Correlation of Somatostatin Receptor 1–5 Expression, [68Ga]Ga-DOTANOC, [18F]F-FDG PET/CT and Clinical Outcome in a Prospective Cohort of Pancreatic Neuroendocrine Neoplasms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. [68Ga]Ga-DOTANOC and [18F]F-FDG-PET/CT Imaging
2.3. Immunohistochemistry
2.4. Scoring of the Staining Results
2.5. Statistical Analysis
3. Results
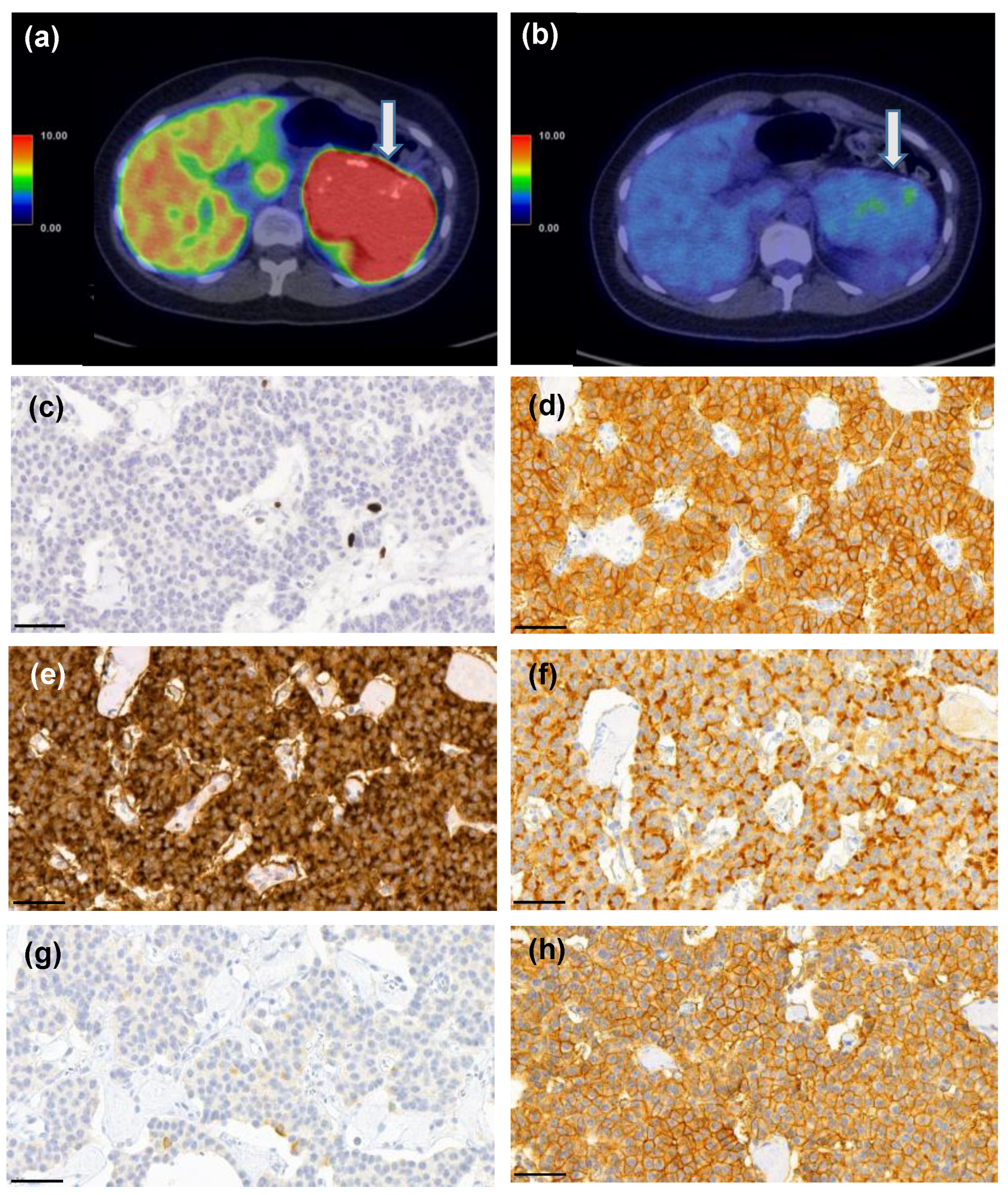
3.1. SSTR2 Expression Correlates with [68Ga]Ga-DOTANOC PET/CT
3.2. SSTR1, 3, 4, 5 Expression Does Not Correlate with [68Ga]Ga-DOTANOC PET/CT
3.3. [68Ga]Ga-DOTANOC-Avid Tumors
3.4. SSTR1-5 Expression and Correlation to [18F]F-FDG PET/CT
3.5. Somatostatin Receptors and Proliferation Index
3.6. SSTR Expression and Tumor Aggressiveness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Man, D.; Wu, J.; Shen, Z.; Zhu, X. Prognosis of patients with neuroendocrine tumor: A SEER database analysis. Cancer Manag Res. 2018, 10, 5629–5638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Inzani, F.; Petrone, G.; Rindi, G. The New World Health Organization Classification for Pancreatic Neuroendocrine Neoplasia. Endocrinol. Metab. Clin. N. Am. 2018, 47, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Talamonti, M.S.; Tomlinson, J.S.; Stewart, A.K.; Winchester, D.P.; Ko, C.Y.; Bentrem, D.J. Prognostic Score Predicting Survival After Resection of Pancreatic Neuroendocrine Tumors. Ann. Surg. 2008, 247, 490–500. [Google Scholar] [CrossRef]
- Johnbeck, C.B.; Knigge, U.P.; Langer, S.W.; Loft, A.; Berthelsen, A.K.; Federspiel, B.; Binderup, T.; Kjaer, A. Prognostic Value of 18F-FLT PET in Patients with Neuroendocrine Neoplasms: A Prospective Head-to-Head Comparison with 18F-FDG PET and Ki-67 in 100 Patients. J. Nucl. Med. 2016, 57, 1851–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binderup, T.; Knigge, U.; Loft, A.; Federspiel, B.; Kjaer, A. 18F-Fluorodeoxyglucose Positron Emission Tomography Predicts Survival of Patients with Neuroendocrine Tumors. Clin. Cancer Res. 2010, 16, 978–985. [Google Scholar] [CrossRef] [Green Version]
- Cingarlini, S.; Ortolani, S.; Salgarello, M.; Butturini, G.; Malpaga, A.; Malfatti, V.; D’Onofrio, M.; Davì, M.V.; Vallerio, P.; Ruzzenente, A.; et al. Role of Combined 68Ga-DOTATOC and 18F-FDG Positron Emission Tomography/Computed Tomography in the Diagnostic Workup of Pancreas Neuroendocrine Tumors. Pancreas 2017, 46, 42–47. [Google Scholar] [CrossRef]
