Nipple Aspirate Fluid at a Glance
Abstract
:Simple Summary
Abstract
1. What Is Nipple Aspirate Fluid?
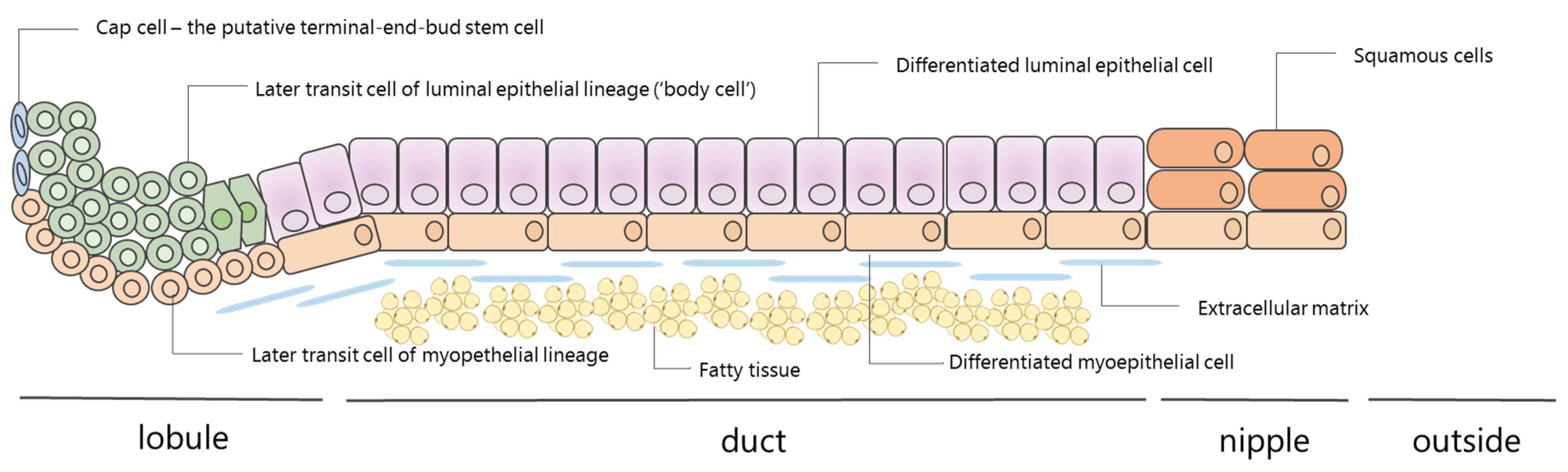
2. How Is NAF Produced?
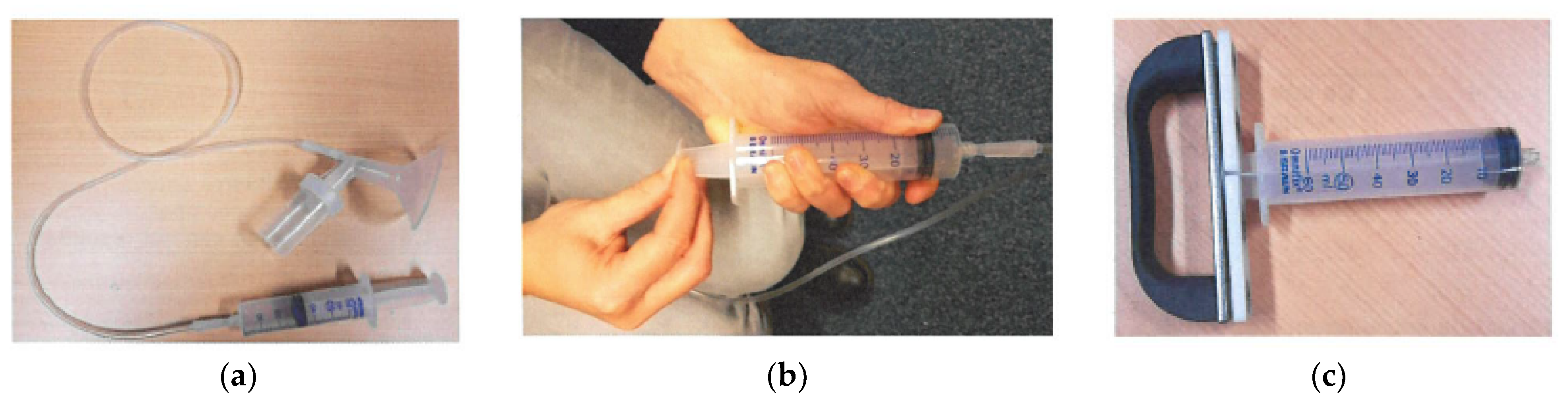
3. How Is NAF Collected?
4. Discomfort Associated with NFA
5. Why Investigate NAF?
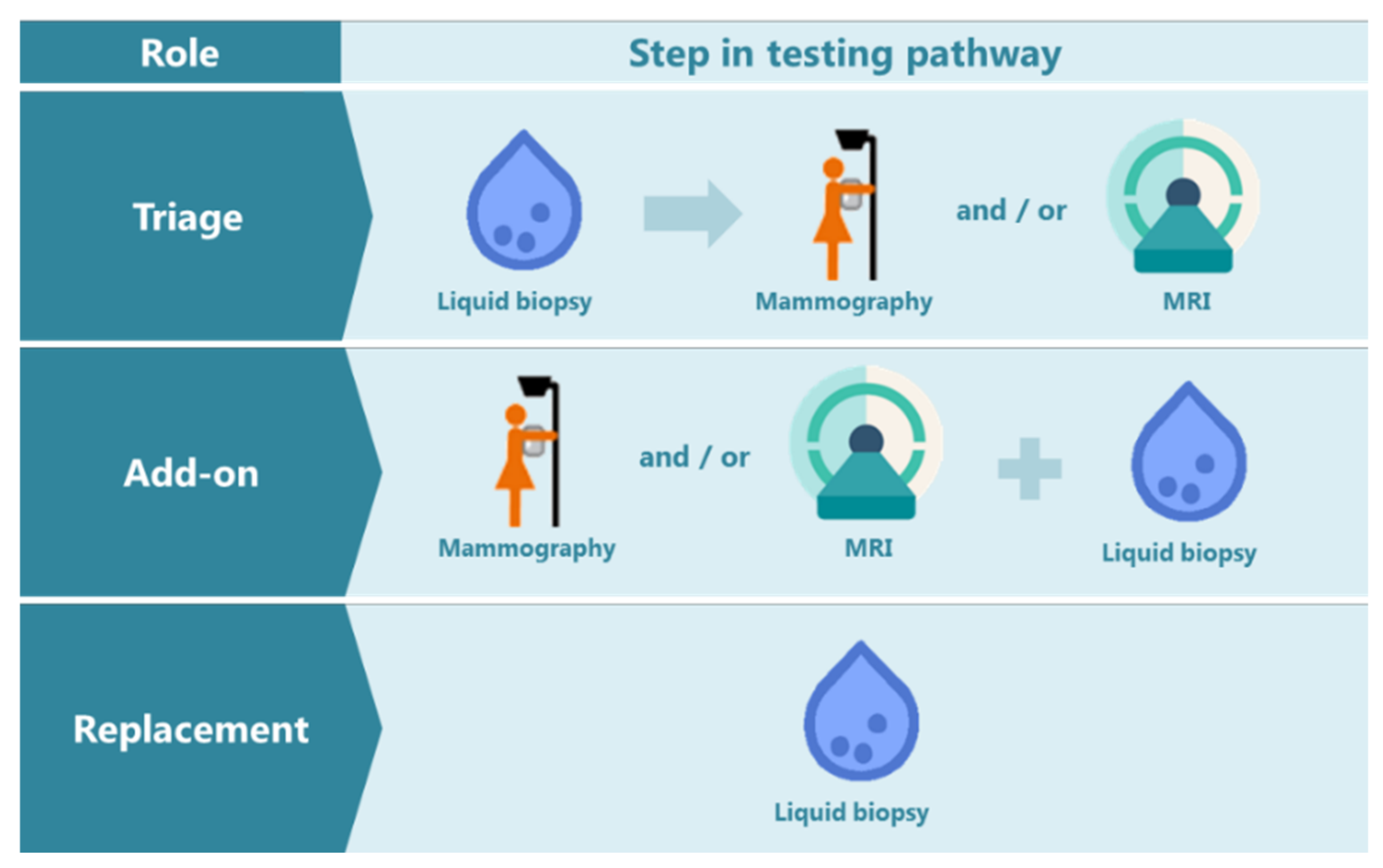
6. How Could NAF Be of Added Value as an Early Breast Cancer Detection Tool?
7. Challenges in Using NAF as a Biomarker Source
8. Historical Overview of Studies Focusing on NAF
- -
- Nipple aspirate fluid (NAF) is a physiological fluid that is produced by the luminal layer of the breast lobules and ducts
- -
- Nipple fluid aspiration (NFA) is well tolerated by women
- -
- NAF can be acquired in the majority of women
- -
- Many biomarkers can be found in NAF, such as DNA, RNA, microRNA and proteins
- -
- Cytology assessment in NAF has low diagnostic accuracy
- -
- NAF originates from the location where breast cancer arises
- -
- NAF can be acquired repeatedly, easily and non-invasively
- -
- Bilateral NAF samples allow intra-patient control analyses
- -
- NAF samples are of low volume, can be viscous, have low cellularity and have different colors, which may affect biomarker analysis
- -
- It is unclear which duct NAF derives from
- -
- For the NFA procedure, use oxytocin nose spray to increase success rate and tolerability for the woman, and use an ergonomic handle for the research nurse performing the procedure
- -
- Combine nipple fluid research with imaging results and include anthropomorphic measures and risk factors for breast cancer
- -
- Development of technologies that are feasible for detection and interpretation of biomarkers in samples that are viscous and of low volume
- -
- To reduce sample viscosity, use mechanical and/or chemical liquefaction in sample processing steps
- -
- Report NAF biomarker results in diagnostic accuracy values in order to be able to interpret their translational role
- -
- Involve medical decision making experts and patient advocate groups to discuss the potential use of liquid biopsies in early detection
- -
- Develop a self-test for NFA collection and interpretation
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shaheed, S.U.; Tait, C.; Kyriacou, K.; Linforth, R.; Salhab, M.; Sutton, C. Evaluation of Nipple Aspirate Fluid as a Diagnostic Tool for Early Detection of Breast Cancer. Clin. Proteom. 2018, 15, 3. [Google Scholar] [CrossRef]
- Waaijer, L.; van Diest, P.; van der Pol, C.C.; Verolme, B.; Hennink, A.; Witkamp, A.J. Ductoscopy for Pathologic Nipple Discharge. Ned. Tijdschr. Voor Geneeskd. 2013, 157, A6358. [Google Scholar]
- Dixon, J.M.; Mansel, R.E. Abc of Breast Diseases. Symptoms Assessment and Guidelines for Referral. BMJ 1994, 309, 722–726. [Google Scholar] [CrossRef] [Green Version]
- Sheiman, L.S.; Levesque, P.H. The In’s and Out’s of Ductography: A Comprehensive Review. Curr. Probl. Diagn. Radiol. 2016, 45, 61–70. [Google Scholar] [CrossRef]
- Ohtake, T.; Kimijima, I.; Fukushima, T.; Yasuda, M.; Sekikawa, K.; Takenoshita, S.; Abe, R. Computer-Assisted Complete Three-Dimensional Reconstruction of the Mammary Ductal/Lobular Systems: Implications of Ductal Anastomoses for Breast-Conserving Surgery. Cancer 2001, 91, 2263–2272. [Google Scholar] [CrossRef]
- Going, J.J.; Moffat, D.F. Escaping from Flatland: Clinical and Biological Aspects of Human Mammary Duct Anatomy in Three Dimensions. J. Pathol. 2004, 203, 538–544. [Google Scholar] [CrossRef]
- Jesinger, R.A. Breast Anatomy for the Interventionalist. Tech. Vasc. Interv. Radiol. 2014, 17, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paine, I.S.; Lewis, M.T. The Terminal End Bud: The Little Engine That Could. J. Mammary Gland. Biol. Neoplasia 2017, 22, 93–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macias, H.; Hinck, L. Mammary Gland Development. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 533–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, B.L.; Love, S.M. The Intraductal Approach to the Breast: Raison D’etre. Breast Cancer Res. 2006, 8, 206. [Google Scholar] [CrossRef] [Green Version]
- Chatterton, R.T., Jr.; Geiger, A.S.; Mateo, E.T.; Helenowski, I.B.; Gann, P.H. Comparison of Hormone Levels in Nipple Aspirate Fluid of Pre- and Postmenopausal Women: Effect of Oral Contraceptives and Hormone Replacement. J. Clin. Endocrinol. Metab. 2005, 90, 1686–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartorius, O.W. The Biochemistry of Breast Cyst Fluids and Duct Secretions. Breast Cancer Res. Treat. 1995, 35, 255–266. [Google Scholar] [CrossRef]
- Petrakis, N.L. Breast Secretory Activity in Nonlactating Women, Postpartum Breast Involution, and the Epidemiology of Breast Cancer. Natl. Cancer Inst. Monogr. 1977, 47, 161–164. [Google Scholar]
- Petrakis, N.L. Physiologic, Biochemical, and Cytologic Aspects of Nipple Aspirate Fluid. Breast Cancer Res. Treat. 1986, 8, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Zangar, R.C.; Varnum, S.M.; Covington, C.Y.; Smith, R.D. A Rational Approach for Discovering and Validating Cancer Markers in Very Small Samples Using Mass Spectrometry and Elisa Microarrays. Dis Markers. 2004, 20, 135–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Groot, J.S.; van Diest, P.J.; van Amersfoort, M.; Vlug, E.J.; Pan, X.; Ter Hoeve, N.D.; Rosing, H.; Beijnen, J.H.; Youssef, S.A.; de Bruin, A.; et al. Intraductal Cisplatin Treatment in a Brca-Associated Breast Cancer Mouse Model Attenuates Tumor Development but Leads to Systemic Tumors in Aged Female Mice. Oncotarget 2017, 8, 60750–60763. [Google Scholar] [CrossRef] [Green Version]
