Simple Summary
Clinical management of advanced stages of primary and secondary liver tumors remains challenging. Combining different treatment approaches to create the most effective therapy for patients is, however, often necessary. With this study we aim to analyze the efficacy and safety of a combined intrahepatic treatment of transarterial radioembolization and CT-guided high-dose-rate interstitial brachytherapy. Our study showed that patients not responding to systemic chemotherapy or suffering from tumor relapse after surgical resection might benefit from a combined minimal-invasive treatment.
Abstract
Purpose: Treatment of patients with primary and secondary liver tumors remains challenging. This study analyzes the efficacy and safety of transarterial radioembolization (TARE) combined with CT-guided high-dose-rate interstitial brachytherapy (CT-HDRBT) for the treatment of primary and secondary liver tumors. Patients and Methods: A total of 77 patients (30 female) with various liver malignancies were treated. Primary endpoints were median overall survival (OS) and time to untreatable progression (TTUP). Additionally, subgroup analyses were performed in consideration of diagnosis and procedure sequence. Median OS and TTUP prediction were estimated using Kaplan–Meier analysis and hazard ratios (HR) were calculated using a multivariate Cox proportional hazard model. Results: A total of 115 CT-HDRBT and 96 TARE procedures were performed with no significant complications recorded. Median OS and TTUP were 29.8 (95% CI 18.1–41.4) and 23.8 (95% CI 9.6–37.9) months. Median OS for hepatocellular carcinoma (HCC)-, cholangiocarcinoma carcinoma (CCA) and colorectal cancer (CRC) patients was 29.8, 29.6 and 34.4 months. Patients starting with TARE had a median OS of 26.0 (95% CI 14.5–37.5) compared to 33.7 (95% CI 21.6–45.8) months for patients starting with CT-HDRBT. Hazard ratio of 1.094 per month was shown for patients starting with CT-HDRBT. Conclusion: Combining TARE and CT-HDRBT is effective and safe for the treatment of advanced stage primary and secondary liver tumors. Our data indicate that early TARE during the disease progression may have a positive effect on survival.
Keywords:
Interventional Radiology; Oncology; SIRT; Ablation; Minimal Invasive; Locoregional therapy 1. Introduction
Besides advancements in oncological therapies, the management of primary and secondary liver malignancies remains challenging. Liver metastases are often fatal, independently of their primary cancer, and the prognosis is poor [1]. Approximately one-third of all oncological patients suffer from metastases at the time of diagnosis, and 50% of patients diagnosed in early-stages subsequently develop metastases in the liver over the course of disease. Although incidences for primary liver cancer have decreased in the last three decades, they still remain high [2]. In 2018, liver cancer was found to be responsible for approximately 780,000 deaths worldwide, accounting for 8% of all cancer-related deaths [3]. For primary malignancies such as hepatocellular carcinoma (HCC) and cholangiocarcinoma carcinoma (CCA), surgical resection remains the therapy of choice but is often impossible due to inaccessibility, number of lesions and tumor distribution [4]. Furthermore, recurrence after resection is common [5,6]. Despite advances in systemic therapies, local treatment approaches using minimally invasive therapies (MIT) have proven to significantly prolong overall survival (OS) in patients with limited metastatic disease to the liver, supporting the concept of oligometastatic disease [7,8].
Transarterial radioembolization (TARE) showed good results of OS for both primary and secondary malignancies of the liver [9,10,11,12]. CT-guided high-dose-rate interstitial brachytherapy (CT-HDRBT) is an ablative technique by which a radioactive source (Iridium 192) is inserted into tumor lesions through catheters which have been implanted in the tumor under CT guidance [13,14]. It is being used by a growing number of centers around the world with excellent treatment results for the treatment of solid primary and secondary tumors [15,16,17,18]. In contrast to radiofrequency ablation (RFA) and microwave ablation (MWA), CT-HDRBT overcomes size limitations and restrictions due to tumor location (e.g., proximity to the liver hilum or vessels).
Both TARE and CT-HDRBT have been combined with a variety of MIT with good results regarding OS and safety [19,20]. Yet no study has evaluated the combination of both treatments. In this study, we evaluated the efficacy and safety of a combined treatment approach of TARE and CT-HDRBT in patients who received at least one TARE and one CT-HDRBT, regardless of diagnosis and pretreatment.
2. Methods
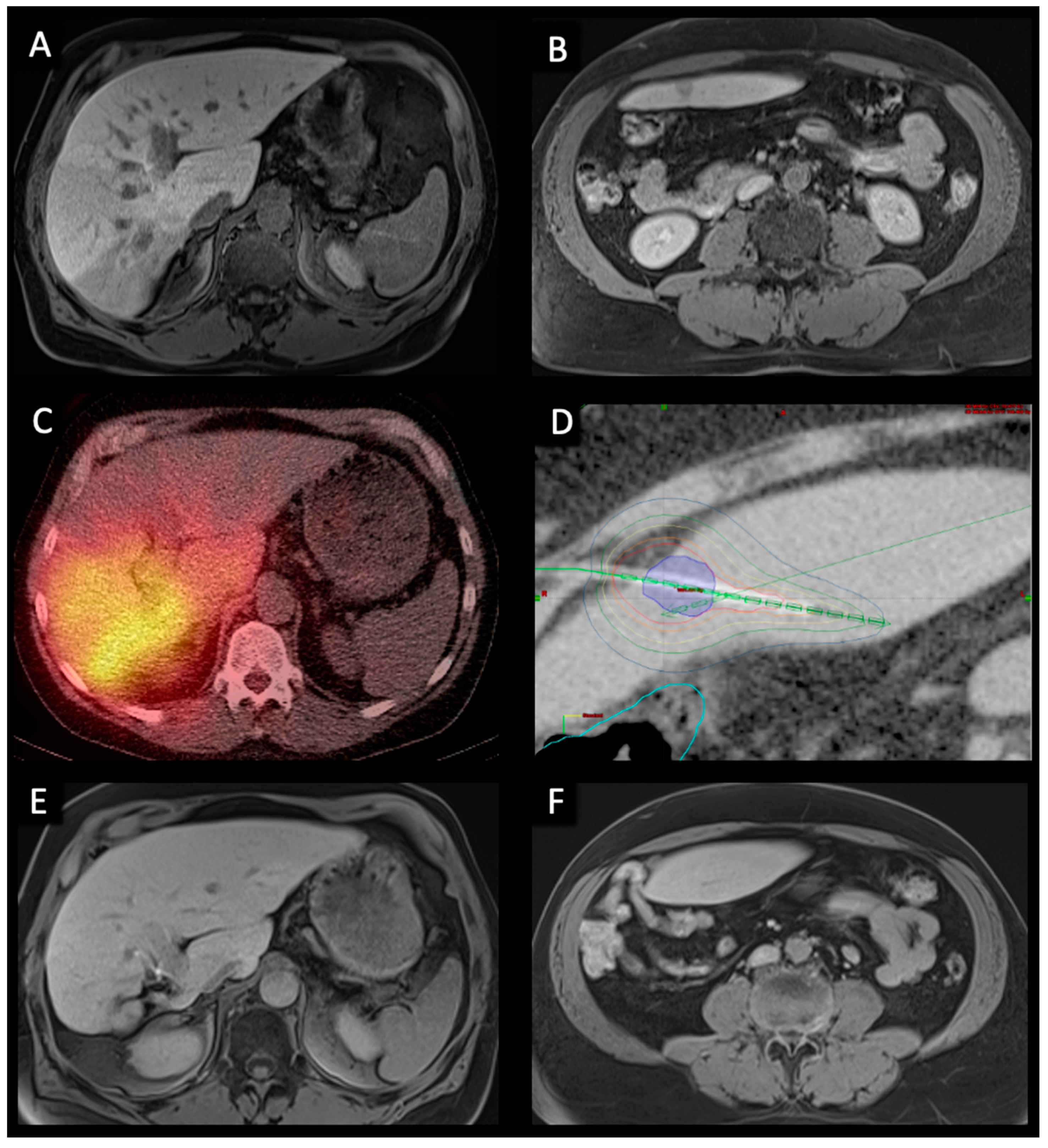
This study was performed in accordance with the standards of the Helsinki Declaration and was approved by the Charité ethical review board (EA4/08917) on 24 May 2017. Between March 2007 and November 2020, a total of 77 patients received at least one TARE and one CT-HDRBT. Written informed consent was obtained from each patient. Before all procedures a contrast enhanced Gd-EOB-DTPA (Primovist, Bayer, Leverkusen, Germany) MRI was acquired (Figure 1A,B). All indications for CT-HDRBT and TARE procedures were confirmed by a multidisciplinary tumor board. Demographics of all patients included are summarized in Table 1.
