Designing the Surface Chemistry of Inorganic Nanocrystals for Cancer Imaging and Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Inorganic Nanocrystals for Cancer Imaging and Therapy
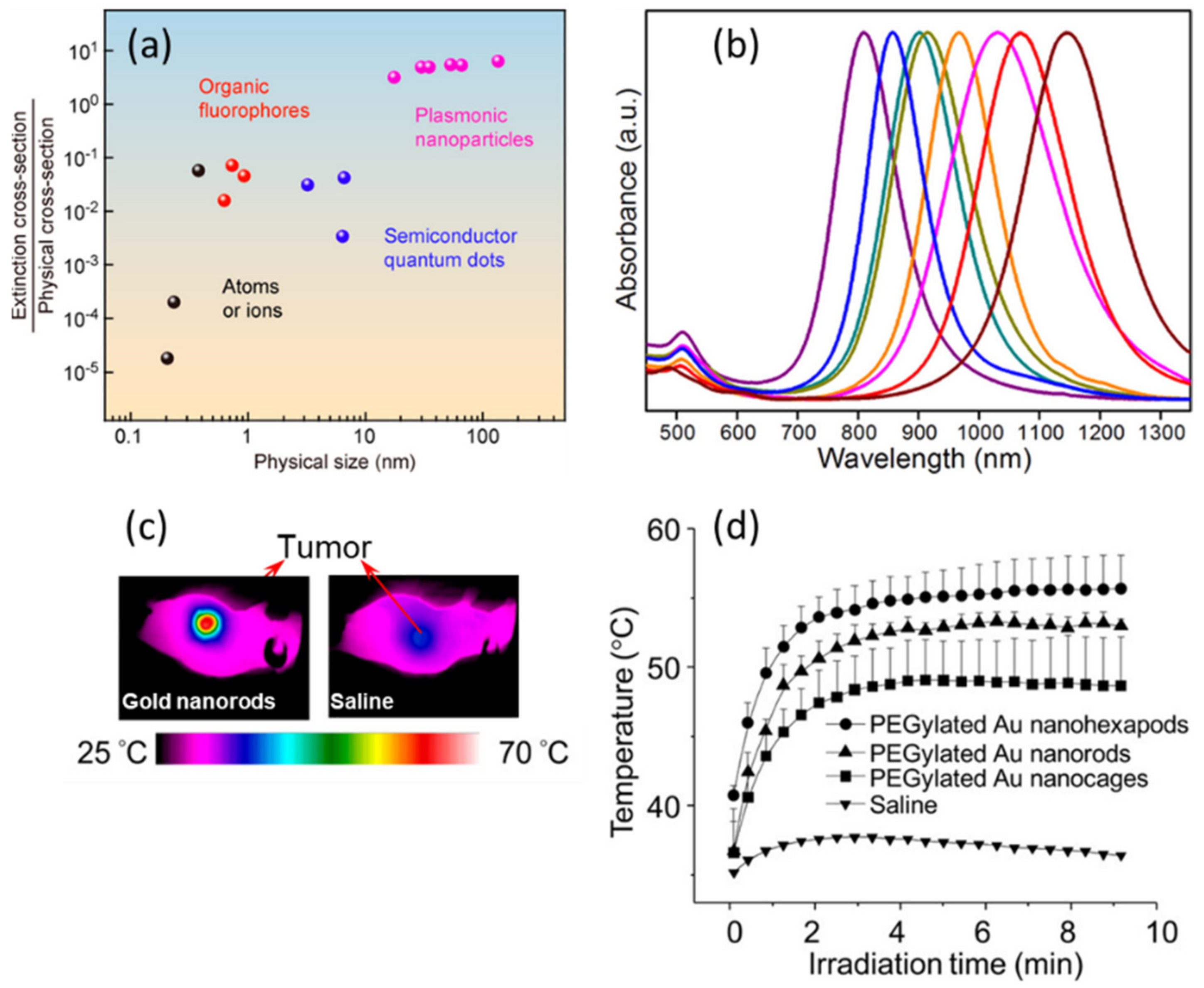
2.1. Gold NPs
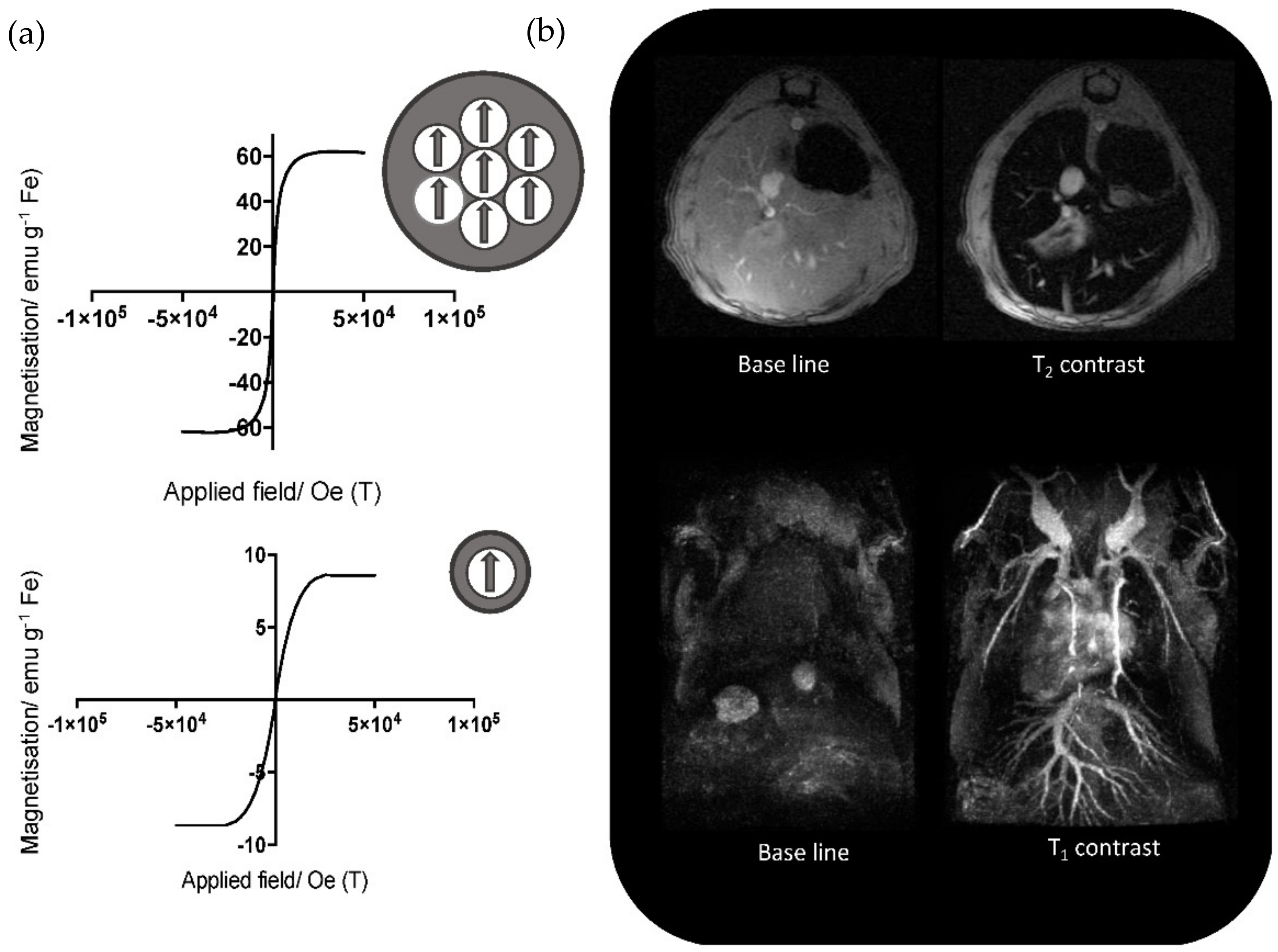
2.2. Magnetic NPs
2.3. Semiconductor Quantum Dots
2.4. Inorganic Nanocrystals as Therapeutic Enhancers
3. Designing Surface Ligands
3.1. Anchoring Functions
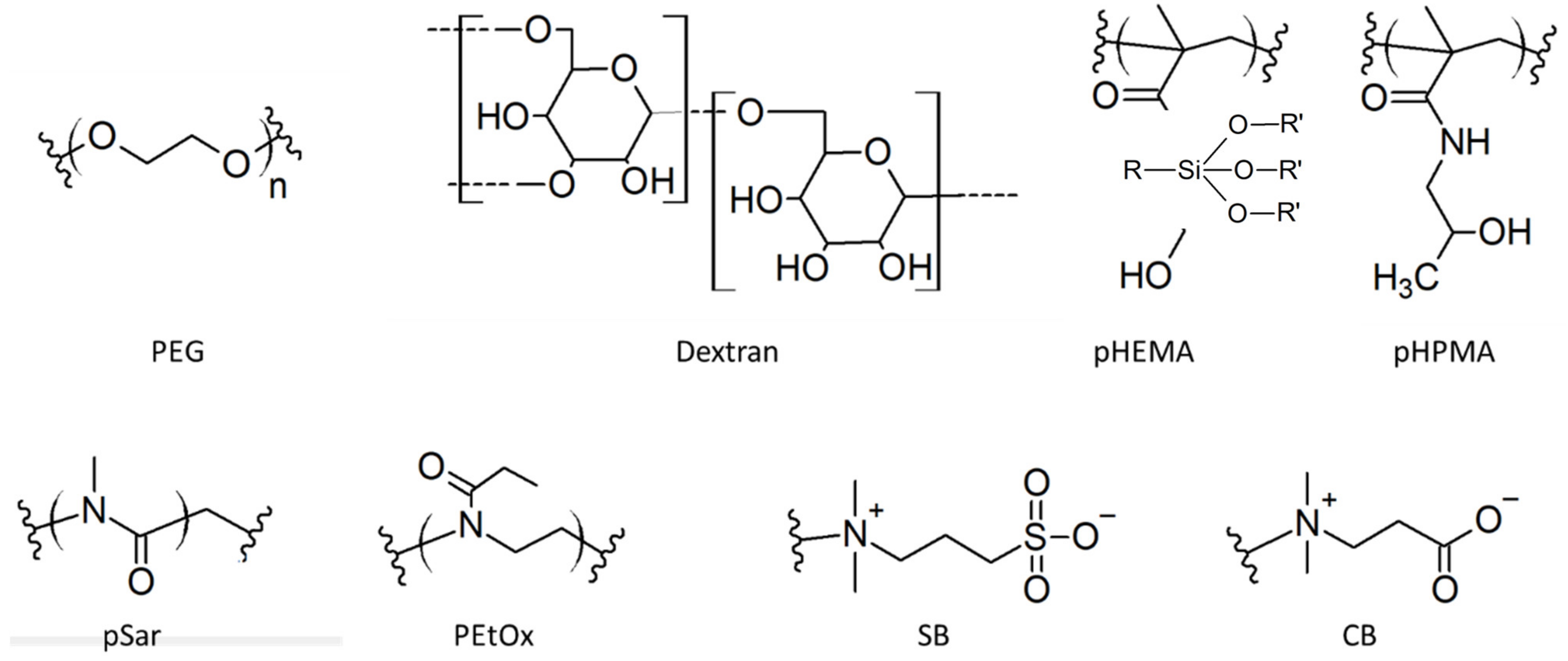
3.2. Hydrophilic Functions
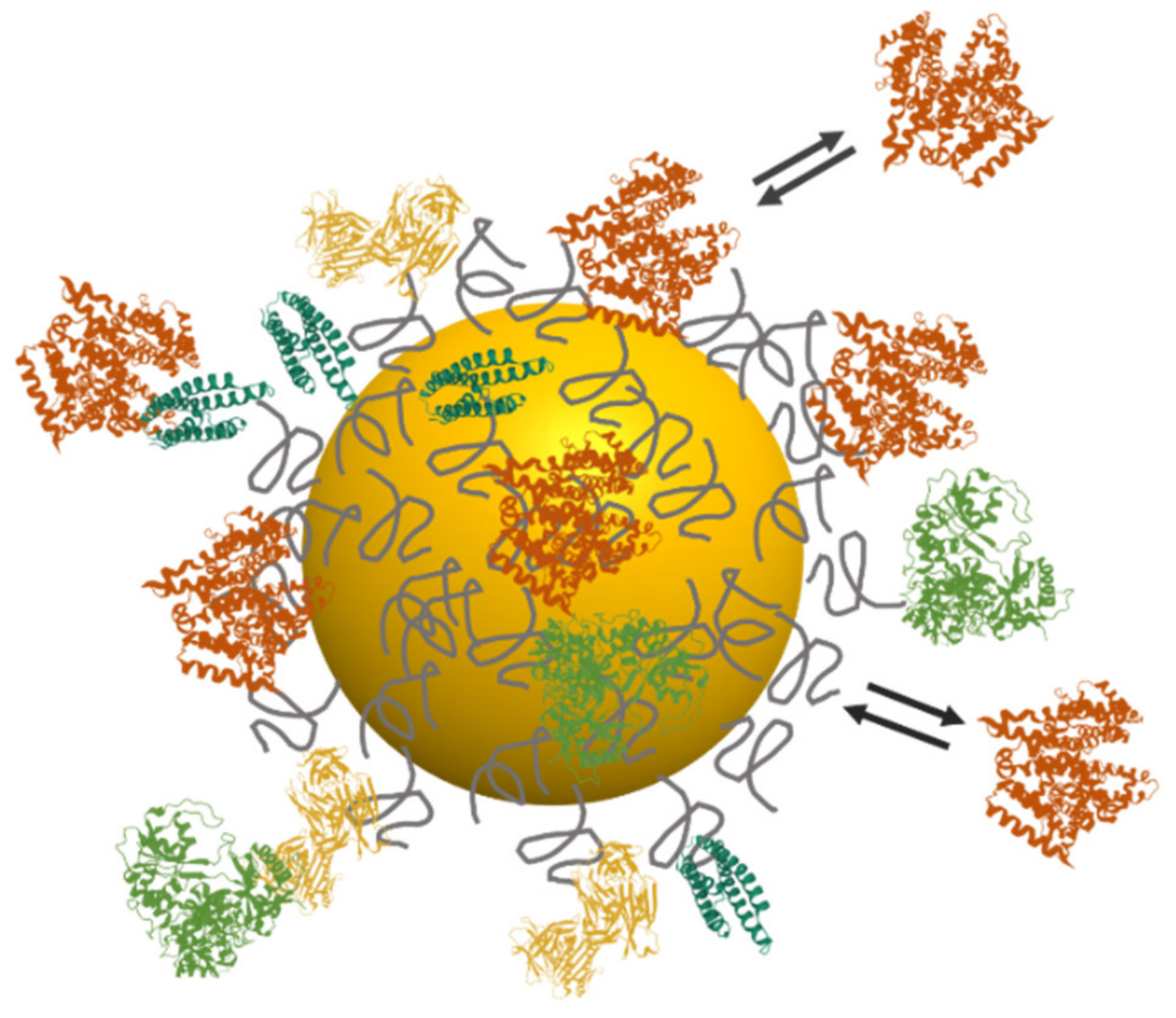
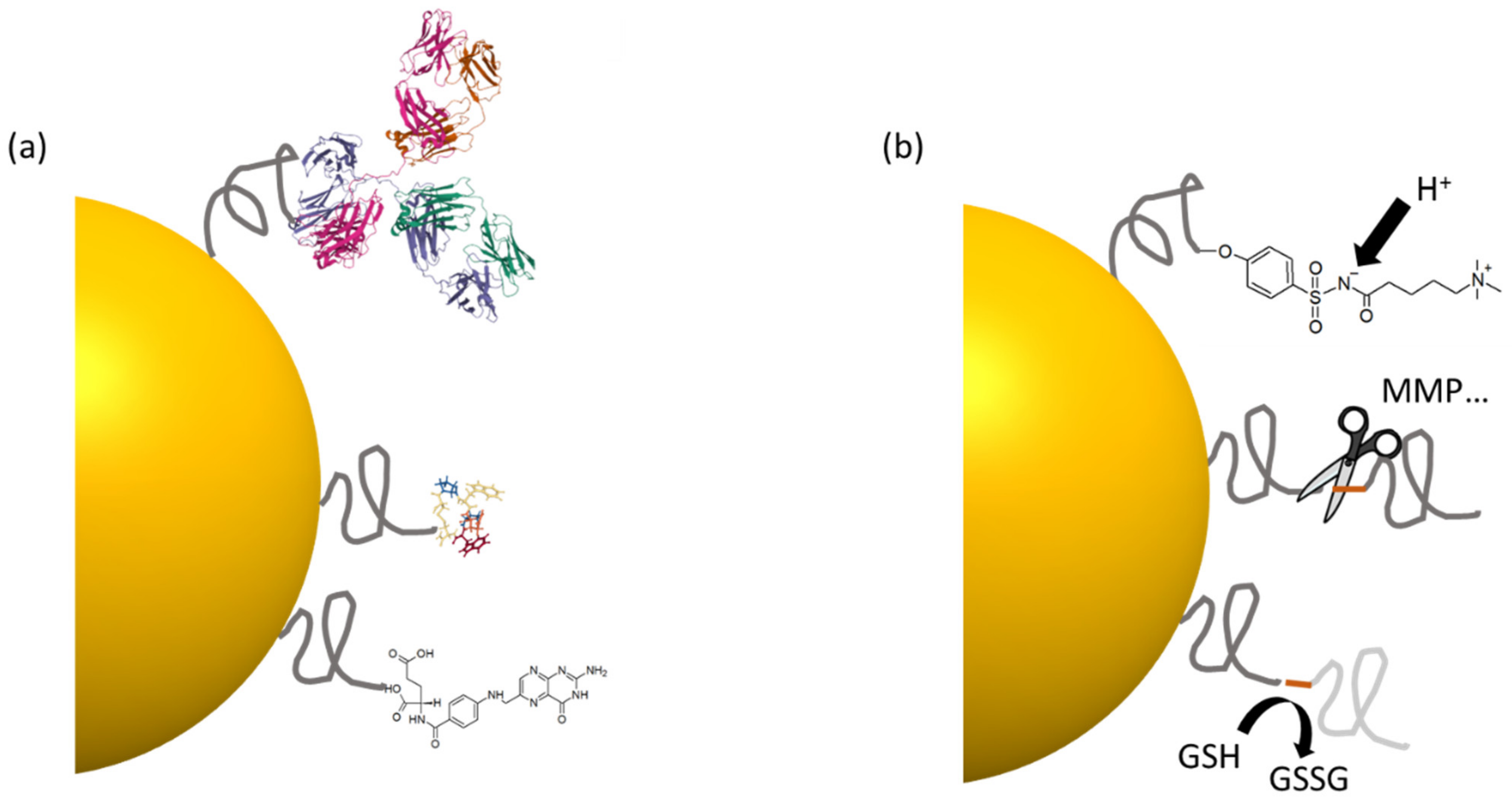
4. Interactions with Surrounding Biomolecules: The Protein Corona
4.1. Formation of the Protein Corona
4.2. Analytical Methods to Characterize the Protein Corona
5. In Vivo Biodistribution and Clinical Outcomes
5.1. Circulation in Blood and Elimination
5.2. Tumor Targeting Strategies
5.2.1. Active Biomolecular Targeting
5.2.2. Tumor Microenvironment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Smith, B.R.; Gambhir, S.S. Nanomaterials for In Vivo Imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef] [PubMed]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Mou, L.; Jiang, X. Surface Chemistry of Gold Nanoparticles for Health-Related Applications. Chem. Sci. 2020, 11, 923–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattoussi, H.; Palui, G.; Na, H.B. Luminescent Quantum Dots as Platforms for Probing in Vitro and in Vivo Biological Processes. Adv. Drug Deliv. Rev. 2012, 64, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer Therapy with Iron Oxide Nanoparticles: Agents of Thermal and Immune Therapies. Adv. Drug Deliv. Rev. 2020, 163–164, 65–83. [Google Scholar] [CrossRef]