- Suijkerbuijk, K.P.; van der Wall, E.; Meijrink, H.; Pan, X.; Borel Rinkes, I.H.; Ausems, M.G.; van Diest, P.J. Successful Oxytocin-Assisted Nipple Aspiration in Women at Increased Risk for Breast Cancer. Fam. Cancer 2010, 9, 321–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delmonico, L.; Areias, V.R.; Pinto, R.C.; Matos Cda, S.; Rosa, M.F.; De Azevedo, C.M.; Alves, G. Protein Identification from Dried Nipple Aspirate Fluid on Guthrie Cards Using Mass Spectrometry. Mol. Med. Rep. 2015, 12, 159–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Gui, G.; Zhang, K.; Twelves, D.; Kliethermes, B.; Sauter, E.R. Proteins and Carbohydrates in Nipple Aspirate Fluid Predict the Presence of Atypia and Cancer in Women Requiring Diagnostic Breast Biopsy. BMC Cancer 2012, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Mannello, F.; Tonti, G.A.; Pederzoli, A.; Simone, P.; Smaniotto, A.; Medda, V. Detection of Superoxide Dismutase-1 in Nipple Aspirate Fluids: A Reactive Oxygen Species-Regulating Enzyme in the Breast Cancer Microenvironment. Clin. Breast Cancer 2010, 10, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Wrensch, M.R.; Petrakis, N.L.; Gruenke, L.D.; Ernster, V.L.; Miike, R.; King, E.B.; Hauck, W.W. Factors Associated with Obtaining Nipple Aspirate Fluid: Analysis of 1428 Women and Literature Review. Breast Cancer Res. Treat. 1990, 15, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.S.; Pang, D.; Wang, F.; Xue, Y.W.; Gao, D.N.; Li, H.; Li, K.; Wang, B.Y.; Wang, D.; Li, H.Y. Nipple Aspirate Fluid Collection, Related Factors and Relationship between Carcinoembryonic Antigen in Nipple Aspirate Fluid and Breast Diseases in Women in Harbin, Prc. Cancer Epidemiol. Prev. Biomark. 2009, 18, 732–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Klemp, J.R.; Simonsen, M.; Welsko, C.M.; Zalles, C.M.; Kimler, B.F.; Fabian, C.J. Failure of High Risk Women to Produce Nipple Aspirate Fluid Does Not Exclude Detection of Cytologic Atypia in Random Periareolar Fine Needle Aspiration Specimens. Breast Cancer Res. Treat. 2004, 87, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Baltzell, K.A.; Wrensch, M.; Sison, J.D. A Descriptive Study of Variables Associated with Obtaining Nipple Aspirate Fluid in a Cohort of Non-Lactating Women. BMC Womens Health 2006, 6, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrakis, N.L.; Barnes, S.; King, E.B.; Lowenstein, J.; Wiencke, J.; Lee, M.M.; Miike, R.; Kirk, M.; Coward, L. Stimulatory Influence of Soy Protein Isolate on Breast Secretion in Pre- and Postmenopausal Women. Cancer Epidemiol. Biomark. Prev. 1996, 5, 785–794. [Google Scholar]
- Klein, P.; Glaser, E.; Grogan, L.; Keane, M.; Lipkowitz, S.; Soballe, P.; Brooks, L.; Jenkins, J.; Steinberg, S.M.; DeMarini, D.M.; et al. Biomarker Assays in Nipple Aspirate Fluid. Breast J. 2001, 7, 378–387. [Google Scholar] [CrossRef]
- Djuric, Z.; Visscher, D.W.; Heilbrun, L.K.; Chen, G.; Atkins, M.; Covington, C.Y. Influence of Lactation History on Breast Nipple Aspirate Fluid Yields and Fluid Composition. Breast J. 2005, 11, 92–99. [Google Scholar] [CrossRef]
- Maskarinec, G.; Morimoto, Y.; Conroy, S.M.; Pagano, I.S.; Franke, A.A. The Volume of Nipple Aspirate Fluid Is Not Affected by 6 Months of Treatment with Soy Foods in Premenopausal Women. J. Nutr. 2011, 141, 626–630. [Google Scholar] [CrossRef]
- Smalley, M.; Ashworth, A. Stem Cells and Breast Cancer: A Field in Transit. Nat. Rev. Cancer. 2003, 3, 832–844. [Google Scholar] [CrossRef]
- Jakub, J.W.; Peled, A.W.; Gray, R.J.; Greenup, R.A.; Kiluk, J.V.; Sacchini, V.; McLaughlin, S.A.; Tchou, J.C.; Vierkant, R.A.; Degnim, A.C.; et al. Oncologic Safety of Prophylactic Nipple-Sparing Mastectomy in a Population with Brca Mutations: A Multi-Institutional Study. JAMA Surg. 2018, 153, 123–129. [Google Scholar] [CrossRef]
- Maskarinec, G.; Ollberding, N.J.; Conroy, S.M.; Morimoto, Y.; Pagano, I.S.; Franke, A.A.; Gentzschein, E.; Stanczyk, F.Z. Estrogen Levels in Nipple Aspirate Fluid and Serum During a Randomized Soy Trial. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1815–1821. [Google Scholar] [CrossRef] [Green Version]
- Dua, R.S.; Isacke, C.M.; Gui, G.P. The Intraductal Approach to Breast Cancer Biomarker Discovery. J. Clin. Oncol. 2006, 24, 1209–1216. [Google Scholar] [CrossRef]
- Menekse, E.; McKolanis, J.; Finn, O.J.; McAuliffe, P.F.; Johnson, R.; Soran, A. Anti-Muc1 Antibody in Nipple Aspirate Fluids Correlates with Tumor Aggressiveness in Breast Cancer: A Feasibility Study. Dis. Markers 2015, 2015, 179689. [Google Scholar] [CrossRef] [Green Version]
- Chatterton, R.T.; Muzzio, M.; Heinz, R.; Gann, P.H.; Khan, S.A. Methodological Considerations in Estrogen Assays of Breast Fluid and Breast Tissue. Steroids 2015, 99 Pt A, 103–107. [Google Scholar] [CrossRef]
