Development of an MRI-Guided Approach to Selective Internal Radiation Therapy Using Holmium-166 Microspheres
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. MR Safety
2.1.1. Radiofrequency (RF)-Induced Heating
2.1.2. Magnetically Induced Displacement and Artefact Size
2.2. Near Real-Time MRI Visibility of Microspheres
2.2.1. Flow Phantom
2.2.2. In Vivo Procedure
2.3. Image Analysis
2.4. Statistics
3. Results
3.1. RF-Induced Heating
3.2. Magnetically Induced Displacement and Artefact Size
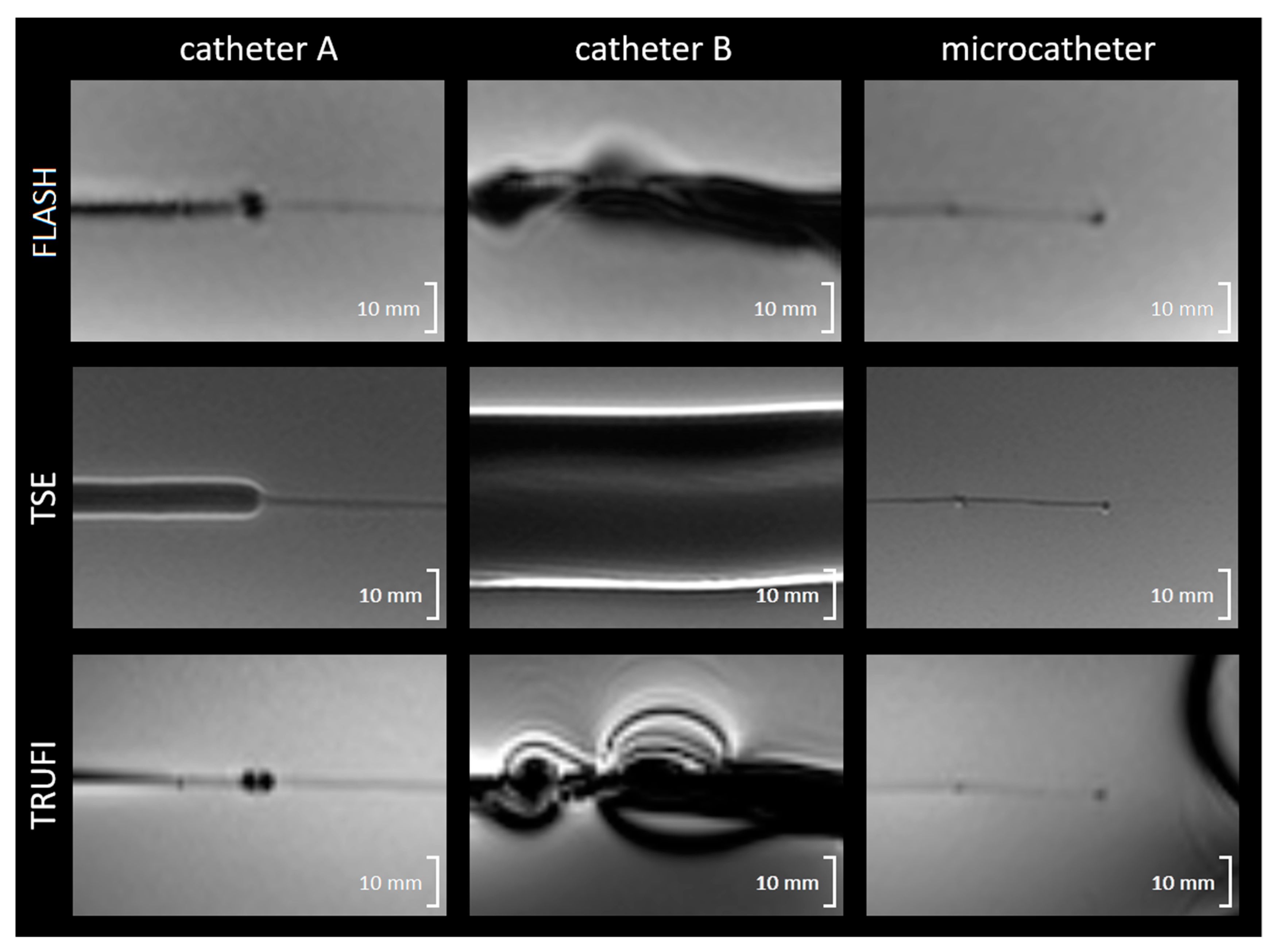
3.3. In Vivo Artefact Size
3.4. Near Real-Time MRI Visibility of Microspheres
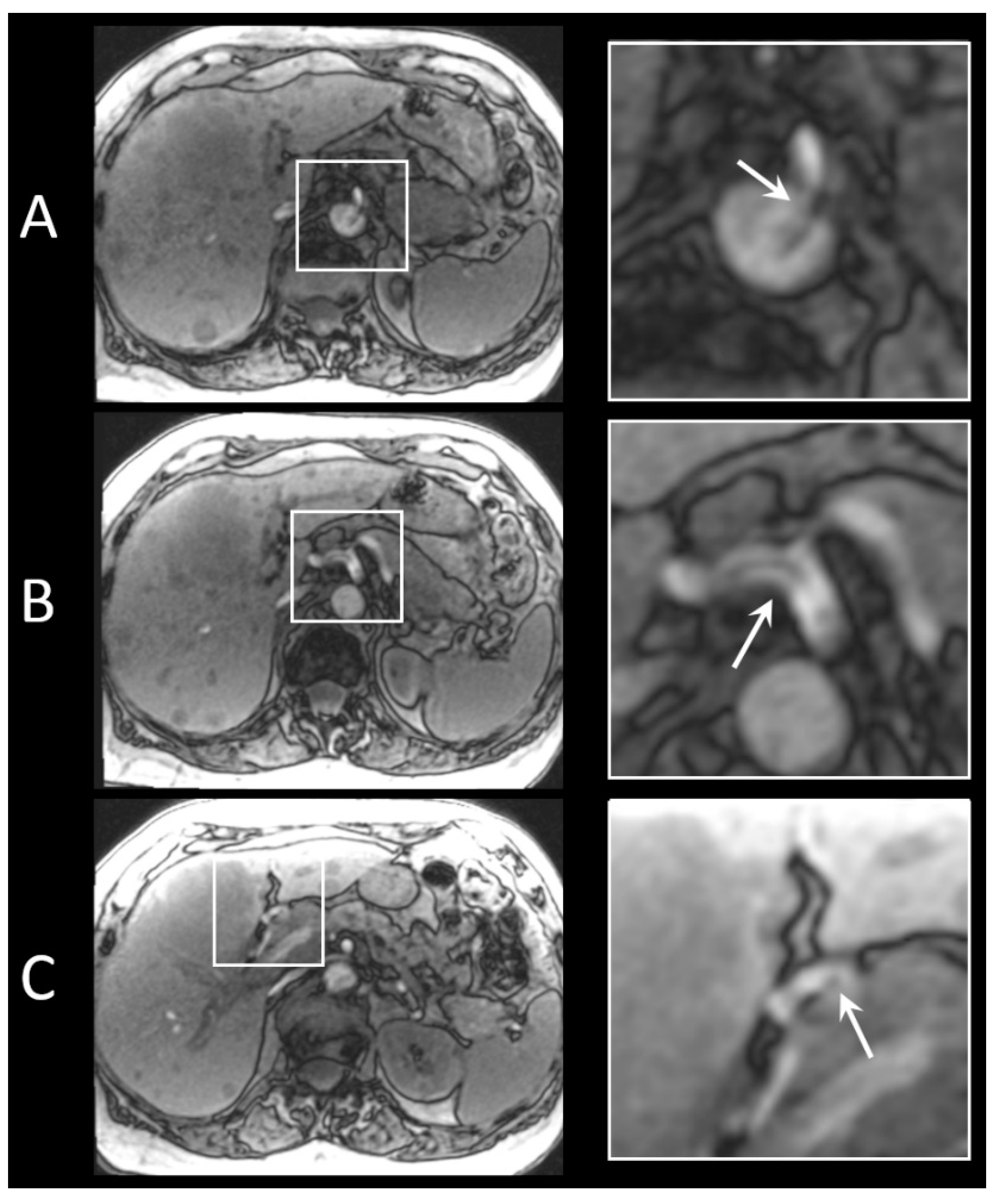
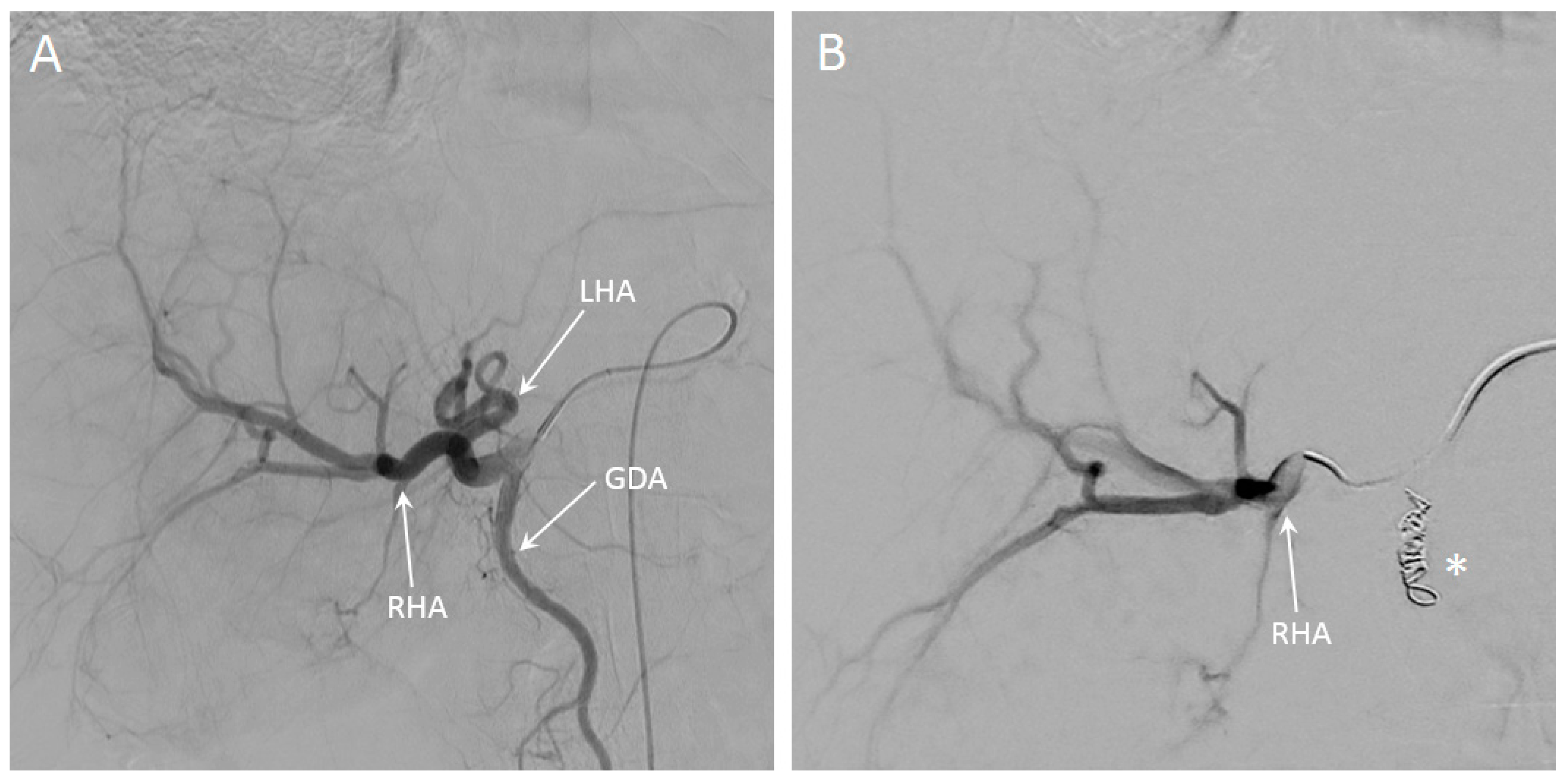
3.5. In Vivo Visibility during Holmium-166 SIRT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levillain, H.; Bagni, O.; Deroose, C.M.; Dieudonne, A.; Gnesin, S.; Grosser, O.S.; Kappadath, S.C.; Kennedy, A.; Kokabi, N.; Liu, D.M.; et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Padia, S.A.; Lam, M.; Bell, J.; Chiesa, C.; Fowers, K.; Hamilton, B.; Herman, J.; Kappadath, S.C.; Leung, T.; et al. Clinical and dosimetric considerations for Y90: Recommendations from an international multidisciplinary working group. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1695–1704. [Google Scholar] [CrossRef]
- Cremonesi, M.; Ferrari, M.; Bartolomei, M.; Orsi, F.; Bonomo, G.; Arico, D.; Mallia, A.; De Cicco, C.; Pedroli, G.; Paganelli, G. Radioembolisation with 90Y-microspheres: Dosimetric and radiobiological investigation for multi-cycle treatment. