Neoadjuvant Treatment Lowers the Risk of Mesopancreatic Fat Infiltration and Local Recurrence in Patients with Pancreatic Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Demographic Data
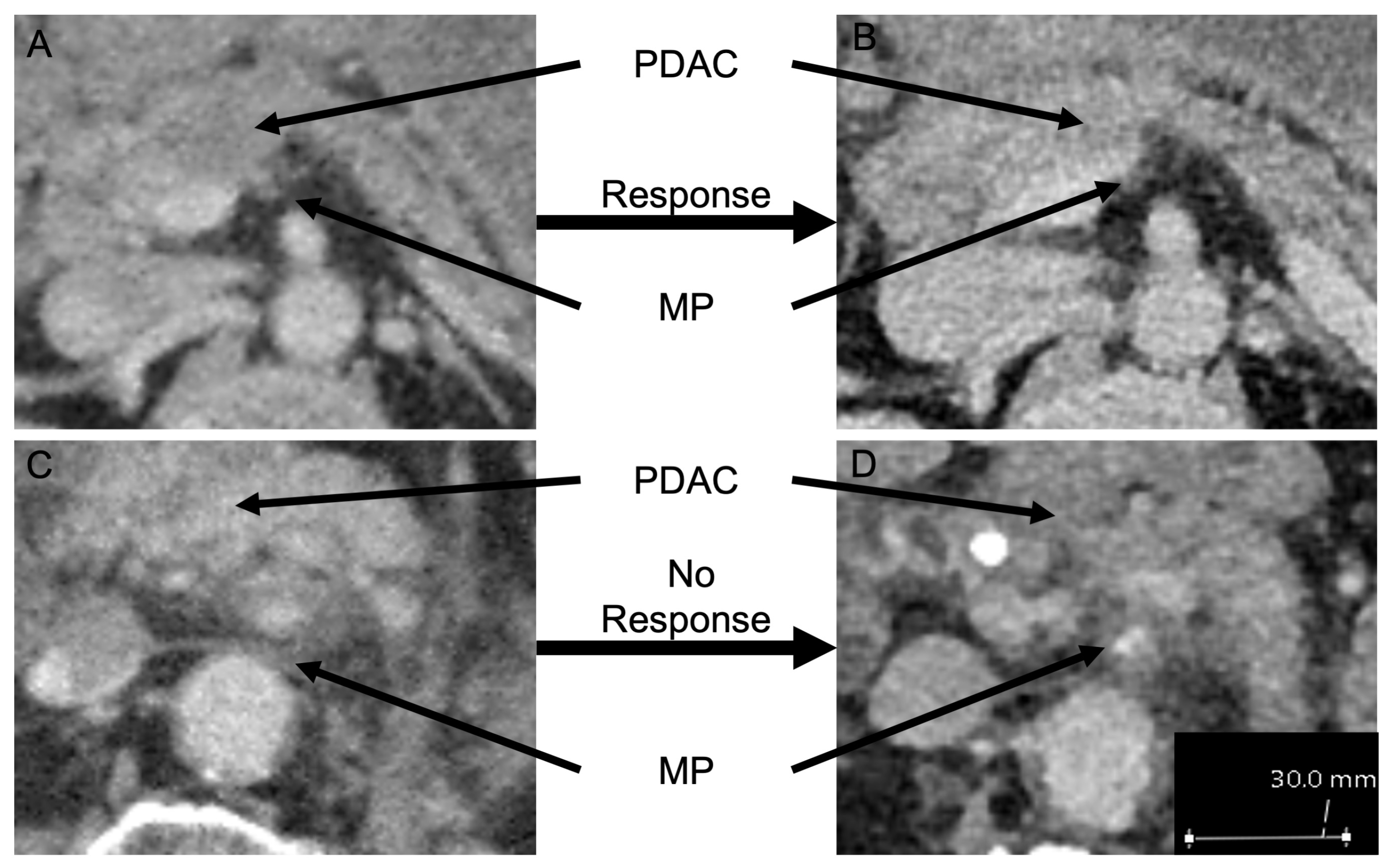
2.2. Radiographic Imaging
2.3. Operative Procedure
2.4. Pathological Analysis
2.5. Postoperative Follow-Up
2.6. Statistics
3. Results
3.1. Demographic Data
3.2. Histopathological Results
3.3. Influence of Clinicopathological Variables
3.4. Mesopancreatic Fat Infiltration in Patients with and without Neoadjuvant Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kenner, B.J.; Chari, S.T.; Maitra, A.; Srivastava, S.; Cleeter, D.F.; Go, V.L.W.; Rothschild, L.J.; Goldberg, A.E. Early detection of pancreatic cancer: A defined future using lessons from other cancers: A white paper. Pancreas 2016, 45, 1073–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kausch, W. Das Carcinom der Papilla duodeni und seine radikale Entfernung. Beitr Klin Chir. 1912, 78, 439–486. [Google Scholar]
- Whipple, A.O.; Parsons, W.B.; Mullins, C.R. Treatment of carcinoma of the ampulla of vater. Ann. Surg. 1935, 102, 763–779. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; OReilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Sinn, M.; Bahra, M.; Liersch, T.; Gellert, K.; Messmann, H.; Bechstein, W.; Waldschmidt, D.; Jacobasch, L.; Wilhelm, M.; Rau, B.M.; et al. CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3330–3337. [Google Scholar] [CrossRef]
- Burris, H.A., 3rd; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef] [Green Version]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Chone, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Jones, R.P.; Psarelli, E.E.; Jackson, R.; Ghaneh, P.; Halloran, C.M.; Palmer, D.H.; Campbell, F.; Valle, J.W.; Faluyi, O.; O’Reilly, D.A.; et al. Patterns of Recurrence After Resection of Pancreatic Ductal Adenocarcinoma: A Secondary Analysis of the ESPAC-4 Randomized Adjuvant Chemotherapy Trial. JAMA Surg 2019, 154, 1038–1048. [Google Scholar] [CrossRef]
- Wagner, M.; Redaelli, C.; Lietz, M.; Seiler, C.A.; Friess, H.; Büchler, M.W. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br. J. Surg. 2004, 91, 586–594. [Google Scholar] [CrossRef]
- Kayahara, M.; Nagakawa, T.; Ueno, K.; Ohta, T.; Takeda, T.; Miyazaki, I. An evaluation of radical resection for pancreatic cancer based on the mode of recurrence as determined by autopsy and diagnostic imaging. Cancer 1993, 72, 2118–2123. [Google Scholar] [CrossRef]
- Hishinuma, S.; Ogata, Y.; Tomikawa, M.; Ozawa, I.; Hirabayashi, K.; Igarashi, S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J. Gastrointest. Surg. 2006, 10, 511–518. [Google Scholar] [CrossRef]
- Verbeke, C.S.; Leitch, D.; Menon, K.V.; McMahon, M.J.; Guillou, P.J.; Anthoney, A. Redefining the R1 resection in pancreatic cancer. Br. J. Surg. 2006, 93, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Esposito, I. Most pancreatic cancer resections are R1 resections. Ann. Surg. Oncol. 2008, 15, 1651–1660. [Google Scholar] [CrossRef]