- Tredwell, G.D.; Miller, J.A.; Chow, H.H.; Thompson, P.A.; Keun, H.C. Metabolomic Characterization of Nipple Aspirate Fluid by (1)H Nmr Spectroscopy and Gc-Ms. J. Proteome Res. 2014, 13, 883–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maskarinec, G.; Hebshi, S.; Custer, L.; Franke, A.A. The Relation of Soy Intake and Isoflavone Levels in Nipple Aspirate Fluid. Eur. J. Cancer Prev. 2008, 17, 67–70. [Google Scholar] [CrossRef] [PubMed]
- King, E.B.; Chew, K.L.; Hom, J.D.; Miike, R.; Wrensch, M.R.; Petrakis, N.L. Multiple Sampling for Increasing the Diagnostic Sensitivity of Nipple Aspirate Fluid for Atypical Cytology. Acta Cytol. 2004, 48, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shao, Z.M.; Beatty, P.; Sartippour, M.; Wang, H.J.; Elashoff, R.; Chang, H.; Brooks, M.N. The Use of Oxytocin in Nipple Fluid Aspiration. Breast J. 2003, 9, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Suijkerbuijk, K.P.; van der Wall, E.; van Diest, P.J. Oxytocin: Bringing Magic into Nipple Aspiration. Ann. Oncol. 2007, 18, 1743–1744. [Google Scholar] [CrossRef] [PubMed]
- de Groot, J.S.; Moelans, C.B.; Elias, S.G.; Hennink, A.; Verolme, B.; Suijkerbuijk, K.P.; Jager, A.; Seynaeve, C.; Bos, P.; Witkamp, A.J.; et al. Repeated Nipple Fluid Aspiration: Compliance and Feasibility Results from a Prospective Multicenter Study. PLoS ONE 2015, 10, e0127895. [Google Scholar] [CrossRef] [Green Version]
- Shaheed, S.U.; Tait, C.; Kyriacou, K.; Mullarkey, J.; Burrill, W.; Patterson, L.H.; Linforth, R.; Salhab, M.; Sutton, C.W. Nipple Aspirate Fluid-a Liquid Biopsy for Diagnosing Breast Health. Proteom. Clin. Appl. 2017, 11, 1700015. [Google Scholar] [CrossRef] [Green Version]
- Phillips, H.A.; Howard, G.C.; Miller, W.R. Pilot Studies on the P53 Gene in Nipple Aspirate Fluid from Patients with Breast Cancer. Breast Cancer Res. Treat. 2000, 61, 139–143. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, W.; Ehya, H.; Lininger, J.; Sauter, E. Microsatellite Changes in Nipple Aspirate Fluid and Breast Tissue from Women with Breast Carcinoma or Its Precursors. Clin. Cancer Res. 2003, 9, 3029–3033. [Google Scholar] [PubMed]
- Krassenstein, R.; Sauter, E.; Dulaimi, E.; Battagli, C.; Ehya, H.; Klein-Szanto, A.; Cairns, P. Detection of Breast Cancer in Nipple Aspirate Fluid by Cpg Island Hypermethylation. Clin. Cancer Res. 2004, 10 Pt 1, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Patuleia, S.I.S.; van Gils, C.H.; Oneto Cao, A.M.; Bakker, M.F.; van Diest, P.J.; van der Wall, E.; Moelans, C.B. The Physiological Microrna Landscape in Nipple Aspirate Fluid: Differences and Similarities with Breast Tissue, Breast Milk, Plasma and Serum. Int. J. Mol. Sci. 2020, 21, 8466. [Google Scholar] [CrossRef] [PubMed]
- Sauter, E.R. Analysis of Nipple Aspirate Fluid for Diagnosis of Breast Cancer: An Alternative to Invasive Biopsy. Expert Rev. Mol. Diagn. 2005, 5, 873–881. [Google Scholar] [CrossRef]
- Mannello, F.; Medda, V.; Tonti, G.A. Protein Profile Analysis of the Breast Microenvironment to Differentiate Healthy Women from Breast Cancer Patients. Expert Rev. Proteomics. 2009, 6, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Jiwa, N.; Gandhewar, R.; Chauhan, H.; Ashrafian, H.; Kumar, S.; Wright, C.; Takats, Z.; Leff, D.R. Diagnostic Accuracy of Nipple Aspirate Fluid Cytology in Asymptomatic Patients: A Meta-Analysis and Systematic Review of the Literature. Ann. Surg. Oncol. 2021, 28, 3751–3760. [Google Scholar] [CrossRef]
- Dabbs, D.J. Mammary Ductal Foam Cells: Macrophage Immunophenotype. Hum. Pathol. 1993, 24, 977–981. [Google Scholar] [CrossRef]
- Lee, M.M.; Petrakis, N.L.; Wrensch, M.R.; King, E.B.; Miike, R.; Sickles, E. Association of Abnormal Nipple Aspirate Cytology and Mammographic Pattern and Density. Cancer Epidemiol. Biomark. Prev. 1994, 3, 33–36. [Google Scholar]
- Scott, W.N.; Miller, W.R. The Mutagenic Activity of Human Breast Secretions. J. Cancer Res. Clin. Oncol. 1990, 116, 499–502. [Google Scholar] [CrossRef]
- Petrakis, N.L.; Maack, C.A.; Lee, R.E.; Lyon, M. Mutagenic Activity in Nipple Aspirates of Human Breast Fluid. Cancer Res. 1980, 40, 188–189. [Google Scholar] [PubMed]
- Isaacs, C.; Cavalli, L.R.; Cohen, Y.; Pennanen, M.; Shankar, L.K.; Freedman, M.; Singh, B.; Liu, M.; Gallagher, A.; Rone, J.D.; et al. Detection of Loh and Mitochondrial DNA Alterations in Ductal Lavage and Nipple Aspirate Fluids from Hngh-Risk Patients. Breast Cancer Res. Treat. 2004, 84, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Jakupciak, J.P.; Maggrah, A.; Maragh, S.; Maki, J.; Reguly, B.; Maki, K.; Wittock, R.; Robinson, K.; Wagner, P.D.; Thayer, R.E.; et al. Facile Whole Mitochondrial Genome Resequencing from Nipple Aspirate Fluid Using Mitochip V2.0. BMC Cancer 2008, 8, 95. [Google Scholar] [CrossRef] [Green Version]
- Moelans, C.B.; Patuleia, S.I.S.; van Gils, C.H.; van der Wall, E.; van Diest, P.J. Application of Nipple Aspirate Fluid Mirna Profiles for Early Breast Cancer Detection and Management. Int. J. Mol. Sci. 2019, 20, 5814. [Google Scholar] [CrossRef] [Green Version]
- Sauter, E.R.; Zhu, W.; Fan, X.J.; Wassell, R.P.; Chervoneva, I.; Du Bois, G.C. Proteomic Analysis of Nipple Aspirate Fluid to Detect Biologic Markers of Breast Cancer. Br. J. Cancer 2002, 86, 1440–1443. [Google Scholar] [CrossRef] [Green Version]