- Beik, J.; Khateri, M.; Khosravi, Z.; Kamrava, S.K.; Kooranifar, S.; Ghaznavi, H.; Shakeri-Zadeh, A. Gold Nanoparticles in Combinatorial Cancer Therapy Strategies. Coord. Chem. Rev. 2019, 387, 299–324. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Wu, Y.; El-Sayed, M.A. Gold-Nanoparticle-Assisted Plasmonic Photothermal Therapy Advances Toward Clinical Application. J. Phys. Chem. C 2019, 123, 15375–15393. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold Nanoparticles (GNPs) in Biomedical and Clinical Applications: A Review. Nano Select 2022, 3, 792–828. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.-P. Magnetic Nanoparticles in Nanomedicine: A Review of Recent Advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef] [Green Version]
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and Cancer Therapy: Perspectives for Application of Nanoparticles in the Treatment of Cancers. J. Cell. Physiol. 2020, 235, 1962–1972. [Google Scholar] [CrossRef]
- Fernandes, N.; Rodrigues, C.F.; Moreira, A.F.; Correia, I.J. Overview of the Application of Inorganic Nanomaterials in Cancer Photothermal Therapy. Biomater. Sci. 2020, 8, 2990–3020. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Altug, H.; Oh, S.-H.; Maier, S.A.; Homola, J. Advances and Applications of Nanophotonic Biosensors. Nat. Nanotechnol. 2022, 17, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Jauffred, L.; Samadi, A.; Klingberg, H.; Bendix, P.M.; Oddershede, L.B. Plasmonic Heating of Nanostructures. Chem. Rev. 2019, 119, 8087–8130. