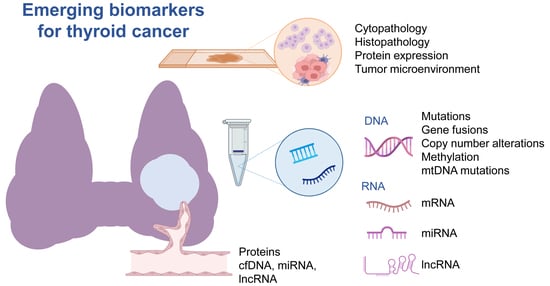
Emerging Biomarkers in Thyroid Practice and Research
Abstract
Simple Summary
Abstract
1. Introduction
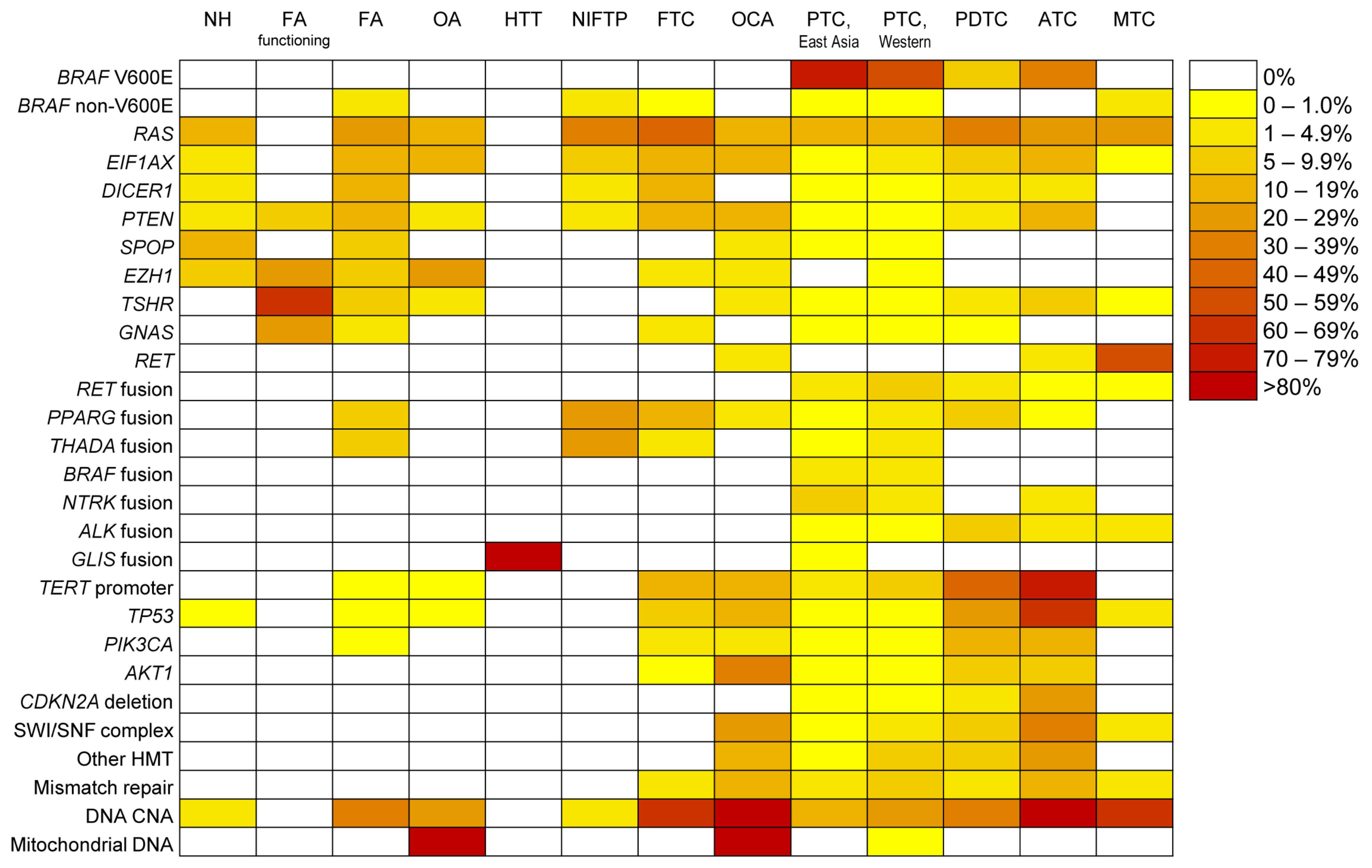
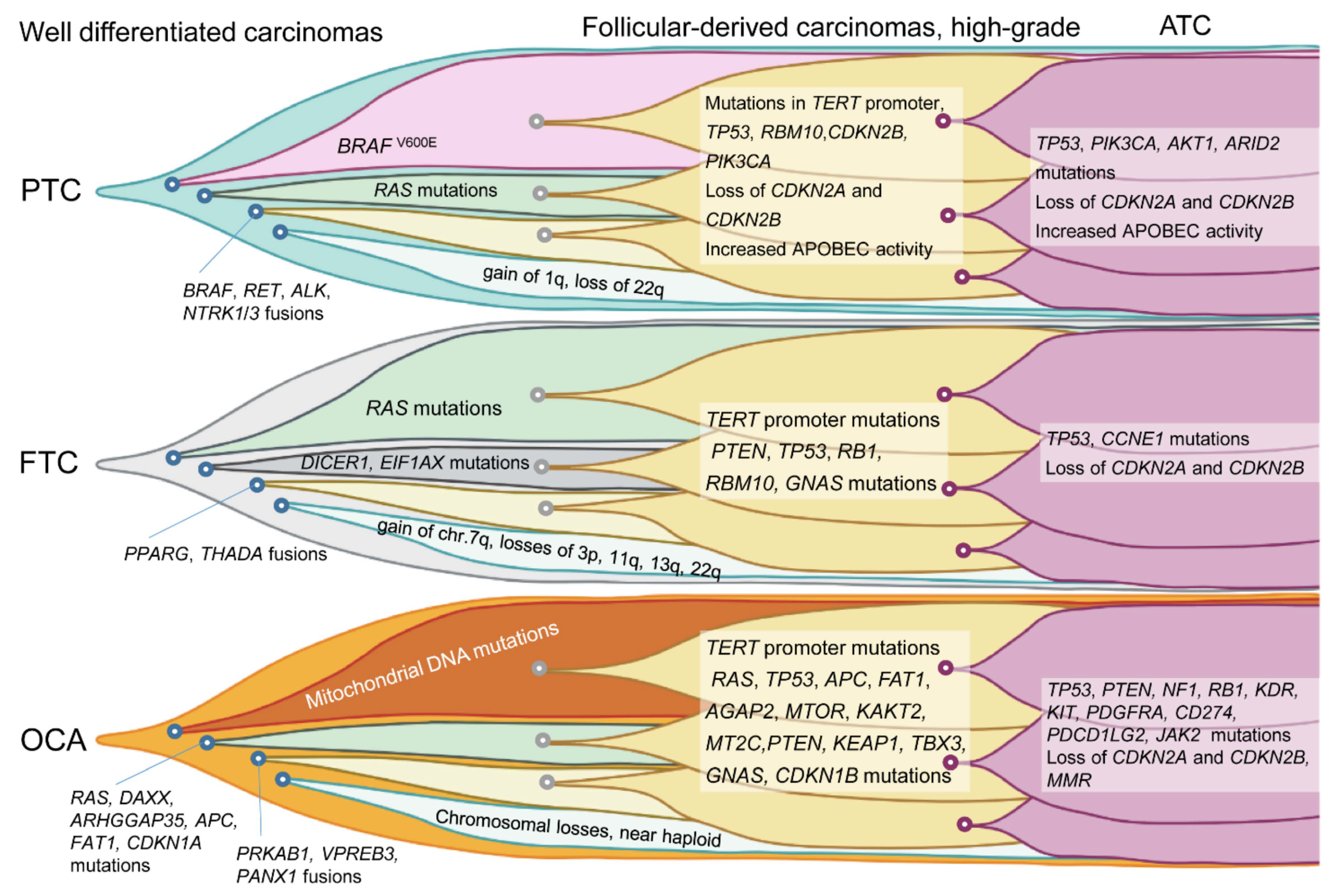
2. Molecular Landscape of Follicular Cell-Derived Thyroid Cancer
2.1. Recently Discovered Molecular Alterations in Thyroid Cancer
2.1.1. Recurrent Promoter Mutations in Thyroid Cancer
2.1.2. ALK
2.1.3. NTRK
2.1.4. DICER1
2.1.5. PTEN
2.1.6. GLIS
2.1.7. EIF1AX
2.2. Epigenetics
2.2.1. DNA Methylation
2.2.2. MicroRNA
2.2.3. lncRNA
2.3. Familial Thyroid Cancer
2.4. Predisposition to Thyroid Cancer
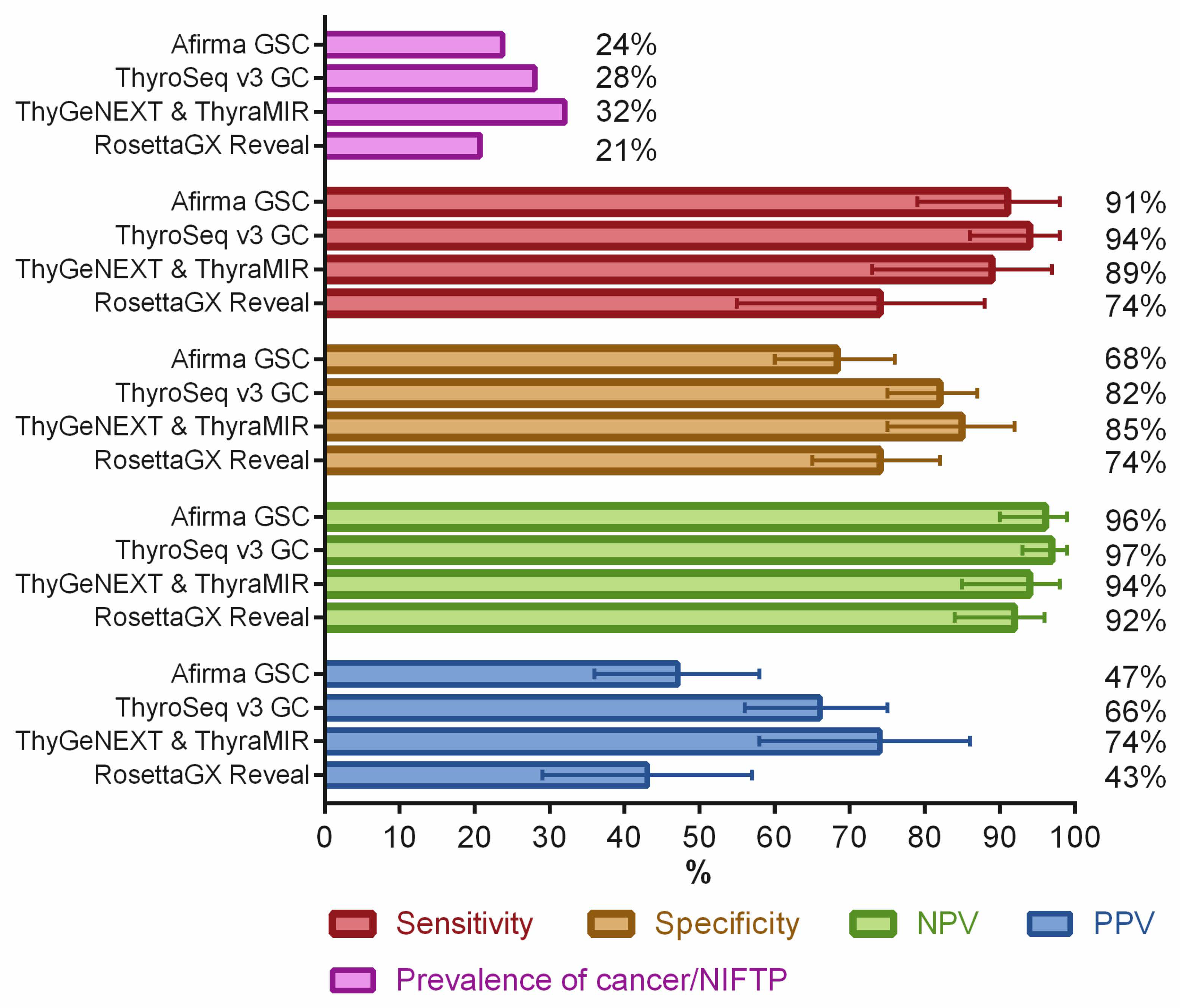
3. Preoperative Molecular Diagnosis of Indeterminate Thyroid Nodules
4. Liquid Biopsy
5. Targeted Therapies in Thyroid Cancer
6. Immunohistochemical Markers
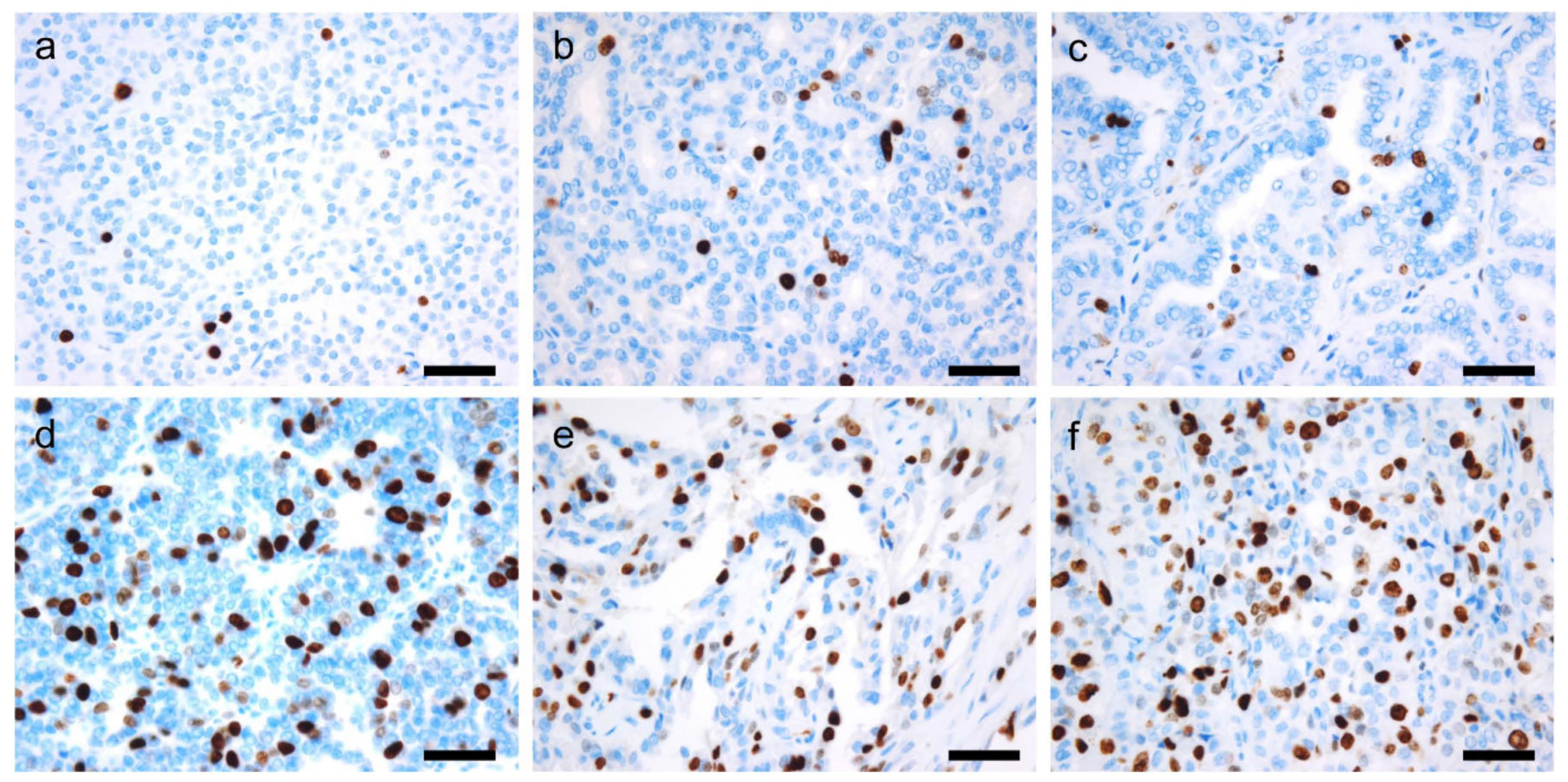
6.1. Ki-67
6.2. Second-Generation Neuroendocrine Markers
6.3. Next-Generation Immunohistochemistry
6.3.1. BRAF V600E (VE1)
6.3.2. RAS
6.3.3. Pan-Trk
6.3.4. β-Catenin
6.3.5. PTEN
6.3.6. ALK
6.4. Tumor Microenvironment
6.4.1. PD-1/PD-L1
6.4.2. CD Markers
6.5. Other IHC Markers with Potential Promise for Targeted Therapy
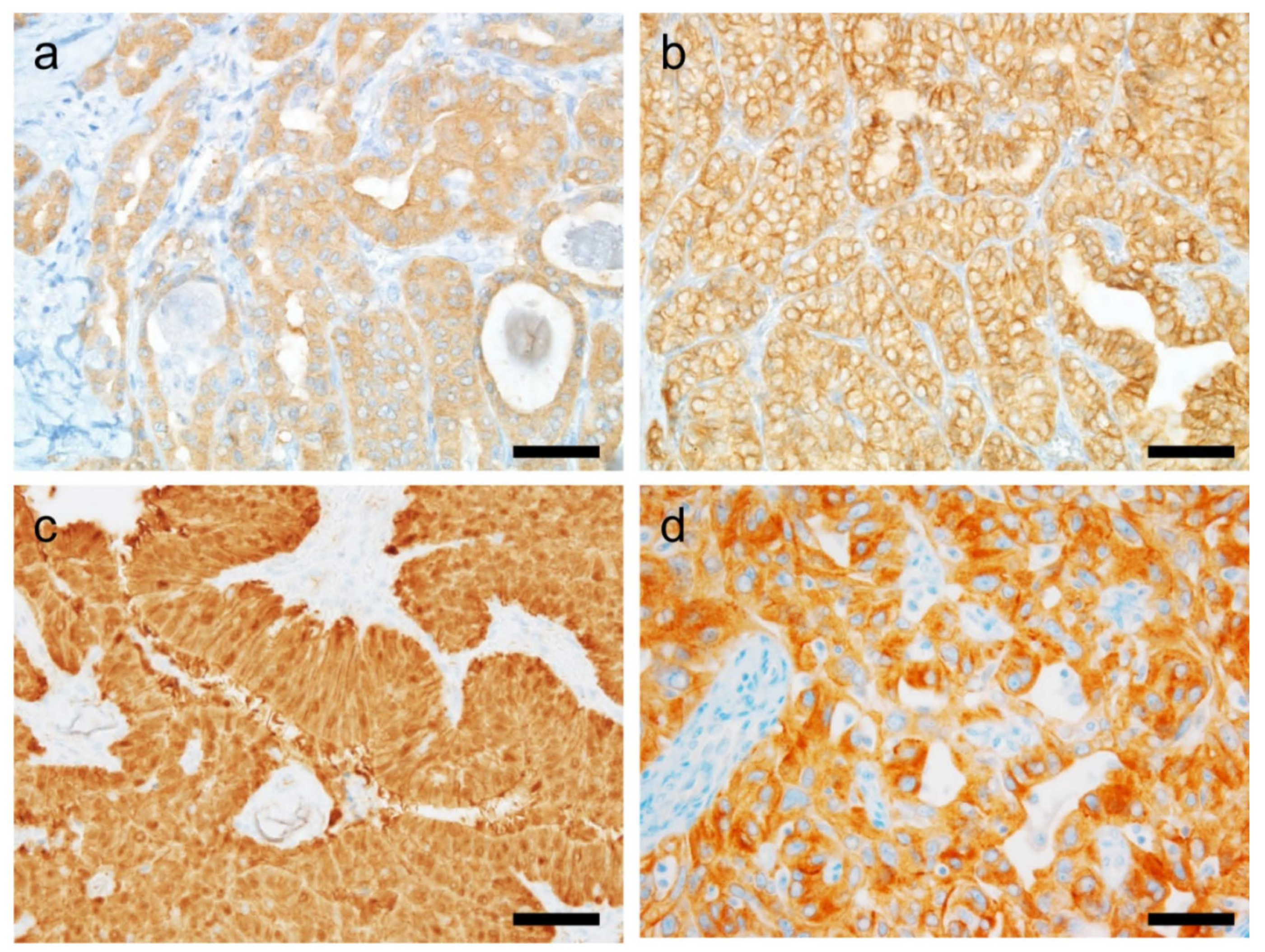
6.5.1. PSMA
6.5.2. MSI/MMR
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guth, S.; Theune, U.; Aberle, J.; Galach, A.; Bamberger, C.M. Very High Prevalence of Thyroid Nodules Detected by High Frequency (13 MHz) Ultrasound Examination. Eur. J. Clin. Investig. 2009, 39, 699–706. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Keh, S.M.; El-Shunnar, S.K.; Palmer, T.; Ahsan, S.F. Incidence of Malignancy in Solitary Thyroid Nodules. J. Laryngol. Otol. 2015, 129, 677–681. [Google Scholar] [CrossRef]
- Kakudo, K.; Bychkov, A.; Bai, Y.; Li, Y.; Liu, Z.; Jung, C.K. The New 4th Edition World Health Organization Classification for Thyroid Tumors, Asian Perspectives. Pathol. Int. 2018, 68, 641–664. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Osamura, R.Y.; Kloppel, G.; Rosai, J. Chapter 2 Tumours of the Thyroid Gland. In WHO Classification of Tumours of Endocrine Organ; International Agency for Research on Cancer (IARC): Lyon, France, 2017; pp. 65–143. [Google Scholar]
- Onenerk, A.M.; Pusztaszeri, M.P.; Canberk, S.; Faquin, W.C. Triage of the Indeterminate Thyroid Aspirate: What Are the Options for the Practicing Cytopathologist? Cancer Cytopathol. 2017, 125, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [CrossRef] [PubMed]
- Yoo, S.-K.; Lee, S.; Kim, S.-J.; Jee, H.-G.; Kim, B.-A.; Cho, H.; Song, Y.S.; Cho, S.W.; Won, J.-K.; Shin, J.-Y.; et al. Comprehensive Analysis of the Transcriptional and Mutational Landscape of Follicular and Papillary Thyroid Cancers. PLoS Genet. 2016, 12, e1006239. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jeon, S.; Kim, T.-M.; Jung, C.K. Immune Gene Signature Delineates a Subclass of Papillary Thyroid Cancer with Unfavorable Clinical Outcomes. Cancers 2018, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-H.; Kim, M.S.; Jung, C.K.; Park, H.-C.; Kim, S.Y.; Liu, J.; Bae, J.-S.; Lee, S.H.; Kim, T.-M.; Lee, S.H.; et al. Mutational Burdens and Evolutionary Ages of Thyroid Follicular Adenoma Are Comparable to Those of Follicular Carcinoma. Oncotarget 2016, 7, 69638–69648. [Google Scholar] [CrossRef]
- Jeong, S.H.; Hong, H.S.; Lee, E.H.; Kwak, J.J.; Lee, J.Y. Analysis of RAS Mutation in Thyroid Nodular Hyperplasia and Follicular Neoplasm in a Korean Population. Endocrinol. Diabetes Metab. 2018, 1, e00040. [Google Scholar] [CrossRef]
- Hernandez-Prera, J.C.; Valderrabano, P.; Creed, J.H.; de la Iglesia, J.V.; Slebos, R.J.C.; Centeno, B.A.; Tarasova, V.; Hallanger-Johnson, J.; Veloski, C.; Otto, K.J.; et al. Molecular Determinants of Thyroid Nodules with Indeterminate Cytology and RAS Mutations. Thyroid 2021, 31, 36–49. [Google Scholar] [CrossRef]
- Gilani, S.M.; Abi-Raad, R.; Garritano, J.; Cai, G.; Prasad, M.L.; Adeniran, A.J. RAS Mutation and Associated Risk of Malignancy in the Thyroid Gland: An FNA Study with Cytology-Histology Correlation. Cancer Cytopathol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and Transcriptomic Hallmarks of Poorly Differentiated and Anaplastic Thyroid Cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef]
- Pozdeyev, N.; Gay, L.M.; Sokol, E.S.; Hartmaier, R.; Deaver, K.E.; Davis, S.; French, J.D.; Borre, P.V.; LaBarbera, D.V.; Tan, A.-C.; et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef]
- Jung, C.K.; Jung, S.-H.; Jeon, S.; Jeong, Y.M.; Kim, Y.; Lee, S.; Bae, J.-S.; Chung, Y.-J. Risk Stratification Using a Novel Genetic Classifier Including PLEKHS1 Promoter Mutations for Differentiated Thyroid Cancer with Distant Metastasis. Thyroid 2020, 30, 1589–1600. [Google Scholar] [CrossRef]
- Kelly, L.M.; Barila, G.; Liu, P.; Evdokimova, V.N.; Trivedi, S.; Panebianco, F.; Gandhi, M.; Carty, S.E.; Hodak, S.P.; Luo, J.; et al. Identification of the Transforming STRN-ALK Fusion as a Potential Therapeutic Target in the Aggressive Forms of Thyroid Cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 4233–4238. [Google Scholar] [CrossRef]
- Kohler, H.; Latteyer, S.; Hönes, G.S.; Theurer, S.; Liao, X.-H.; Christoph, S.; Zwanziger, D.; Schulte, J.H.; Kero, J.; Undeutsch, H.; et al. Increased Anaplastic Lymphoma Kinase Activity Induces a Poorly Differentiated Thyroid Carcinoma in Mice. Thyroid 2019, 29, 1438–1446. [Google Scholar] [CrossRef]
- Panebianco, F.; Nikitski, A.V.; Nikiforova, M.N.; Kaya, C.; Yip, L.; Condello, V.; Wald, A.I.; Nikiforov, Y.E.; Chiosea, S.I. Characterization of Thyroid Cancer Driven by Known and Novel ALK Fusions. Endocr. Relat. Cancer 2019, 26, 803–814. [Google Scholar] [CrossRef]
- Arndt, A.; Steinestel, K.; Rump, A.; Sroya, M.; Bogdanova, T.; Kovgan, L.; Port, M.; Abend, M.; Eder, S. Anaplastic Lymphoma Kinase (ALK) Gene Rearrangements in Radiation-Related Human Papillary Thyroid Carcinoma after the Chernobyl Accident. J. Pathol. Clin. Res. 2018, 4, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.; Fraser, S.; Toon, C.W.; Clarkson, A.; Sioson, L.; Farzin, M.; Cussigh, C.; Aniss, A.; O’Neill, C.; Watson, N.; et al. A Detailed Clinicopathologic Study of ALK-Translocated Papillary Thyroid Carcinoma. Am. J. Surg. Pathol. 2015, 39, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Pekova, B.; Sykorova, V.; Dvorakova, S.; Vaclavikova, E.; Moravcova, J.; Katra, R.; Astl, J.; Vlcek, P.; Kodetova, D.; Vcelak, J.; et al. RET, NTRK, ALK, BRAF, and MET Fusions in a Large Cohort of Pediatric Papillary Thyroid Carcinomas. Thyroid 2020, 30, 1771–1780. [Google Scholar] [CrossRef]
- Latteyer, S.; Tiedje, V.; König, K.; Ting, S.; Heukamp, L.C.; Meder, L.; Schmid, K.W.; Führer, D.; Moeller, L.C. Targeted Next-Generation Sequencing for TP53, RAS, BRAF, ALK and NF1 Mutations in Anaplastic Thyroid Cancer. Endocrine 2016, 54, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Murugan, A.K.; Xing, M. Anaplastic Thyroid Cancers Harbor Novel Oncogenic Mutations of the ALK Gene. Cancer Res. 2011, 71, 4403–4411. [Google Scholar] [CrossRef]