- Schlitter, E. Definition of Microscopic Tumor Clearance (R0) in Pancreatic Cancer Resections. Cancers 2010, 2, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Häberle, L.; Esposito, I. Circumferential resection margin (CRM) in pancreatic cancer. Surg. Pract. Sci. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Safi, S.A.; Haeberle, L.; Fluegen, G.; Lehwald-Tywuschik, N.; Krieg, A.; Keitel, V.; Luedde, T.; Esposito, I.; Rehders, A.; Knoefel, W.T. Mesopancreatic excision for pancreatic ductal adenocarcinoma improves local disease control and survival. Pancreatol. Off. J. Int. Assoc. Pancreatol. (IAP) 2021, 21, 787–795. [Google Scholar] [CrossRef]
- Heald, R.J.; Ryall, R.D. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986, 1, 1479–1482. [Google Scholar] [CrossRef]
- Hohenberger, W.; Weber, K.; Matzel, K.; Papadopoulos, T.; Merkel, S. Standardized surgery for colonic cancer: Complete mesocolic excision and central ligation–technical notes and outcome. Colorectal Dis. Off. J. Assoc. Coloproctol. Great Br. Irel. 2009, 11, 354–364; discussion 364–365. [Google Scholar] [CrossRef]
- Inoue, Y.; Saiura, A.; Yoshioka, R.; Ono, Y.; Takahashi, M.; Arita, J.; Takahashi, Y.; Koga, R. Pancreatoduodenectomy With Systematic Mesopancreas Dissection Using a Supracolic Anterior Artery-first Approach. Ann. Surg. 2015, 262, 1092–1101. [Google Scholar] [CrossRef]
- Shimizu, A.; Hirono, S.; Tani, M.; Kawai, M.; Okada, K.; Miyazawa, M.; Kitahata, Y.; Nakamura, Y.; Noda, T.; Yokoyama, S.; et al. Coexpression of MUC16 and mesothelin is related to the invasion process in pancreatic ductal adenocarcinoma. Cancer Sci. 2012, 103, 739–746. [Google Scholar] [CrossRef]
- Gerashchenko, T.S.; Novikov, N.M.; Krakhmal, N.V.; Zolotaryova, S.Y.; Zavyalova, M.V.; Cherdyntseva, N.V.; Denisov, E.V.; Perelmuter, V.M. Markers of Cancer Cell Invasion: Are They Good Enough? J. Clin. Med. 2019, 8, 1092. [Google Scholar] [CrossRef] [Green Version]
- Safi, S.A.; Haeberle, L.; Heuveldop, S.; Kroepil, P.; Fung, S.; Rehders, A.; Keitel, V.; Luedde, T.; Fuerst, G.; Esposito, I.; et al. Pre-Operative MDCT Staging Predicts Mesopancreatic Fat Infiltration—A Novel Marker for Neoadjuvant Treatment? Cancers 2021, 13, 4361. [Google Scholar] [CrossRef]
- Werner, J.; Combs, S.E.; Springfeld, C.; Hartwig, W.; Hackert, T.; Buchler, M.W. Advanced-stage pancreatic cancer: Therapy options. Nat. Rev. Clin. Oncol. 2013, 10, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Bockhorn, M.; Uzunoglu, F.G.; Adham, M.; Imrie, C.; Milicevic, M.; Sandberg, A.A.; Asbun, H.J.; Bassi, C.; Büchler, M.; Charnley, R.M.; et al. Borderline resectable pancreatic cancer: A consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014, 155, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Schneitler, S.; Kröpil, P.; Riemer, J.; Antoch, G.; Knoefel, W.T.; Häussinger, D.; Graf, D. Metastasized pancreatic carcinoma with neoadjuvant FOLFIRINOX therapy and R0 resection. World J. Gastroenterol. 