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Chen, J.; Au, L.; Lu, X.; Li, X.; Xia, Y. Gold Nanocages for Biomedical Applications. Adv. Mater. 2007, 19, 3177–3184. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Au, L.; Li, X.; Xia, Y. Facile Synthesis of Ag Nanocubes and Au Nanocages. Nat. Protoc. 2007, 2, 2182–2190. [Google Scholar] [CrossRef]
- Li, S.; Huang, H.; Shao, L.; Wang, J. How to Utilize Excited Plasmon Energy Efficiently. ACS Nano 2021, 15, 10759–10768. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-Based Lateral Flow Biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef] [Green Version]
- Thakor, A.S.; Jokerst, J.; Zavaleta, C.; Massoud, T.F.; Gambhir, S.S. Gold Nanoparticles: A Revival in Precious Metal Administration to Patients. Nano Lett. 2011, 11, 4029–4036. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.W. State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis. Pharmaceutics 2020, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Chen, Y.; Su, Y.; Huang, Y.; Chen, R.; Lei, J. Application of Silver Nanoparticle-Based SERS Spectroscopy for DNA Analysis in Radiated Nasopharyngeal Carcinoma Cells. J. Raman Spectrosc. 2013, 44, 680–685. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, X.; Tang, P.; Zhang, D.; Tian, J.; Zhong, L. Gold Nanoparticle (AuNP)-Based Surface-Enhanced Raman Scattering (SERS) Probe of Leukemic Lymphocytes. Plasmonics 2016, 11, 1361–1368. [Google Scholar] [CrossRef]