- Majala, S.; Seppanen, H.; Kemppainen, J.; Sundstrom, J.; Schalin-Jantti, C.; Gullichsen, R.; Schildt, J.; Mustonen, H.; Vesterinen, T.; Arola, J.; et al. Prediction of the aggressiveness of non-functional pancreatic neuroendocrine tumors based on the dual-tracer PET/CT. EJNMMI Res. 2019, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Portela-Gomes, G.M.; Stridsberg, M.; Grimelius, L.; Oberg, K.; Janson, E.T. Expression of the five different somatostatin receptor subtypes in endocrine cells of the pancreas. Appl. Immunohistochem. Mol. Morphol. 2000, 8, 126–132. [Google Scholar] [CrossRef]
- Brunner, P.; Jörg, A.-C.; Glatz, K.; Bubendorf, L.; Radojewski, P.; Umlauft, M.; Marincek, N.; Spanjol, P.-M.; Krause, T.; Dumont, R.A.; et al. The prognostic and predictive value of sstr2-immunohistochemistry and sstr2-targeted imaging in neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2016, 44, 468–475. [Google Scholar] [CrossRef] [Green Version]
- Mehta, S.; de Reuver, P.R.; Gill, P.; Andrici, J.; D’Urso, L.; Mittal, A.; Pavlakis, N.; Clarke, S.; Samra, J.S.; Gill, A.J. Somatostatin Receptor SSTR-2a Expression Is a Stronger Predictor for Survival Than Ki-67 in Pancreatic Neuroendocrine Tumors. Medicine 2015, 94, e1281. [Google Scholar] [CrossRef]
- Okuwaki, K.; Kida, M.; Mikami, T.; Yamauchi, H.; Imaizumi, H.; Miyazawa, S.; Iwai, T.; Takezawa, M.; Saegusa, M.; Watanabe, M.; et al. Clinicopathologic characteristics of pancreatic neuroendocrine tumors and relation of somatostatin receptor type 2A to outcomes. Cancer 2013, 119, 4094–4102. [Google Scholar] [CrossRef]
- Qian, Z.R.; Li, T.; Ter-Minassian, M.; Yang, J.; Chan, J.A.; Brais, L.K.; Masugi, Y.; Thiaglingam, A.; Brooks, N.; Nishihara, R.; et al. Association Between Somatostatin Receptor Expression and Clinical Outcomes in Neuroendocrine Tumors. Pancreas 2016, 45, 1386–1393. [Google Scholar] [CrossRef] [Green Version]
- Song, K.B.; Kim, S.C.; Kim, J.H.; Seo, D.-W.; Hong, S.-M.; Park, K.-M.; Hwang, D.W.; Lee, J.H.; Lee, Y.-J. Prognostic Value of Somatostatin Receptor Subtypes in Pancreatic Neuroendocrine Tumors. Pancreas 2016, 45, 187–192. [Google Scholar] [CrossRef]
- Kaemmerer, D.; Peter, L.; Lupp, A.; Schulz, S.; Sänger, J.; Prasad, V.; Kulkarni, H.; Haugvik, S.-P.; Hommann, M.; Baum, R.P. Molecular imaging with 68Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Partelli, S.; Bartsch, D.K.; Capdevila, J.; Chen, J.; Knigge, U.; Niederle, B.; Nieveen van Dijkum, E.J.M.; Pape, U.F.; Pascher, A.; Ramage, J.; et al. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2017, 105, 255–265. [Google Scholar] [CrossRef]
