Comparing Outcomes of Oligometastases Treated with Hypofractionated Image-Guided Radiotherapy (HIGRT) with a Simultaneous Integrated Boost (SIB) Technique versus Metastasis Alone: A Multi-Institutional Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patient Selection
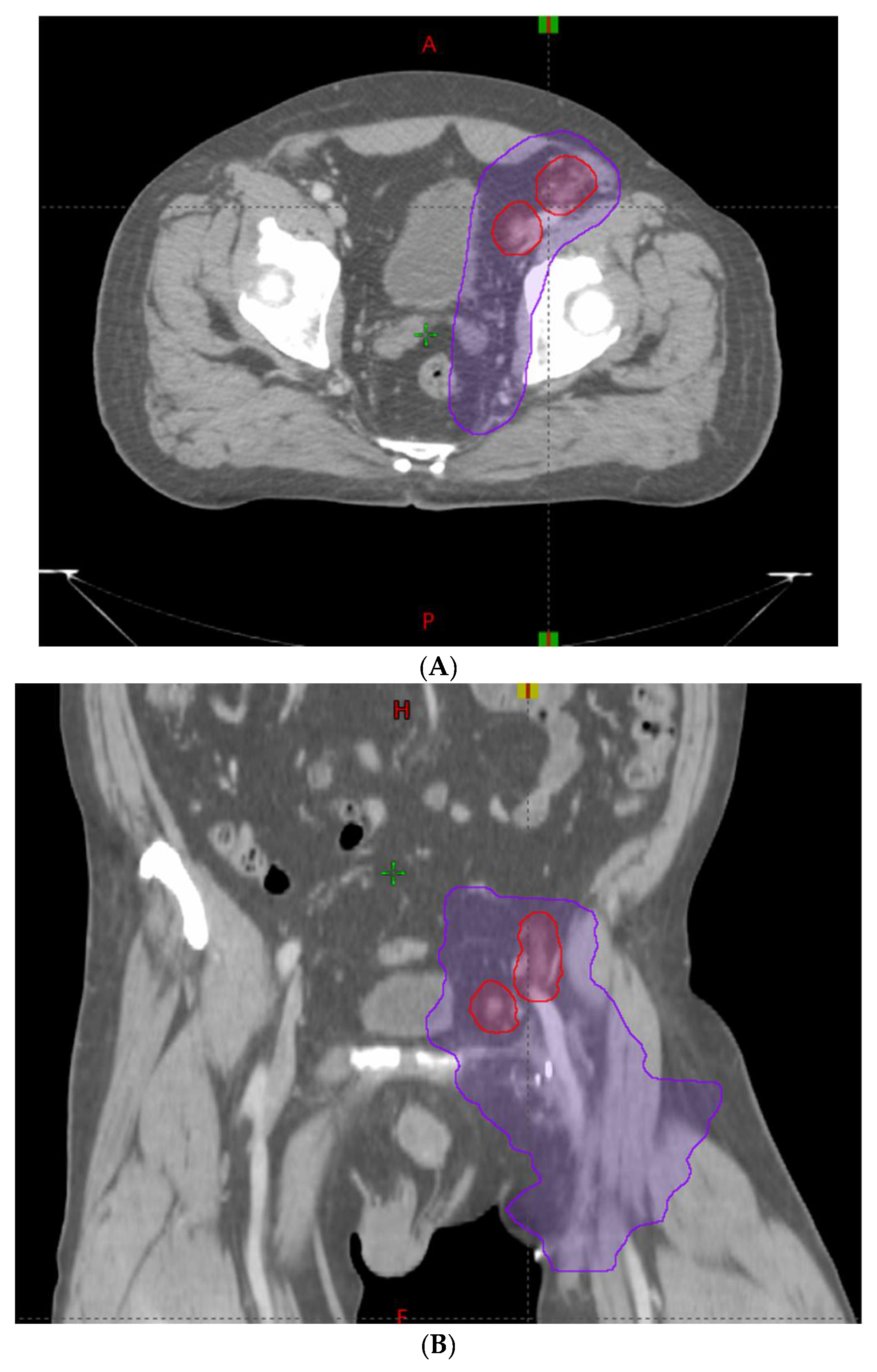
2.2. Treatment Technique
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Toxicity and Pain Analysis
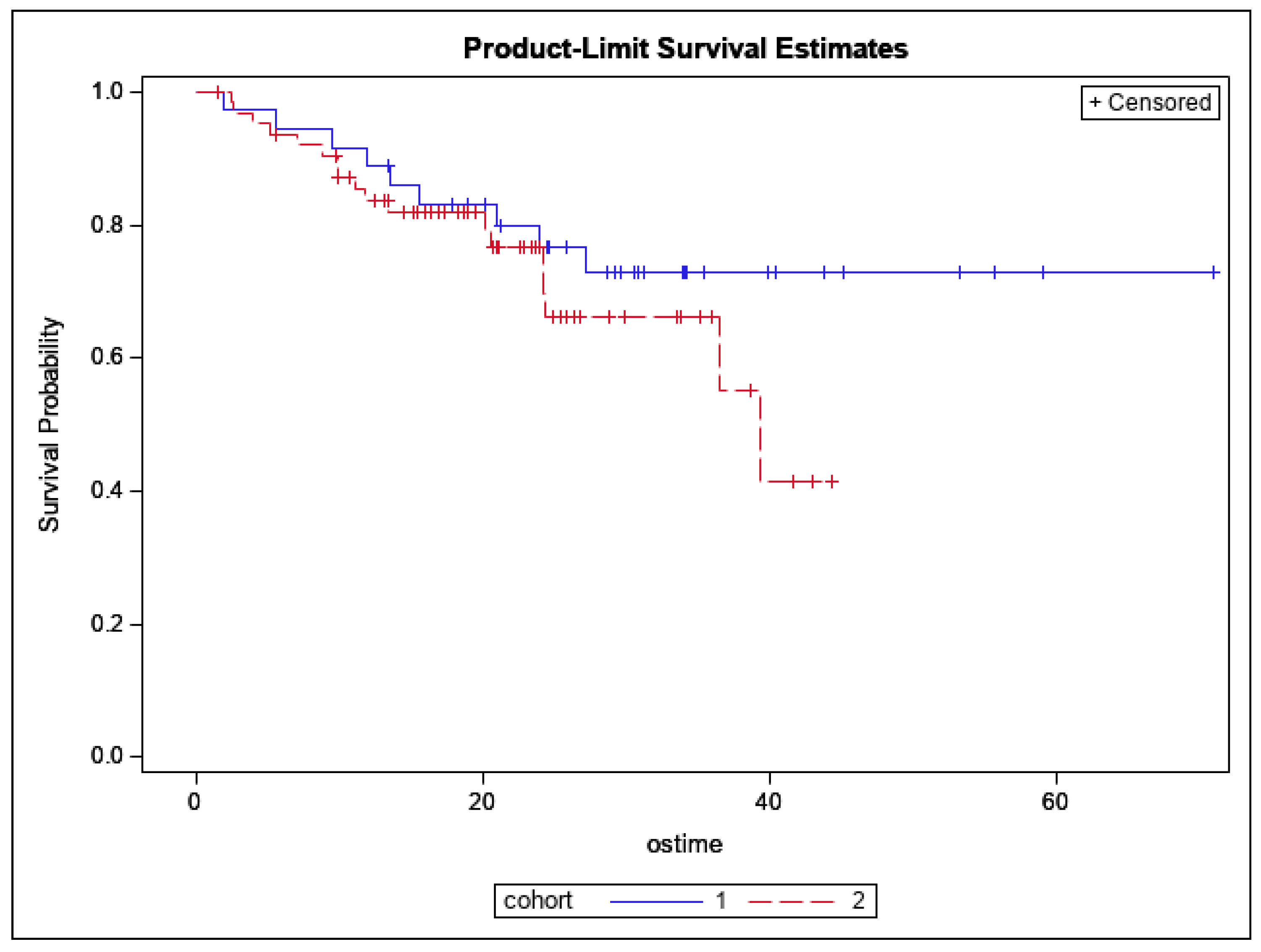
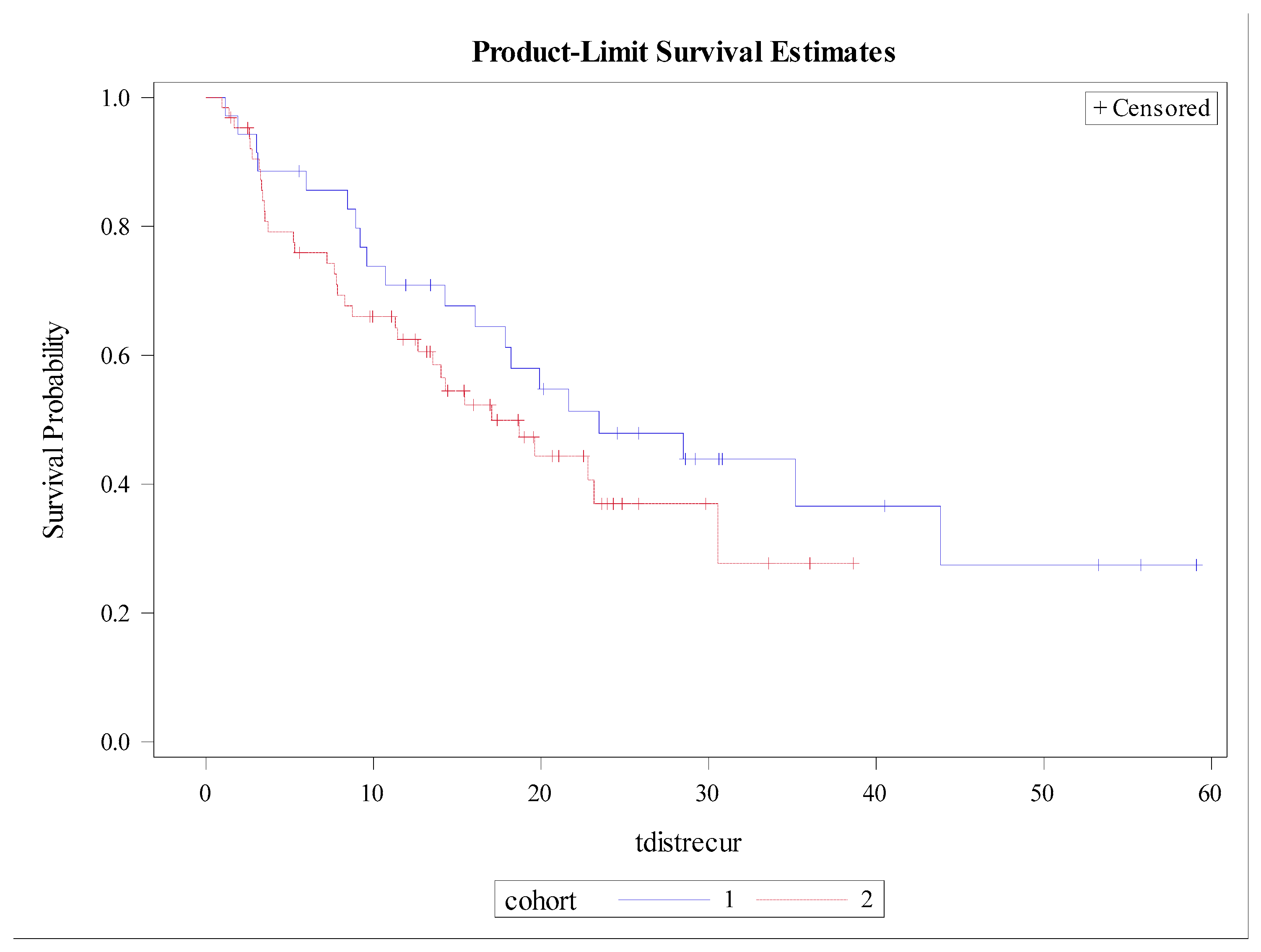
3.3. Patterns of Recurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, U.; Buyse, M.; Friedel, G.; Ginsberg, R.J.; Girard, P.; Goldstraw, P.; Johnston, M.; McCormack, P.; Pass, H.; Putnam, J.B., Jr.; et al. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J. Thorac. Cardiovasc. Surg. 1997, 113, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Fong, Y.; Cohen, A.M.; Fortner, J.G.; Enker, W.E.; Turnbull, A.D.; Coit, D.G.; Marrero, A.M.; Prasad, M.; Blumgart, L.H.; Brennan, M.F. Liver resection for colorectal metastases. J. Clin. Oncol. 1997, 15, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.R.; Blumenschein, G.R., Jr.; Lee, J.J.; Hernandez, M.; Ye, R.; Camidge, D.R.; Doebele, R.C.; Skoulidis, F.; Gaspar, L.E.; Gibbons, D.L.; et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016, 17, 1672–1682. [Google Scholar] [CrossRef] [Green Version]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Iyengar, P.; Wardak, Z.; Gerber, D.E.; Tumati, V.; Ahn, C.; Hughes, R.S.; Dowell, J.E.; Cheedella, N.; Nedzi, L.; Westover, K.D.; et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e173501. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef]
- Al-Hallaq, H.A.; Chmura, S.; Salama, J.K.; Winter, K.A.; Robinson, C.G.; Pisansky, T.M.; Borges, V.; Lowenstein, J.R.; McNulty, S.; Galvin, J.M.; et al. Rationale of technical requirements for NRG-BR001: The first NCI-sponsored trial of SBRT for the treatment of multiple metastases. Pract. Radiat. Oncol. 2016, 6, e291–e298. [Google Scholar] [CrossRef] [Green Version]