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, N.J.M.; Arntz, M.J.; Gil Arranja, A.; Roosen, J.; Nijsen, J.F.W. The various therapeutic applications of the medical isotope holmium-166: A narrative review. EJNMMI Radiopharm. Chem. 2019, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Prince, J.F.; van den Bosch, M.; Nijsen, J.F.W.; Smits, M.L.J.; van den Hoven, A.F.; Nikolakopoulos, S.; Wessels, F.J.; Bruijnen, R.C.G.; Braat, M.; Zonnenberg, B.A.; et al. Efficacy of Radioembolization with (166)Ho-Microspheres in Salvage Patients with Liver Metastases: A Phase 2 Study. J. Nucl. Med. 2018, 59, 582–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kafrouni, M.; Allimant, C.; Fourcade, M.; Vauclin, S.; Guiu, B.; Mariano-Goulart, D.; Ben Bouallegue, F. Analysis of differences between (99m)Tc-MAA SPECT- and (90)Y-microsphere PET-based dosimetry for hepatocellular carcinoma selective internal radiation therapy. EJNMMI Res. 2019, 9, 62. [Google Scholar] [CrossRef] [Green Version]
- Smits, M.L.J.; Dassen, M.G.; Prince, J.F.; Braat, A.; Beijst, C.; Bruijnen, R.C.G.; de Jong, H.; Lam, M. The superior predictive value of (166)Ho-scout compared with (99m)Tc-macroaggregated albumin prior to (166)Ho-microspheres radioembolization in patients with liver metastases. Eur. J. Nucl. Med. Mol. Imaging 2019, 47, 798–806. [Google Scholar] [CrossRef] [Green Version]
- van den Hoven, A.F.; Rosenbaum, C.E.; Elias, S.G.; de Jong, H.W.; Koopman, M.; Verkooijen, H.M.; Alavi, A.; van den Bosch, M.A.; Lam, M.G. Insights into the Dose-Response Relationship of Radioembolization with Resin 90Y-Microspheres: A Prospective Cohort Study in Patients with Colorectal Cancer Liver Metastases. J. Nucl. Med. 2016, 57, 1014–1019. [Google Scholar] [CrossRef] [Green Version]