- Paweletz, C.P.; Trock, B.; Pennanen, M.; Tsangaris, T.; Magnant, C.; Liotta, L.A.; Petricoin, E.F., 3rd. Proteomic Patterns of Nipple Aspirate Fluids Obtained by Seldi-Tof: Potential for New Biomarkers to Aid in the Diagnosis of Breast Cancer. Dis. Markers 2001, 17, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coombes, K.R.; Fritsche, H.A., Jr.; Clarke, C.; Chen, J.N.; Baggerly, K.A.; Morris, J.S.; Xiao, L.C.; Hung, M.C.; Kuerer, H.M. Quality Control and Peak Finding for Proteomics Data Collected from Nipple Aspirate Fluid by Surface-Enhanced Laser Desorption and Ionization. Clin. Chem. 2003, 49, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Sauter, E.R.; Davis, W.; Qin, W.; Scanlon, S.; Mooney, B.; Bromert, K.; Folk, W.R. Identification of a Beta-Casein-Like Peptide in Breast Nipple Aspirate Fluid That Is Associated with Breast Cancer. Biomark Med. 2009, 3, 577–588. [Google Scholar] [CrossRef] [Green Version]
- Alexander, H.; Stegner, A.L.; Wagner-Mann, C.; Du Bois, G.C.; Alexander, S.; Sauter, E.R. Proteomic Analysis to Identify Breast Cancer Biomarkers in Nipple Aspirate Fluid. Clin. Cancer Res. 2004, 10, 7500–7510. [Google Scholar] [CrossRef] [Green Version]
- Varnum, S.M.; Covington, C.C.; Woodbury, R.L.; Petritis, K.; Kangas, L.J.; Abdullah, M.S.; Pounds, J.G.; Smith, R.D.; Zangar, R.C. Proteomic Characterization of Nipple Aspirate Fluid: Identification of Potential Biomarkers of Breast Cancer. Breast Cancer Res. Treat. 2003, 80, 87–97. [Google Scholar] [CrossRef]
- Hsiung, R.; Zhu, W.; Klein, G.; Qin, W.; Rosenberg, A.; Park, P.; Rosato, E.; Sauter, E. High Basic Fibroblast Growth Factor Levels in Nipple Aspirate Fluid Are Correlated with Breast Cancer. Cancer J. 2002, 8, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, R.T., Jr.; Geiger, A.S.; Khan, S.A.; Helenowski, I.B.; Jovanovic, B.D.; Gann, P.H. Variation in Estradiol, Estradiol Precursors, and Estrogen-Related Products in Nipple Aspirate Fluid from Normal Premenopausal Women. Cancer Epidemiol. Biomark. Prev. 2004, 13, 928–935. [Google Scholar]
- Sauter, E.R.; Tichansky, D.S.; Chervoneva, I.; Diamandis, E.P. Circulating Testosterone and Prostate-Specific Antigen in Nipple Aspirate Fluid and Tissue Are Associated with Breast Cancer. Environ. Health Perspect. 2002, 110, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, J.L.; Chang, H.; Barsky, S.H.; Nguyen, M. Breast-Cancer Diagnosis with Nipple Fluid Bfgf. Lancet 2000, 356, 567. [Google Scholar] [CrossRef]
- Sauter, E.R.; Garofalo, C.; Hewett, J.; Hewett, J.E.; Morelli, C.; Surmacz, E. Leptin Expression in Breast Nipple Aspirate Fluid (Naf) and Serum Is Influenced by Body Mass Index (Bmi) but Not by the Presence of Breast Cancer. Horm. Metab. Res. 2004, 36, 336–340. [Google Scholar] [PubMed]
- Sauter, E.R.; Daly, M.; Linahan, K.; Ehya, H.; Engstrom, P.F.; Bonney, G.; Ross, E.A.; Yu, H.; Diamandis, E. Prostate-Specific Antigen Levels in Nipple Aspirate Fluid Correlate with Breast Cancer Risk. Cancer Epidemiol. Biomark. Prev. 1996, 5, 967–970. [Google Scholar]
- Zhao, Y.; Verselis, S.J.; Klar, N.; Sadowsky, N.L.; Kaelin, C.M.; Smith, B.; Foretova, L.; Li, F.P. Nipple Fluid Carcinoembryonic Antigen and Prostate-Specific Antigen in Cancer-Bearing and Tumor-Free Breasts. J. Clin. Oncol. 2001, 19, 1462–1467. [Google Scholar] [CrossRef]
- Sauter, E.R.; Klein, G.; Wagner-Mann, C.; Diamandis, E.P. Prostate-Specific Antigen Expression in Nipple Aspirate Fluid Is Associated with Advanced Breast Cancer. Cancer Detect. Prev. 2004, 28, 27–31. [Google Scholar] [CrossRef]
- Zubor, P.; Kubatka, P.; Kajo, K.; Dankova, Z.; Polacek, H.; Bielik, T.; Kudela, E.; Samec, M.; Liskova, A.; Vlcakova, D.; et al. Why the Gold Standard Approach by Mammography Demands Extension by Multiomics? Application of Liquid Biopsy Mirna Profiles to Breast Cancer Disease Management. Int. J. Mol. Sci. 2019, 20, 2878. [Google Scholar] [CrossRef] [Green Version]
- Jiwa, N.; Takats, Z.; Leff, D.R.; Sutton, C. Breast Health Screening: A Uk-Wide Questionnaire. BMJ Nutr. Prev. Health 2021, 4, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Leeflang, M.M.; Deeks, J.J.; Takwoingi, Y.; Macaskill, P. Cochrane Diagnostic Test Accuracy Reviews. Syst. Rev. 2013, 2, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uitnodigingsfolder ‘Bevolkingsonderzoek Borstkanker’. Available online: https://www.rivm.nl/documenten/folder-bevolkingsonderzoek-borstkanker-nl (accessed on 12 November 2021).
- Mannello, F.; Tonti, G.A.; Canestrari, F. Nutrients and Nipple Aspirate Fluid Composition: The Breast Microenvironment Regulates Protein Expression and Cancer Aetiology. Genes Nutr. 2008, 3, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Patuleia, S.I.S.; van der Wall, E.; van Gils, C.H.; Bakker, M.F.; Jager, A.; Voorhorst-Ogink, M.M.; van Diest, P.J.; Moelans, C.B. The Changing Microrna Landscape by Color and Cloudiness: A Cautionary Tale for Nipple Aspirate Fluid Biomarker Analysis. Cell. Oncol. 2021, 44, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Qiashredder Homogenizer. Available online: https://www.qiagen.com/us/products/instruments-and-automation/accessories/qiashredder/ (accessed on 16 December 2021).