- Salama, J.K.; Milano, M.T. Radical irradiation of extracranial oligometastases. J. Clin. Oncol. 2014, 32, 2902–2912. [Google Scholar] [CrossRef] [Green Version]
- Chang, E.L.; Shiu, A.S.; Mendel, E.; Mathews, L.A.; Mahajan, A.; Allen, P.K.; Weinberg, J.S.; Brown, B.W.; Wang, X.S.; Woo, S.Y.; et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J. Neurosurg. Spine 2007, 7, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Koyfman, S.A.; Djemil, T.; Burdick, M.J.; Woody, N.; Balagamwala, E.H.; Reddy, C.A.; Angelov, L.; Suh, J.H.; Chao, S.T. Marginal recurrence requiring salvage radiotherapy after stereotactic body radiotherapy for spinal metastases. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.D.; Palta, M.; Williamson, H.; Price, J.G.; Czito, B.G.; Salama, J.K.; Moravan, M.J. Hypofractionated Image-Guided Radiation Therapy with Simultaneous-Integrated Boost Technique for Limited Metastases: A Multi-Institutional Analysis. Front. Oncol. 2019, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Milano, M.T.; Katz, A.W.; Schell, M.C.; Philip, A.; Okunieff, P. Descriptive analysis of oligometastatic lesions treated with curative-intent stereotactic body radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Swanick, C.W.; Allen, P.K.; Gomez, D.R.; Welsh, J.W.; Liao, Z.; Balter, P.A.; Chang, J.Y. Stereotactic ablative radiotherapy (SABR) using 70 Gy in 10 fractions for non-small cell lung cancer: Exploration of clinical indications. Radiother. Oncol. 2014, 112, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Chance, W.W.; Nguyen, Q.N.; Mehran, R.; Welsh, J.W.; Gomez, D.R.; Balter, P.; Komaki, R.; Liao, Z.; Chang, J.Y. Stereotactic ablative radiotherapy for adrenal gland metastases: Factors influencing outcomes, patterns of failure, and dosimetric thresholds for toxicity. Pract. Radiat. Oncol. 2017, 7, e195–e203. [Google Scholar] [CrossRef]