- Li, C.-F.; Wu, X.-L.; Wang, J.-J.; Wang, K.; Zhang, S.-Y.; Huang, J.-J.; Hu, H.-Z.; Zheng, H. ALK-1-Positive Inflammatory Myofibroblastic Tumor of the Thyroid Complicated by Hashimoto’s Thyroiditis: Report of a Rare Case and a Literature Review. Diagn. Pathol. 2020, 15, 58. [Google Scholar] [CrossRef]
- Weiss, L.M.; Funari, V.A. NTRK Fusions and Trk Proteins: What Are They and How to Test for Them. Hum. Pathol. 2021, 112, 59–69. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chen, J.-Y.; Huang, C.-J.; Chen, H.-S.; Yang, A.-H.; Hang, J.-F. Detection of NTRK1/3 Rearrangements in Papillary Thyroid Carcinoma Using Immunohistochemistry, Fluorescent In Situ Hybridization, and Next-Generation Sequencing. Endocr. Pathol. 2020, 31, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Okamura, R.; Boichard, A.; Kato, S.; Sicklick, J.K.; Bazhenova, L.; Kurzrock, R. Analysis of NTRK Alterations in Pan-Cancer Adult and Pediatric Malignancies: Implications for NTRK-Targeted Therapeutics. JCO Precis. Oncol. 2018, 2018, 1–20. [Google Scholar] [CrossRef]
- Solomon, J.P.; Linkov, I.; Rosado, A.; Mullaney, K.; Rosen, E.Y.; Frosina, D.; Jungbluth, A.A.; Zehir, A.; Benayed, R.; Drilon, A.; et al. NTRK Fusion Detection across Multiple Assays and 33,997 Cases: Diagnostic Implications and Pitfalls. Mod. Pathol. 2020, 33, 38–46. [Google Scholar] [CrossRef]
- Rabes, H.M.; Demidchik, E.P.; Sidorow, J.D.; Lengfelder, E.; Beimfohr, C.; Hoelzel, D.; Klugbauer, S. Pattern of Radiation-Induced RET and NTRK1 Rearrangements in 191 Post-Chernobyl Papillary Thyroid Carcinomas: Biological, Phenotypic, and Clinical Implications. Clin. Cancer Res. 2000, 6, 1093–1103. [Google Scholar] [PubMed]
- Leeman-Neill, R.J.; Kelly, L.M.; Liu, P.; Brenner, A.V.; Little, M.P.; Bogdanova, T.I.; Evdokimova, V.N.; Hatch, M.; Zurnadzy, L.Y.; Nikiforova, M.N.; et al. ETV6-NTRK3 Is a Common Chromosomal Rearrangement in Radiation-Associated Thyroid Cancer. Cancer 2014, 120, 799–807. [Google Scholar] [CrossRef]
- Prasad, M.L.; Vyas, M.; Horne, M.J.; Virk, R.K.; Morotti, R.; Liu, Z.; Tallini, G.; Nikiforova, M.N.; Christison-Lagay, E.R.; Udelsman, R.; et al. NTRK Fusion Oncogenes in Pediatric Papillary Thyroid Carcinoma in Northeast United States. Cancer 2016, 122, 1097–1107. [Google Scholar] [CrossRef]
- Chu, Y.-H.; Dias-Santagata, D.; Farahani, A.A.; Boyraz, B.; Faquin, W.C.; Nosé, V.; Sadow, P.M. Clinicopathologic and Molecular Characterization of NTRK-Rearranged Thyroid Carcinoma (NRTC). Mod. Pathol. 2020, 33, 2186–2197. [Google Scholar] [CrossRef]
- Seethala, R.R.; Chiosea, S.I.; Liu, C.Z.; Nikiforova, M.; Nikiforov, Y.E. Clinical and Morphologic Features of ETV6-NTRK3 Translocated Papillary Thyroid Carcinoma in an Adult Population Without Radiation Exposure. Am. J. Surg. Pathol. 2017, 41, 446–457. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Approves Larotrectinib for Solid Tumors with NTRK Gene Fusions. Available online: Https://www.fda.gov/Drugs/Fda-Approves-Larotrectinib-Solid-Tumors-Ntrk-Gene-Fusions (accessed on 6 October 2021).
- Thunders, M.; Delahunt, B. Gene of the Month: DICER1: Ruler and Controller. J. Clin. Pathol. 2021, 74, 69–72. [Google Scholar] [CrossRef]
- Bae, J.-S.; Jung, S.-H.; Hirokawa, M.; Bychkov, A.; Miyauchi, A.; Lee, S.; Chung, Y.-J.; Jung, C.K. High Prevalence of DICER1 Mutations and Low Frequency of Gene Fusions in Pediatric Follicular-Patterned Tumors of the Thyroid. Endocr. Pathol. 2021, 32, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Priest, J.R.; Duchaine, T.F. DICER1: Mutations, MicroRNAs and Mechanisms. Nat. Rev. Cancer 2014, 14, 662–672. [Google Scholar] [CrossRef]
- Khan, N.E.; Bauer, A.J.; Schultz, K.A.P.; Doros, L.; Decastro, R.M.; Ling, A.; Lodish, M.B.; Harney, L.A.; Kase, R.G.; Carr, A.G.; et al. Quantification of Thyroid Cancer and Multinodular Goiter Risk in the DICER1 Syndrome: A Family-Based Cohort Study. J. Clin. Endocrinol. Metab. 2017, 102, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- De Kock, L.; Bah, I.; Revil, T.; Bérubé, P.; Wu, M.K.; Sabbaghian, N.; Priest, J.R.; Ragoussis, J.; Foulkes, W.D. Deep Sequencing Reveals Spatially Distributed Distinct Hot Spot Mutations in DICER1-Related Multinodular Goiter. J. Clin. Endocrinol. Metab. 2016, 101, 3637–3645. [Google Scholar] [CrossRef]
- Oliver-Petit, I.; Bertozzi, A.-I.; Grunenwald, S.; Gambart, M.; Pigeon-Kerchiche, P.; Sadoul, J.-L.; Caron, P.J.; Savagner, F. Multinodular Goitre Is a Gateway for Molecular Testing of DICER1 Syndrome. Clin. Endocrinol. 2019, 91, 669–675. [Google Scholar] [CrossRef]
- Rio Frio, T.; Bahubeshi, A.; Kanellopoulou, C.; Hamel, N.; Niedziela, M.; Sabbaghian, N.; Pouchet, C.; Gilbert, L.; O’Brien, P.K.; Serfas, K.; et al. DICER1 Mutations in Familial Multinodular Goiter with and without Ovarian Sertoli-Leydig Cell Tumors. JAMA 2011, 305, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Gullo, I.; Batista, R.; Rodrigues-Pereira, P.; Soares, P.; Barroca, H.; do Bom-Sucesso, M.; Sobrinho-Simões, M. Multinodular Goiter Progression Toward Malignancy in a Case of DICER1 Syndrome: Histologic and Molecular Alterations. Am. J. Clin. Pathol. 2018, 149, 379–386. [Google Scholar] [CrossRef]
- Lee, Y.A.; Im, S.-W.; Jung, K.C.; Chung, E.-J.; Shin, C.H.; Kim, J.-I.; Park, Y.J. Predominant DICER1 Pathogenic Variants in Pediatric Follicular Thyroid Carcinomas. Thyroid 2020, 30, 1120–1131. [Google Scholar] [CrossRef]
- Wasserman, J.D.; Sabbaghian, N.; Fahiminiya, S.; Chami, R.; Mete, O.; Acker, M.; Wu, M.K.; Shlien, A.; de Kock, L.; Foulkes, W.D. DICER1 Mutations Are Frequent in Adolescent-Onset Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2018, 103, 2009–2015. [Google Scholar] [CrossRef]
- Juhlin, C.C.; Stenman, A.; Zedenius, J. Macrofollicular Variant Follicular Thyroid Tumors Are DICER1 Mutated and Exhibit Distinct Histological Features. Histopathology 2021, 79, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Chernock, R.D.; Rivera, B.; Borrelli, N.; Hill, D.A.; Fahiminiya, S.; Shah, T.; Chong, A.-S.; Aqil, B.; Mehrad, M.; Giordano, T.J.; et al. Poorly Differentiated Thyroid Carcinoma of Childhood and Adolescence: A Distinct Entity Characterized by DICER1 Mutations. Mod. Pathol. 2020, 33, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Ghossein, C.A.; Dogan, S.; Farhat, N.; Landa, I.; Xu, B. Expanding the Spectrum of Thyroid Carcinoma with Somatic DICER1 Mutation: A Survey of 829 Thyroid Carcinomas Using MSK-IMPACT next-Generation Sequencing Platform. Virchows Arch. 2021, 1–10. [Google Scholar] [CrossRef]