- Papanicolaou, G.N.; Bader, G.M.; Holmquist, D.G.; Falk, E.A. Cytologic Evaluation of Breast Secretions. Ann. N. Y. Acad. Sci. 1956, 63, 1409–1421. [Google Scholar] [CrossRef]
- Fleming, R.M. Cytological Studies in Lesions of the Breast: Findings in Nipple Secretions and Aspirates from Tumors. South Med. J. 1955, 48, 74–78. [Google Scholar] [CrossRef]
- Sartorius, O.W.; Smith, H.S.; Morris, P.; Benedict, D.; Friesen, L. Cytologic Evaluation of Breast Fluid in the Detection of Breast Disease. J. Natl. Cancer Inst. 1977, 59, 1073–1080. [Google Scholar] [CrossRef]
- Petrakis, N.L.; Mason, L.; Lee, R.; Sugimoto, B.; Pawson, S.; Catchpool, F. Association of Race, Age, Menopausal Status, and Cerumen Type with Breast Fluid Secretion in Nonlactating Women, as Determined by Nepple Aspiration. J. Natl. Cancer Inst. 1975, 54, 829–834. [Google Scholar]
- Petrakis, N.L.; Ernster, V.L.; Sacks, S.T.; King, E.B.; Schweitzer, R.J.; Hunt, T.K.; King, M.C. Epidemiology of Breast Fluid Secretion: Association with Breast Cancer Risk Factors and Cerumen Type. J. Natl. Cancer Inst. 1981, 67, 277–284. [Google Scholar] [PubMed]
- Petrakis, N.L.; King, E.B.; Lee, M.; Miike, R. Cerumen Phenotype and Proliferative Epithelium in Breast Fluids of U.S.-Born Vs. Immigrant Asian Women: A Possible Genetic-Environmental Interaction. Breast Cancer Res. Treat. 1990, 16, 279–285. [Google Scholar] [CrossRef]
- Lee, M.M.; Wrensch, M.R.; Miike, R.; Petrakis, N.L. The Association of Dietary Fat with Ability to Obtain Breast Fluid by Nipple Aspiration. Cancer Epidemiol. Biomark. Prev. 1992, 1, 277–280. [Google Scholar]
- Petrakis, N.L.; Lee, M.M.; Wrensch, M.R.; Ernster, V.L.; Miike, R.; Koo, L.C.; Ho, J.C. Birthplace and Yield of Nipple Aspirate Fluid in Chinese Women. Cancer Epidemiol. Biomark. Prev. 1998, 7, 835–839. [Google Scholar]
- Petrakis, N.L.; Ernster, V.L.; King, E.B.; Sacks, S.T. Epithelial Dysplasia in Nipple Aspirates of Breast Fluid: Association with Family History and Other Breast Cancer Risk Factors. J. Natl. Cancer Inst. 1982, 68, 9–13. [Google Scholar] [PubMed]
- King, E.B.; Chew, K.L.; Petrakis, N.L.; Ernster, V.L. Nipple Aspirate Cytology for the Study of Breast Cancer Precursors. J. Natl. Cancer Inst. 1983, 71, 1115–1121. [Google Scholar] [PubMed]
- Tice, J.A.; Miike, R.; Adduci, K.; Petrakis, N.L.; King, E.; Wrensch, M.R. Nipple Aspirate Fluid Cytology and the Gail Model for Breast Cancer Risk Assessment in a Screening Population. Cancer Epidemiol. Biomark. Prev. 2005, 14, 324–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrakis, N.L. Genetic-Environmental Interactions in Relation to Low Dose Studies: A Possible Model from Breast Cancer. Environ. Health Perspect. 1981, 42, 97–102. [Google Scholar] [CrossRef]
- King, E.B.; Kromhout, L.K.; Chew, K.L.; Mayall, B.H.; Petrakis, N.L.; Jensen, R.H.; Young, I.T. Analytic Studies of Foam Cells from Breast Cancer Precursors. Cytometry 1984, 5, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Gruenke, L.D.; Wrensch, M.R.; Petrakis, N.L.; Miike, R.; Ernster, V.L.; Craig, J.C. Breast Fluid Cholesterol and Cholesterol Epoxides: Relationship to Breast Cancer Risk Factors and Other Characteristics. Cancer Res. 1987, 47, 5483–5487. [Google Scholar]
- Petrakis, N.L.; Lim, M.L.; Miike, R.; Lee, R.E.; Morris, M.; Lee, L.; Mason, L. Nipple Aspirate Fluids in Adult Nonlactating Women--Lactose Content, Cationic Na+, K+, Na+/K+ Ratio, and Coloration. Breast Cancer Res. Treat. 1989, 13, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, N.L. Aspo Distinguished Achievement Award Lecture. Studies on the Epidemiology and Natural History of Benign Breast Disease and Breast Cancer Using Nipple Aspirate Fluid. Cancer Epidemiol. Biomark. Prev. 1993, 2, 3–10. [Google Scholar]
- Mannello, F.; Tonti, G.A.; Medda, V.; Pederzoli, A.; Sauter, E.R. Increased Shedding of Soluble Fragments of P-Cadherin in Nipple Aspirate Fluids from Women with Breast Cancer. Cancer Sci. 2008, 99, 2160–2169. [Google Scholar] [CrossRef]
- Mannello, F.; Tonti, G.A.; Medda, V. Protein Oxidation in Breast Microenvironment: Nipple Aspirate Fluid Collected from Breast Cancer Women Contains Increased Protein Carbonyl Concentration. Cell. Oncol. 2009, 31, 383–392. [Google Scholar] [CrossRef]
- Mannello, F.; Qin, W.; Zhu, W.; Fabbri, L.; Tonti, G.A.; Sauter, E.R. Nipple Aspirate Fluids from Women with Breast Cancer Contain Increased Levels of Group Iia Secretory Phospholipase A2. Breast Cancer Res. Treat. 2008, 111, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F.; Tonti, G.A.; Pagliarani, S.; Benedetti, S.; Canestrari, F.; Zhu, W.; Qin, W.; Sauter, E.R. The 8-Epimer of Prostaglandin F(2alpha), a Marker of Lipid Peroxidation and Oxidative Stress, Is Decreased in the Nipple Aspirate Fluid of Women with Breast Cancer. Int. J. Cancer 2007, 120, 1971–1976. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F.; Tonti, G.A.; Simone, P.; Ligi, D.; Medda, V. Iron-Binding Proteins and C-Reactive Protein in Nipple Aspirate Fluids: Role of Iron-Driven Inflammation in Breast Cancer Microenvironment? Am. J. Transl. Res. 2010, 3, 100–113. [Google Scholar]
- Mannello, F.; Ligi, D.; Canale, M. Aluminium, Carbonyls and Cytokines in Human Nipple Aspirate Fluids: Possible Relationship between Inflammation, Oxidative Stress and Breast Cancer Microenvironment. J. Inorg. Biochem. 2013, 128, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F.; Tonti, G.A.; Medda, V.; Simone, P.; Darbre, P.D. Analysis of Aluminium Content and Iron Homeostasis in Nipple Aspirate Fluids from Healthy Women and Breast Cancer-Affected Patients. J. Appl. Toxicol. 2011, 31, 262–269. [Google Scholar] [CrossRef]
- Darbre, P.D.; Pugazhendhi, D.; Mannello, F. Aluminium and Human Breast Diseases. J. Inorg. Biochem. 2011, 105, 1484–1488. [Google Scholar] [CrossRef]
- Qin, W.; Zhu, W.; Hewett, J.E.; Rottinghaus, G.; Chen, Y.C.; Flynn, J.T.; Kliethermes, B.; Mannello, F.; Sauter, E.R. Upa Is Upregulated by High Dose Celecoxib in Women at Increased Risk of Developing Breast Cancer. BMC Cancer 2008, 8, 298. [Google Scholar] [CrossRef] [Green Version]
- Darbre, P.D.; Mannello, F.; Exley, C. Aluminium and Breast Cancer: Sources of Exposure, Tissue Measurements and Mechanisms of Toxicological Actions on Breast Biology. J. Inorg. Biochem. 2013, 128, 257–261. [Google Scholar] [CrossRef]
- Mannello, F.; Ligi, D. Resolving Breast Cancer Heterogeneity by Searching Reliable Protein Cancer Biomarkers in the Breast Fluid Secretome. BMC Cancer 2013, 13, 344. [Google Scholar] [CrossRef] [Green Version]
- Mannello, F.; Medda, V.; Smaniotto, A.; Tonti, G.A. Intracrinology of Breast Microenvironment: Hormonal Status in Nipple Aspirate Fluid and Its Relationship to Breast Cancer. Expert Rev. Endocrinol. Metab. 2009, 4, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F. New Horizon for Breast Cancer Biomarker Discoveries: What Might the Liquid Biopsy of Nipple Aspirate Fluid Hold? Proteom. Clin. Appl. 2017, 11, 1700060. [Google Scholar] [CrossRef]
- Sauter, E.R.; Diamandis, E.P. Prostate-Specific Antigen Levels in Nipple Aspirate Fluid. J. Clin. Oncol. 2001, 19, 3160. [Google Scholar] [CrossRef] [PubMed]
- Sauter, E.R.; Lininger, J.; Magklara, A.; Hewett, J.E.; Diamandis, E.P. Association of Kallikrein Expression in Nipple Aspirate Fluid with Breast Cancer Risk. Int. J. Cancer 2004, 108, 588–591. [Google Scholar] [CrossRef]
- Sauter, E.R.; Babb, J.; Daly, M.; Engstrom, P.F.; Ehya, H.; Malick, J.; Diamandis, E. Prostate-Specific Antigen Production in the Female Breast: Association with Progesterone. Cancer Epidemiol. Biomark. Prev. 1998, 7, 315–320. [Google Scholar]
- Sauter, E.R.; Chervoneva, I.; Diamandis, A.; Khosravi, J.M.; Litwin, S.; Diamandis, E.P. Prostate-Specific Antigen and Insulin-Like Growth Factor Binding Protein-3 in Nipple Aspirate Fluid Are Associated with Breast Cancer. Cancer Detect. Prev. 2002, 26, 149–157. [Google Scholar] [CrossRef]
- Pavlou, M.P.; Kulasingam, V.; Sauter, E.R.; Kliethermes, B.; Diamandis, E.P. Nipple Aspirate Fluid Proteome of Healthy Females and Patients with Breast Cancer. Clin. Chem. 2010, 56, 848–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Zhu, W.; Schlatter, L.; Miick, R.; Loy, T.S.; Atasoy, U.; Hewett, J.E.; Sauter, E.R. Increased Expression of the Inflammatory Protein Ykl-40 in Precancers of the Breast. Int. J. Cancer 2007, 121, 1536–1542. [Google Scholar] [CrossRef]