- Ost, P.; Jereczek-Fossa, B.A.; Van As, N.; Zilli, T.; Tree, A.; Henderson, D.; Orecchia, R.; Casamassima, F.; Surgo, A.; Miralbell, R.; et al. Pattern of Progression after Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Nodal Recurrences. Clin. Oncol. 2016, 28, e115–e120. [Google Scholar] [CrossRef]
- Decaestecker, K.; De Meerleer, G.; Lambert, B.; Delrue, L.; Fonteyne, V.; Claeys, T.; De Vos, F.; Huysse, W.; Hautekiet, A.; Maes, G.; et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat. Oncol. 2014, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, Q.N.; Shiu, A.S.; Rhines, L.D.; Wang, H.; Allen, P.K.; Wang, X.S.; Chang, E.L. Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1185–1192. [Google Scholar] [CrossRef]
- Gerszten, P.C.; Burton, S.A.; Ozhasoglu, C.; Welch, W.C. Radiosurgery for spinal metastases: Clinical experience in 500 cases from a single institution. Spine 2007, 32, 193–199. [Google Scholar] [CrossRef]
- Sogono, P.; Bressel, M.; David, S.; Shaw, M.; Chander, S.; Chu, J.; Plumridge, N.; Byrne, K.; Hardcastle, N.; Kron, T.; et al. Safety, Efficacy, and Patterns of Failure After Single-Fraction Stereotactic Body Radiation Therapy (SBRT) for Oligometastases. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 756–763. [Google Scholar] [CrossRef]
- Chi, A.; Liao, Z.; Nguyen, N.P.; Xu, J.; Stea, B.; Komaki, R. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: Clinical implications. Radiother. Oncol. 2010, 94, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kotsakis, A.; Sarra, E.; Peraki, M.; Koukourakis, M.; Apostolaki, S.; Souglakos, J.; Mavromanomakis, E.; Vlachonikolis, J.; Georgoulias, V. Docetaxel-induced lymphopenia in patients with solid tumors: A prospective phenotypic analysis. Cancer 2000, 89, 1380–1386. [Google Scholar] [CrossRef]