- van Roekel, C.; Bastiaannet, R.; Smits, M.L.J.; Bruijnen, R.C.; Braat, A.; de Jong, H.; Elias, S.G.; Lam, M. Dose-effect relationships of holmium-166 radioembolization in colorectal cancer. J. Nucl. Med. 2020. [Google Scholar] [CrossRef]
- Bastiaannet, R.; van Roekel, C.; Smits, M.L.J.; Elias, S.G.; van Amsterdam, W.A.C.; Doan, D.T.; Prince, J.F.; Bruijnen, R.C.G.; de Jong, H.W.A.M.; Lam, M.G.E.H. First evidence for a dose-response relationship in patients treated with 166Ho-radioembolization: A prospective study. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2019, 61, 608–612. [Google Scholar] [CrossRef]
- Witt, J.S.; Rosenberg, S.A.; Bassetti, M.F. MRI-guided adaptive radiotherapy for liver tumours: Visualising the future. Lancet Oncol 2020, 21, e74–e82. [Google Scholar] [CrossRef]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; de Baere, T.; Assenat, E.; Tacher, V.; Robert, C.; Terroir-Cassou-Mounat, M.; et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): A randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Roosen, J.; Klaassen, N.J.M.; Westlund Gotby, L.E.L.; Overduin, C.G.; Verheij, M.; Konijnenberg, M.W.; Nijsen, J.F.W. To 1000 Gy and back again: A systematic review on dose-response evaluation in selective internal radiation therapy for primary and secondary liver cancer. Eur. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.L.; Elschot, M.; van den Bosch, M.A.; van de Maat, G.H.; van het Schip, A.D.; Zonnenberg, B.A.; Seevinck, P.R.; Verkooijen, H.M.; Bakker, C.J.; de Jong, H.W.; et al. In vivo dosimetry based on SPECT and MR imaging of 166Ho-microspheres for treatment of liver