2015, 21, 6384–6390. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, J.M.; Heh, V.; Pawlik, T.M.; Ejaz, A.; Dillhoff, M.; Tsung, A.; Williams, T.; Abushahin, L.; Bridges, J.F.P.; Santry, H. Neoadjuvant Therapy for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2020, 9, 1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhir, M.; Malhotra, G.K.; Sohal, D.P.S.; Hein, N.A.; Smith, L.M.; O’Reilly, E.M.; Bahary, N.; Are, C. Neoadjuvant treatment of pancreatic adenocarcinoma: A systematic review and meta-analysis of 5520 patients. World J. Surg. Oncol. 2017, 15, 183. [Google Scholar] [CrossRef] [Green Version]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouche, O.; Guimbaud, R.; Becouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardiere, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Lee, J.-C.; Yang, S.Y.; Kim, J.; Hwang, J.-H. Neoadjuvant therapy versus upfront surgery in resectable pancreatic cancer according to intention-to-treat and per-protocol analysis: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 15662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motoi, F.; Kosuge, T.; Ueno, H.; Yamaue, H.; Satoi, S.; Sho, M.; Honda, G.; Matsumoto, I.; Wada, K.; Furuse, J.; et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn. J. Clin. Oncol. 2019, 49, 190–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cong, L.; Liu, Q.; Zhang, R.; Cui, M.; Zhang, X.; Gao, X.; Guo, J.; Dai, M.; Zhang, T.; Liao, Q.; et al. Tumor size classification of the 8(th) edition of TNM staging system is superior to that of the 7(th) edition in predicting the survival outcome of pancreatic cancer patients after radical resection and adjuvant chemotherapy. Sci. Rep. 2018, 8, 10383. [Google Scholar] [CrossRef] [PubMed]
- Seufferlein, T.; Porzner, M.; Becker, T.; Budach, V.; Ceyhan, G.; Esposito, I.; Fietkau, R.; Follmann, M.; Friess, H.; Galle, P.; et al. S3-guideline exocrine pancreatic cancer. Z. Gastroenterol. 2013, 51, 1395–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbeke, C.; Häberle, L.; Lenggenhager, D.; Esposito, I. Pathology assessment of pancreatic cancer following neoadjuvant treatment: Time to move on. Pancreatol. Off. J. Int. Assoc. Pancreatol. (IAP) 2018, 18, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, Y.; Tanaka, T.; Nishi, T.; Monma, H.; Yano, S.; Tajima, Y. Appraisal of a total meso-pancreatoduodenum excision with pancreaticoduodenectomy for pancreatic head carcinoma. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2012, 38, 574–579. [Google Scholar] [CrossRef]