- Sauter, E.R.; Wagner-Mann, C.; Ehya, H.; Klein-Szanto, A. Biologic Markers of Breast Cancer in Nipple Aspirate Fluid and Nipple Discharge Are Associated with Clinical Findings. Cancer Detect. Prev. 2007, 31, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.R.; Sauter, E.R.; Quinn, T.P.; Deutscher, S.L. Thomsen-Friedenreich and Tn Antigens in Nipple Fluid: Carbohydrate Biomarkers for Breast Cancer Detection. Clin. Cancer Res. 2005, 11 Pt 1, 6868–6871. [Google Scholar] [CrossRef] [Green Version]
- Sauter, E.R.; Shan, S.; Hewett, J.E.; Speckman, P.; Du Bois, G.C. Proteomic Analysis of Nipple Aspirate Fluid Using Seldi-Tof-Ms. Int. J. Cancer 2005, 114, 791–796. [Google Scholar] [CrossRef]
- Qin, W.; Zhu, W.; Wagner-Mann, C.; Sauter, E.R. Nipple Aspirate Fluid Expression of Urokinase-Type Plasminogen Activator, Plasminogen Activator Inhibitor-1, and Urokinase-Type Plasminogen Activator Receptor Predicts Breast Cancer Diagnosis and Advanced Disease. Ann. Surg. Oncol. 2003, 10, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhu, W.; Wagner-Mann, C.; Folk, W.; Sauter, E.R. Association of Upa, Pat-1, and Upar in Nipple Aspirate Fluid (Naf) with Breast Cancer. Cancer J. 2003, 9, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.; Trott, P.A.; Morris, L.; Coleman, N.; Sauter, E.; Eeles, R.A. Cellular Characteristics of Nipple Aspiration Fluid During the Menstrual Cycle in Healthy Premenopausal Women. Cytopathology 2001, 12, 184–196. [Google Scholar] [CrossRef]
- Qin, W.; Holick, M.F.; Sorensen, W.; Walker, C.R.; Sauter, E.R. Vitamin D3 Treatment Influences Pge2 and Tgfbeta in Normal and Increased Breast Cancer Risk Women. Anticancer Res. 2016, 36, 5347–5353. [Google Scholar] [CrossRef] [Green Version]
- Sauter, E.R. Using Organ Specific and Circulatory Biofluids to Screen Individuals at High Risk for Breast Cancer Presents Unique Challenges and Opportunities. Cancer Epidemiol. Biomark. Prev. 2021, 30, 429–431. [Google Scholar] [CrossRef]
- de Groot, J.S.; Moelans, C.B.; Elias, S.G.; Jo Fackler, M.; van Domselaar, R.; Suijkerbuijk, K.P.; Witkamp, A.J.; Sukumar, S.; van Diest, P.J.; van der Wall, E. DNA Promoter Hypermethylation in Nipple Fluid: A Potential Tool for Early Breast Cancer Detection. Oncotarget 2016, 7, 24778–24791. [Google Scholar] [CrossRef] [Green Version]
- de Groot, J.S.; Pan, X.; Meeldijk, J.; van der Wall, E.; van Diest, P.J.; Moelans, C.B. Validation of DNA Promoter Hypermethylation Biomarkers in Breast Cancer--a Short Report. Cell. Oncol. 2014, 37, 297–303. [Google Scholar] [CrossRef]
- Early Detection of Hereditary Breast Cancer by Monitoring Microrna Expression in Nipple Aspirate Fluid. Available online: https://www.trialregister.nl/trial/8661 (accessed on 3 September 2021).
- Breast Cancer Biomarkers in Nipple Aspirate Fluid and Blood in Healthy Women. Available online: https://www.trialregister.nl/trial/8987 (accessed on 3 September 2021).
- The Ornament Study: A Multicenter, Cross Sectional, Study to Assess Microrna Expression in Nipple Aspirated Fluid, Blood and Tumor Material in Women with Primary Breast Cancer Compared with Healthy Controls. Available online: https://www.trialregister.nl/trial/6031 (accessed on 3 September 2021).
- Patuleia, S.I.S.; Hagenaars, S.C.; Moelans, C.B.; Ausems Mgem van Gils, C.H.; Tollenaar Raem van Diest, P.J.; Mesker, W.E.; van der Wall, E. Lessons Learned from Setting up a Prospective, Longitudinal, Multicenter Study with Women at High Risk for Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2021, 30, 441–449. [Google Scholar] [CrossRef] [PubMed]
- George, A.L.; Shaheed, S.U.; Sutton, C.W. High-Throughput Proteomic Profiling of Nipple Aspirate Fluid from Breast Cancer Patients Compared with Non-Cancer Controls: A Step Closer to Clinical Feasibility. J. Clin. Med. 2021, 10, 2243. [Google Scholar] [CrossRef]
- Jiwa, N.; Takats, Z.; Leff, D.R. Aso Author Reflections: Diagnostic Accuracy of Nipple Aspirate Fluid Cytology in Asymptomatic Patients and Its Predictive Validity on Future Risk of Breast Cancer: A Meta-Analysis and Systematic Review of the Literature. Ann. Surg. Oncol. 2021, 28, 3761–3762. [Google Scholar] [CrossRef]
- Nipple Aspirate Fluid in Detecting Breast Cancer. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03715959?term=NAF%2C+nipple+aspirate+fluid&recrs=ab&draw=2&rank=1 (accessed on 3 September 2021).
- Physical Activity and Dietary Counseling and Supervised Group Exercise for First-Time Pregnant Women—A Feasibility Study of a Controlled Trial. Available online: https://www.isrctn.com/ISRCTN21512277?q=nipple%20aspirate%20fluid&filters=&sort=&offset=1&totalResults=1&page=1&pageSize=10&searchType=basic-search (accessed on 3 September 2021).
- Martinez, J.A.; Chalasani, P.; Thomson, C.A.; Roe, D.; Altbach, M.; Galons, J.P.; Stopeck, A.; Thompson, P.A.; Villa-Guillen, D.E.; Chow, H.H. Phase Ii Study of Metformin for Reduction of Obesity-Associated Breast Cancer Risk: A Randomized Controlled Trial Protocol. BMC Cancer 2016, 16, 500. [Google Scholar] [CrossRef] [Green Version]
- Kelsey, J.L.; Bernstein, L. Epidemiology and Prevention of Breast Cancer. Annu. Rev. Public Health 1996, 17, 47–67. [Google Scholar] [CrossRef]
- Phillips, H.A.; Howard, G.C.; Miller, W.R. Nipple Aspirate Fluid in Relation to Breast Cancer. Breast 1999, 8, 169–174. [Google Scholar] [CrossRef]
- Klein, P.M.; Lawrence, J.A. Lavage and Nipple Aspiration of Breast Ductal Fluids: A Source of Biomarkers for Environmental Mutagenesis. Environ. Mol. Mutagen. 2002, 39, 127–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabian, C.J.; Kimler, B.F. Breast Cancer Chemoprevention: Current Challenges and a Look toward the Future. Clin. Breast Cancer 2002, 3, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Dooley, W.C. Ductal Lavage, Nipple Aspiration, and Ductoscopy for Breast Cancer Diagnosis. Curr. Oncol. Rep. 2003, 5, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A. The Role of Ductal Lavage in the Management of Women at High Risk for Breast Carcinoma. Curr. Treat. Options Oncol. 2004, 5, 145–151. [Google Scholar] [CrossRef]
- Kenney, P.J.; Ellison, M.C. Ductal Lavage in the Screening of High-Risk Women. Curr. Oncol. Rep. 2004, 6, 69–73. [Google Scholar] [CrossRef]
- Khan, S.A.; Bhandare, D.; Chatterton, R.T., Jr. The Local Hormonal Environment and Related Biomarkers in the Normal Breast. Endocr.-Relat. Cancer 2005, 12, 497–510. [Google Scholar] [CrossRef]
- King, B.L.; Love, S.M.; Rochman, S.; Kim, J.A. The Fourth International Symposium on the Intraductal Approach to Breast Cancer, Santa Barbara, California, 10–13 March 2005. Breast Cancer Res. 2005, 7, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Coombes, K.R.; Tsavachidis, S.; Morris, J.S.; Baggerly, K.A.; Hung, M.C.; Kuerer, H.M. Improved Peak Detection and Quantification of Mass Spectrometry Data Acquired from Surface-Enhanced Laser Desorption and Ionization by Denoising Spectra with the Undecimated Discrete Wavelet Transform. Proteomics 2005, 5, 4107–4117. [Google Scholar] [CrossRef]
- Fabian, C.J.; Kimler, B.F.; Mayo, M.S.; Khan, S.A. Breast-Tissue Sampling for Risk Assessment and Prevention. Endocr.-Relat. Cancer 2005, 12, 185–213. [Google Scholar] [CrossRef] [Green Version]
- Escobar, P.F.; Crowe, J.P.; Matsunaga, T.; Mokbel, K. The Clinical Applications of Mammary Ductoscopy. Am. J. Surg. 2006, 191, 211–215. [Google Scholar] [CrossRef]
- Hu, S.; Loo, J.A.; Wong, D.T. Human Body Fluid Proteome Analysis. Proteomics 2006, 6, 6326–6353. [Google Scholar] [CrossRef]
- Lang, J.E.; Kuerer, H.M. Breast Ductal Secretions: Clinical Features, Potential Uses, and Possible Applications. Cancer Control. 2007, 14, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Ruhlen, R.L.; Sauter, E.R. Proteomics of Nipple Aspirate Fluid, Breast Cyst Fluid, Milk, and Colostrum. Proteom. Clin. Appl. 2007, 1, 845–852. [Google Scholar] [CrossRef]
- Ruhlen, R.L.; Sauter, E.R. Proteomic Analysis of Breast Tissue and Nipple Aspirate Fluid for Breast Cancer Detection. Biomark Med. 2007, 1, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Maurya, P.; Meleady, P.; Dowling, P.; Clynes, M. Proteomic Approaches for Serum Biomarker Discovery in Cancer. Anticancer Res. 2007, 27, 1247–1255. [Google Scholar] [PubMed]