malignancies. J. Nucl. Med. 2013, 54, 2093–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Maat, G.H.; Seevinck, P.R.; Elschot, M.; Smits, M.L.; de Leeuw, H.; van Het Schip, A.D.; Vente, M.A.; Zonnenberg, B.A.; de Jong, H.W.; Lam, M.G.; et al. MRI-based biodistribution assessment of holmium-166 poly(L-lactic acid) microspheres after radioembolisation. Eur. Radiol. 2013, 23, 827–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radosa, C.G.; Radosa, J.C.; Grosche-Schlee, S.; Zophel, K.; Plodeck, V.; Kuhn, J.P.; Kotzerke, J.; Hoffmann, R.T. Holmium-166 Radioembolization in Hepatocellular Carcinoma: Feasibility and Safety of a New Treatment Option in Clinical Practice. Cardiovasc. Intervent. Radiol. 2019, 42, 405–412. [Google Scholar] [CrossRef]
- Real-time Imaging of Holmium Radioembolization: A Feasibility Study (Emeritus); Clinicaltrials.Gov Identifier NCT04269499. Available online: https://clinicaltrials.gov/ct2/show/NCT04269499 (accessed on 10 October 2021).
- Clogenson, H.C.M.; Van den Dobbelsteen, J.J. Catheters and guide wires for interventional MRI: Are we there yet? Imaging Med. 2016, 8, 39–43. [Google Scholar]
- van Rooij, R.; Braat, A.; de Jong, H.; Lam, M. Simultaneous (166)Ho/(99m)Tc dual-isotope SPECT with Monte Carlo-based downscatter correction for automatic liver dosimetry in radioembolization. EJNMMI Phys. 2020, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Elschot, M.; Smits, M.L.; Nijsen, J.F.; Lam, M.G.; Zonnenberg, B.A.; van den Bosch, M.A.; Viergever, M.A.; de Jong, H.W. Quantitative Monte Carlo-based holmium-166 SPECT reconstruction. Med. Phys. 2013, 40, 112502. [Google Scholar] [CrossRef]