- Chmura, S.; Winter, K.A.; Robinson, C.; Pisansky, T.M.; Borges, V.; Al-Hallaq, H.; Matuszak, M.; Park, S.S.; Yi, S.; Hasan, Y.; et al. Evaluation of Safety of Stereotactic Body Radiotherapy for the Treatment of Patients with Multiple Metastases: Findings from the NRG-BR001 Phase 1 Trial. JAMA Oncol. 2021, 7, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Poon, I.; Erler, D.; Dagan, R.; Redmond, K.J.; Foote, M.; Badellino, S.; Biswas, T.; Louie, A.V.; Lee, Y.; Atenafu, E.G.; et al. Evaluation of Definitive Stereotactic Body Radiotherapy and Outcomes in Adults with Extracranial Oligometastasis. JAMA Netw. Open 2020, 3, e2026312. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, I.; Bibault, J.E.; Dewas, S.; Kramar, A.; Mirabel, X.; Prevost, B.; Lacornerie, T.; Jerraya, H.; Lartigau, E. A single-institution study of stereotactic body radiotherapy for patients with unresectable visceral pulmonary or hepatic oligometastases. Radiat. Oncol. 2012, 7, 164. [Google Scholar] [CrossRef] [Green Version]
- Rusthoven, K.E.; Kavanagh, B.D.; Burri, S.H.; Chen, C.; Cardenes, H.; Chidel, M.A.; Pugh, T.J.; Kane, M.; Gaspar, L.E.; Schefter, T.E. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J. Clin. Oncol. 2009, 27, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Rusthoven, K.E.; Kavanagh, B.D.; Cardenes, H.; Stieber, V.W.; Burri, S.H.; Feigenberg, S.J.; Chidel, M.A.; Pugh, T.J.; Franklin, W.; Kane, M.; et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J. Clin. Oncol. 2009, 27, 1572–1578. [Google Scholar] [CrossRef] [Green Version]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef] [Green Version]
- Beckham, T.H.; Yang, T.J.; Gomez, D.; Tsai, C.J. Metastasis-directed therapy for oligometastasis and beyond. Br. J. Cancer 2021, 124, 136–141. [Google Scholar] [CrossRef]