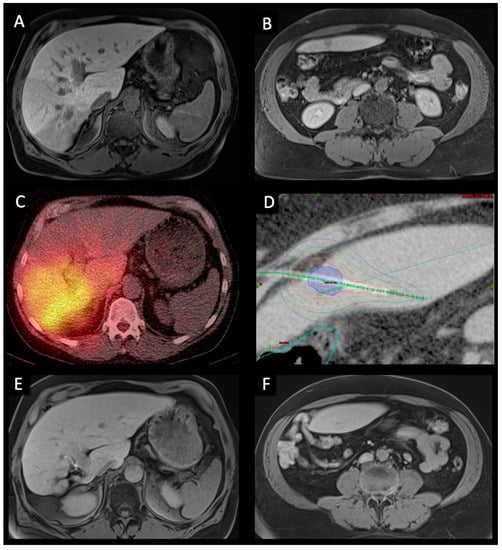
Figure 1.
Pre-, peri-, and post-interventional imaging of a 56-year-old male with bilobar HCC treated with right lobar TARE and left lobar CT-HDRBT at intervals of six weeks. (A,B). Pre-interventional transversal contrast-enhanced MRI in the hepatobiliary excretion phase showing a large infiltrative HCC in segment VI/VII as well as a smaller contralateral metastasis in segment III. (C). Post-TARE PET/CT scan showing good radiopharmaceutical distribution of Y-90 spheres in the right liver. (D). CT-HDRBT peri-interventional 3D-irradiation plan using contrast-enhanced CT after CT-guided positioning of the afterloading catheter. Visible tumor borders were defined as the clinical target volume (CTV) (blue area). Dose distribution was adjusted by 3D-treatment planning. The planned minimal enclosing dose was 20 Gy. Isodose irradiation lines surround the CTV. The colon was marked (light blue line) to minimize collateral radiation. (E,F). 36-months post-interventional transversal contrast-enhanced MRI examination in the hepatobiliary excretion phase showing complete response to treatment as well as hypertrophy of the right liver lobe.

Table 1.
Patient cohort characteristics.
2.1. CT-HDRBT
CT-HDRBT is used in our institution for the treatment of unresectable liver only or dominant tumors or liver metastases. Criteria for performing the procedure are: (1) Liver function Child–Pugh Class A or B, (2) total bilirubin< 2 mg/dL, (3) platelet count >50,000/nL, (4) prothrombin time (PT) > 50%, and (5) partial thromboplastin time (PTT) < 50 s. If necessary, the haemostasis was improved. If present, ascites was drained before treatment to avoid bleeding. Exclusion criteria for CT-HDRBT include (1) any evidence of progressive extrahepatic tumor spread and (2) more than five intrahepatic tumor lesions. Of note, for CT-HDRBT there is no limit regarding the maximum size of a treated lesion [21].
All patients were treated under conscious sedation using midazolam and fentanyl. After local anaesthesia, the tumor lesion was punctured using a 17 G needle under CT-guidance. A flexible 6F angiographic sheath (Radiofocus™, Terumo, Japan) was then introduced into the hepatic target lesion over a stiff guide wire (Amplatz™, Boston Scientific, Boston, MA, USA) using the Seldinger technique. The guide wire was then removed and a closed-ended 6F afterloading catheter (Primed™, Halberstadt Medizintechnik GmbH, Halberstadt, Germany) was inserted through the sheath. Eventually, a CT scan of the liver was acquired to confirm correct catheter positions for three-dimensional radiation planning (Brachyvision, Varian Medical Systems, Palo Alto, CA, USA). Catheters, clinical target volume (CTV), and potential risk structures were plotted semi-automatically. Radiation target dose of the CTV was 20 Gy (Figure 1D). Maximum doses above 50 Gy were allowed in the tumor center. All irradiations were completed as single-fraction in afterloading technique using an Iridium-192 radiation source with a nominal activity of 370 Gbq. After irradiation, all catheters were carefully removed, and the puncture tracts sealed using thrombogenic sponge torpedoes (Gelfoam® absorbable gelatin sponge, USP, Pfizer, New York, NY, USA) to minimize the risk of bleeding [15,22,23].
2.2. TARE
Generally, all liver tumors (both primary and metastatic) are potentially suitable for TARE and are generally considered for treatment if they fall into a subset that are (1) chemotherapy-refractory, (2) too advanced or technically not suitable for ablation and liver surgery. Patients are not treated if they show rapidly progressive extra-hepatic disease with no strategy available for an adequate disease control. At our institution the most common indication is third or subsequent line liver-only or liver-dominant chemotherapy-refractory metastatic colorectal cancer. General exclusion criteria are: (1) life expectancy >12 weeks, (2) ECOG/WHO performance status 0–2, and (3) adequate liver function (i.e., <bilirubin 34 µmol/L, i.e., 2.0 mg/dL).
