinib in a patient with ALK rearrangement, ctDNA analysis showed persistence of ALK rearrangement (estimated AF 30–45% vs. 40–54% before lorlatinib) and disappearance of acquired G1202R, concurrent with SCLC transformation. |
| Alì 2016 [77] | 1 | PCR | Evidence of EGFR T790M in ctDNA concurrent with transformed SCLC in tissue biopsy harboring EGFR activating mutation, but not T790M. |
| Piotroska 2015 [65] | 1 | Beaming (EGFR) | Increasing levels of EGFR activating mutation, with T790M levels remaining suppressed, at the time of progression with SCLC transformation. |
| Han 2017 [72] ABS | 11 | NGS | 3/11 patients developed EGFR T790M mutation in the post-transformation ctDNA rather than in their tissue samples. |
| CTC | |||
| Zhu 2020 [76] | 14 | Aptamer-modified PEG-PLGA-nanofiber microfluidic system for CTC capture, and single-cell sequencing | Histological transformation was reflected by CTC phenotype change from TTF1+, NapsinA+, CK7+, P63- toward CD56+, CgA+, and Syn+, with a significant reduction (p < 0.05) of the mean nuclear size of CTCs. 14/14 patients showed the same molecular characteristics for EGFR, RB1, and TP53 between CTC and tissue samples. |
| Ni 2013 [75] | 1 | CellSearch and Single-Cell Exome Sequencing in CTC | EGFR del19 was identified in tSCLC biopsy as well as in CTCs. PIK3CA, RB1, and TP53 mutations were identified in tSCLC tissue biopsy and CTCs, with higher abundance than in the original NSCLC tissue. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizzutilo, E.G.; Pedrani, M.; Amatu, A.; Ruggieri, L.; Lauricella, C.; Veronese, S.M.; Signorelli, D.; Cerea, G.; Giannetta, L.; Siena, S.; et al. Liquid Biopsy for Small Cell Lung Cancer either De Novo or Transformed: Systematic Review of Different Applications and Meta-Analysis. Cancers 2021, 13, 2265. https://doi.org/10.3390/cancers13092265
Pizzutilo EG, Pedrani M, Amatu A, Ruggieri L, Lauricella C, Veronese SM, Signorelli D, Cerea G, Giannetta L, Siena S, et al. Liquid Biopsy for Small Cell Lung Cancer either De Novo or Transformed: Systematic Review of Different Applications and Meta-Analysis. Cancers. 2021; 13(9):2265. https://doi.org/10.3390/cancers13092265
Chicago/Turabian StylePizzutilo, Elio Gregory, Martino Pedrani, Alessio Amatu, Lorenzo Ruggieri, Calogero Lauricella, Silvio Marco Veronese, Diego Signorelli, Giulio Cerea, Laura Giannetta, Salvatore Siena, and et al. 2021. "Liquid Biopsy for Small Cell Lung Cancer either De Novo or Transformed: Systematic Review of Different Applications and Meta-Analysis" Cancers 13, no. 9: 2265. https://doi.org/10.3390/cancers13092265
APA StylePizzutilo, E. G., Pedrani, M., Amatu, A., Ruggieri, L., Lauricella, C., Veronese, S. M., Signorelli, D., Cerea, G., Giannetta, L., Siena, S., & Sartore-Bianchi, A. (2021). Liquid Biopsy for Small Cell Lung Cancer either De Novo or Transformed: Systematic Review of Different Applications and Meta-Analysis. Cancers, 13(9), 2265. https://doi.org/10.3390/cancers13092265