- Fionda, B.; Iezzi, R.; Tagliaferri, L. Evolutionary game theory and oligometastatic patient: Considering the role of interventional oncology. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7272–7274. [Google Scholar] [CrossRef]
- Lievens, Y.; Guckenberger, M.; Gomez, D.; Hoyer, M.; Iyengar, P.; Kindts, I.; Romero, A.M.; Nevens, D.; Palma, D.; Park, C.; et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother. Oncol. 2020, 148, 157–166. [Google Scholar] [CrossRef] [PubMed]



| HIGRT-SIB | HIGRT-MA | |
|---|---|---|
| Age, Median (range) | 69 | 66 |
| Gender, n (%) | ||
| Female | 10 (10%) | 20 (20%) |
| Male | 52 (51%) | 19 (19%) |
| Primary Tumor Site, n (%) | ||
| Prostate | 36 (40%) | 14 (30%) |
| Breast | 0 (0%) | 9 (20%) |
| Lung | 15 (17%) | 6 (13%) |
| Gastrointestinal | 14 (16%) | 3 (7%) |
| Kidney | 2 (2%) | 6 (13%) |
| Thyroid | 3 (3%) | 3 (7%) |
| Skin | 3 (3%) | 0 (0%) |
| Head and Neck | 1 (1%) | 0 (0%) |
| Testicle | 1 (1%) | 0 (0%) |
| Gynecologic | 1 (1%) | 0 (0%) |
| Other | 14 (16%) | 5 (10%) |
| Months from diagnosis to first metastasis, median (IQR) | 44.75 (IQR: 11.9–73.1) | 55.1 (IQR: 0–55.1) |
| Number of active metastases at the time of HIGRT, n (%) | ||
| 1 | 39 (43%) | 27 (59%) |
| 2 | 35 (39%) | 13 (28%) |
| 3 | 7 (8%) | 6 (13%) |
| 4 | 1 (1%) | 0 (0%) |
| 5 | 8 (9%) | 0 (0%) |
| TREATED METASTASIS-SPECIFIC VARIABLE | ||
| HIGRT target, n (%) | ||
| Lymph node metastasis | 48 (53%) | 6 (13%) |
| Painful osseous metastasis | 22 (24%) | 11 (24%) |
| Non-painful osseous metastasis | 20 (23%) | 29 (63%) |
| HIGRT anatomic location, n (%) | ||
| Abdominopelvic | 46 (51%) | 12 (26%) |
| Spine | 16 (18%) | 19 (41%) |
| Sternum or rib | 11 (12%) | 6 (13%) |
| Supraclavicular fossa, mediastinum, or axilla | 16 (18%) | 7 (15%) |
| Extremity | 1 (1%) | 2 (5%) |