TARE is a two-step procedure consisting of evaluation and therapy procedure, which has been described in detail previously [24,25,26]. Briefly, TARE evaluation contained an angiographic evaluation of the hepatic vasculature as well as, if needed, coil embolization of the gastroduodenal artery and the right gastric artery to prevent potential extrahepatic deposition of radioactive material. Subsequently, technetium-99 m labelled macroaggregated albumin acting as a surrogate marker was injected in the left and right hepatic artery. Afterwards, a single photon emission CT was performed to identify potentially extrahepatic uptake. Moreover, the CT scan serves as a tool to evaluate lung and gastrointestinal-tract shunt fractions. Approximately two weeks after evaluation, patients were prepared for treatment session. A Gd-EOB-DTPA (Primovist®, Bayer, Leverkusen, Germany) MRI was acquired to quantify liver, as well as tumor, volumes. Required dosage of resin Yttrium-90 (Y-90) microspheres (Sirtex, North Sydney, NSW, Australia) was calculated based on the dosimetric (partition) model [25]. After successful injection of the particles, a Y-90 PET/CT scan was acquired to determine the radiopharmaceutical distribution (Figure 1C).
2.3. Follow Up
Follow-up routine included MRI, clinical visits as well as a multidisciplinary tumor board case discussion. The MRI was obtained 6- and 12-weeks post-procedure before prolonging the interval to 3 months. Six months post-procedure chest imaging was included in the routine biannually. In case of stable disease or remission, this cycle was maintained for 18 months, before reducing MRI scans to biannual appointments (Figure 1E,F). MRI evaluation was performed by two board-certified radiologists in consensus. In case of tumor progression, all therapeutic approaches were performed in accordance with the multidisciplinary tumor board.
2.4. Endpoints and Statistical Analysis
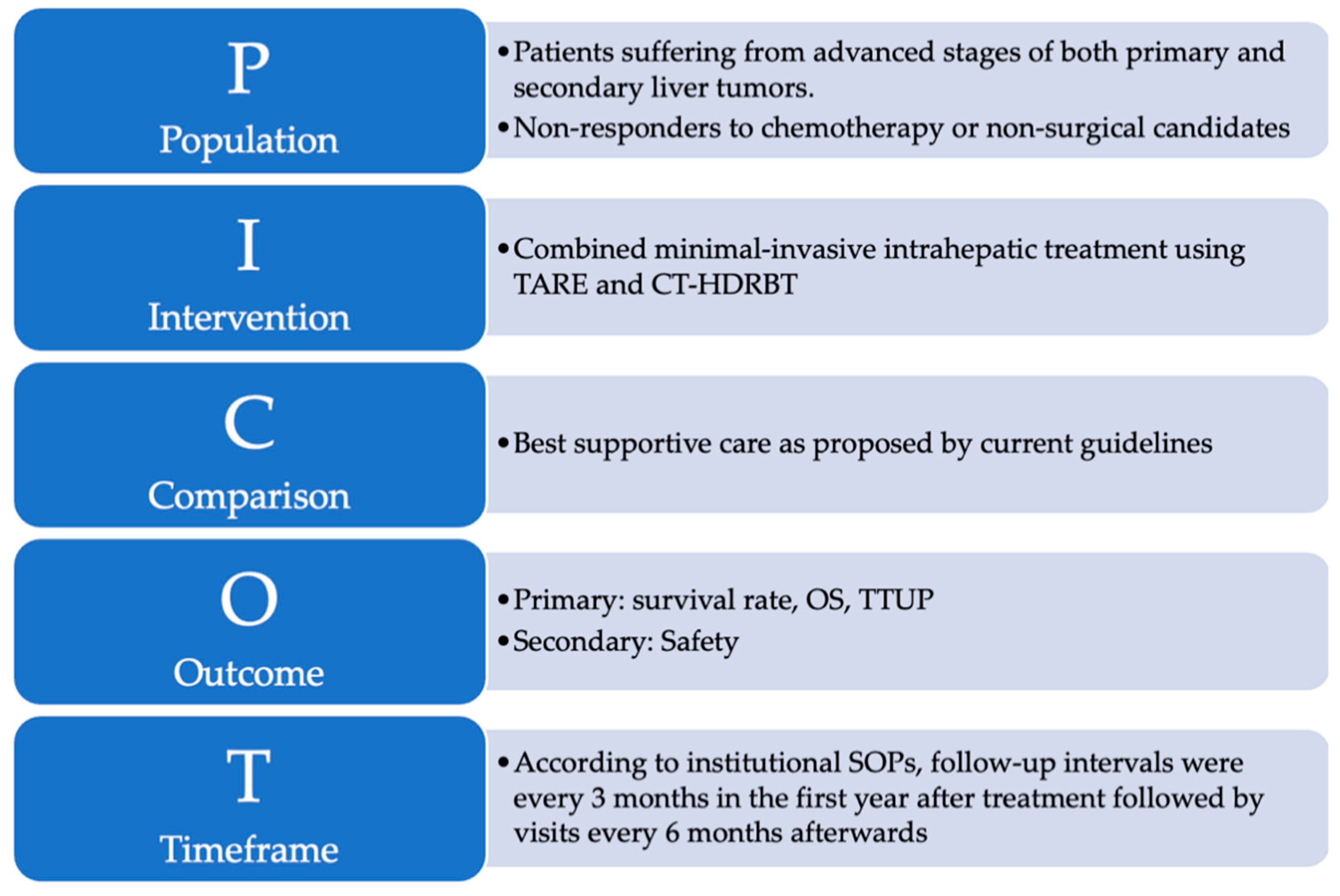
Primary endpoints were median OS and time to untreatable progression (TTUP). TTUP was defined as the time from the first treatment with either TARE or CT-HDRBT to the exhaustion of all local therapy approaches [27]. Data collection ended in December 2020. Time of death was determined by using the internal hospital information system, searching for obituaries, and contacting general practitioners. Additionally, subgroup analyses were performed depending on the most frequent diagnoses and in consideration of the procedure sequences. Complications were classified according to the standards of the Society of Interventional Radiology [28]. The study design is graphically summarized using the PICOT format in Figure 2 [29].
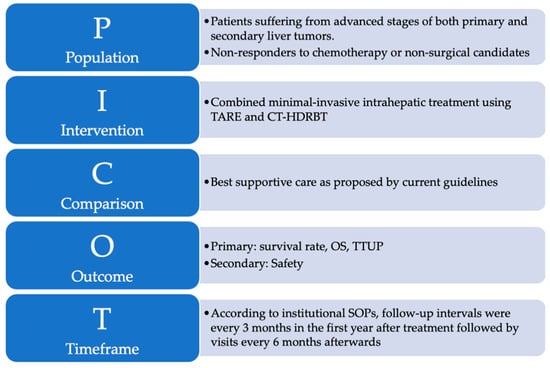
Figure 2.
The PICOT format is a helpful and reader-friendly approach for summarizing research questions that explore the effect of treatment interventions: (P)–Population refers to the sample of subjects in this study. (I)–Intervention refers to the treatment that was provided to subjects enrolled in this study. (C)–Comparison identifies the reference group of patients to compare with the treatment intervention. (O)–Outcome represents the outcome parameters of this study. (T)–Time describes the duration of data collection.