- Aimoto, T.; Mizutani, S.; Kawano, Y.; Matsushita, A.; Yamashita, N.; Suzuki, H.; Uchida, E. Left posterior approach pancreaticoduodenectomy with total mesopancreas excision and circumferential lymphadenectomy around the superior mesenteric artery for pancreatic head carcinoma. J. Nippon. Med Sch./Nippon. Ika Daigaku Zasshi 2013, 80, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Bertelsen, C.A.; Neuenschwander, A.U.; Jansen, J.E.; Tenma, J.R.; Wilhelmsen, M.; Kirkegaard-Klitbo, A.; Iversen, E.R.; Bols, B.; Ingeholm, P.; Rasmussen, L.A.; et al. 5-year outcome after complete mesocolic excision for right-sided colon cancer: A population-based cohort study. Lancet Oncol. 2019, 20, 1556–1565. [Google Scholar] [CrossRef]
- Bokey, L.; Chapuis, P.H.; Chan, C.; Stewart, P.; Rickard, M.J.; Keshava, A.; Dent, O.F. Long-term results following an anatomically based surgical technique for resection of colon cancer: A comparison with results from complete mesocolic excision. Colorectal Dis. Off. J. Assoc. Coloproctol. Great Br. Irel. 2016, 18, 676–683. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.F.; Bagante, F.; Chakedis, J.; Moris, D.; Beal, E.W.; Weiss, M.; Popescu, I.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; et al. Perioperative and Long-Term Outcome for Intrahepatic Cholangiocarcinoma: Impact of Major Versus Minor Hepatectomy. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2017, 21, 1841–1850. [Google Scholar] [CrossRef]
- Glimelius, B.; Tiret, E.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24 (Suppl. S6), vi81–vi88. [Google Scholar] [CrossRef]
- Hölscher, A.H.; Gockel, I.; Porschen, R. Updated German S3 guidelines on esophageal cancer and supplements from a surgical perspective. Der Chir. Z. Fur Alle Geb. Der Oper. Medizen 2019, 90, 398–402. [Google Scholar] [CrossRef]
- Da Costa, W.L., Jr.; Tran Cao, H.S.; Sheetz, K.H.; Gu, X.; Norton, E.C.; Massarweh, N.N. Comparative Effectiveness of Neoadjuvant Therapy and Upfront Resection for Patients with Resectable Pancreatic Adenocarcinoma: An Instrumental Variable Analysis. Ann. Surg. Oncol. 2021, 28, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Rieser, C.J.; Narayanan, S.; Bahary, N.; Bartlett, D.L.; Lee, K.K.; Paniccia, A.; Smith, K.; Zureikat, A.H. Optimal management of patients with operable pancreatic head cancer: A Markov decision analysis. J. Surg. Oncol. 2021, 124, 801–809. [Google Scholar] [CrossRef]
- Yoo, C.; Shin, S.H.; Kim, K.P.; Jeong, J.H.; Chang, H.M.; Kang, J.H.; Lee, S.S.; Park, D.H.; Song, T.J.; Seo, D.W.; et al. Clinical Outcomes of Conversion Surgery after Neoadjuvant Chemotherapy in Patients with Borderline Resectable and Locally Advanced Unresectable Pancreatic Cancer: A Single-Center, Retrospective Analysis. Cancers 2019, 11, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, Q.P.; van Dam, J.L.; Bonsing, B.A.; Bos, H.; Bosscha, K.P.; Coene, P.; van Eijck, C.H.J.; de Hingh, I.; Karsten, T.M.; van der Kolk, M.B.; et al. Total neoadjuvant FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for resectable and borderline resectable pancreatic cancer (PREOPANC-2 trial): Study protocol for a nationwide multicenter randomized controlled trial. BMC Cancer 2021, 21, 300. [Google Scholar] [CrossRef]