| Greatest diameter of largest metastasis in cm, Median, (IQR) | 2.6 (1.7–3.6) | 2.7 (1.8–3.3) |
| GTV in cm3, Median (IQR) | 11.6 (3.9–20.6) | 5.6 (2.3–17.3) |
| PTVboost in cm3, Median (IQR) | 28.3 (11.9–54.2) | 34.3 (18.7–73.9) |
| PTVelect in cm3, Median (IQR) | 229.2 (111.6–346.4) | N/A |
| Dose to PTVelect in Gy, Median (IQR) | 30 (30–30) | N/A |
| Dose to PTVboost in Gy, Median (IQR) | 50 (50–50) | 30 (18–50) |
| HIGRT Fractions, Median (IQR) | 10 (10–10) | 7 (1–10) |
| HIGRT duration in days, Median (IQR) | 13 (11–14) | 10.5 (0–13) |
| All Patients Toxicity | Acute Grade 1–2 N (%) | Acute Grade ≥ 3 N (%) | Late Grade 1–2 N (%) | Late Grade ≥ 3 N (%) |
|---|---|---|---|---|
| GI 1 | 31 (29) | 0 (0) | 3 (2) | 0 (0) |
| GU 2 | 2 (2) | 0 (0) | 0 (0) | 0 (0) |
| Hematologic | 2 (2) | 1 (1) | 0 (0) | 0 (0) |
| Neurologic | 5 (5) | 0 (0) | 3 (2) | 4 (3) |
| Respiratory | 2 (2) | 0 (0) | 0 (0) | 0 (0) |
| General | 52 (48) | 0 (0) | 4 (4) | 0 (0) |
| Pain Flare | 5 (5) | 0 (0) |
| Toxicity | Acute Grade 1–2 N (%) | Acute Grade ≥ 3 N (%) | Late Grade 1–2 N (%) | Late Grade ≥ 3 N (%) |
|---|---|---|---|---|
| GI 1 | 4 (10) | 0 (0) | 0 (0) | 0 (0) |
| GU 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hematologic | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Neurologic | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Respiratory | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| General | 9 (23) | 0 (0) | 1 (3) | 0 (0) |
| Pain Flare | 0 (0) | 0 (0) |
| Toxicity | Acute Grade 1–2 N (%) | Acute Grade ≥ 3 N (%) | Late Grade 1–2 N (%) | Late Grade ≥ 3 N (%) |
|---|---|---|---|---|
| GI 1 | 27 (40) | 0 (0) | 3 (4) | 0 (0) |
| GU 2 | 2 (3) | 0 (0) | 0 (0) | 0 (0) |
| Hematologic | 2 (3) | 1 (1) | 0 (0) | 0 (0) |
| Neurologic | 4 (6) | 0 (0) | 3 (4) | 4 (6) |
| Respiratory | 2 (3) | 0 (0) | 0 (0) | 0 (0) |
| General | 43 (63) | 0 (0) | 3 (4) | 0 (0) |