Statistical analysis was conducted using SPSS (Statistical Package for the Social Sciences, version 27.0). Testing for normality was performed with the Shapiro–Wilk test. Normally-distributed continuous data were presented as the mean and standard deviation (SD) and non-normally distributed data were expressed as median and interquartile range (IQR) or range. Kaplan–Meier curves were used to analyse and visualize OS and TTUP. For the subgroup analysis, groups were compared using the nonparametric Mann–Whitney U test and Chi-squared test. Furthermore, a Cox regression model with time-dependent and time-independent covariates was used to analyze effects on survival. p-values of <0.05 were considered significant.
3. Results
The study population included 30 women and 47 men ranging in age from 22 to 85 years (median 63 years). The most common diagnoses were HCC (n = 37), colorectal carcinoma (CRC, n = 13), CCA (n = 9) and neuroendocrine tumor (NET, n = 9). Multiple patients underwent liver surgery (n = 20) as well as chemotherapy (n = 43), mostly before MIT. Furthermore, a majority of patients underwent other MITs such as transarterial chemoembolization (TACE) or RFA. Demographic characteristics are summarized in Table 1.
3.1. Procedures and Adverse Events
Our patients received a total of 115 CT-HDRBT treatments. Fifty-three patients were treated only once, fifteen were treated twice and nine patients had more than three treatments. A total of eight mild and two moderate adverse events were recorded: five patients developed free perihepatic fluid, two patients experienced nausea and one patient showed elevated temperatures post-treatment. All mild complications were treated pharmaceutically without any intervention needed. We recorded one moderate complication in a patient with a pneumothorax after CT-HDRBT, which was treated with a pleural drainage. The patient was discharged two days after treatment without any discomfort. A second patient with a moderate adverse event developed hyperbilirubinemia combined with severe pain and was therefore transferred to the department of gastroenterology where he was recompensated.
A total of 96 TARE therapies were performed. Fourteen patients underwent a sequential procedure with about six weeks in between treatments. Five patients received two TARE procedures. The mean activity delivered to the patient was 1.22 GBq (SD 0.54). Nine mild, one moderate and one severe AE occurred. The nine mild AE consisted of nausea (n = 8) and mild fever (n = 8) that were treated pharmacologically. One patient with a mild complication suffered from post-interventional ascites that was treated with an abdominal ascites drainage. No further intervention was necessary. The one patient with a severe AE suffered from a pseudoaneurysm at the puncture site of the femoral access, which was successfully treated using ultrasound-guided thrombin injection.
3.2. Primary Outcomes
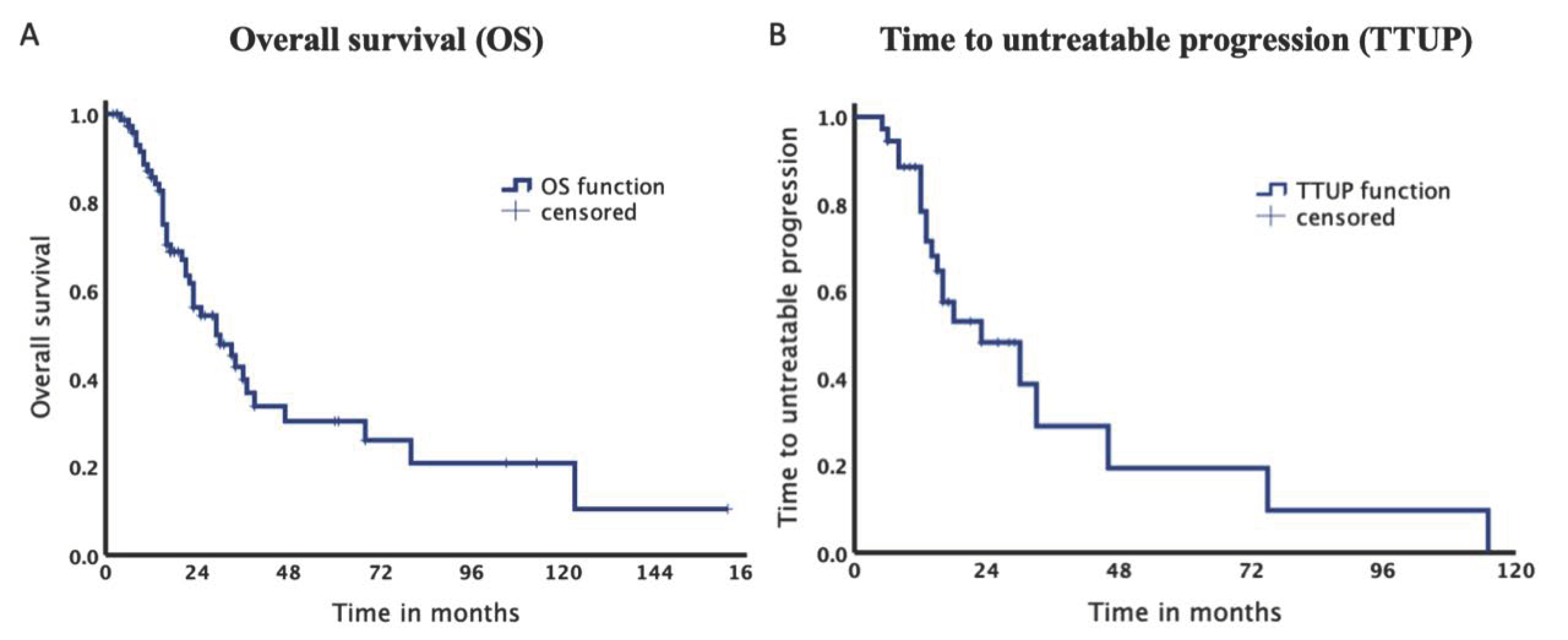
The total survival rate after 12, 24 and 36 months was 85.7%, 56.2% and 39.8%, respectively (Figure 3). Median OS was 29.8 months (95% confidence interval [CI] 18.16–41.42). Median TTUP was 23.8 months (95% CI 9.61–37.93).
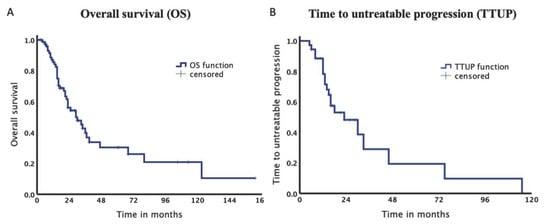
Figure 3.
Kaplan–Meier Curves of the entire collective. (A). Median overall survival was 29.8 months. (B). Median time to untreatable progression was 23.8 months.