- LaKind, J.S.; Wilkins, A.A.; Bates, M.N. Human Breast Biomonitoring and Environmental Chemicals: Use of Breast Tissues and Fluids in Breast Cancer Etiologic Research. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 525–540. [Google Scholar] [CrossRef] [Green Version]
- Suijkerbuijk, K.P.; van der Wall, E.; Vooijs, M.; van Diest, P.J. Molecular Analysis of Nipple Fluid for Breast Cancer Screening. Pathobiology 2008, 75, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F. Analysis of the Intraductal Microenvironment for the Early Diagnosis of Breast Cancer: Identification of Biomarkers in Nipple-Aspirate Fluids. Expert Opin. Med. Diagn. 2008, 2, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Debald, M.; Wolfgarten, M.; Walgenbach-Brunagel, G.; Kuhn, W.; Braun, M. Non-Invasive Proteomics-Thinking About Personalized Breast Cancer Screening and Treatment. EPMA J. 2010, 1, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Green, V.L. Breast Cancer Risk Assessment, Prevention, and the Future. Obstet Gynecol Clin North Am. 2013, 40, 525–549. [Google Scholar] [CrossRef]
- Maskarinec, G. The Human Mammary Gland as a Target for Isoflavones: How Does the Relation Vary in Individuals with Different Ethnicity? Planta Med. 2013, 79, 554–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaaij-Visser, T.B.; de Wit, M.; Lam, S.W.; Jimenez, C.R. The Cancer Secretome, Current Status and Opportunities in the Lung, Breast and Colorectal Cancer Context. Biochim Biophys Acta. 2013, 1834, 2242–2258. [Google Scholar] [CrossRef]
- Masood, S. Development of a Novel Approach for Breast Cancer Prediction and Early Detection Using Minimally Invasive Procedures and Molecular Analysis: How Cytomorphology Became a Breast Cancer Risk Predictor. Breast J. 2015, 21, 82–96. [Google Scholar] [CrossRef]
- Hornberger, J.; Chen, S.C.; Li, Q.; Kakad, P.; Quay, S.C. Proliferative Epithelial Disease Identified in Nipple Aspirate Fluid and Risk of Developing Breast Cancer: A Systematic Review. Curr. Med. Res. Opin. 2015, 31, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Parida, S.; Sharma, D. The Power of Small Changes: Comprehensive Analyses of Microbial Dysbiosis in Breast Cancer. Biochim Biophys Acta Rev Cancer. 2019, 1871, 392–405. [Google Scholar] [CrossRef]
- Li, J.; Guan, X.; Fan, Z.; Ching, L.M.; Li, Y.; Wang, X.; Cao, W.M.; Liu, D.X. Non-Invasive Biomarkers for Early Detection of Breast Cancer. Cancers 2020, 12, 2767. [Google Scholar] [CrossRef] [PubMed]

| First Author (Year) (Reference) | NFA | Pap Smear | Mammography | MRI | Breast-Feeding | Breast Examination | Cohort | Number of Women |
|---|---|---|---|---|---|---|---|---|
| Klein et al. (2001) [26] | 2.4 | 2.2 | 4.6 | N.R. | 6.8 | N.R. | H | 25 |
| Suijkerbuijk et al. (2007) [39] | 1.3 | N.R. | 4.3 | N.R. | 1.9 | N.R. | H | 67 |
| Suijkerbuijk et al. (2010) [17] | 0.6 | N.R. | 4.9 | 2.6 | 1.8 | 1.1 | HR | 90 |
| de Groot et al. (2015) [40] | 0.7 | N.R. | 5.2 | 3.55 | 2.5 | 1.15 | HR | 451 |
| Molecules or Molecular Changes Detected in NAF | Technique | References | ||
|---|---|---|---|---|
| Technique Used in NAF | Advantage(s) | Limitation(s) | ||
| DNA mutagens | Ames test | Simple to perform Inexpensive | Relatively long time to perform analysis | [26,51,52] |
| Methylation changes in DNA | Methylation-specific PCR (MSP) technique | High sensitivity and reproducibility of quantitative measurements High resolution of target regions High-throughput capability | False-positive results Variability of results due to assay conditions | [44] |
| Mutations in mitochondrial DNA | Polymerase chain reaction (PCR) | Accurate quantitation High molecular sensitivity Ease of use | Low/medium throughput | [53] |
| Microsatellite markers in DNA to investigate loss of heterozygosity or microsatellite instability alterations | [43,53] | |||
| Mutations in the mitochondrial genome (mtgenome) | Sequencing the entire mitochondrial genome and mitochondrial resequencing array 2.0 (MCv2) | High sensitivity Automation, which leads to cost benefits in reagents, labor, time-to-results, ease, and accuracy of data interpretation | Data interpretation | [54] |
| RNA and microRNA | Real time quantitative reverse transcription PCR (RT-qPCR) | High sensitivity and specificity High dynamic range Suitable for quantification | Low/medium throughput | [45,55] |
| Proteome | Mass spectrometry Surface-enhanced laser desorption/ionization (SELDI)-TOF technique and Matrix-Assisted Laser Desorption Ionization (MALDI)-TOF mass spectrometry | Extremely sensitive (feasible with very small sample quantities) Able to identify unknown components in liquid samples Very precise, rapid, and sensitive Rapid turnaround time | Costly Requires a skilled technician Generated data require expertise for interpretation | [56,57,58,59] |
| 2D polyacrylamide gel electrophoresis (PAGE) | Allows accurate analysis of thousands of proteins in a single run | Many sample-handling steps Limited reproducibility Not automated for high throughput analysis | [60] | |
| Liquid chromatography | Extremely quick and efficient | Is subject to greater peak or band-broadening and therefore to lower resolution | [61] | |
| Enzyme-linked immunosorbent assay (ELISA) | Simple procedure High specificity and sensitivity Easily automated | Is subject to high background which affects the sensitivity of the assay | [60] | |
| Biochemical substances (e.g., α-lactalbumin, immunoglobulins, lipids, fatty acids, proteins, cholesterol, and cholesterol oxidation products) | Gas liquid chromatography (GLC, for lipids) | High efficiency: GLC allows the separation of sample components in a reasonable time | Only for thermally stable and volatile compounds One time use per sample as it leads to sample destruction | [14] |
| Fluorescence technique of Tappel (for lipid peroxidation) | N.A. (not used anymore) | N.A. (not used anymore) | [14] | |
| Immunoelectrophoresis (for alpha-lactalbumin) and rocket immunoelectrophoresis (RIE, for immunoglobulins) | Good stability of the reagents Performance similar to ELISA | Time-consuming | [14] | |
| Hormones (e.g., estrogens, androgens, progesterone, dehydroepiandrosterone Sulfate (DHEAS), prolactin, growth hormone and leptin, and the growth factors epidermal growth factor, transforming growth factor-α, vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF)) | Immunoassays (such as enzyme-linked immunosorbent assay (ELISA)) | High efficiency | Antibody instability Insufficient blocking of immobilized antigen can lead to false results | [62,63,64,65,66] |
| Tumor antigens (e.g., carcinoembryonic antigen (CEA) and prostate-specific antigen (PSA)) | Immunoassays (fluorescence immunoassay (FIA) for PSA, immunoenzymometric assay for CEA) | Highly sensitive and highly specific High efficiency | Interference of filtration step in final emission of FIA | [64,67,68,69] |
| Title of the Clinical Study | Characteristics of Participants | Aims Related to Nipple Aspirate Fluid Samples | Status | Source | Reference |
|---|---|---|---|---|---|
| Nipple Aspirate Fluid in Detecting Breast Cancer | Participants are healthy volunteers >40 years who undergo collection of nipple aspirate fluid from both breasts. | Nipple aspiration fluid samples will be compared between breast cancer participants and healthy participants. will perform the logistic regression model for each biomarker that shows any difference between the breast cancer patients and healthy individuals. Then we will include multiple biomarkers in one model while controlling for confounders. | Recruiting | Clinicaltrials.gov (accessed on 25 December 2021) | [128] |
| Early detection of Hereditary Breast Cancer by Monitoring MicroRNA expression in Nipple Aspirate Fluid | Women at high risk of developing breast cancer | - To establish biomarker profiles in NAF, follow them in time and establish a correlation with breast cancer development. - To determine threshold values of these biomarkers that point to a significant risk of imminent breast cancer development thereby indicating the right time of prophylactic breast surgery. (Trial NL8661) | Recruitment stopped in 2021. Lab analyses ongoing. | trialregister.nl (accessed on 25 December 2021) | [122] |