- Tam, N.C.M.; McVeigh, P.Z.; MacDonald, T.D.; Farhadi, A.; Wilson, B.C.; Zheng, G. Porphyrin–Lipid Stabilized Gold Nanoparticles for Surface Enhanced Raman Scattering Based Imaging. Bioconj. Chem. 2012, 23, 1726–1730. [Google Scholar] [CrossRef] [PubMed]
- Quynh, L.M.; Nam, N.H.; Kong, K.; Nhung, N.T.; Notingher, I.; Henini, M.; Luong, N.H. Surface-Enhanced Raman Spectroscopy Study of 4-ATPon Gold Nanoparticles for Basal Cell CarcinomaFingerprint Detection. J. Electron. Mater. 2016, 45, 2563–2568. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Cole, A.J.; Van de Sompel, D.; Gambhir, S.S. Gold Nanorods for Ovarian Cancer Detection with Photoacoustic Imaging and Resection Guidance via Raman Imaging in Living Mice. ACS Nano 2012, 6, 10366–10377. [Google Scholar] [CrossRef] [Green Version]
- Mantri, Y.; Jokerst, J.V. Engineering Plasmonic Nanoparticles for Enhanced Photoacoustic Imaging. ACS Nano 2020, 14, 9408–9422. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer Cell Imaging and Photothermal Therapy in the Near-Infrared Region by Using Gold Nanorods. J. Am. Chem. Soc 2006, 128, 2115–2120. [Google Scholar] [CrossRef]
- Liu, Y.; Ashton, J.R.; Moding, E.J.; Yuan, H.; Register, J.K.; Fales, A.M.; Choi, J.; Whitley, M.J.; Zhao, X.; Qi, Y. A Plasmonic Gold Nanostar Theranostic Probe for In Vivo Tumor Imaging and Photothermal Therapy. Theranostics 2015, 5, 946–960. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Chen, D.; Li, L.; Liu, T.; Tan, L.; Wu, X.; Tang, F. Multifunctional Gold Nanoshells on Silica Nanorattles: A Platform for the Combination of Photothermal Therapy and Chemotherapy with Low Systemic Toxicity. Angew. Chem. Int. Ed. 2011, 50, 891–895. [Google Scholar] [CrossRef]