- Nijsen, J.F.; Seppenwoolde, J.H.; Havenith, T.; Bos, C.; Bakker, C.J.; van het Schip, A.D. Liver tumors: MR imaging of radioactive holmium microspheres--phantom and rabbit study. Radiology 2004, 231, 491–499. [Google Scholar] [CrossRef]
- Seppenwoolde, J.H.; Bartels, L.W.; van der Weide, R.; Nijsen, J.F.; van het Schip, A.D.; Bakker, C.J. Fully MR-guided hepatic artery catheterization for selective drug delivery: A feasibility study in pigs. J. Magn. Reson. Imaging 2006, 23, 123–129. [Google Scholar] [CrossRef] [PubMed]
- American Society for Testing of Materials. ASTM-F2182-11: Standard Test Method for Measurement of Radiofrequency induced Heating on or Near Passive Implants during Magnetic Resonance Imaging (2011); American Society for Testing of Materials: West Conshohocken, PA, USA, 2011. [Google Scholar]
- American Society for Testing of Materials. ASTM-F2052-06: Standard Test Method for Measurement of Magnetically Induced Displacement Force on Medical Devices in the Magnetic Resonance Environment (2006); American Society for Testing of Materials: West Conshohocken, PA, USA, 2006. [Google Scholar]
- American Society for Testing of Materials. ASTM-F2119-07: Standard Test Method for Evaluation of MR Image Artifacts from Passive Implants (2007); American Society for Testing of Materials: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Winter, L.; Seifert, F.; Zilberti, L.; Murbach, M.; Ittermann, B. MRI-Related Heating of Implants and Devices: A Review. J. Magn. Reson. Imaging 2020. [Google Scholar] [CrossRef]
- Cordis, TEMPO™ 4F & 5F: Catheters with Neon™ Tips Brochure. Available online: https://www.cordis.com (accessed on 18 January 2021).