- Krenning, E.P.; Valkema, R.; Kooij, P.P.; Breeman, W.A.; Bakker, W.H.; deHerder, W.W.; vanEijck, C.H.; Kwekkeboom, D.J.; deJong, M.; Pauwels, S. Scintigraphy and radionuclide therapy with [indium-111-labelled-diethyl triamine penta-acetic acid-D-Phe1]-octreotide. Ital. J. Gastroenterol. Hepatol. 1999, 31, S219–S223. [Google Scholar]
- Chan, D.L.; Pavlakis, N.; Schembri, G.; Bernard, E.J.; Hsiao, E.; Hayes, A.; Barnes, T.; Diakos, C.; Khasraw, M.; Samra, J.; et al. Dual Somatostatin Receptor/FDG PET/CT Imaging in Metastatic Neuroendocrine Tumours: Proposal for a Novel Grading Scheme with Prognostic Significance. Theranostics 2017, 7, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Elston, M.S.; Meyer-Rochow, G.Y.; Conaglen, H.M.; Clarkson, A.; Clifton-Bligh, R.J.; Conaglen, J.V.; Gill, A.J. Increased SSTR2A and SSTR3 expression in succinate dehydrogenase–deficient pheochromocytomas and paragangliomas. Hum. Pathol. 2015, 46, 390–396. [Google Scholar] [CrossRef]
- Körner, M.; Waser, B.; Schonbrunn, A.; Perren, A.; Reubi, J.C. Somatostatin Receptor Subtype 2A Immunohistochemistry Using a New Monoclonal Antibody Selects Tumors Suitable for In Vivo Somatostatin Receptor Targeting. Am. J. Surg. Pathol. 2012, 36, 242–252. [Google Scholar] [CrossRef] [Green Version]
- Remes, S.M.; Tuominen, V.J.; Helin, H.; Isola, J.; Arola, J. Grading of Neuroendocrine Tumors with Ki-67 Requires High-quality Assessment Practices. Am. J. Surg. Pathol. 2012, 36, 1359–1363. [Google Scholar] [CrossRef]
- Kaemmerer, D.; Wirtz, R.M.; Fischer, E.K.; Hommann, M.; Sänger, J.; Prasad, V.; Specht, E.; Baum, R.P.; Schulz, S.; Lupp, A. Analysis of Somatostatin Receptor 2A Immunohistochemistry, RT-qPCR, and In Vivo PET/CT Data in Patients with Pancreatic Neuroendocrine Neoplasm. Pancreas 2015, 44, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Miederer, M.; Seidl, S.; Buck, A.; Scheidhauer, K.; Wester, H.-J.; Schwaiger, M.; Perren, A. Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2008, 36, 48–52. [Google Scholar] [CrossRef]
- Olsen, I.H.; Langer, S.W.; Federspiel, B.H.; Oxbøl, J.; Loft, A.; Berthelsen, A.K.; Mortensen, J.; Oturai, P.; Knigge, U.; Kjaer, A. 68Ga-DOTATOC PET and gene expression profile in patients with neuroendocrine carcinomas: Strong correlation between PET tracer uptake and gene expression of somatostatin receptor subtype 2. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 59–72. [Google Scholar]
- Haug, A.; Assmann, G.; Rist, C.; Tiling, R.; Schmidt, G.; Bartenstein, P.; Hacker, M. Quantifizierung der Somatostatinrezeptorexpression neuroendokriner Tumoren mit der 68Ga-DOTATATE-PET/CT. Der Radiol. 2010, 50, 349–354. [Google Scholar] [CrossRef]