- Schultz, K.A.P.; Williams, G.M.; Kamihara, J.; Stewart, D.R.; Harris, A.K.; Bauer, A.J.; Turner, J.; Shah, R.; Schneider, K.; Schneider, K.W.; et al. DICER1 and Associated Conditions: Identification of At-Risk Individuals and Recommended Surveillance Strategies. Clin. Cancer Res. 2018, 24, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Witkowski, L.; Stoehr, R.; Cuenca, J.C.C.; González-Muller, C.A.; Brütting, A.; Bährle, M.; Mantsopoulos, K.; Amin, R.M.S.; Hartmann, A.; et al. Malignant Teratoid Tumor of the Thyroid Gland: An Aggressive Primitive Multiphenotypic Malignancy Showing Organotypical Elements and Frequent DICER1 Alterations-Is the Term “Thyroblastoma” More Appropriate? Virchows Arch. 2020, 477, 787–798. [Google Scholar] [CrossRef]
- Rooper, L.M.; Bynum, J.P.; Miller, K.P.; Lin, M.T.; Gagan, J.; Thompson, L.D.R.; Bishop, J.A. Recurrent DICER1 Hotspot Mutations in Malignant Thyroid Gland Teratomas: Molecular Characterization and Proposal for a Separate Classification. Am. J. Surg. Pathol. 2020, 44, 826–833. [Google Scholar] [CrossRef]
- Pilarski, R.; Burt, R.; Kohlman, W.; Pho, L.; Shannon, K.M.; Swisher, E. Cowden Syndrome and the PTEN Hamartoma Tumor Syndrome: Systematic Review and Revised Diagnostic Criteria. J. Natl. Cancer Inst. 2013, 105, 1607–1616. [Google Scholar] [CrossRef]
- Yehia, L.; Eng, C. PTEN Hamartoma Tumor Syndrome. In GeneReviews®; University of Washington: Seattle, WA, USA, 2001; pp. 1993–2021. [Google Scholar]
- Harach, H.R.; Soubeyran, I.; Brown, A.; Bonneau, D.; Longy, M. Thyroid Pathologic Findings in Patients with Cowden Disease. Ann. Diagn. Pathol. 1999, 3, 331–340. [Google Scholar] [CrossRef]
- Laury, A.R.; Bongiovanni, M.; Tille, J.-C.; Kozakewich, H.; Nosé, V. Thyroid Pathology in PTEN-Hamartoma Tumor Syndrome: Characteristic Findings of a Distinct Entity. Thyroid 2011, 21, 135–144. [Google Scholar] [CrossRef]
- Milas, M.; Mester, J.; Metzger, R.; Shin, J.; Mitchell, J.; Berber, E.; Siperstein, A.E.; Eng, C. Should Patients with Cowden Syndrome Undergo Prophylactic Thyroidectomy? Surgery 2012, 152, 1201–1210. [Google Scholar] [CrossRef]
- Alvarez-Nuñez, F.; Bussaglia, E.; Mauricio, D.; Ybarra, J.; Vilar, M.; Lerma, E.; de Leiva, A.; Matias-Guiu, X. Thyroid Neoplasia Study Group PTEN Promoter Methylation in Sporadic Thyroid Carcinomas. Thyroid 2006, 16, 17–23. [Google Scholar] [CrossRef]
- Beg, S.; Siraj, A.K.; Jehan, Z.; Prabakaran, S.; Al-Sobhi, S.S.; Al-Dawish, M.; Al-Dayel, F.; Al-Kuraya, K.S. PTEN Loss Is Associated with Follicular Variant of Middle Eastern Papillary Thyroid Carcinoma. Br. J. Cancer 2015, 112, 1938–1943. [Google Scholar] [CrossRef] [PubMed]
- Dahia, P.L.; Marsh, D.J.; Zheng, Z.; Zedenius, J.; Komminoth, P.; Frisk, T.; Wallin, G.; Parsons, R.; Longy, M.; Larsson, C.; et al. Somatic Deletions and Mutations in the Cowden Disease Gene, PTEN, in Sporadic Thyroid Tumors. Cancer Res. 1997, 57, 4710–4713. [Google Scholar] [PubMed]
- Halachmi, N.; Halachmi, S.; Evron, E.; Cairns, P.; Okami, K.; Saji, M.; Westra, W.H.; Zeiger, M.A.; Jen, J.; Sidransky, D. Somatic Mutations of the PTEN Tumor Suppressor Gene in Sporadic Follicular Thyroid Tumors. Genes Chromosomes Cancer 1998, 23, 239–243. [Google Scholar] [CrossRef]
- Rurale, G.; Marelli, F.; Duminuco, P.; Persani, L. Glis3 as a Critical Regulator of Thyroid Primordium Specification. Thyroid 2020, 30, 277–289. [Google Scholar] [CrossRef]
- Kang, H.S.; Kumar, D.; Liao, G.; Lichti-Kaiser, K.; Gerrish, K.; Liao, X.-H.; Refetoff, S.; Jothi, R.; Jetten, A.M. GLIS3 Is Indispensable for TSH/TSHR-Dependent Thyroid Hormone Biosynthesis and Follicular Cell Proliferation. J. Clin. Investig. 2017, 127, 4326–4337. [Google Scholar] [CrossRef] [PubMed]
- Scoville, D.W.; Kang, H.S.; Jetten, A.M. Transcription Factor GLIS3: Critical Roles in Thyroid Hormone Biosynthesis, Hypothyroidism, Pancreatic Beta Cells and Diabetes. Pharmacol. Ther. 2020, 215, 107632. [Google Scholar] [CrossRef] [PubMed]
- Pinto, K.; Chetty, R. Gene of the Month: GLIS1-3. J. Clin. Pathol. 2020, 73, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, M.N.; Nikitski, A.V.; Panebianco, F.; Kaya, C.; Yip, L.; Williams, M.; Chiosea, S.I.; Seethala, R.R.; Roy, S.; Condello, V.; et al. GLIS Rearrangement Is a Genomic Hallmark of Hyalinizing Trabecular Tumor of the Thyroid Gland. Thyroid 2019, 29, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Basili, T.; Dopeso, H.; Kim, S.H.; Ferrando, L.; Pareja, F.; Da Cruz Paula, A.; da Silva, E.M.; Stylianou, A.; Maroldi, A.; Marchiò, C.; et al. Oncogenic Properties and Signaling Basis of the PAX8-GLIS3 Fusion Gene. Int. J. Cancer 2020, 147, 2253–2264. [Google Scholar] [CrossRef]
- Kunstman, J.W.; Juhlin, C.C.; Goh, G.; Brown, T.C.; Stenman, A.; Healy, J.M.; Rubinstein, J.C.; Choi, M.; Kiss, N.; Nelson-Williams, C.; et al. Characterization of the Mutational Landscape of Anaplastic Thyroid Cancer via Whole-Exome Sequencing. Hum. Mol. Genet. 2015, 24, 2318–2329. [Google Scholar] [CrossRef] [PubMed]
- Karunamurthy, A.; Panebianco, F.; Hsiao, S.J.; Vorhauer, J.; Nikiforova, M.N.; Chiosea, S.; Nikiforov, Y.E. Prevalence and Phenotypic Correlations of EIF1AX Mutations in Thyroid Nodules. Endocr. Relat. Cancer 2016, 23, 295–301. [Google Scholar] [CrossRef]
- Alzahrani, A.S.; Murugan, A.K.; Qasem, E.; Alswailem, M.M.; AlGhamdi, B.; Moria, Y.; Al-Hindi, H. Absence of EIF1AX, PPM1D, and CHEK2 Mutations Reported in Thyroid Cancer Genome Atlas (TCGA) in a Large Series of Thyroid Cancer. Endocrine 2019, 63, 94–100. [Google Scholar] [CrossRef]
- Nicolson, N.G.; Murtha, T.D.; Dong, W.; Paulsson, J.O.; Choi, J.; Barbieri, A.L.; Brown, T.C.; Kunstman, J.W.; Larsson, C.; Prasad, M.L.; et al. Comprehensive Genetic Analysis of Follicular Thyroid Carcinoma Predicts Prognosis Independent of Histology. J. Clin. Endocrinol. Metab. 2018, 103, 2640–2650. [Google Scholar] [CrossRef]
- Simões-Pereira, J.; Moura, M.M.; Marques, I.J.; Rito, M.; Cabrera, R.A.; Leite, V.; Cavaco, B.M. The Role of EIF1AX in Thyroid Cancer Tumourigenesis and Progression. J. Endocrinol. Investig. 2019, 42, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, G.P.; Davidson, N.R.; Leach, S.D.; Zhao, Z.; Lowe, S.W.; Lee, G.; Landa, I.; Nagarajah, J.; Saqcena, M.; Singh, K.; et al. EIF1AX and RAS Mutations Cooperate to Drive Thyroid Tumorigenesis through ATF4 and C-MYC. Cancer Discov. 2019, 9, 264–281. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Ezzat, S. The Epigenetic Landscape of Differentiated Thyroid Cancer. Mol. Cell. Endocrinol. 2018, 469, 3–10. [Google Scholar] [CrossRef]
- Sasanakietkul, T.; Murtha, T.D.; Javid, M.; Korah, R.; Carling, T. Epigenetic Modifications in Poorly Differentiated and Anaplastic Thyroid Cancer. Mol. Cell. Endocrinol. 2018, 469, 23–37. [Google Scholar] [CrossRef]
- Zhang, K.; Li, C.; Liu, J.; Tang, X.; Li, Z. DNA Methylation Alterations as Therapeutic Prospects in Thyroid Cancer. J. Endocrinol. Investig. 2019, 42, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Zafon, C.; Gil, J.; Pérez-González, B.; Jordà, M. DNA Methylation in Thyroid Cancer. Endocr. Relat. Cancer 2019, 26, R415–R439. [Google Scholar] [CrossRef]