- Terumo Europe, Radifocus® Angiographic Catheter Brochure. Available online: https://www.terume-europe.com/ (accessed on 18 January 2021).
- Terumo Europe, Progreat® Micro Catheter System Brochure. Available online: https://www.terume-europe.com/ (accessed on 18 January 2021).
- Quirem Medical BV. Instructions for Use Ho-166-PLLA Microspheres, Version LS-1101-10; Quirem Medical BV: Deventer, The Netherlands, 2017. [Google Scholar]
- Drescher, R.; Seifert, P.; Guhne, F.; Aschenbach, R.; Kuhnel, C.; Freesmeyer, M. Radioembolization With Holmium-166 Polylactic Acid Microspheres: Distribution of Residual Activity in the Delivery Set and Outflow Dynamics During Planning and Treatment Procedures. J. Endovasc. Ther. 2021, 28, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Settecase, F.; Martin, A.J.; Lillaney, P.; Losey, A.; Hetts, S.W. Magnetic Resonance-Guided Passive Catheter Tracking for Endovascular Therapy. Magn. Reson. Imaging Clin. 2015, 23, 591–605. [Google Scholar] [CrossRef] [Green Version]
- Gutjahr, R.; Bakker, R.C.; Tiessens, F.; van Nimwegen, S.A.; Schmidt, B.; Nijsen, J.F.W. Quantitative dual-energy CT material decomposition of holmium microspheres: Local concentration determination evaluated in phantoms and a rabbit tumor model. Eur. Radiol. 2021, 31, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Bakker, R.C.; Bastiaannet, R.; van Nimwegen, S.A.; Barten-van Rijbroek, A.D.; Van Es, R.J.J.; Rosenberg, A.J.W.P.; de Jong, H.W.A.M.; Lam, M.G.E.H.; Nijsen, J.F.W. Feasibility of CT quantification of intratumoural (166)Ho-microspheres. Eur. Radiol. Exp. 2020, 4, 29. [Google Scholar] [CrossRef]
- Bakker, R.C.; van Es, R.J.J.; Rosenberg, A.; van Nimwegen, S.A.; Bastiaannet, R.; de Jong, H.; Nijsen, J.F.W.; Lam, M. Intratumoral injection of radioactive holmium-166 microspheres in recurrent head and neck squamous cell carcinoma: Preliminary results of first use. Nucl. Med. Commun. 2018, 39, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Arranja, A.G.; Hennink, W.E.; Chassagne, C.; Denkova, A.G.; Nijsen, J.F.W. Preparation and characterization of inorganic radioactive holmium-166 microspheres for internal radionuclide therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110244. [Google Scholar] [CrossRef]
- Arranja, A.G.; Hennink, W.E.; Denkova, A.G.; Hendrikx, R.W.A.; Nijsen, J.F.W. Radioactive holmium phosphate microspheres for cancer treatment. Int. J. Pharm. 2018, 548, 73–81. [Google Scholar] [CrossRef] [PubMed]







| Name | Sequence Type | TE/TR (ms) | Flip Angle (°) | Total Slices (n) | Slice Thickness (mm) | Field of View (mm × mm) | Matrix Size | Acquisition Time (min:sec) | Time-Averaged RF-Power (W) | Whole-Body SAR (W/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| T1 VIBE | Spoiled gradient echo | 1.91/4.81 | 6 | 26 | 2.5 | 300 × 300 | 192 × 192 | 1:30 | 20.6 | 0.26 |