- Müssig, K.; Öksüz, M.Ö.; Dudziak, K.; Ueberberg, B.; Wehrmann, M.; Horger, M.; Schulz, S.; Häring, H.; Pfannenberg, C.; Bares, R.; et al. Association of Somatostatin Receptor 2 Immunohistochemical Expression with [111In]-DTPA Octreotide Scintigraphy and [68Ga]-DOTATOC PET/CT in Neuroendocrine Tumors. Horm. Metab. Res. 2010, 42, 599–606. [Google Scholar] [CrossRef]
- Kimura, N.; Pilichowska, M.; Date, F.; Kimura, I.; Schindler, M. Immunohistochemical expression of somatostatin type 2A receptor in neuroendocrine tumors. Clin Cancer Res 1999, 5, 3483–3487. [Google Scholar]
- Appetecchia, M.; Baldelli, R. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumours, current aspects and new perspectives. J. Exp. Clin. Cancer Res. 2010, 29, 19. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Xiao, X.; Li, Y.; Zhou, T. Somatostatin analogues inhibit cancer cell proliferation in an SSTR2-dependent manner via both cytostatic and cytotoxic pathways. Oncol. Rep. 2009, 21, 379–386. [Google Scholar]
- Strowski, M.Z.; Kohler, M.; Chen, H.Y.; Trumbauer, M.E.; Li, Z.; Szalkowski, D.; Gopal-Truter, S.; Fisher, J.K.; Schaeffer, J.M.; Blake, A.D.; et al. Somatostatin receptor subtype 5 regulates insulin secretion and glucose homeostasis. Mol. Endocrinol. 2003, 17, 93–106. [Google Scholar] [CrossRef]
- Hofland, L.J.; van der Hoek, J.; Feelders, R.; van Aken, M.O.; van Koetsveld, P.M.; Waaijers, M.; Sprij-Mooij, D.; Bruns, C.; Weckbecker, G.; de Herder, W.W.; et al. The multi-ligand somatostatin analogue SOM230 inhibits ACTH secretion by cultured human corticotroph adenomas via somatostatin receptor type 5. Eur. J. Endocrinol. 2005, 152, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Bruns, C.; Lewis, I.; Briner, U.; Meno-Tetang, G.; Weckbecker, G. SOM230: A novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur. J. Endocrinol. 2002, 146, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Vesterinen, T.; Leijon, H.; Mustonen, H.; Remes, S.; Knuuttila, A.; Salmenkivi, K.; Vainio, P.; Arola, J.; Haglund, C. Somatostatin Receptor Expression Is Associated with Metastasis and Patient Outcome in Pulmonary Carcinoid Tumors. J. Clin. Endocrinol. Metab. 2019, 104, 2083–2093. [Google Scholar] [CrossRef] [Green Version]
- Righi, L.; Volante, M.; Tavaglione, V.; Bille, A.; Daniele, L.; Angusti, T.; Inzani, F.; Pelosi, G.; Rindi, G.; Papotti, M. Somatostatin receptor tissue distribution in lung neuroendocrine tumours: A clinicopathologic and immunohistochemical study of 218 ‘clinically aggressive’ cases. Ann. Oncol. 