- Park, J.-L.; Jeon, S.; Seo, E.-H.; Bae, D.H.; Jeong, Y.M.; Kim, Y.; Bae, J.S.; Kim, S.-K.; Jung, C.K.; Kim, Y.S. Comprehensive DNA Methylation Profiling Identifies Novel Diagnostic Biomarkers for Thyroid Cancer. Thyroid 2020, 30, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Keelawat, S.; Thorner, P.S.; Shuangshoti, S.; Bychkov, A.; Kitkumthorn, N.; Rattanatanyong, P.; Boonyayothin, W.; Poumsuk, U.; Ruangvejvorachai, P.; Mutirangura, A. Detection of Global Hypermethylation in Well-Differentiated Thyroid Neoplasms by Immunohistochemical (5-Methylcytidine) Analysis. J. Endocrinol. Investig. 2015, 38, 725–732. [Google Scholar] [CrossRef]
- Celano, M.; Rosignolo, F.; Maggisano, V.; Pecce, V.; Iannone, M.; Russo, D.; Bulotta, S. MicroRNAs as Biomarkers in Thyroid Carcinoma. Int. J. Genom. 2017, 2017, 6496570. [Google Scholar] [CrossRef]
- Chruścik, A.; Lam, A.K. Clinical Pathological Impacts of MicroRNAs in Papillary Thyroid Carcinoma: A Crucial Review. Exp. Mol. Pathol. 2015, 99, 393–398. [Google Scholar] [CrossRef]
- Aragon Han, P.; Weng, C.-H.; Khawaja, H.T.; Nagarajan, N.; Schneider, E.B.; Umbricht, C.B.; Witwer, K.W.; Zeiger, M.A. MicroRNA Expression and Association with Clinicopathologic Features in Papillary Thyroid Cancer: A Systematic Review. Thyroid 2015, 25, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Boufraqech, M.; Klubo-Gwiezdzinska, J.; Kebebew, E. MicroRNAs in the Thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 603–619. [Google Scholar] [CrossRef]
- Park, J.-L.; Kim, S.-K.; Jeon, S.; Jung, C.-K.; Kim, Y.-S. MicroRNA Profile for Diagnostic and Prognostic Biomarkers in Thyroid Cancer. Cancers 2021, 13, 632. [Google Scholar] [CrossRef]
- Visone, R.; Russo, L.; Pallante, P.; De Martino, I.; Ferraro, A.; Leone, V.; Borbone, E.; Petrocca, F.; Alder, H.; Croce, C.M.; et al. MicroRNAs (MiR)-221 and MiR-222, Both Overexpressed in Human Thyroid Papillary Carcinomas, Regulate P27Kip1 Protein Levels and Cell Cycle. Endocr. Relat. Cancer 2007, 14, 791–798. [Google Scholar] [CrossRef]
- Czajka, A.A.; Wójcicka, A.; Kubiak, A.; Kotlarek, M.; Bakuła-Zalewska, E.; Koperski, Ł.; Wiechno, W.; Jażdżewski, K. Family of MicroRNA-146 Regulates RARβ in Papillary Thyroid Carcinoma. PLoS ONE 2016, 11, e0151968. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jian, W.; Wei, C.; Song, H.; Gu, Y.; Luo, Y.; Fang, L. Down-Regulation of MiR-181b Promotes Apoptosis by Targeting CYLD in Thyroid Papillary Cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 7672–7680. [Google Scholar] [PubMed]
- Wojtas, B.; Ferraz, C.; Stokowy, T.; Hauptmann, S.; Lange, D.; Dralle, H.; Musholt, T.; Jarzab, B.; Paschke, R.; Eszlinger, M. Differential MiRNA Expression Defines Migration and Reduced Apoptosis in Follicular Thyroid Carcinomas. Mol. Cell. Endocrinol. 2014, 388, 1–9. [Google Scholar] [CrossRef]
- Dettmer, M.; Vogetseder, A.; Durso, M.B.; Moch, H.; Komminoth, P.; Perren, A.; Nikiforov, Y.E.; Nikiforova, M.N. MicroRNA Expression Array Identifies Novel Diagnostic Markers for Conventional and Oncocytic Follicular Thyroid Carcinomas. J. Clin. Endocrinol. Metab. 2013, 98, E1–E7. [Google Scholar] [CrossRef]
- Sun, D.; Han, S.; Liu, C.; Zhou, R.; Sun, W.; Zhang, Z.; Qu, J. Microrna-199a-5p Functions as a Tumor Suppressor via Suppressing Connective Tissue Growth Factor (CTGF) in Follicular Thyroid Carcinoma. Med. Sci. Monit. 2016, 22, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Jikuzono, T.; Kawamoto, M.; Yoshitake, H.; Kikuchi, K.; Akasu, H.; Ishikawa, H.; Hirokawa, M.; Miyauchi, A.; Tsuchiya, S.; Shimizu, K.; et al. The MiR-221/222 Cluster, MiR-10b and MiR-92a Are Highly Upregulated in Metastatic Minimally Invasive Follicular Thyroid Carcinoma. Int. J. Oncol. 2013, 42, 1858–1868. [Google Scholar] [CrossRef]
- Fuziwara, C.S.; Kimura, E.T. MicroRNA Deregulation in Anaplastic Thyroid Cancer Biology. Int. J. Endocrinol. 2014, 2014, 743450. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.; Jackson, N.; Gundara, J.S.; Zhao, J.; Gill, A.J.; Delbridge, L.; Robinson, B.G.; Sidhu, S.B. MicroRNA Profiling of Sporadic and Hereditary Medullary Thyroid Cancer Identifies Predictors of Nodal Metastasis, Prognosis, and Potential Therapeutic Targets. Clin. Cancer Res. 2011, 17, 4772–4781. [Google Scholar] [CrossRef]
- Pennelli, G.; Galuppini, F.; Barollo, S.; Cavedon, E.; Bertazza, L.; Fassan, M.; Guzzardo, V.; Pelizzo, M.R.; Rugge, M.; Mian, C. The PDCD4/MiR-21 Pathway in Medullary Thyroid Carcinoma. Hum. Pathol. 2015, 46, 50–57. [Google Scholar] [CrossRef]
- Chu, Y.-H.; Lloyd, R.V. Medullary Thyroid Carcinoma: Recent Advances Including MicroRNA Expression. Endocr. Pathol. 2016, 27, 312–324. [Google Scholar] [CrossRef]
- Lupo, M.A.; Walts, A.E.; Sistrunk, J.W.; Giordano, T.J.; Sadow, P.M.; Massoll, N.; Campbell, R.; Jackson, S.A.; Toney, N.; Narick, C.M.; et al. Multiplatform Molecular Test Performance in Indeterminate Thyroid Nodules. Diagn. Cytopathol. 2020, 48, 1254–1264. [Google Scholar] [CrossRef]
- Zhang, R.; Hardin, H.; Chen, J.; Guo, Z.; Lloyd, R.V. Non-Coding RNAs in Thyroid Cancer. Endocr. Pathol. 2016, 27, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Sui, F.; Ji, M.; Hou, P. Long Non-Coding RNAs in Thyroid Cancer: Biological Functions and Clinical Significance. Mol. Cell. Endocrinol. 2018, 469, 11–22. [Google Scholar] [CrossRef]
- Murugan, A.K.; Munirajan, A.K.; Alzahrani, A.S. Long Noncoding RNAs: Emerging Players in Thyroid Cancer Pathogenesis. Endocr. Relat. Cancer 2018, 25, R59–R82. [Google Scholar] [CrossRef]
- Sedaghati, M.; Kebebew, E. Long Noncoding RNAs in Thyroid Cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Rahner, N.; Steinke, V. Hereditary Cancer Syndromes. Dtsch. Arztebl. Int. 2008, 105, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Nosé, V. Familial Follicular Cell Tumors: Classification and Morphological Characteristics. Endocr. Pathol. 2010, 21, 219–226. [Google Scholar] [CrossRef]
- LiVolsi, V.A.; Baraban, E.; Baloch, Z.W. Familial Thyroid Carcinoma: The Road Less Traveled in Thyroid Pathology—An Update. Diagn. Histopathol. 2017, 23, 366–377. [Google Scholar] [CrossRef]
- Hińcza, K.; Kowalik, A.; Kowalska, A. Current Knowledge of Germline Genetic Risk Factors for the Development of Non-Medullary Thyroid Cancer. Genes 2019, 10, 482. [Google Scholar] [CrossRef]
- Bann, D.V.; Jin, Q.; Sheldon, K.E.; Houser, K.R.; Nguyen, L.; Warrick, J.I.; Baker, M.J.; Broach, J.R.; Gerhard, G.S.; Goldenberg, D. Genetic Variants Implicate Dual Oxidase-2 in Familial and Sporadic Nonmedullary Thyroid Cancer. Cancer Res. 2019, 79, 5490–5499. [Google Scholar] [CrossRef] [PubMed]
- Nosé, V. Familial Thyroid Cancer: A Review. Mod. Pathol. 2011, 24 (Suppl. 2), S19–S33. [Google Scholar] [CrossRef]