3.3. Subgroup Analysis
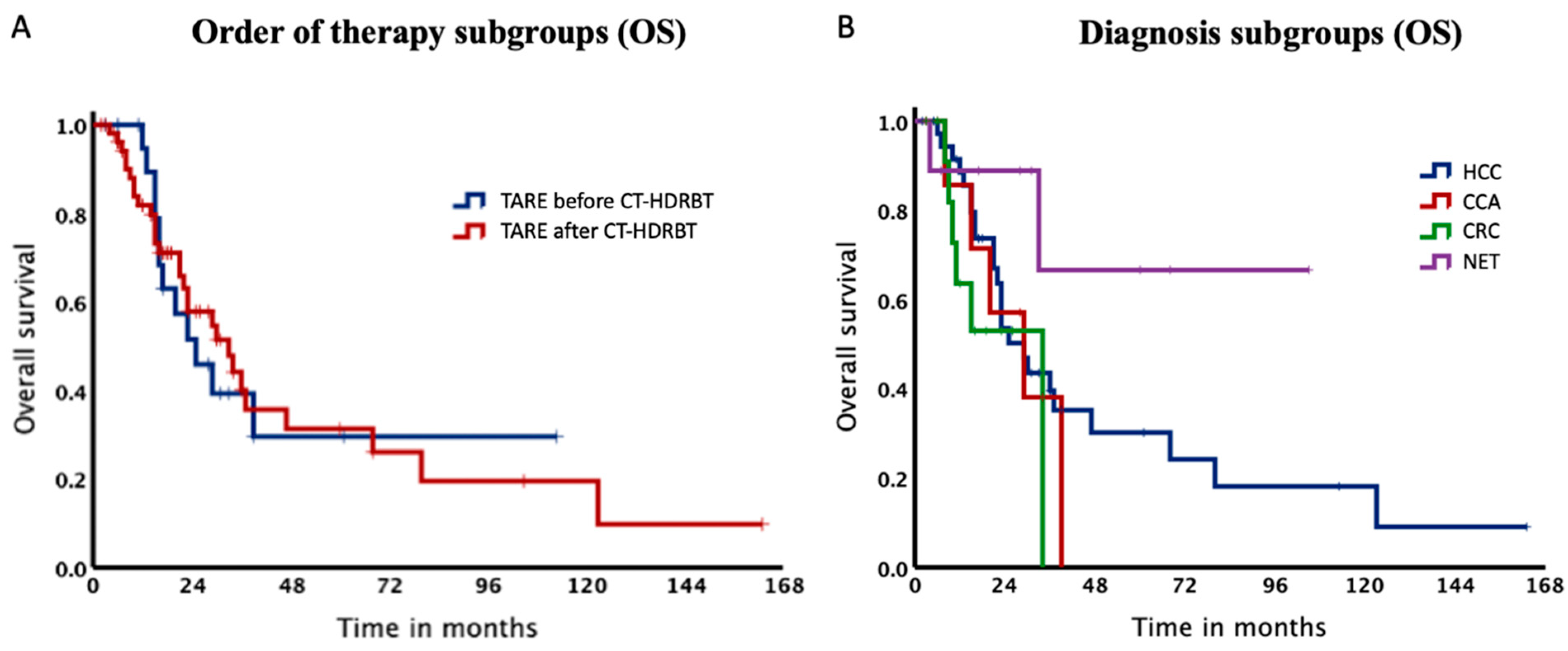
Baseline characteristics of our study subdivided depending on procedure sequence are summarized in Table 2. Patients starting with TARE showed a significantly shorter median duration before switching therapies than patients treated with CT-HDRBT first (p = 0.037). The total survival rate in the TARE before CT-HDRBT group after 12, 24 and 36 months was 94.7%, 51.7% and 39.4% with a median OS of 26.0 months. In contrast, for patients, who received CT-HDRBT before TARE survival rates were 81.9%, 58.0% and 40.2% after 12, 24 and 36 months with a median OS of 33.7 months. Log-Rank test could not be calculated since the proportional hazard assumption was violated (Figure 4A).

Table 2.
Subgroup characteristics regarding procedure sequence.
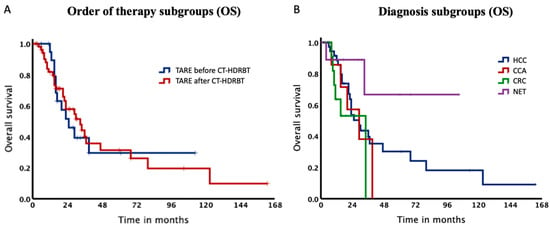
Figure 4.
Kaplan–Meier Curves of the subgroups. (A). Median overall survival was 26.0 months for patients, who received TARE before CT-HDRBT and 33.7 months, when receiving TARE after CT-HDRBT. (B). Kaplan–Meier Curves of our subgroups. Median overall survival for HCC, CCA and CRC was 29.8, 29.6 and 34.4 months.
Therefore, we computed our stratification factor TARE before or after CT-HDRBT as a time-dependent covariate and included it in our Cox regression model. We additionally added TARE before or after CT-HDRBT as a time-independent covariate and the time between TARE and CT-HDRBT in our model. Per month passed, the risk of death increased by 1.094 (95% CI 1.027–1.165, p = 0.005) times for patients starting with CT-HDRBT compared to patients starting with TARE (time-dependent covariate). When not taking into account the time passed, no difference between the groups could be observed (time-independent covariate). Further, we could show that patients with less time between their therapies showed a lower OS with a Hazard Ratio of 0.922 (95%CI 0.889–0.956, p = 0.00001). Thus, the risk of death decreased almost 8% per month in between the two therapies.
Additionally, the cohort was stratified according to diagnoses with a median OS of 29.8, 29.6 and 34.4 months for HCC, CCA and CRC, respectively (Table 3). Of note, OS rate for NET did not drop below 65% (Figure 4) and therefore it was not possible to estimate the median OS for this subgroup. Survival rates after 12, 24 and 36 months for HCC patients were 88.5%, 53.6% and 39.6%.

Table 3.
Subgroup patient characteristics according to diagnoses.
4. Discussion
This study has three main findings. First, the concept of combining TARE with CT-HDRBT is an effective treatment for advanced-stage liver tumors. Second, it can be applied safely to a broad field of patients including extensive pretreatments and multiple tumor entities. Thirdly, our data indicate that an early TARE in the course of the disease might have a positive effect on survival.
The management of advanced stages of both primary and secondary liver tumors remains challenging and combining different approaches in order to create the most effective treatment for patients is often a clinical necessity. The lack of standardized treatments in these patients is met by a broad variety of minimal-invasive procedures [30]. The herein presented first analysis of a combined treatment of TARE and CT-HDRBT supports an individual combination of multiple minimal-invasive therapy approaches in order to maximize treatment success and the quality of patient care.
Merging therapeutic effects to maximize response to treatment is a clinical reality in the field of interventional radiology. Multiple studies report the efficacy and safety of combinations of minimal-invasive therapies such as TACE or TARE plus local ablative therapies such as RFA, MWA or CT-HDRBT.