2010, 21, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Zamora, V.; Cabanne, A.; Salanova, R.; Bestani, C.; Domenichini, E.; Marmissolle, F.; Giacomi, N.; O’Connor, J.; Mendez, G.; Roca, E.; et al. Immunohistochemical expression of somatostatin receptors in digestive endocrine tumours. Dig. Liver. Dis. 2010, 42, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Remes, S.M.; Leijon, H.L.; Vesterinen, T.J.; Arola, J.T.; Haglund, C.H. Immunohistochemical Expression of Somatostatin Receptor Subtypes in a Panel of Neuroendocrine Neoplasias. J. Histochem. Cytochem. 2019, 67, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Kulaksiz, H.; Eissele, R.; Rossler, D.; Schulz, S.; Hollt, V.; Cetin, Y.; Arnold, R. Identification of somatostatin receptor subtypes 1, 2A, 3, and 5 in neuroendocrine tumours with subtype specific antibodies. Gut 2002, 50, 52–60. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B.; Liu, Q.; Laissue, J.A.; Schonbrunn, A. Subcellular distribution of somatostatin sst2A receptors in human tumors of the nervous and neuroendocrine systems: Membranous versus intracellular location. J. Clin. Endocrinol. Metab. 2000, 85, 3882–3891. [Google Scholar] [CrossRef]







| No. Patients | 21 |
|---|---|
| No. tumors | 35 |
| Sex, Male, n (%) | 13 (62) |
| Age y, mean (SD) | 54.9 (18.1) |
| BMI, mean (SD) | 25.8 (4.2) |
| Asymptomatic, n (%) | 18 (86) |
| MEN1 syndrome, n (%) | 7 (33) |
| P/S-CgA, n (%) | |
| Strongly positive | 3 (14) |
| Weakly positive | 11 (53) |
| Negative | 7 (33) |
| S-PP (pmol/L), median (IQR) | 91 (28.5–252.0) |
| S-5HIAA (nmol/L), median (IQR) | 70 (51.5–95.5) |
| Tumor size (mm), median (IQR) | 20 (10–32.5) |
| Tumor localization, n (%) | |
| Caput | 6 (28) |
| Corpus | 1 (5) |
| Cauda | 10 (48) |
| Multiple | 4 (19) |
| Type of surgery, n (%) | |
| Total pancreatectomy | 2 (10) |
| Pancreaticoduodenectomy | 4 (20) |
| Distal pancreatectomy ± splenectomy | 13 (65) |
| Enucleation | 1 (5) |
| Type of surgery, n (%) | |
| Open | 13 (65) |
| Minimally invasive | 7 (35) |
| Grade, n (%) | |
| G1 | 22 (63) |
| G2 | 12 (34) |
| G3 NEN | 1 (3) |
| Non-Aggressive Tumors | Aggressive Tumors |
|---|---|
| G1 tumors without any metastases | G2 tumors |
| G3 tumors | |
| Any tumor with lymph node metastases | |
| Any tumor with distant metastases |
| Antibody | Clone | Supplier | Dilution | Incubation (min) | Pre-Treatment | Detection |
|---|---|---|---|---|---|---|
| Ki-67 | MIB-1 | Dako (M7240) | 1:100 | 32 | CC1 std | ultraView |
| SSTR1 | UMB7 | Abcam a (ab137083) | 1:500 | 45 | Tris-EDTA pH 9.0 | EnVision |
| SSTR2 b | UMB1 | Abcam (ab134152) | 1:300 | 32 | CC1 std | Optiview |