- Liang, B.; Ding, H.; Huang, L.; Luo, H.; Zhu, X. GWAS in Cancer: Progress and Challenges. Mol. Genet. Genom. 2020, 295, 537–561. [Google Scholar] [CrossRef] [PubMed]
- Saenko, V.A.; Rogounovitch, T.I. Genetic Polymorphism Predisposing to Differentiated Thyroid Cancer: A Review of Major Findings of the Genome-Wide Association Studies. Endocrinol. Metab. 2018, 33, 164–174. [Google Scholar] [CrossRef]
- Liyanarachchi, S.; Gudmundsson, J.; Ferkingstad, E.; He, H.; Jonasson, J.G.; Tragante, V.; Asselbergs, F.W.; Xu, L.; Kiemeney, L.A.; Netea-Maier, R.T.; et al. Assessing Thyroid Cancer Risk Using Polygenic Risk Scores. Proc. Natl. Acad. Sci. USA 2020, 117, 5997–6002. [Google Scholar] [CrossRef]
- Matsuse, M.; Takahashi, M.; Mitsutake, N.; Nishihara, E.; Hirokawa, M.; Kawaguchi, T.; Rogounovitch, T.; Saenko, V.; Bychkov, A.; Suzuki, K.; et al. The FOXE1 and NKX2-1 Loci Are Associated with Susceptibility to Papillary Thyroid Carcinoma in the Japanese Population. J. Med. Genet. 2011, 48, 645–648. [Google Scholar] [CrossRef]
- Bychkov, A.; Saenko, V.; Nakashima, M.; Mitsutake, N.; Rogounovitch, T.; Nikitski, A.; Orim, F.; Yamashita, S. Patterns of FOXE1 Expression in Papillary Thyroid Carcinoma by Immunohistochemistry. Thyroid 2013, 23, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Nikitski, A.V.; Rogounovitch, T.I.; Bychkov, A.; Takahashi, M.; Yoshiura, K.-I.; Mitsutake, N.; Kawaguchi, T.; Matsuse, M.; Drozd, V.M.; Demidchik, Y.; et al. Genotype Analyses in the Japanese and Belarusian Populations Reveal Independent Effects of Rs965513 and Rs1867277 but Do Not Support the Role of FOXE1 Polyalanine Tract Length in Conferring Risk for Papillary Thyroid Carcinoma. Thyroid 2017, 27, 224–235. [Google Scholar] [CrossRef]
- Rogounovitch, T.I.; Bychkov, A.; Takahashi, M.; Mitsutake, N.; Nakashima, M.; Nikitski, A.V.; Hayashi, T.; Hirokawa, M.; Ishigaki, K.; Shigematsu, K.; et al. The Common Genetic Variant Rs944289 on Chromosome 14q13.3 Associates with Risk of Both Malignant and Benign Thyroid Tumors in the Japanese Population. Thyroid 2015, 25, 333–340. [Google Scholar] [CrossRef]
- Zidane, M.; Cazier, J.-B.; Chevillard, S.; Ory, C.; Schlumberger, M.; Dupuy, C.; Deleuze, J.-F.; Boland, A.; Haddy, N.; Lesueur, F.; et al. Genetic Susceptibility to Radiation-Related Differentiated Thyroid Cancers: A Systematic Review of Literature. Endocr. Relat. Cancer 2019, 26, R583–R596. [Google Scholar] [CrossRef]
- Jung, C.K.; Hong, S.; Bychkov, A.; Kakudo, K. The Use of Fine-Needle Aspiration (FNA) Cytology in Patients with Thyroid Nodules in Asia: A Brief Overview of Studies from the Working Group of Asian Thyroid FNA Cytology. J. Pathol. Transl. Med. 2017, 51, 571–578. [Google Scholar] [CrossRef]
- Vuong, H.G.; Chung, D.G.B.; Ngo, L.M.; Bui, T.Q.; Hassell, L.; Jung, C.K.; Kakudo, K.; Bychkov, A. The Use of the Bethesda System for Reporting Thyroid Cytopathology in Pediatric Thyroid Nodules: A Meta-Analysis. Thyroid 2021, 31, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.N.; Yip, L.; Lubitz, C.C.; Grubbs, E.G.; Miller, B.S.; Shen, W.; Angelos, P.; Chen, H.; Doherty, G.M.; Fahey, T.J.; et al. The American Association of Endocrine Surgeons Guidelines for the Definitive Surgical Management of Thyroid Disease in Adults. Ann. Surg. 2020, 271, e21–e93. [Google Scholar] [CrossRef] [PubMed]
- Labourier, E.; Shifrin, A.; Busseniers, A.E.; Lupo, M.A.; Manganelli, M.L.; Andruss, B.; Wylie, D.; Beaudenon-Huibregtse, S. Molecular Testing for MiRNA, MRNA, and DNA on Fine-Needle Aspiration Improves the Preoperative Diagnosis of Thyroid Nodules with Indeterminate Cytology. J. Clin. Endocrinol. Metab. 2015, 100, 2743–2750. [Google Scholar] [CrossRef]
- Patel, K.N.; Angell, T.E.; Babiarz, J.; Barth, N.M.; Blevins, T.; Duh, Q.-Y.; Ghossein, R.A.; Harrell, R.M.; Huang, J.; Kennedy, G.C.; et al. Performance of a Genomic Sequencing Classifier for the Preoperative Diagnosis of Cytologically Indeterminate Thyroid Nodules. JAMA Surg. 2018, 153, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Steward, D.L.; Carty, S.E.; Sippel, R.S.; Yang, S.P.; Sosa, J.A.; Sipos, J.A.; Figge, J.J.; Mandel, S.; Haugen, B.R.; Burman, K.D.; et al. Performance of a Multigene Genomic Classifier in Thyroid Nodules with Indeterminate Cytology: A Prospective Blinded Multicenter Study. JAMA Oncol. 2019, 5, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Lithwick-Yanai, G.; Dromi, N.; Shtabsky, A.; Morgenstern, S.; Strenov, Y.; Feinmesser, M.; Kravtsov, V.; Leon, M.E.; Hajdúch, M.; Ali, S.Z.; et al. Multicentre Validation of a MicroRNA-Based Assay for Diagnosing Indeterminate Thyroid Nodules Utilising Fine Needle Aspirate Smears. J. Clin. Pathol. 2017, 70, 500–507. [Google Scholar] [CrossRef]
- Tonozzi, T.R.; Kammesheidt, A.; Braunstein, G.D.; Braunstein, G.D. Liquid Biopsies in Endocrine Neoplasia—A Systematic Review. US Endocrinol. 2019, 15, 39. [Google Scholar] [CrossRef]
- Sato, A.; Tanabe, M.; Tsuboi, Y.; Niwa, T.; Shinozaki-Ushiku, A.; Seto, Y.; Murakami, Y. Circulating Tumor DNA Harboring the BRAFV600E Mutation May Predict Poor Outcomes of Primary Papillary Thyroid Cancer Patients. Thyroid 2021, 31, 1822–1828. [Google Scholar] [CrossRef]
- Allin, D.M.; Shaikh, R.; Carter, P.; Thway, K.; Sharabiani, M.T.A.; Gonzales-de-Castro, D.; O’Leary, B.; Garcia-Murillas, I.; Bhide, S.; Hubank, M.; et al. Circulating Tumour DNA Is a Potential Biomarker for Disease Progression and Response to Targeted Therapy in Advanced Thyroid Cancer. Eur. J. Cancer 2018, 103, 165–175. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, J.R.; Wang, Y.; Iyer, P.; Cote, G.J.; Busaidy, N.L.; Dadu, R.; Zafereo, M.; Williams, M.D.; Ferrarotto, R.; et al. Clinical Utility of Circulating Cell-Free DNA Mutations in Anaplastic Thyroid Carcinoma. Thyroid 2021, 31, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, C.C.; Parangi, S.; Holm, T.M.; Bernasconi, M.J.; Schalck, A.P.; Suh, H.; Economopoulos, K.P.; Gunda, V.; Donovan, S.E.; Sadow, P.M.; et al. Detection of Circulating BRAF(V600E) in Patients with Papillary Thyroid Carcinoma. J. Mol. Diagn. 2016, 18, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Samsonov, R.; Burdakov, V.; Shtam, T.; Radzhabova, Z.; Vasilyev, D.; Tsyrlina, E.; Titov, S.; Ivanov, M.; Berstein, L.; Filatov, M.; et al. Plasma Exosomal MiR-21 and MiR-181a Differentiates Follicular from Papillary Thyroid Cancer. Tumor Biol. 2016, 37, 12011–12021. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Zhao, J.-T.; Gundara, J.; Serpell, J.; Bach, L.A.; Sidhu, S. Papillary Thyroid Cancer-Derived Exosomes Contain MiRNA-146b and MiRNA-222. J. Surg. Res. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- NIH National Cancer Institute. Drugs Approved for Thyroid Cancer. Available online: Https://Www.Cancer.Gov/about-Cancer/Treatment/Drugs/Thyroid (accessed on 1 November 2021).