For the treatment of advanced HCC, systemic chemotherapy can be distinguished into targeted therapies and immunotherapies. As a result of promising studies, tyrosine kinase inhibitors such as sorefenib and lenvatinib were granted approval in first-line therapy whereas checkpoint inhibitors such as nivolumab are considered as second-line therapy [31,32]. However, the efficacy of TKI is limited by the development of drug resistance. In this context, major neuronal isoform of (RAS)/Rapidly Accelerated Fibrosarcoma protein (RAF)/mitogen-activated and extracellular-signal regulated kinase (MEK)/extracellular-signal regulated kinases (ERK) pathways play a central role [33]. Hence, most large randomized multicenter studies showed disease control in only about 50% of cases, still lacking robust evidence for significant survival benefits. Moreover, especially treatment with tyrosine kinase inhibitors is known to be linked to severe limitations of quality of life [34]. In light of these caveats, therapies using local treatment approaches are today a clinical reality. A retrospective case control study with 240 patients showed an advantage in combining TACE with RFA compared to RFA alone [35]. In 2020, Wang et al. examined 183 patients with a recurrence of HCC, who were either treated with TACE alone or with RFA/MWA and TACE combined [36]. After propensity score matching, there were two groups including 65 patients each with no significant difference in their baseline characteristics. The TACE-Ablation group had 1, 3 and 5-year OS rates of 81.2%, 52.4%, 41.6% compared to the TACE-alone group with only 64.9%, 36.6%, 30.2%, showing a clear advantage for combined treatments. A previously conducted study examined 47 patients, who were treated with TACE or TAE combined with CT-HDRBT [19]. The TAE group achieved a median OS of 32.3 months and the TACE group 28.9 months. The 37 HCC patients in our cohort achieved a median OS of 29.8 months, confirming these results.
Regarding CCA, the recently presented MISPHEC Trial was conducted in seven centers in France and included 41 patients with unresectable CCA [37]. The first-line treatment encompassed chemotherapy (gemcitabine + cisplatin) and TARE in combination. This prospective study showed a median OS of 22 months.
CT-HDRBT in the context of CCA has been investigated in multiple studies in the past [38,39,40]. In a recently published study, 61 CCA patients received 96 CT-HDRBTs in total [38]. The study reported a median OS of 15.5 months for lesions smaller than 4 cm (n = 18) and 10.0 months for larger lesions (n = 43). We can report a median OS of 29 months for CCA patients indicating a very good response to a combined therapy of TARE and CT-HDRBT.
Regarding CRC metastases, a conducted study evaluated 23 patients who were treated with TACE using Irinotecan-loaded microspheres and CT-HDRBT in combination [41]. The authors highlighted the overall safety and good feasibility of this procedure and reported median OS of eight months. TARE is known for being safe, which we can confirm by only recording one AE in 15 sessions for 13 patients. A recently published study examined 131 patients with CRC metastases and showed a median OS of 10.7 months [42]. We can report a median OS of 34 months for our CRC patient subgroup indicating very good response to treatment in our cohort.
A retrospective study evaluating the efficacy of TARE on NET metastatic to the liver analysed 40 patients treated with 56 sessions in total [43]. The authors report a median OS of 24.7 months. Survival rates of patients with NET metastatic to the liver treated with CT-HDRBT is reported with a 5-year OS rate of 63% [44]. In spite of the small patient numbers in this patient subgroup, results indicate that a combination of TARE and CT-HDRBT seems reasonable, especially since our nine patients’ total survival rate remained above 65%.
Nine patients with liver metastases from other origins than previously described were also included in our study. We did not notice any abnormalities in safety and feasibility.
The Cox regression model shows a significant survival benefit for patients treated with TARE early in the course of disease. Hence, we assume that TARE sufficiently stabilises tumor progression. This result stands in contrast to the clinical reality, where most patients receive TARE rather late in the course of disease. Even though, it is not infrequently observed that an earlier TARE shows good results in both short- and long-term outcomes [42,43,45].
The present study demonstrates that TARE and CT-HDRBT can effectively be combined in patients suffering from advanced primary and secondary liver tumors. According to current guidelines, most of the patients in our cohort would have only qualified for best supportive care since they were non-responders to chemotherapy or suffered from tumor relapse after surgical resection. Yet, treating these patients by deviating from current guidelines is a clinical reality and this study therefore addresses a topic that might be of interest beyond the field of interventional radiology. This unique combination merges an unselective, whole liver approach of TARE with a focused and high-dose approach of CT-HDRBT. With combining both treatment principles, we are able to treat advanced tumors of various origins successfully with excellent outcomes regarding median OS, TTUP and no significant complications. We believe that with the development of new targeted chemo- and immunotherapies the need for a combination of treatment strategies will emerge in the very near future. In this context the present study focusses on one potential element of future therapies designed for patients suffering from cancer in advanced stages.
This study has several limitations. Firstly, it has a retrospective single-center design. This might limit transferability to other oncological centers. Moreover, while the heterogeneity of our study population provided previously mentioned advantages, it nevertheless might weaken comparability. Statistical calculations were furthermore limited by small numbers within the analysed subgroups. The results of this study are not based on a robust statistical dataset, especially in tumors that are not commonly treated using TARE and ablation such as metastases from pancreatic or breast cancer. However, since a combination of TARE with any other MIT is generally rarely performed, it is unlikely to find a much larger patient cohort. In order to confirm the findings of this study statistically powered clinical trials will be necessary in the future.
5. Conclusions
A combination of TARE and CT-HDRBT offers an effective and safe treatment approach for a broad range of advanced primary and secondary liver malignancies. The promising median OS and TTUP presented in this study are encouraging regarding the use of different treatment combinations according to the individual course of diseases. Finally, the herein presented results indicate that a treatment with TARE early in the course of disease might be beneficial with regard to survival outcomes.