- Yuan, H.; Fales, A.M.; Vo-Dinh, T. TAT Peptide-Functionalized Gold Nanostars: Enhanced Intracellular Delivery and Efficient NIR Photothermal Therapy Using Ultralow Irradiance. J. Am. Chem. Soc. 2012, 134, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, T.; Pons, T.; Hou, X.; Van Do, K.; Caron, B.; Rigal, M.; Di Benedetto, M.; Palpant, B.; Leboeuf, C.; Janin, A.; et al. Pulsed-Laser Irradiation of Multifunctional Gold Nanoshells to Overcome Trastuzumab Resistance in HER2-Overexpressing Breast Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, N.; Jiang, Y.-W.; Zhang, X.; Wu, H.; Myers, J.N.; Liu, P.; Jin, H.; Gu, N.; He, N.; Wu, F.-G. Enhanced Radiosensitization of Gold Nanospikes via Hyperthermia in Combined Cancer Radiation and Photothermal Therapy. ACS Appl. Mater. Interfaces 2016, 8, 28480–28494. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Li, W.; Peng, J.; Yang, Q.; Li, H.; Wei, Y.; Zhang, X.; Qian, Z. Combined Cancer Photothermal-Chemotherapy Based on Doxorubicin/Gold Nanorod-Loaded Polymersomes. Theranostics 2015, 5, 345–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vankayala, R.; Huang, Y.K.; Kalluru, P.; Chiang, C.S.; Hwang, K.C. First Demonstration of Gold Nanorods-Mediated Photodynamic Therapeutic Destruction of Tumors via near Infra-Red Light Activation. Small 2014, 10, 1612–1622. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, R.; Gao, F.; Wang, Y.; Jiang, X.; Gao, X. Plasmon-Mediated Generation of Reactive Oxygen Species from near-Infrared Light Excited Gold Nanocages for Photodynamic Therapy in Vitro. ACS Nano 2014, 8, 7260–7271. [Google Scholar] [CrossRef]