| T2 TSE | Turbo spin echo | 110/4490 | 150 | 31 | 3.0 | 160 × 160 | 256 × 230 | 1:30 | 113.2 | 1.52 |
| T2 HASTE | Half fourier single-shot turbo spin echo | 102/3000 | 180 | 29 | 5.0 | 266 × 266 | 256 × 256 | 1:30 | 151.5 | 2.00 |
| TRUFI | Balanced steady state free precession | 2.13/4.26 | 64 | 16 | 3.0 | 269 × 278 | 256 × 248 | 1:30 | 145.4 | 1.92 |
| TRUFI RFmax | Balanced steady state free precession | 2.13/4.26 | 40 | 16 | 3.0 | 280 × 280 | 256 × 256 | 1:30 | 146.7 | 1.96 |
| Sequence | Insertion Depth: 70 cm | Insertion Depth: 65 cm | Insertion Depth: 60 cm | |||
|---|---|---|---|---|---|---|
| ∆T (°C), mc− | ∆T 9(°C), mc+ | ∆T (°C), mc− | ∆T (°C), mc+ | ∆T (°C), mc− | ∆T (°C), mc+ | |
| T1 VIBE | 0.00 (−0.1–0.1) | 0.03 (0.0–0.1) | 0.00 (0.0–0.0) | 0.00 (−0.1–0.1) | 0.03 (−0.2–0.3) | 0.00 (−0.3–0.2) |
| T2 TSE | 0.00 (−0.1–0.1) | 0.17 (0.0–0.3) | 0.00 (0.0–0.0) | 0.13 (0.1–0.2) | −0.03 (−0.2–0.1) | 0.13 (0.0–0.3) |
| T2 HASTE | 0.00 (−0.1–0.1) | −0.03 (−0.1–0.1) | 0.00 (0.0–0.0) | 0.13 (0.1–0.2) | −0.03 (−0.1–0.1) | 0.10 (0.0–0.2) |
| TRUFI | 0.00 (−0.1–0.1) | 0.37 (0.3–0.5) | 0.03 (0.0–0.1) | 0.27 (0.2–0.4) | 0.00 (0.0–0.0) | 0.40 (0.4–0.4) |
| TRUFI RFmax | 0.03 (0.0–0.1) | 0.33 (0.3–0.4) | 0.03 (0.0–0.1) | 0.67 (0.6–0.7) | −0.03 (−0.1–0.1) | 0.50 (0.5–0.5) |
| Sequence | Catheter A | Catheter B | Microcatheter |
|---|---|---|---|
| FLASH | 5.5/3.1 | 21.9 | 2.3/4.7 |
| TSE | 8.1/1.9 | 36.9 | 1.6/2.8 |
| TRUFI | 5.9/2.4 | 30.9 | 2.4/2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roosen, J.; Arntz, M.J.; Janssen, M.J.R.; de Jong, S.F.; Fütterer, J.J.; Overduin, C.G.; Nijsen, J.F.W. Development of an MRI-Guided Approach to Selective Internal Radiation Therapy Using Holmium-166 Microspheres. Cancers 2021, 13, 5462. https://doi.org/10.3390/cancers13215462
Roosen J, Arntz MJ, Janssen MJR, de Jong SF, Fütterer JJ, Overduin CG, Nijsen JFW. Development of an MRI-Guided Approach to Selective Internal Radiation Therapy Using Holmium-166 Microspheres. Cancers. 2021; 13(21):5462. https://doi.org/10.3390/cancers13215462
Chicago/Turabian StyleRoosen, Joey, Mark J. Arntz, Marcel J. R. Janssen, Sytse F. de Jong, Jurgen J. Fütterer, Christiaan G. Overduin, and J. Frank W. Nijsen. 2021. "Development of an MRI-Guided Approach to Selective Internal Radiation Therapy Using Holmium-166 Microspheres" Cancers 13, no. 21: 5462. https://doi.org/10.3390/cancers13215462
APA StyleRoosen, J., Arntz, M. J., Janssen, M. J. R., de Jong, S. F., Fütterer, J. J., Overduin, C. G., & Nijsen, J. F. W. (2021). Development of an MRI-Guided Approach to Selective Internal Radiation Therapy Using Holmium-166 Microspheres. Cancers, 13(21), 5462. https://doi.org/10.3390/cancers13215462