- Safi, S.A.; Rehders, A.; Haeberle, L.; Fung, S.; Lehwald, N.; Esposito, I.; Ziayee, F.; Krieg, A.; Knoefel, W.T.; Fluegen, G. Para-aortic lymph nodes and ductal adenocarcinoma of the pancreas: Distant neighbors? Surgery 2021, 170, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Strobel, O.; Hank, T.; Hinz, U.; Bergmann, F.; Schneider, L.; Springfeld, C.; Jäger, D.; Schirmacher, P.; Hackert, T.; Büchler, M.W. Pancreatic Cancer Surgery: The New R-status Counts. Ann. Surg. 2017, 265, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A. Contemporary trials evaluating neoadjuvant therapy for resectable pancreatic cancer. J. Surg. Oncol. 2021, 123, 1423–1431. [Google Scholar] [CrossRef]
- Rutter, C.E.; Park, H.S.; Corso, C.D.; Lester-Coll, N.H.; Mancini, B.R.; Yeboa, D.N.; Johung, K.L. Addition of radiotherapy to adjuvant chemotherapy is associated with improved overall survival in resected pancreatic adenocarcinoma: An analysis of the National Cancer Data Base. Cancer 2015, 121, 4141–4149. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, D.; Chen, Y.; Li, X.; Cao, P.; Cao, D. Network meta-analysis comparing neoadjuvant chemoradiation, neoadjuvant chemotherapy and upfront surgery in patients with resectable, borderline resectable, and locally advanced pancreatic ductal adenocarcinoma. Radiat. Oncol. 2019, 14, 120. [Google Scholar] [CrossRef]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.M.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.; Koch, M.; Debus, J.; Hohler, T.; Galle, P.R.; Buchler, M.W. Colorectal cancer. Lancet 2005, 365, 153–165. [Google Scholar] [CrossRef]
- Vogl, T.J.; Pereira, P.L.; Helmberger, T.; Schreyer, A.G.; Schmiegel, W.; Fischer, S.; Herzog, C. Updated S3 Guidelines—Diagnosis and Treatment of Colorectal Carcinoma: Relevance for Radiological Diagnosis and Intervention. RoFo Fortschr. Dem Geb. Rontgenstrahlen Nukl. 2019, 191, 298–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Age in Years | ||
|---|---|---|
| Median (Range) | 66 Years (41–80) | |
| Sex | n | % |
| Male | 16 | 59.3 |
| Female | 11 | 40.7 |
| T-stage | ||
| ypT0 | 2 | 7.4 |
| ypT1 | 3 | 11.1 |
| ypT2 | 13 | 48.1 |
| ypT3 | 9 | 33.3 |
| N-stage | ||
| N0 | 11 | 40.7 |
| N1 | 10 | 37 |
| N2 | 6 | 22.2 |
| Grading | ||
| G1/G2 | 18 | 66.6 |
| G3 | 9 | 33.3 |
| Pn | ||
| Pn0 | 9 | 33.3 |
| Pn1 | 18 | 66.6 |
| L | ||
| L0 | 20 | 74.1 |
| L1 | 7 | 25.9 |
| V | ||
| V0 | 21 | 77.8 |
| V1 | 6 | 22.2 |
| R-status | ||
| R0(CRM−) | 17 | 62.9 |
| R1/R0(CRM+) | 10 | 37.1 |
| MPI | ||
| Positive | 17 | 62.9 |
| negative | 10 | 37.1 |
| No Mesopancreatic Fat Infiltration | Mesopancreatic Fat Infiltration | p-Value | |||
|---|---|---|---|---|---|
| n = 10 | n = 17 | ||||
| Treatment response | n | % | n | % | 0.003 ** |