Author Contributions
Conceptualization, F.N.F. and B.G.; methodology, F.N.F., M.K. and B.G.; software, M.J.R. and T.A.A.; validation, U.F., G.B., T.M. and G.F.T.; formal analysis, F.N.F. and M.J.R.; investigation, F.N.F.; resources, F.N.F.; B.G. and F.C.; data curation, F.N.F.; writing—original draft preparation, F.N.F. and M.J.R.; writing—review and editing, T.A.A., T.M., G.F.T. and U.F.; visualization, F.N.F. and M.J.R.; supervision, F.C.; project administration, B.G.; funding acquisition, F.N.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was performed in accordance with the standards of the Helsinki Declaration and was approved by the Charité ethical review board (EA4/08917) on 24 May 2017.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Acknowledgments
We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité-Universitätsmedizin Berlin.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, S.; Feng, Y.; Swinnen, J.; Oyen, R.; Li, Y.; Ni, Y. Incidence and prognosis of liver metastasis at diagnosis: A pan-cancer population-based study. Am. J. Cancer Res. 2020, 10, 1477–1517. [Google Scholar]
- Liu, Z.; Suo, C.; Mao, X.; Jiang, Y.; Jin, L.; Zhang, T.; Chen, X. Global incidence trends in primary liver cancer by age at diagnosis, sex, region, and etiology, 1990–2017. Cancer 2020, 126, 2267–2278. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Heinrich, S.; Lang, H. Hepatic resection for primary and secondary liver malignancies. Innov. Surg. Sci. 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Le Linn, Y.; Chee, M.Y.; Koh, Y.; Teo, J.; Cheow, P.; Chow, P.K.H.; Chan, C.; Chung, A.Y.F.; Ooi, L.L.P.J.; Goh, B.K.P. Actual 10-year survivors and 10-year recurrence free survivors after primary liver resection for hepatocellular carcinoma in the 21st century: A single institution contemporary experience. J. Surg. Oncol. 2020, 123, 214–221. [Google Scholar] [CrossRef]
- Rizzo, A.; Brandi, G. Pitfalls, challenges, and updates in adjuvant systemic treatment for resected biliary tract cancer. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 547–554. [Google Scholar] [CrossRef]
- Fong, Y.; Cohen, A.M.; Fortner, J.G.; Enker, W.E.; Turnbull, A.D.; Coit, D.G.; Marrero, A.M.; Prasad, M.; Blumgart, L.H.; Brennan, M.F. Liver resection for colorectal metastases. J. Clin. Oncol. 1997, 15, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.C.; Primrose, J.N.; Colquitt, J.L.; Garden, O.J.; Poston, G.J.; Rees, M. Surgical resection of hepatic metastases from colorectal cancer: A systematic review of published studies. Br. J. Cancer 2006, 94, 982–999. [Google Scholar] [CrossRef]
- Hirsch, R.D.; Mills, C.; Sawhney, R.; Sood, S.; Bird, V.; Mishra, G.; Dev, A.; Kemp, W.; Lubel, J.; Roberts, S.K.; et al. SIRT Compared with DEB-TACE for Hepatocellular Carcinoma: A Real-world Study (the SITAR Study). J. Gastrointest. Cancer 2020, 52, 907–914. [Google Scholar] [CrossRef]
- Garrean, S.; Muhs, A.; Bui, J.T.; Blend, M.J.; Owens, C.; Helton, W.S.; Espat, N.J. Complete eradication of hepatic metastasis from colorectal cancer by Yttrium-90 SIRT. World J. Gastroenterol. 2007, 13, 3016–3019. [Google Scholar] [CrossRef]
- Zarva, A.; Mohnike, K.; Damm, R.; Ruf, J.; Seidensticker, R.; Ulrich, G.; Seidensticker, M.; Pech, M.; Ricke, J.; Amthauer, H. Safety of Repeated Radioembolizations in Patients with Advanced Primary and Secondary Liver Tumors and Progressive Disease After First Selective Internal Radiotherapy. J. Nucl. Med. 2014, 55, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Zhou, L.; Dorfman, R.G.; Liu, L.; Cai, R.; Jiang, C.; Tang, D.; Wang, Y.; Zou, X.; et al. SIRT3 elicited an anti-Warburg effect through HIF1alpha/PDK1/PDHA1 to inhibit cholangiocarcinoma tumorigenesis. Cancer Med. 2019, 8, 2380–2391. [Google Scholar] [CrossRef] [PubMed]
- Ricke, J.; Wust, P.; Stohlmann, A.; Beck, A.; Cho, C.H.; Pech, M.; Wieners, G.; Spors, B.; Werk, M.; Rosner, C.; et al. CT-guided interstitial brachytherapy of liver malignancies alone or in combination with thermal ablation: Phase I-II results of a novel technique. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, T.; Mohnike, K.; Hass, P.; Seidensticker, R.; Göppner, D.; Dudeck, O.; Streitparth, F.; Ricke, J. Efficacy and safety of image-guided interstitial single fraction high-dose-rate brachytherapy in the management of metastatic malignant melanoma. J. Contemp. Brachytherapy 2015, 2, 154–160. [Google Scholar] [CrossRef]
- Collettini, F.; Schnapauff, D.; Poellinger, A.; Denecke, T.; Schott, E.; Berg, T.; Wust, P.; Hamm, B.; Gebauer, B. Hepatocellular carcinoma: Computed-tomography-guided high-dose-rate brachytherapy (CT-HDRBT) ablation of large (5–7 cm) and very large (>7 cm) tumours. Eur. Radiol. 2011, 22, 1101–1109. [Google Scholar] [CrossRef]
- Wang, W.; Song, Z.; Ye, J.; Wang, Y.; Li, Y. Computed tomography-guided iodine-125 brachytherapy for unresectable hepatocellular carcinoma. J. Cancer Res. Ther. 2019, 15, 1553–1560. [Google Scholar] [CrossRef]
- Collettini, F.; Schreiber, N.; Schnapauff, D.; Denecke, T.; Wust, P.; Schott, E.; Hamm, B.; Gebauer, B. CT-guided high-dose-rate brachytherapy of unresectable hepatocellular carcinoma. Strahlenther. Und Onkol. 2014, 191, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Schnapauff, D.; Tegel, B.R.; Powerski, M.J.; Colletini, F.; Hamm, B.; Gebauer, B. Interstitial Brachytherapy in Combination With Previous Transarterial Embolization in Patients With Unresectable Hepatocellular Carcinoma. Anticancer Res. 2019, 39, 1329–1336. [Google Scholar] [CrossRef]
- Wang, T.-H.; Huang, P.-I.; Hu, Y.-W.; Lin, K.-H.; Liu, C.-S.; Lin, Y.-Y.; Liu, C.-A.; Tseng, H.-S.; Liu, Y.-M.; Lee, R.-C. Combined Yttrium-90 microsphere selective internal radiation therapy and external beam radiotherapy in patients with hepatocellular carcinoma: From clinical aspects to dosimetry. PLoS ONE 2018, 13, e0190098. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, T.; Ricke, J.; Gebauer, B.; Streitparth, F. Image-guided high-dose-rate brachytherapy of malignancies in various inner organs-technique, indications, and perspectives. J. Contemp. Brachytherapy 2016, 8, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Denecke, T.; Stelter, L.; Schnapauff, D.; Steffen, I.; Sinn, B.; Schott, E.; Seidensticker, R.; Puhl, G.; Gebauer, B.; Lopez Hänninen, E.; et al. CT-guided Interstitial Brachytherapy of Hepatocellular Carcinoma before Liver Transplantation: An Equivalent Alternative to Transarterial Chemoembolization? Eur. Radiol. 2015, 25, 2608–2616. [Google Scholar] [CrossRef]