- Kimura, S. Thyroid-Specific Transcription Factors and Their Roles in Thyroid Cancer. J. Thyroid Res. 2011, 2011, 710213. [Google Scholar] [CrossRef]
- Cuylen, S.; Blaukopf, C.; Politi, A.Z.; Müller-Reichert, T.; Neumann, B.; Poser, I.; Ellenberg, J.; Hyman, A.A.; Gerlich, D.W. Ki-67 Acts as a Biological Surfactant to Disperse Mitotic Chromosomes. Nature 2016, 535, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, M.; Nielsen, T.O.; A’Hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. Assessment of Ki67 in Breast Cancer: Recommendations from the International Ki67 in Breast Cancer Working Group. J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations from the International Ki67 in Breast Cancer Working Group. J. Natl. Cancer Inst. 2021, 113, 808–819. [Google Scholar] [CrossRef]
- Saad, A.G.; Kumar, S.; Ron, E.; Lubin, J.H.; Stanek, J.; Bove, K.E.; Nikiforov, Y.E. Proliferative Activity of Human Thyroid Cells in Various Age Groups and Its Correlation with the Risk of Thyroid Cancer after Radiation Exposure. J. Clin. Endocrinol. Metab. 2006, 91, 2672–2677. [Google Scholar] [CrossRef] [PubMed]
- Bychkov, A. Epithelial Hyperplasia Is Responsible for the Compensatory Enlargement of Remaining Thyroid Lobe after Thyroidectomy. Eur. Arch. Otorhinolaryngol. 2018, 275, 2417–2419. [Google Scholar] [CrossRef]
- Kakudo, K.; Wakasa, T.; Ohta, Y.; Yane, K.; Ito, Y.; Yamashita, H. Prognostic Classification of Thyroid Follicular Cell Tumors Using Ki-67 Labeling Index: Risk Stratification of Thyroid Follicular Cell Carcinomas. Endocr. J. 2015, 62, 1–12. [Google Scholar] [CrossRef]
- Hirokawa, M.; Sugitani, I.; Kakudo, K.; Sakamoto, A.; Higashiyama, T.; Sugino, K.; Toda, K.; Ogasawara, S.; Yoshimoto, S.; Hasegawa, Y.; et al. Histopathological Analysis of Anaplastic Thyroid Carcinoma Cases with Long-Term Survival: A Report from the Anaplastic Thyroid Carcinoma Research Consortium of Japan. Endocr. J. 2016, 63, 441–447. [Google Scholar] [CrossRef]
- Kakudo, K.; Asai, M.; Bai, Y. How to Confirm or Deny High-Risk Thyroid Carcinoma Is a Challenge for Pathologists. Pathol. Int. 2017, 67, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Hirokawa, M.; Miyauchi, A.; Masuoka, H.; Yabuta, T.; Fukushima, M.; Kihara, M.; Higashiyama, T.; Kobayashi, K.; Miya, A. Prognostic Impact of Ki-67 Labeling Index in Minimally Invasive Follicular Thyroid Carcinoma. Endocr. J. 2016, 63, 913–917. [Google Scholar] [CrossRef]
- Fuchs, T.L.; Nassour, A.J.; Glover, A.; Sywak, M.S.; Sidhu, S.B.; Delbridge, L.W.; Clifton-Bligh, R.J.; Gild, M.L.; Tsang, V.; Robinson, B.G.; et al. A Proposed Grading Scheme for Medullary Thyroid Carcinoma Based on Proliferative Activity (Ki-67 and Mitotic Count) and Coagulative Necrosis. Am. J. Surg. Pathol. 2020, 44, 1419–1428. [Google Scholar] [CrossRef]
- Kakudo, K.; Liu, Z.; Bai, Y.; Li, Y.; Kitayama, N.; Satoh, S.; Nakashima, M.; Jung, C.K. How to Identify Indolent Thyroid Tumors Unlikely to Recur and Cause Cancer Death Immediately after Surgery—Risk Stratification of Papillary Thyroid Carcinoma in Young Patients. Endocr. J. 2021, 68, 871–880. [Google Scholar] [CrossRef]
- Tang, J.; Gui, C.; Qiu, S.; Wang, M. The Clinicopathological Significance of Ki67 in Papillary Thyroid Carcinoma: A Suitable Indicator? World J. Surg. Oncol. 2018, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Matsuse, M.; Yabuta, T.; Saenko, V.; Hirokawa, M.; Nishihara, E.; Suzuki, K.; Yamashita, S.; Miyauchi, A.; Mitsutake, N. TERT Promoter Mutations and Ki-67 Labeling Index as a Prognostic Marker of Papillary Thyroid Carcinomas: Combination of Two Independent Factors. Sci. Rep. 2017, 7, 41752. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.N.; Siikanen, J.; Hedman, C.; Juhlin, C.C.; Ihre Lundgren, C. Pre-Therapeutic Measurements of Iodine Avidity in Papillary and Poorly Differentiated Thyroid Cancer Reveal Associations with Thyroglobulin Expression, Histological Variants and Ki-67 Index. Cancers 2021, 13, 3627. [Google Scholar] [CrossRef]
- Hirokawa, M.; Matsuda, K.; Kudo, T.; Higuchi, M.; Suzuki, A.; Takada, N.; Nakashima, M.; Miyauchi, A. Cribriform-Morular Variant of Papillary Thyroid Carcinoma Shows High Ki-67 Labeling Indices, despite Its Excellent Prognosis. Pathobiology 2019, 86, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Mian, C.; Pennelli, G.; Barollo, S.; Cavedon, E.; Nacamulli, D.; Vianello, F.; Negro, I.; Pozza, G.; Boschin, I.M.; Pelizzo, M.R.; et al. Combined RET and Ki-67 Assessment in Sporadic Medullary Thyroid Carcinoma: A Useful Tool for Patient Risk Stratification. Eur. J. Endocrinol. 2011, 164, 971–976. [Google Scholar] [CrossRef]
- Frank-Raue, K.; Machens, A.; Leidig-Bruckner, G.; Rondot, S.; Haag, C.; Schulze, E.; Lorenz, A.; Kreissl, M.C.; Dralle, H.; Raue, F.; et al. Prevalence and Clinical Spectrum of Nonsecretory Medullary Thyroid Carcinoma in a Series of 839 Patients with Sporadic Medullary Thyroid Carcinoma. Thyroid 2013, 23, 294–300. [Google Scholar] [CrossRef]
- Xu, B.; Fuchs, T.L.; Ahmadi, S.; Alghamdi, M.; Alzumaili, B.; Bani, M.-A.; Baudin, E.; Chou, A.; De Leo, A.; Fagin, J.A.; et al. International Medullary Thyroid Carcinoma Grading System: A Validated Grading System for Medullary Thyroid Carcinoma. J. Clin. Oncol. 2021, 40, 96–104. [Google Scholar] [CrossRef]
- Agaimy, A.; Erlenbach-Wünsch, K.; Konukiewitz, B.; Schmitt, A.M.; Rieker, R.J.; Vieth, M.; Kiesewetter, F.; Hartmann, A.; Zamboni, G.; Perren, A.; et al. ISL1 Expression Is Not Restricted to Pancreatic Well-Differentiated Neuroendocrine Neoplasms, but Is Also Commonly Found in Well and Poorly Differentiated Neuroendocrine Neoplasms of Extrapancreatic Origin. Mod. Pathol. 2013, 26, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.Y.; Kang, M.; De Peralta-Venturina, M.; Fan, X. Diagnostic Utility of INSM1 in Medullary Thyroid Carcinoma. Int. J. Surg. Pathol. 2021, 29, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Juhlin, C.C. Second-Generation Neuroendocrine Immunohistochemical Markers: Reflections from Clinical Implementation. Biology 2021, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, D. A Study of FoxA1 Expression in Thyroid Tumors. Hum. Pathol. 2017, 65, 217–224. [Google Scholar] [CrossRef]
- Hornick, J.L. Replacing Molecular Genetic Testing with Immunohistochemistry Using Antibodies That Recognize the Protein Products of Gene Rearrangements: “Next-Generation” Immunohistochemistry. Am. J. Surg. Pathol. 2021, 45, 584–586. [Google Scholar] [CrossRef]
- Singarayer, R.; Mete, O.; Perrier, L.; Thabane, L.; Asa, S.L.; Van Uum, S.; Ezzat, S.; Goldstein, D.P.; Sawka, A.M. A Systematic Review and Meta-Analysis of the Diagnostic Performance of BRAF V600E Immunohistochemistry in Thyroid Histopathology. Endocr. Pathol. 2019, 30, 201–218. [Google Scholar] [CrossRef]
- Choden, S.; Keelawat, S.; Jung, C.K.; Bychkov, A. VE1 Immunohistochemistry Improves the Limit of Genotyping for Detecting BRAFV600E Mutation in Papillary Thyroid Cancer. Cancers 2020, 12, 596. [Google Scholar] [CrossRef]
- Choden, S.; Keelawat, S.; Jung, C.K.; Bychkov, A. An Affordable Immunohistochemical Approach to Estimate the Prevalence of BRAFV600E in Large Cohort Studies-Establishing the Baseline Rate of BRAF Mutation in an Institutional Series of Papillary Thyroid Carcinoma from Thailand. Gland Surg. 2020, 9, 1867–1877. [Google Scholar] [CrossRef]
- Dias-Santagata, D.; Su, Y.; Hoang, M.P. Immunohistochemical Detection of NRASQ61R Mutation in Diverse Tumor Types. Am. J. Clin. Pathol. 2016, 145, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Oishi, N.; Kondo, T.; Vuong, H.G.; Nakazawa, T.; Mochizuki, K.; Kasai, K.; Inoue, T.; Tahara, I.; Hirokawa, M.; Miyauchi, A.; et al. Immunohistochemical Detection of NRAS(Q61R) Protein in Follicular-Patterned Thyroid Tumors. Hum. Pathol. 2016, 53, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Reagh, J.; Bullock, M.; Andrici, J.; Turchini, J.; Sioson, L.; Clarkson, A.; Watson, N.; Sheen, A.; Lim, G.; Delbridge, L.; et al. NRASQ61R Mutation-Specific Immunohistochemistry Also Identifies the HRASQ61R Mutation in Medullary Thyroid Cancer and May Have a Role in Triaging Genetic Testing for MEN2. Am. J. Surg. Pathol. 2017, 41, 75–81. [Google Scholar] [CrossRef]
- Lasota, J.; Kowalik, A.; Felisiak-Golabek, A.; Inaguma, S.; Wang, Z.-F.; Pięciak, L.; Zięba, S.; Pęksa, R.; Kopczynski, J.; Okoń, K.; et al. SP174, NRAS Q61R Mutant-Specific Antibody, Cross-Reacts With KRAS Q61R Mutant Protein in Colorectal Carcinoma. Arch. Pathol. Lab. Med. 2017, 141, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Marotta, V.; Bifulco, M.; Vitale, M. Significance of RAS Mutations in Thyroid Benign Nodules and Non-Medullary Thyroid Cancer. Cancers 2021, 13, 3785. [Google Scholar] [CrossRef] [PubMed]
- Hechtman, J.F.; Benayed, R.; Hyman, D.M.; Drilon, A.; Zehir, A.; Frosina, D.; Arcila, M.E.; Dogan, S.; Klimstra, D.S.; Ladanyi, M.; et al. Pan-Trk Immunohistochemistry Is an Efficient and Reliable Screen for the Detection of NTRK Fusions. Am. J. Surg. Pathol. 2017, 41, 1547–1551. [Google Scholar] [CrossRef]
- Andrici, J.; Gill, A.J.; Hornick, J.L. Next Generation Immunohistochemistry: Emerging Substitutes to Genetic Testing? Semin. Diagn. Pathol. 2018, 35, 161–169. [Google Scholar] [CrossRef]
- Rebecchini, C.; Nobile, A.; Piana, S.; Sarro, R.; Bisig, B.; Gerasimos, S.P.; Saglietti, C.; Matter, M.; Marino, L.; Bongiovanni, M. Papillary Thyroid Carcinoma with Nodular Fasciitis-like Stroma and β-Catenin Mutations Should Be Renamed Papillary Thyroid Carcinoma with Desmoid-Type Fibromatosis. Mod. Pathol. 2017, 30, 236–245. [Google Scholar] [CrossRef]
- Papathomas, T.G.; Nosé, V. New and Emerging Biomarkers in Endocrine Pathology. Adv. Anat. Pathol. 2019, 26, 198–209. [Google Scholar] [CrossRef]
- Pulido, R.; Mingo, J.; Gaafar, A.; Nunes-Xavier, C.E.; Luna, S.; Torices, L.; Angulo, J.C.; López, J.I. Precise Immunodetection of PTEN Protein in Human Neoplasia. Cold Spring Harb. Perspect. Med. 2019, 9, a036293. [Google Scholar] [CrossRef]
- Barletta, J.A.; Bellizzi, A.M.; Hornick, J.L. Immunohistochemical Staining of Thyroidectomy Specimens for PTEN Can Aid in the Identification of Patients with Cowden Syndrome. Am. J. Surg. Pathol. 2011, 35, 1505–1511. [Google Scholar] [CrossRef]
- Marchetti, A.; Di Lorito, A.; Pace, M.V.; Iezzi, M.; Felicioni, L.; D’Antuono, T.; Filice, G.; Guetti, L.; Mucilli, F.; Buttitta, F. ALK Protein Analysis by IHC Staining after Recent Regulatory Changes: A Comparison of Two Widely Used Approaches, Revision of the Literature, and a New Testing Algorithm. J. Thorac. Oncol. 2016, 11, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Human Services. Letter to Ventana Medical Systems on Approval of Premarket Authorization Application for the VENTANA ALK (D5F3) CDx Assay. Available online: Http://Www.Accessdata.Fda.Gov/Cdrh_docs/Pdf14/P140025a.Pdf (accessed on 6 October 2021).