| CAP 0 | 2 | 20 | 0 | 0 | |
| CAP 1 | 4 | 40 | 1 | 5.9 | |
| CAP 2 | 4 | 40 | 11 | 64.7 | |
| CAP 3 | 0 | 0 | 5 | 29.4 | |
| R-status | 0.031 * | ||||
| R0(CRM−) | 8 | 80 | 9 | 52.9 | |
| R1/R0(CRM+) | 2 | 20 | 8 | 47.1 | |
| R0(CRM−) | R1/R0(CRM+) | p-Value | |||
|---|---|---|---|---|---|
| n = 17 | n = 10 | ||||
| Treatment Response | n | % | n | % | 0.042 ** |
| CAP 0 | 2 | 11.8 | 0 | 0 | |
| CAP 1 | 5 | 29.4 | 0 | 0 | |
| CAP 2 | 9 | 52.9 | 6 | 60 | |
| CAP 3 | 1 | 5.8 | 4 | 40 | |
| MDCT Tumor Response | MDCT No Tumor Response | p-Value | |||
|---|---|---|---|---|---|
| n = 17 | n = 10 | ||||
| Treatment response | n | % | n | % | 0.122 |
| CAP 0 and 1 | 6 | 35.3 | 1 | 10 | |
| CAP 2 and 3 | 11 | 64.7 | 9 | 90 | |
| MP Infiltration | * 0.042 | ||||
| positive | 8 | 47.1 | 9 | 90 | |
| negative | 9 | 52.9 | 1 | 10 | |
| R-status | 0.692 | ||||
| R0(CRM−) | 11 | 64.7 | 6 | 60 | |
| R1/R0(CRM+) | 6 | 35.3 | 4 | 40 | |
| MP Status | Neoadjuvant and Surgery | Upfront Surgery | p-Value | ||
|---|---|---|---|---|---|
| n = 27 | n = 173 | ||||
| MP Infiltration | 0.039 | ||||
| positive | 17 | 62.9 | 131 | 75.7 | |
| negative | 10 | 37.1 | 42 | 24.3 | |
| Therapy Modality | No Metastases | Systemic Relapse | Local Recurrence | p-Value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Neoadjuvant | 5 | 18.5 | 18 | 66.7 | 2 | 7.4 | 0.04 |
| n = 27 | |||||||
| Upfront surgery | 63 | 36.4 | 81 | 46.8 | 29 | 16.8 | |
| n = 173 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safi, S.-A.; Haeberle, L.; Rehders, A.; Fung, S.; Vaghiri, S.; Roderburg, C.; Luedde, T.; Ziayee, F.; Esposito, I.; Fluegen, G.; et al. Neoadjuvant Treatment Lowers the Risk of Mesopancreatic Fat Infiltration and Local Recurrence in Patients with Pancreatic Cancer. Cancers 2022, 14, 68. https://doi.org/10.3390/cancers14010068
Safi S-A, Haeberle L, Rehders A, Fung S, Vaghiri S, Roderburg C, Luedde T, Ziayee F, Esposito I, Fluegen G, et al. Neoadjuvant Treatment Lowers the Risk of Mesopancreatic Fat Infiltration and Local Recurrence in Patients with Pancreatic Cancer. Cancers. 2022; 14(1):68. https://doi.org/10.3390/cancers14010068
Chicago/Turabian StyleSafi, Sami-Alexander, Lena Haeberle, Alexander Rehders, Stephen Fung, Sascha Vaghiri, Christoph Roderburg, Tom Luedde, Farid Ziayee, Irene Esposito, Georg Fluegen, and et al. 2022. "Neoadjuvant Treatment Lowers the Risk of Mesopancreatic Fat Infiltration and Local Recurrence in Patients with Pancreatic Cancer" Cancers 14, no. 1: 68. https://doi.org/10.3390/cancers14010068
APA StyleSafi, S. -A., Haeberle, L., Rehders, A., Fung, S., Vaghiri, S., Roderburg, C., Luedde, T., Ziayee, F., Esposito, I., Fluegen, G., & Knoefel, W. T. (2022). Neoadjuvant Treatment Lowers the Risk of Mesopancreatic Fat Infiltration and Local Recurrence in Patients with Pancreatic Cancer. Cancers, 14(1), 68. https://doi.org/10.3390/cancers14010068