- Mohnike, K.; Wieners, G.; Schwartz, F.; Seidensticker, M.; Pech, M.; Ruehl, R.; Wust, P.; Lopez-Hänninen, E.; Gademann, G.; Peters, N.; et al. Computed tomography-guided high-dose-rate brachytherapy in hepatocellular carcinoma: Safety, efficacy, and effect on survival. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 172–179. [Google Scholar] [CrossRef]
- Moctezuma-Velazquez, C.; Montano-Loza, A.J.; Meza-Junco, J.; Burak, K.; Ma, M.; Bain, V.G.; Kneteman, N.; Sarlieve, P.; Owen, R.J. Selective Internal Radiation Therapy for Hepatocellular Carcinoma Across the Barcelona Clinic Liver Cancer Stages. Dig. Dis. Sci. 2020, 66, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Prompers, L.; Bucerius, J.; Brans, B.; Temur, Y.; Berger, L.; Mottaghy, F.M. Selective internal radiation therapy (SIRT) in primary or secondary liver cancer. Methods 2011, 55, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Thurston, K.G. Radioembolization with 90yttrium microspheres: A state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 2: Special topics. J. Vasc. Interv. Radiol. 2006, 17, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, B.; Wang, Y.; Zhang, J.; Wu, Y.; Fan, W.; Li, J. Time to untreatable progression is an appropriate surrogate endpoint for overall survival in patients with hepatocellular carcinoma after transarterial chemoembolization. J. Cancer Res. Ther. 2020, 16, 301–308. [Google Scholar] [CrossRef]
- Khalilzadeh, O.; Baerlocher, M.O.; Shyn, P.B.; Connolly, B.L.; Devane, A.M.; Morris, C.S.; Cohen, A.M.; Midia, M.; Thornton, R.H.; Gross, K.; et al. Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee. J. Vasc. Interv. Radiol. 2017, 28, 1432–1437.e3. [Google Scholar] [CrossRef]
- Riva, J.J.; Malik, K.M.; Burnie, S.J.; Endicott, A.R.; Busse, J. What is your research question? An introduction to the PICOT format for clinicians. J. Can. Chiropr. Assoc. 2012, 56, 167–171. [Google Scholar]
- Hass, P.; Mohnike, K. Extending the Frontiers beyond Thermal Ablation by Radiofrequency Ablation: SBRT, Brachytherapy, SIRT (Radioembolization). Viszeralmedizin 2014, 30, 245–252. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Gnoni, A.; Licchetta, A.; Memeo, R.; Argentiero, A.; Solimando, A.G.; Longo, V.; Delcuratolo, S.; Brunetti, O. Role of BRAF in Hepatocellular Carcinoma: A Rationale for Future Targeted Cancer Therapies. Medicina 2019, 55, 754. [Google Scholar] [CrossRef]
- Le Grazie, M.; Biagini, M.R.; Tarocchi, M.; Polvani, S.; Galli, A. Chemotherapy for hepatocellular carcinoma: The present and the future. World J. Hepatol. 2017, 9, 907–920. [Google Scholar] [CrossRef]
- Peng, Z.-W.; Chen, M.-S.; Liang, H.-H.; Gao, H.-J.; Zhang, Y.; Li, J.-Q.; Lau, W. A case-control study comparing percutaneous radiofrequency ablation alone or combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. Eur. J. Surg. Oncol. (EJSO) 2010, 36, 257–263. [Google Scholar] [CrossRef]
- Wang, C.; Liao, Y.; Qiu, J.; Yuan, Y.; Zhang, Y.; Li, K.; Zou, R.; Wang, Y.; Zuo, D.; He, W.; et al. Transcatheter arterial chemoembolization alone or combined with ablation for recurrent intermediate-stage hepatocellular carcinoma: A propensity score matching study. J. Oncol. 2020, 146, 2669–2680. [Google Scholar] [CrossRef]
- Edeline, J.; Touchefeu, Y.; Guiu, B.; Farge, O.; Tougeron, D.; Baumgaertner, I.; Ayav, A.; Campillo-Gimenez, B.; Beuzit, L.; Pracht, M.; et al. Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 51–59. [Google Scholar] [CrossRef]
- Jonczyk, M.; Collettini, F.; Schnapauff, D.; Geisel, D.; Boning, G.; Feldhaus, F.; Denecke, T.; Wieners, G.; Hamm, B.; Gebauer, B. Cholangiocarcinoma: CT-guided High-Dose Rate Brachytherapy (CT-HDRBT) for Limited (<4 cm) and Large (>4 cm) Tumors. Anticancer Res. 2018, 38, 5843–5852. [Google Scholar] [CrossRef] [PubMed]
- Kamphues, C.; Seehofer, D.; Collettini, F.; Bahra, M.; Neuhaus, P.; Wust, P.; Denecke, T.; Gebauer, B.; Schnapauff, D. Preliminary experience with CT-guided high-dose rate brachytherapy as an alternative treatment for hepatic recurrence of cholangiocarcinoma. HPB 2012, 14, 791–797. [Google Scholar] [CrossRef][Green Version]
- Schnapauff, D.; Denecke, T.; Grieser, C.; Colletini, F.; Seehofer, D.; Sinn, M.; Banzer, J.; Lopez-Hänninen, E.; Hamm, B.; Wust, P.; et al. Computed Tomography-Guided Interstitial HDR Brachytherapy (CT-HDRBT) of the Liver in Patients with Irresectable Intrahepatic Cholangiocarcinoma. Cardiovasc. Interv. Radiol. 2011, 35, 581–587. [Google Scholar] [CrossRef]
- Collettini, F.; Jonczyk, M.; Meddeb, A.; Wieners, G.; Geisel, D.; Schnapauff, D.; Gebauer, B. Feasibility and Safety of CT-Guided High-Dose-Rate Brachytherapy Combined with Transarterial Chemoembolization Using Irinotecan-Loaded Microspheres for the Treatment of Large, Unresectable Colorectal Liver Metastases. J. Vasc. Interv. Radiol. 2019, 31, 315–322. [Google Scholar] [CrossRef]
- Weiner, A.A.; Gui, B.; Newman, N.B.; Nosher, J.L.; Yousseff, F.; Lu, S.-E.; Foltz, G.M.; Carpizo, D.; Lowenthal, J.; Zuckerman, D.A.; et al. Predictors of Survival after Yttrium-90 Radioembolization for Colorectal Cancer Liver Metastases. J. Vasc. Interv. Radiol. 2018, 29, 1094–1100. [Google Scholar] [CrossRef]
- Barbier, C.E.; Garske-Román, U.; Sandström, M.; Nyman, R.; Granberg, D. Selective internal radiation therapy in patients with progressive neuroendocrine liver metastases. Eur. J. Nucl. Med. Mol. Imaging 2015, 43, 1425–1431. [Google Scholar] [CrossRef]
- Schippers, A.C.; Collettini, F.; Steffen, I.G.; Wieners, G.; Denecke, T.; Pavel, M.; Wust, P.; Gebauer, B. Initial Experience with CT–Guided High-Dose-Rate Brachytherapy in the Multimodality Treatment of Neuroendocrine Tumor Liver Metastases. J. Vasc. Interv. Radiol. 2017, 28, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, O.N.; Soydal, C.; Lacin, S.; Ozkan, E.; Bilgic, S. Selective intraarterial radionuclide therapy with Yttrium-90 (Y-90) microspheres for unresectable primary and metastatic liver tumors. World J. Surg. Oncol. 2011, 9, 86. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).