- He, L.; Mao, C.; Brasino, M.; Harguindey, A.; Park, W.; Goodwin, A.P.; Cha, J.N. TiO2-Capped Gold Nanorods for Plasmon-Enhanced Production of Reactive Oxygen Species and Photothermal Delivery of Chemotherapeutic Agents. ACS Appl. Mater. Interfaces 2018, 10, 27965–27971. [Google Scholar] [CrossRef]
- Chuang, C.C.; Chen, Y.N.; Wang, Y.Y.; Huang, Y.C.; Lin, S.Y.; Huang, R.Y.; Jang, Y.Y.; Yang, C.C.; Huang, Y.F.; Chang, C.W. Stem Cell-Based Delivery of Gold/Chlorin E6 Nanocomplexes for Combined Photothermal and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 30021–30030. [Google Scholar] [CrossRef]
- Peng, C.; Zheng, L.; Chen, Q.; Shen, M.; Guo, R.; Wang, H.; Cao, X.; Zhang, G.; Shi, X. PEGylated Dendrimer-Entrapped Gold Nanoparticles for in Vivo Blood Pool and Tumor Imaging by Computed Tomography. Biomaterials 2012, 33, 1107–1119. [Google Scholar] [CrossRef]
- Dou, Y.; Guo, Y.; Li, X.; Li, X.; Wang, S.; Wang, L.; Lv, G.; Zhang, X.; Wang, H.; Gong, X.; et al. Size-Tuning Ionization to Optimize Gold Nanoparticles for Simultaneous Enhanced CT Imaging and Radiotherapy. ACS Nano 2016, 10, 2536–2548. [Google Scholar] [CrossRef]
- Ye, X.; Zheng, C.; Chen, J.; Gao, Y.; Murray, C.B. Using Binary Surfactant Mixtures to Simultaneously Improve the Dimensional Tunability and Monodispersity in the Seeded Growth of Gold Nanorods. Nano Lett. 2013, 13, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Black, K.C.L.; Luehmann, H.; Li, W.; Zhang, Y.; Cai, X.; Wan, D.; Liu, S.