- Poltavets, V.; Kochetkova, M.; Pitson, S.M.; Samuel, M.S. The Role of the Extracellular Matrix and Its Molecular and Cellular Regulators in Cancer Cell Plasticity. Front. Oncol. 2018, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Fallahi, P.; Galdiero, M.R.; Ruffilli, I.; Elia, G.; Ragusa, F.; Paparo, S.R.; Patrizio, A.; Mazzi, V.; Varricchi, G.; et al. Immune and Inflammatory Cells in Thyroid Cancer Microenvironment. Int. J. Mol. Sci. 2019, 20, 4413. [Google Scholar] [CrossRef]
- French, J.D. Immunotherapy for Advanced Thyroid Cancers - Rationale, Current Advances and Future Strategies. Nat. Rev. Endocrinol. 2020, 16, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Loffredo, S.; Marone, G.; Modestino, L.; Fallahi, P.; Ferrari, S.M.; de Paulis, A.; Antonelli, A.; Galdiero, M.R. The Immune Landscape of Thyroid Cancer in the Context of Immune Checkpoint Inhibition. Int. J. Mol. Sci. 2019, 20, 3934. [Google Scholar] [CrossRef]
- Qing, W.; Fang, W.-Y.; Ye, L.; Shen, L.-Y.; Zhang, X.-F.; Fei, X.-C.; Chen, X.; Wang, W.-Q.; Li, X.-Y.; Xiao, J.-C.; et al. Density of Tumor-Associated Macrophages Correlates with Lymph Node Metastasis in Papillary Thyroid Carcinoma. Thyroid 2012, 22, 905–910. [Google Scholar] [CrossRef]
- Ryder, M.; Ghossein, R.A.; Ricarte-Filho, J.C.M.; Knauf, J.A.; Fagin, J.A. Increased Density of Tumor-Associated Macrophages Is Associated with Decreased Survival in Advanced Thyroid Cancer. Endocr. Relat. Cancer 2008, 15, 1069–1074. [Google Scholar] [CrossRef]
- Cristinziano, L.; Modestino, L.; Loffredo, S.; Varricchi, G.; Braile, M.; Ferrara, A.L.; de Paulis, A.; Antonelli, A.; Marone, G.; Galdiero, M.R. Anaplastic Thyroid Cancer Cells Induce the Release of Mitochondrial Extracellular DNA Traps by Viable Neutrophils. J. Immunol. 2020, 204, 1362–1372. [Google Scholar] [CrossRef]
- Liu, C.-L.; Lee, J.-J.; Liu, T.-P.; Chang, Y.-C.; Hsu, Y.-C.; Cheng, S.-P. Blood Neutrophil-to-Lymphocyte Ratio Correlates with Tumor Size in Patients with Differentiated Thyroid Cancer. J. Surg. Oncol. 2013, 107, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Angell, T.E.; Lechner, M.G.; Smith, A.M.; Martin, S.E.; Groshen, S.G.; Maceri, D.R.; Singer, P.A.; Epstein, A.L. Circulating Myeloid-Derived Suppressor Cells Predict Differentiated Thyroid Cancer Diagnosis and Extent. Thyroid 2016, 26, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.L.; Morari, E.C.; Guihen, A.C.T.; Razolli, D.; Gerhard, R.; Nonogaki, S.; Soares, F.A.; Vassallo, J.; Ward, L.S. Infiltration of a Mixture of Immune Cells May Be Related to Good Prognosis in Patients with Differentiated Thyroid Carcinoma. Clin. Endocrinol. 2012, 77, 918–925. [Google Scholar] [CrossRef]
- Fozzatti, L.; Cheng, S.-Y. Tumor Cells and Cancer-Associated Fibroblasts: A Synergistic Crosstalk to Promote Thyroid Cancer. Endocrinol. Metab. 2020, 35, 673–680. [Google Scholar] [CrossRef]
- Shin, M.H.; Kim, J.; Lim, S.A.; Kim, J.; Lee, K.-M. Current Insights into Combination Therapies with MAPK Inhibitors and Immune Checkpoint Blockade. Int. J. Mol. Sci. 2020, 21, 2531. [Google Scholar] [CrossRef]
- Ulisse, S.; Tuccilli, C.; Sorrenti, S.; Antonelli, A.; Fallahi, P.; D’Armiento, E.; Catania, A.; Tartaglia, F.; Amabile, M.I.; Giacomelli, L.; et al. PD-1 Ligand Expression in Epithelial Thyroid Cancers: Potential Clinical Implications. Int. J. Mol. Sci. 2019, 20, 1405. [Google Scholar] [CrossRef]
- Sul, J.; Blumenthal, G.M.; Jiang, X.; He, K.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Patients with Metastatic Non-Small Cell Lung Cancer Whose Tumors Express Programmed Death-Ligand 1. Oncologist 2016, 21, 643–650. [Google Scholar] [CrossRef]
- Cheung, C.C.; Barnes, P.; Bigras, G.; Boerner, S.; Butany, J.; Calabrese, F.; Couture, C.; Deschenes, J.; El-Zimaity, H.; Fischer, G.; et al. Fit-For-Purpose PD-L1 Biomarker Testing for Patient Selection in Immuno-Oncology: Guidelines For Clinical Laboratories From the Canadian Association of Pathologists-Association Canadienne Des Pathologistes (CAP-ACP). Appl. Immunohistochem. Mol. Morphol. 2019, 27, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Paver, E.C.; Cooper, W.A.; Colebatch, A.J.; Ferguson, P.M.; Hill, S.K.; Lum, T.; Shin, J.-S.; O’Toole, S.; Anderson, L.; Scolyer, R.A.; et al. Programmed Death Ligand-1 (PD-L1) as a Predictive Marker for Immunotherapy in Solid Tumours: A Guide to Immunohistochemistry Implementation and Interpretation. Pathology 2021, 53, 141–156. [Google Scholar] [CrossRef]
- Girolami, I.; Pantanowitz, L.; Mete, O.; Brunelli, M.; Marletta, S.; Colato, C.; Trimboli, P.; Crescenzi, A.; Bongiovanni, M.; Barbareschi, M.; et al. Programmed Death-Ligand 1 (PD-L1) Is a Potential Biomarker of Disease-Free Survival in Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis of PD-L1 Immunoexpression in Follicular Epithelial Derived Thyroid Carcinoma. Endocr. Pathol. 2020, 31, 291–300. [Google Scholar] [CrossRef]
- Aghajani, M.J.; Roberts, T.L.; Yang, T.; McCafferty, C.E.; Caixeiro, N.J.; DeSouza, P.; Niles, N. Elevated Levels of Soluble PD-L1 Are Associated with Reduced Recurrence in Papillary Thyroid Cancer. Endocr. Connect. 2019, 8, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Schürch, C.M.; Roelli, M.A.; Forster, S.; Wasmer, M.-H.; Brühl, F.; Maire, R.S.; Di Pancrazio, S.; Ruepp, M.-D.; Giger, R.; Perren, A.; et al. Targeting CD47 in Anaplastic Thyroid Carcinoma Enhances Tumor Phagocytosis by Macrophages and Is a Promising Therapeutic Strategy. Thyroid 2019, 29, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Xia, J.; Li, J.; Xu, J. CD47 Is Associated with the Up-Regulation of the PD-1 Oncogenic Signaling Pathway. Int. J. Clin. Exp. Pathol. 2018, 11, 5612–5621. [Google Scholar] [PubMed]
- Courageot, M.-P.; Duca, L.; Martiny, L.; Devarenne-Charpentier, E.; Morjani, H.; El Btaouri, H. Thrombospondin-1 Receptor CD47 Overexpression Contributes to P-Glycoprotein-Mediated Multidrug Resistance Against Doxorubicin in Thyroid Carcinoma FTC-133 Cells. Front. Oncol. 2020, 10, 551228. [Google Scholar] [CrossRef]
- Cameselle-Teijeiro, J.M.; Eloy, C.; Sobrinho-Simões, M. Pitfalls in Challenging Thyroid Tumors: Emphasis on Differential Diagnosis and Ancillary Biomarkers. Endocr. Pathol. 2020, 31, 197–217. [Google Scholar] [CrossRef]
- Oh, E.J.; Bychkov, A.; Cho, H.; Kim, T.-M.; Bae, J.S.; Lim, D.-J.; Jung, C.K. Prognostic Implications of CD10 and CD15 Expression in Papillary Thyroid Carcinoma. Cancers 2020, 12, 1413. [Google Scholar] [CrossRef]
- Nakazawa, T.; Kondo, T.; Vuong, H.G.; Odate, T.; Kawai, M.; Tahara, I.; Kasai, K.; Inoue, T.; Oishi, N.; Mochizuki, K.; et al. High Expression of CD10 in Anaplastic Thyroid Carcinomas. Histopathology 2018, 73, 492–499. [Google Scholar] [CrossRef]
- Ameri, F.; Mokhtari, M. Diagnostic Value of CD-10 Marker in Differentiating of Papillary Thyroid Carcinoma from Benign Thyroid Lesions. Adv. Biomed. Res. 2014, 3, 206. [Google Scholar] [CrossRef]
- Ohta, M.; Ookoshi, T.; Naiki, H.; Imamura, Y. HBME-1 and CD15 Immunocytochemistry in the Follicular Variant of Thyroid Papillary Carcinoma: Immunocytochemistry in Thyroid Cancer. Pathol. Int. 2015, 65, 119–125. [Google Scholar] [CrossRef][Green Version]
- Imamura, Y.; Fukuda, M. CD15 (C3D-1) Immunoreactivity in Normal, Benign, and Malignant Thyroid Lesions. Appl. Immunohistochem. 1998, 6, 181–186. [Google Scholar] [CrossRef]
- Mai, K.T.; Ford, J.C.; Yazdi, H.M.; Garth Perkins, D.; Susan Commons, A. Immunohistochemical Study of Papillary Thyroid Carcinoma and Possible Papillary Thyroid Carcinoma-Related Benign Thyroid Nodules. Pathol. Res. Pract. 2000, 196, 533–540. [Google Scholar] [CrossRef]
- Bychkov, A.; Jung, C.K. Aberrant Expression of CD20 in Thyroid Cancer and Its Clinicopathologic Significance. Hum. Pathol. 2018, 71, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Seto, M.; Miyoshi, H.; Okada, S.; Yokoyama, S.; Ohshima, A.; Ohshima, K. Papillary Thyroid Carcinoma Expressing CD20: CD20-Positive Papillary Thyroid Carcinoma. Pathol. Int. 2017, 67, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M.; Villari, D.; Ruggeri, R.M.; Quattrocchi, E.; Fragetta, F.; Simone, A.; Scarfi, R.; Magro, G.; Batolo, D.; Trimarchi, F.; et al. Expression of CD30 Ligand and CD30 Receptor in Normal Thyroid and Benign and Malignant Thyroid Nodules. Thyroid 2001, 11, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Koo, J.S. Immunohistochemical Analysis of Cancer Stem Cell Marker Expression in Papillary Thyroid Cancer. Front. Endocrinol. 2019, 10, 523. [Google Scholar] [CrossRef]
- Gasbarri, A.; Martegani, M.P.; Del Prete, F.; Lucante, T.; Natali, P.G.; Bartolazzi, A. Galectin-3 and CD44v6 Isoforms in the Preoperative Evaluation of Thyroid Nodules. JCO 1999, 17, 3494–3502. [Google Scholar] [CrossRef]
- Bartolazzi, A.; Gasbarri, A.; Papotti, M.; Bussolati, G.; Lucante, T.; Khan, A.; Inohara, H.; Marandino, F.; Orlandi, F.; Nardi, F.; et al. Application of an Immunodiagnostic Method for Improving Preoperative Diagnosis of Nodular Thyroid Lesions. Lancet 2001, 357, 1644–1650. [Google Scholar] [CrossRef]
- Tastekin, E.; Keskin, E.; Can, N.; Canberk, S.; Mut, A.; Erdogan, E.; Asa, N.; Güldiken, S.; Sezer, A.; Azatcam, M. CD56, CD57, HBME1, CK19, Galectin-3 and P63 Immunohistochemical Stains in Differentiating Diagnosis of Thyroid Benign/Malign Lesions and NIFTP. Pol. J. Pathol. 2019, 70, 286–294. [Google Scholar] [CrossRef]
- Solmaz, O.A. Diagnostic Importance of CD56 with Fine-Needle Aspiration Cytology in Suspected Papillary Thyroid Carcinoma Cases. CytoJournal 2018, 15, 3. [Google Scholar] [CrossRef]
- Pyo, J.-S.; Kim, D.-H.; Yang, J. Diagnostic Value of CD56 Immunohistochemistry in Thyroid Lesions. Int. J. Biol. Markers 2018, 33, 161–167. [Google Scholar] [CrossRef]
- Khan, A.; Baker, S.P.; Patwardhan, N.A.; Pullman, J.M. CD57 (Leu-7) Expression Is Helpful in Diagnosis of the Follicular Variant of Papillary Thyroid Carcinoma. Virchows Arch. 1998, 432, 427–432. [Google Scholar] [CrossRef]
- Jeong, Y.M.; Cho, H.; Kim, T.-M.; Kim, Y.; Jeon, S.; Bychkov, A.; Jung, C.K. CD73 Overexpression Promotes Progression and Recurrence of Papillary Thyroid Carcinoma. Cancers 2020, 12, 3042. [Google Scholar] [CrossRef] [PubMed]
- Folpe, A.L.; Lloyd, R.V.; Bacchi, C.E.; Rosai, J. Spindle Epithelial Tumor With Thymus-like Differentiation: A Morphologic, Immunohistochemical, and Molecular Genetic Study of 11 Cases. Am. J. Surg. Pathol. 2009, 33, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Aydin, O.; Yildiz, L.; Kefeli, M.; Kandemir, B. CD117 Expression in Normal, Neoplastic, Inflammatory, and Reactive Lesions of the Thyroid. Pathol. Res. Pract. 2008, 204, 359–365. [Google Scholar] [CrossRef]