| SSTR3 | UMB5 | Abcam (ab137026) | 1:7000 | 60 | Citrate pH 6.0 | EnVision |
| SSTR4 | sstr4 | Bio-Rad c (MCA5922) | 1:500 | 30 | Citrate pH 6.0 | EnVision |
| SSTR5 | UMB4 | Abcam (ab109495) | 1:1000 | 30 | Citrate pH 6.0 | EnVision |
| Factor | SSTR1 | SSTR2 | SSTR3 | SSTR4 | SSTR5 |
|---|---|---|---|---|---|
| [68Ga]Ga-DOTANOC PET/CT (positive vs. negative) | no a | positivity associated, p = 0.043 b | no a | no a | no a |
| [68Ga]Ga-DOTANOC SUVmax | no a | no a | no a | no a | no a |
| [18F]F-FDG PET/CT (positive vs. negative) | no a | no a | no a | no a | no a |
| [18F]F-FDG SUVmax | no a | no a | no a | no a | membranous expression negativity associated, p = 0.033 b |
| Grade | no a | no a | no a | no a | negativity associated, p = 0.004 b |
| Ki-67 | no a | no a | no a | no a | negativity associated, p = 0.038 b |
| Lymph node SSTR status | no a | no a | cytoplasmic staining positively associated, p = 0.031 c | no a | no a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majala, S.; Vesterinen, T.; Seppänen, H.; Mustonen, H.; Sundström, J.; Schalin-Jäntti, C.; Gullichsen, R.; Schildt, J.; Kemppainen, J.; Arola, J.; et al. Correlation of Somatostatin Receptor 1–5 Expression, [68Ga]Ga-DOTANOC, [18F]F-FDG PET/CT and Clinical Outcome in a Prospective Cohort of Pancreatic Neuroendocrine Neoplasms. Cancers 2022, 14, 162. https://doi.org/10.3390/cancers14010162
Majala S, Vesterinen T, Seppänen H, Mustonen H, Sundström J, Schalin-Jäntti C, Gullichsen R, Schildt J, Kemppainen J, Arola J, et al. Correlation of Somatostatin Receptor 1–5 Expression, [68Ga]Ga-DOTANOC, [18F]F-FDG PET/CT and Clinical Outcome in a Prospective Cohort of Pancreatic Neuroendocrine Neoplasms. Cancers. 2022; 14(1):162. https://doi.org/10.3390/cancers14010162
Chicago/Turabian StyleMajala, Susanna, Tiina Vesterinen, Hanna Seppänen, Harri Mustonen, Jari Sundström, Camilla Schalin-Jäntti, Risto Gullichsen, Jukka Schildt, Jukka Kemppainen, Johanna Arola, and et al. 2022. "Correlation of Somatostatin Receptor 1–5 Expression, [68Ga]Ga-DOTANOC, [18F]F-FDG PET/CT and Clinical Outcome in a Prospective Cohort of Pancreatic Neuroendocrine Neoplasms" Cancers 14, no. 1: 162. https://doi.org/10.3390/cancers14010162
APA StyleMajala, S., Vesterinen, T., Seppänen, H., Mustonen, H., Sundström, J., Schalin-Jäntti, C., Gullichsen, R., Schildt, J., Kemppainen, J., Arola, J., & Kauhanen, S. (2022). Correlation of Somatostatin Receptor 1–5 Expression, [68Ga]Ga-DOTANOC, [18F]F-FDG PET/CT and Clinical Outcome in a Prospective Cohort of Pancreatic Neuroendocrine Neoplasms. Cancers, 14(1), 162. https://doi.org/10.3390/cancers14010162