| Pain Flare | 5 (7) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shenker, R.F.; Price, J.G.; Jacobs, C.D.; Palta, M.; Czito, B.G.; Mowery, Y.M.; Kirkpatrick, J.P.; Boyer, M.J.; Oyekunle, T.; Niedzwiecki, D.; et al. Comparing Outcomes of Oligometastases Treated with Hypofractionated Image-Guided Radiotherapy (HIGRT) with a Simultaneous Integrated Boost (SIB) Technique versus Metastasis Alone: A Multi-Institutional Analysis. Cancers 2022, 14, 2403. https://doi.org/10.3390/cancers14102403
Shenker RF, Price JG, Jacobs CD, Palta M, Czito BG, Mowery YM, Kirkpatrick JP, Boyer MJ, Oyekunle T, Niedzwiecki D, et al. Comparing Outcomes of Oligometastases Treated with Hypofractionated Image-Guided Radiotherapy (HIGRT) with a Simultaneous Integrated Boost (SIB) Technique versus Metastasis Alone: A Multi-Institutional Analysis. Cancers. 2022; 14(10):2403. https://doi.org/10.3390/cancers14102403
Chicago/Turabian StyleShenker, Rachel F., Jeremy G. Price, Corbin D. Jacobs, Manisha Palta, Brian G. Czito, Yvonne M. Mowery, John P. Kirkpatrick, Matthew J. Boyer, Taofik Oyekunle, Donna Niedzwiecki, and et al. 2022. "Comparing Outcomes of Oligometastases Treated with Hypofractionated Image-Guided Radiotherapy (HIGRT) with a Simultaneous Integrated Boost (SIB) Technique versus Metastasis Alone: A Multi-Institutional Analysis" Cancers 14, no. 10: 2403. https://doi.org/10.3390/cancers14102403
APA StyleShenker, R. F., Price, J. G., Jacobs, C. D., Palta, M., Czito, B. G., Mowery, Y. M., Kirkpatrick, J. P., Boyer, M. J., Oyekunle, T., Niedzwiecki, D., Song, H., & Salama, J. K. (2022). Comparing Outcomes of Oligometastases Treated with Hypofractionated Image-Guided Radiotherapy (HIGRT) with a Simultaneous Integrated Boost (SIB) Technique versus Metastasis Alone: A Multi-Institutional Analysis. Cancers, 14(10), 2403. https://doi.org/10.3390/cancers14102403