| Breast Cancer Biomarkers in Nipple Aspirate Fluid and Blood in Healthy Women | Participants are healthy volunteers ≥45 years | The main study parameters is the degree and patterns of microRNA expression in NAF and blood of healthy women, and to compare this with the pattern of women with breast cancer (ORNAMENT study, Trial NL6031) | Recruitment stopped in 2021. Lab analyses ongoing. | trialregister.nl (accessed on 25 December 2021) | [123] |
| The ORNAMENT study: A multicenter, crOss sectional, study to assess microRNA expression in Nipple Aspirated Fluid, blood and tuMor material in women with primary brEast caNcer compared with healthy conTrols. | Women with pathologically established non-metastasized invasive breast carcinoma | To assess the microRNA expression levels in nipple aspiration fluid obtained just before primary surgery. These will be compared to the microRNA expression levels in NAF obtained from healthy controls (Trial NL8987). | Recruitment stopped in 2021. Lab analyses ongoing. | trialregister.nl (accessed on 25 December 2021) | [124] |
| Physical activity and dietary counseling and supervised group exercise for first-time pregnant women—a feasibility study of a controlled trial | Women who gave birth and stopped breastfeeding | Secondary outcome: Levels of selected breast cancer risk markers (hormones, growth factors) in blood and nipple aspirate fluid (only in postpartum women) | Active, but not recruiting | ISRCTN registry | [129] |
| Phase II study of metformin for reduction of obesity-associated breast cancer risk: a randomized controlled trial protocol | Eligible participants will be randomized to receive metformin 850 mg BID (n = 75) or placebo (n = 75) for 12 months. | Exploratory outcomes: changes in metabolomic profiles in plasma and nipple aspirate fluid. | Not recruiting | PubMed | [130] |
| Type | Title | Year | Author(s) | References |
|---|---|---|---|---|
| R/W | Physiologic, biochemical, and cytologic aspects of nipple aspirate fluid. | 1986 | Petrakis | [14] |
| SR | Factors associated with obtaining nipple aspirate fluid: analysis of 1428 women and literature review. | 1990 | Wrensch et al. | [21] |
| R | Epidemiology and prevention of breast cancer. | 1996 | Kelsey and Bernstein | [131] |
| R | Nipple aspirate fluid in relation to breast cancer. | 1999 | Phillips et al. | [132] |
| R | Lavage and nipple aspiration of breast ductal fluids: a source of biomarkers for environmental mutagenesis | 2002 | Klein and Lawrence | [133] |
| R | Breast cancer chemoprevention: current challenges and a look toward the future. | 2002 | Fabian and Kimler | [134] |
| R | Ductal lavage, nipple aspiration, and ductoscopy for breast cancer diagnosis | 2003 | Dooley WC | [135] |
| R | The role of ductal lavage in the management of women at high risk for breast carcinoma | 2004 | Khan SA | [136] |
| R | Ductal lavage in the screening of high-risk women | 2004 | Kenney PJ, Ellison MC | [137] |
| R | The local hormonal environment and related biomarkers in the normal breast | 2005 | Khan SA et al. | [138] |
| R | The Fourth International Symposium on the Intraductal Approach to Breast Cancer, Santa Barbara, California, 10–13 March 2005 | 2005 | King BL, Love SM et al. | [139] |
| R | Improved peak detection and quantification of mass spectrometry data acquired from surface-enhanced laser desorption and ionization by denoising spectra with the undecimated discrete wavelet transform. | 2005 | Coombes et al. | [140] |
| R | Breast-tissue sampling for risk assessment and prevention. | 2005 | Fabian, C J et al. | [141] |
| R | The intraductal approach to breast cancer biomarker discovery | 2006 | Dua et al. | [32] |
| R | The clinical applications of mammary ductoscopy | 2006 | Escobar PF et al. | [142] |
| R | Human body fluid proteome analysis | 2006 | Hu, Shen et al. | [143] |
| R | Breast ductal secretions: clinical features, potential uses, and possible applications | 2007 | Lang JE, Kuerer HM. | [144] |
| R | Proteomics of nipple aspirate fluid, breast cyst fluid, milk, and colostrum. | 2007 | Ruhlen and Sauter | [145] |
| R | Proteomic analysis of breast tissue and nipple aspirate fluid for breast cancer detection. | 2007 | Ruhlen and Sauter | [146] |
| R | Proteomic approaches for serum biomarker discovery in cancer. | 2007 | Maurya et al. | [147] |
| R | Human breast biomonitoring and environmental chemicals: use of breast tissues and fluids in breast cancer etiologic research. | 2007 | LaKind et al. | [148] |
| R | Molecular analysis of nipple fluid for breast cancer screening | 2008 | Suijkerbuijk et al. | [149] |
| R | Analysis of the intraductal microenvironment for the early diagnosis of breast cancer: identification of biomarkers in nipple-aspirate fluids. | 2008 | Mannello | [150] |
| R | Nutrients and nipple aspirate fluid composition: the breast microenvironment regulates protein expression and cancer aetiology. | 2008 | Mannello, et al. | [74] |
| R | Increased shedding of soluble fragments of P-cadherin in nipple aspirate fluids from women with breast cancer. | 2008 | Mannello et al. | [93] |
| R | Intracrinology of breast microenvironment: hormonal status in nipple aspirate fluid and its relationship to breast cancer. | 2009 | Mannello et al. | [104] |
| R | Protein profile analysis of the breast microenvironment to differentiate healthy women from breast cancer patients. | 2009 | Mannello et al. | [47] |
| R | Non-invasive proteomics-thinking about personalized breast cancer screening and treatment | 2010 | Debald M et al. | [151] |
| R | Breast cancer risk assessment, prevention, and the future. | 2013 | Green, Victoria L | [152] |
| R | Aluminium and breast cancer: Sources of exposure, tissue measurements and mechanisms of toxicological actions on breast biology. | 2013 | Darbre et al. | [102] |
| R | Resolving breast cancer heterogeneity by searching reliable protein cancer biomarkers in the breast fluid secretome. | 2013 | Mannello and Ligi | [103] |
| R | The human mammary gland as a target for isoflavones: how does the relation vary in individuals with different ethnicity? | 2013 | Maskarinec, Gertraud | [153] |
| R | The cancer secretome, current status and opportunities in the lung, breast and colorectal cancer context. | 2013 | Schaaij-Visser et al. | [154] |
| R | Development of a novel approach for breast cancer prediction and early detection using minimally invasive procedures and molecular analysis: how cytomorphology became a breast cancer risk predictor. | 2015 | Masood, Shahla | [155] |
| SR | Proliferative epithelial disease identified in nipple aspirate fluid and risk of developing breast cancer: a systematic review. | 2015 | Hornberger et al. | [156] |
| R | The In’s and Out’s of Ductography: A Comprehensive Review. | 2016 | Sheiman et al. | [4] |
| R | Evaluation of nipple aspirate fluid as a diagnostic tool for early detection of breast cancer. | 2018 | Shaheed et al. | [1] |
| R | The power of small changes: Comprehensive analyses of microbial dysbiosis in breast cancer. | 2019 | Parida, Sheetal and Sharma, Dipali | [157] |
| SR | Diagnostic Accuracy of Nipple Aspirate Fluid Cytology in Asymptomatic Patients: A Meta-analysis and Systematic Review of the Literature | 2020 | Jiwa N et al. | [48] |
| R | Lessons Learned from Setting Up a Prospective, Longitudinal, Multicenter Study with Women at High Risk for Breast Cancer | 2020/2021 | Patuleia SIS, Hagenaars SC et al. | [125] |
| R | Non-Invasive Biomarkers for Early Detection of Breast Cancer | 2020 | Li J et al. | [158] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patuleia, S.I.S.; Suijkerbuijk, K.P.M.; van der Wall, E.; van Diest, P.J.; Moelans, C.B. Nipple Aspirate Fluid at a Glance. Cancers 2022, 14, 159. https://doi.org/10.3390/cancers14010159
Patuleia SIS, Suijkerbuijk KPM, van der Wall E, van Diest PJ, Moelans CB. Nipple Aspirate Fluid at a Glance. Cancers. 2022; 14(1):159. https://doi.org/10.3390/cancers14010159
Chicago/Turabian StylePatuleia, Susana I. S., Karijn P. M. Suijkerbuijk, Elsken van der Wall, Paul J. van Diest, and Cathy B. Moelans. 2022. "Nipple Aspirate Fluid at a Glance" Cancers 14, no. 1: 159. https://doi.org/10.3390/cancers14010159
APA StylePatuleia, S. I. S., Suijkerbuijk, K. P. M., van der Wall, E., van Diest, P. J., & Moelans, C. B. (2022). Nipple Aspirate Fluid at a Glance. Cancers, 14(1), 159. https://doi.org/10.3390/cancers14010159