- Pusztaszeri, M.P.; Sadow, P.M.; Faquin, W.C. CD117: A Novel Ancillary Marker for Papillary Thyroid Carcinoma in Fine-Needle Aspiration Biopsies: CD117 in Papillary Thyroid Carcinoma. Cancer Cytopathol. 2014, 122, 596–603. [Google Scholar] [CrossRef]
- Murakawa, T.; Tsuda, H.; Tanimoto, T.; Tanabe, T.; Kitahara, S.; Matsubara, O. Expression of KIT, EGFR, HER-2 and Tyrosine Phosphorylation in Undifferentiated Thyroid Carcinoma: Implication for a New Therapeutic Approach. Pathol. Int. 2005, 55, 757–765. [Google Scholar] [CrossRef]
- Abrosimov, A.; Saenko, V.; Meirmanov, S.; Nakashima, M.; Rogounovitch, T.; Shkurko, O.; Lushnikov, E.; Mitsutake, N.; Namba, H.; Yamashita, S. The Cytoplasmic Expression of MUC1 in Papillary Thyroid Carcinoma of Different Histological Variants and Its Correlation with Cyclin D1 Overexpression. Endocr. Pathol. 2007, 18, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Morari, E.C.; Silva, J.R.; Guilhen, A.C.T.; Cunha, L.L.; Marcello, M.A.; Soares, F.A.; Vassallo, J.; Ward, L.S. Muc-1 Expression May Help Characterize Thyroid Nodules but Does Not Predict Patients’ Outcome. Endocr. Pathol. 2010, 21, 242–249. [Google Scholar] [CrossRef]
- Renaud, F.; Gnemmi, V.; Devos, P.; Aubert, S.; Crépin, M.; Coppin, L.; Ramdane, N.; Bouchindhomme, B.; d’Herbomez, M.; Van Seuningen, I.; et al. MUC1 Expression in Papillary Thyroid Carcinoma Is Associated with BRAF Mutation and Lymph Node Metastasis; the Latter Is the Most Important Risk Factor of Relapse. Thyroid 2014, 24, 1375–1384. [Google Scholar] [CrossRef]
- Liu, H.; Moy, P.; Kim, S.; Xia, Y.; Rajasekaran, A.; Navarro, V.; Knudsen, B.; Bander, N.H. Monoclonal Antibodies to the Extracellular Domain of Prostate-Specific Membrane Antigen Also React with Tumor Vascular Endothelium. Cancer Res. 1997, 57, 3629–3634. [Google Scholar]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-Specific Membrane Antigen Expression in Normal and Malignant Human Tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Bychkov, A.; Vutrapongwatana, U.; Tepmongkol, S.; Keelawat, S. PSMA Expression by Microvasculature of Thyroid Tumors—Potential Implications for PSMA Theranostics. Sci. Rep. 2017, 7, 5202. [Google Scholar] [CrossRef]
- Heitkötter, B.; Steinestel, K.; Trautmann, M.; Grünewald, I.; Barth, P.; Gevensleben, H.; Bögemann, M.; Wardelmann, E.; Hartmann, W.; Rahbar, K.; et al. Neovascular PSMA Expression Is a Common Feature in Malignant Neoplasms of the Thyroid. Oncotarget 2018, 9, 9867–9874. [Google Scholar] [CrossRef]
- Sollini, M.; di Tommaso, L.; Kirienko, M.; Piombo, C.; Erreni, M.; Lania, A.G.; Erba, P.A.; Antunovic, L.; Chiti, A. PSMA Expression Level Predicts Differentiated Thyroid Cancer Aggressiveness and Patient Outcome. EJNMMI Res. 2019, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Ciappuccini, R.; Saguet-Rysanek, V.; Giffard, F.; Licaj, I.; Dorbeau, M.; Clarisse, B.; Poulain, L.; Bardet, S. PSMA Expression in Differentiated Thyroid Cancer: Association with Radioiodine, 18FDG Uptake, and Patient Outcome. J. Clin. Endocrinol. Metab. 2021, 106, 3536–3545. [Google Scholar] [CrossRef]
- Genutis, L.K.; Tomsic, J.; Bundschuh, R.A.; Brock, P.L.; Williams, M.D.; Roychowdhury, S.; Reeser, J.W.; Frankel, W.L.; Alsomali, M.; Routbort, M.J.; et al. Microsatellite Instability Occurs in a Subset of Follicular Thyroid Cancers. Thyroid 2019, 29, 523–529. [Google Scholar] [CrossRef]
- Migdalska-Sęk, M.; Czarnecka, K.H.; Kusiński, M.; Pastuszak-Lewandoska, D.; Nawrot, E.; Kuzdak, K.; Brzeziańska-Lasota, E. Clinicopathological Significance of Overall Frequency of Allelic Loss (OFAL) in Lesions Derived from Thyroid Follicular Cell. Mol. Diagn. Ther. 2019, 23, 369–382. [Google Scholar] [CrossRef]
- Wong, K.S.; Lorch, J.H.; Alexander, E.K.; Nehs, M.A.; Nowak, J.A.; Hornick, J.L.; Barletta, J.A. Clinicopathologic Features of Mismatch Repair-Deficient Anaplastic Thyroid Carcinomas. Thyroid 2019, 29, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Paulsson, J.O.; Backman, S.; Wang, N.; Stenman, A.; Crona, J.; Thutkawkorapin, J.; Ghaderi, M.; Tham, E.; Stålberg, P.; Zedenius, J.; et al. Whole-Genome Sequencing of Synchronous Thyroid Carcinomas Identifies Aberrant DNA Repair in Thyroid Cancer Dedifferentiation. J. Pathol. 2020, 250, 183–194. [Google Scholar] [CrossRef] [PubMed]
- De la Chapelle, A.; Hampel, H. Clinical Relevance of Microsatellite Instability in Colorectal Cancer. J. Clin. Oncol. 2010, 28, 3380–3387. [Google Scholar] [CrossRef] [PubMed]


| Familial Thyroid Cancer | Germline Mutation | Histology | References |
|---|---|---|---|
| Familial non-MTC | HABP2, SRRM2, FOXE1, DUOX2, SRGAP1, TITF-1/NKX2.1, MNG1, PTCSC3, and CHEK2 | NH, FA, PTC, and FTC | [103,104] |
| Familial PTC with papillary renal cell neoplasia | PRN | PTC | [101,102,105] |
| Familial adenomatous polyposis | APC | PTC-CMV | [101,102,105] |
| Cowden syndrome | PTEN, SDHB-D, PIK3CA, AKT1, KLLN, and SEC23B | PTC-FV, FTC, FA, NH, and C-cell hyperplasia | [52,53,54,101,102,105] |
| Carney complex | PRKAR1 α | PTC, FTC, FA, and NH | [101,102,105] |
| Werner syndrome | WRN | PTC, FTC, and ATC | [101,102,105] |
| McCune–Albright syndrome | GNAS | PTC, FTC, and FA with papillary growth | [102] |
| DICER1 syndrome | DICER1 | NH, PTC, and FTC | [39] |
| MEN and FMTC | RET | MTC | [102,105] |
| Drugs | Thyroid Cancers | Targets |
|---|---|---|
| Multikinase Inhibitors | ||
| Sorafenib | RAI-refractory DTC | VEGFR, PDGFR, and BRAF |
| Lenvatinib | RAI-refractory DTC | VEGFR, FGFR, PDGFR, c-Kit, and RET |
| Vandetanib | MTC | VEGFR2, EGFR, and RET |
| Cabozantinib | MTC | c-MET, RET, VEGFR2, and AXL |
| BRAF kinase inhibitors | ||
| Vemurafenib | BRAF V600E mutated cancer | BRAF V600E and CRAF-1 |
| Dabrafenib | BRAF V600E mutated ATC | BRAF V600E and CRAF |
| MEK inhibitors | ||
| Selumetinib | RAI-refractory DTC | MEK1 and MEK2 |
| Trametinib combined with dabrafenib | ATC | MEK1 and MEK2 |
| NTRK inhibitors | ||
| Larotrectinib and entrectinib | NTRK fusion-positive cancer | TrkA, TrkB, and TrkC |
| RET kinase inhibitors | ||
| Selpercatinib (LOXO-292) | RET mutation or fusion-positive cancer | RET, RET mutants V804M, and G810R |
| Pralsetinib (BLU-667) | Advanced or metastatic RET-mutant MTC and RET-fusion-positive thyroid cancer | RET, RET mutants V804L, V804M, M918T, and CCDC6-RET fusion |
| Molecular Alteration | Target Protein (Clone) | Tumor Type | Utility |
|---|---|---|---|
| BRAF V600E | BRAF V600E (clone VE1) | Subset of PTC, PDTC, and ATC | Diagnostic, prognostic, and predictive |
| APC (germline or somatic) or CTNNB1 | β-catenin | Cribriform-morular PTC and PTC with fibromatosis/ fasciitis-like stroma | Diagnostic |
| RAS mutations | Pan-RAS Q61R (clone SP174), including NRAS Q61R, KRAS Q61R, and HRAS Q61R | FA, OA, FTC, OCA, NIFTP, subset of PTC, hyperplastic nodules, and MTC | Diagnostic |
| PTEN inactivation | PTEN | PTEN hamartoma tumor syndrome, FA, FTC, follicular variant of PTC, NIFTP, hyperplastic nodules, PDTC, ATC, OA, and OCA | Diagnostic |
| NTRK rearrangements | Pan-TRK | PTC and secretory carcinoma | Diagnostic and predictive |
| ALK rearrangement | ALK (clones 5A4 and D5F3) | PTC, PDTC, ATC, and MTC | Diagnostic and predictive |
| CD Marker | Gene Symbol | Gene Name | Alias Gene Symbols | Normal Thyroid | Benign Nodules | NIFTP | Malignancy | Prognostic Factor | References |
|---|---|---|---|---|---|---|---|---|---|
| CD5 | CD5 | CD5 molecule | LEU1 and T1 | 0% | 0% | 0% | ITC (100%) | n/d | [191] |
| CD10 | MME | Membrane metalloendopeptidase | CALLA, CD10, and NEP | 0% | 0–22% | n/d | PTC (30–47%, F), FTC (27%, F) ATC (96%, D), and MTC (0%) | n/s | [192,193,194] |
| CD15 | FUT4 | Fucosyltransferase 4 | CD15, FCT3A, ELFT, and FUC-TIV | 0% | 0–10% | n/d | PTC (57–85%), FTC (4–40%), MTC (20%), and ATC (0%) | Excellent therapeutic outcomes to RAI in PTC | [192,195,196,197] |
| CD20 | MS4A1 | Membrane spanning 4-domains A1 | CD20, B1, Bp35, and MS4A2 | 0% | 0% | n/d | PTC (8–23%), PDTC (13%), ATC (0%), and MTC (0%) | n/s | [198,199] |
| CD30 | TNFRSF8 | TNF receptor superfamily member 8 | CD30, D1S166E, and KI-1 | 0% | <40% | n/d | PTC (67%), FTC (7%), ATC (33%), and MTC (67%) | n/d | [200] |
| CD44 | CD44 | CD44 molecule (Indian blood group) | MIC4, MDU2, MDU3, IN, MC56, Pgp1, CD44R, HCELL, and CSPG8 | 0% | n/d | n/d | PTC (80%) | Shorter PFS in PTC | [201] |
| CD44v6 | 0% | 30–40% | n/d | PTC (70–97%) FTC (80–90%), PDTC (55%), ATC (40–75%), and MTC (14%) | n/d | [202,203] | |||
| CD56 | NCAM1 | Neural cell adhesion molecule 1 | NCAM and CD56 | 100% | >90% | 10–100% | PTC (<20%) and FTC (20–90%) | n/s | [204,205,206] |
| CD57 | B3GAT1 | Beta-1,3-glucuronyltransferase 1 | CD57, LEU7, GlcAT-P, HNK-1, and NK-1 | 0% | 10–20% | 85% | PTC (>90%), FTC (>90%) | n/d | [197,204,207] |
| CD73 | NT5E | 5′-nucleotidase ecto | NT5, CD73, eN, eNT, and CALJA | 0% | n/d | n/d | PTC (72%) | Shorter RFS in PTC | [208] |
| CD99 | CD99 | CD99 molecule (Xg blood group) | MIC2 | 0% | 0% | 0% | SETTLE (75%) | n/d | [191,209] |
| CD117 | KIT | KIT proto-oncogene and receptor tyrosine kinase | PBT, CD117, SCFR, and C-Kit | 8–100% | 8–100% | n/d | PTC (0–71%), FTC (47%), ATC (40%), ITC (100%), and SETTLE (75%) | n/s | [191,209,210,211,212] |
| CD166 | ALCAM | Activated leukocyte cell adhesion molecule | CD166 and MEMD | 0% | n/d | n/d | PTC (12%) | Shorter PFS in PTC | [201] |
| CD227 | MUC1 | Mucin 1, cell surface associated | PUM, MCKD1, CD227, PEM, ADMCKD, ADMCKD1, MCKD, and MCD | 6% | 21–30% | n/d | PTC (49–80%), FTC (49%) | Adverse prognosis in PTC (conflicting data) | [213,214,215] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarwal, S.; Bychkov, A.; Jung, C.-K. Emerging Biomarkers in Thyroid Practice and Research. Cancers 2022, 14, 204. https://doi.org/10.3390/cancers14010204
Agarwal S, Bychkov A, Jung C-K. Emerging Biomarkers in Thyroid Practice and Research. Cancers. 2022; 14(1):204. https://doi.org/10.3390/cancers14010204
Chicago/Turabian StyleAgarwal, Shipra, Andrey Bychkov, and Chan-Kwon Jung. 2022. "Emerging Biomarkers in Thyroid Practice and Research" Cancers 14, no. 1: 204. https://doi.org/10.3390/cancers14010204
APA StyleAgarwal, S., Bychkov, A., & Jung, C.-K. (2022). Emerging Biomarkers in Thyroid Practice and Research. Cancers, 14(1), 204. https://doi.org/10.3390/cancers14010204