-Y.; Li, M.; Kim, P.; et al. Comparison Study of Gold Nanohexapods, Nanorods, and Nanocages for Photothermal Cancer Treatment. ACS Nano 2013, 7, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S. Recent Progress on Magnetic Iron Oxide Nanoparticles: Synthesis, Surface Functional Strategies and Biomedical Applications. Sci. Technol. Adv. Mater. 2015, 16, 23501. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Jeon, M.; Halbert, M.V.; Stephen, Z.R.; Zhang, M. Iron Oxide Nanoparticles as T1 Contrast Agents for Magnetic Resonance Imaging: Fundamentals, Challenges, Applications, and Prospectives. Adv. Mater. 2021, 33, 1906539. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H.; Felix, R. Magnetic Fluid Hyperthermia (MFH): Cancer Treatment with AC Magnetic Field Induced Excitation of Biocompatible Superparamagnetic Nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic Fluid Hyperthermia: Focus on Superparamagnetic Iron Oxide Nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- Chang, D.; Lim, M.; Goos, J.A.C.M.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharmacol. 2018, 9, 831. [Google Scholar] [CrossRef] [Green Version]
- Cotin, G.; Blanco-Andujar, C.; Nguyen, D.V.; Affolter, C.; Boutry, S.; Boos, A.; Ronot, P.; Uring-Lambert, B.; Choquet, P.; Zorn, P.E.; et al. Dendron Based Antifouling, MRI and Magnetic Hyperthermia Properties of Different Shaped Iron Oxide Nanoparticles. Nanotechnology 2019, 30, 37. [Google Scholar] [CrossRef]
- Fernández-Barahona, I.; Muñoz-Hernando, M.; Ruiz-Cabello, J.; Herranz, F.; Pellico, J. Iron Oxide Nanoparticles: An Alternative for Positive Contrast in Magnetic Resonance Imaging. Inorganics 2020, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum Dot Bioconjugates for Imaging, Labelling and Sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Dahan, M.; Lévi, S.; Luccardini, C.; Rostaing, P.; Riveau, B.; Triller, A. Diffusion Dynamics of Glycine Receptors Revealed by Single-Quantum Dot Tracking. Science 2003, 302, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Bouccara, S.; Sitbon, G.; Fragola, A.; Loriette, V.; Lequeux, N.; Pons, T. Enhancing Fluorescence in Vivo Imaging Using Inorganic Nanoprobes. Curr. Opin. Biotechnol. 2015, 34, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Pons, T.; Pic, E.; Lequeux, N.; Cassette, E.; Bezdetnaya, L.; Guillemin, F.; Marchal, F.; Dubertret, B. Cadmium-Free CuInS2/ZnS Quantum Dots for Sentinel Lymph Node Imaging with Reduced Toxicity. ACS Nano 2010, 4, 2531–2538. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In Vivo Cancer Targeting and Imaging with Semiconductor Quantum Dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lim, Y.T.; Soltesz, E.G.; De Grand, A.M.; Lee, J.; Nakayama, A.; Parker, J.A.; Mihaljevic, T.; Laurence, R.G.; Dor, D.M.; et al. Near-Infrared Fluorescent Type II Quantum Dots for Sentinel Lymph Node Mapping. Nat. Biotechnol. 2004, 22, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Ballou, B.; Ernst, L.A.; Andreko, S.; Harper, T.; Fitzpatrick, J.A.J.; Waggoner, A.S.; Bruchez, M.P. Sentinel Lymph Node Imaging Using Quantum Dots in Mouse Tumor Models. Bioconjug. Chem. 2007, 18, 389–396. [Google Scholar] [CrossRef]
- Helle, M.; Cassette, E.; Bezdetnaya, L.; Pons, T.; Leroux, A.; Plénat, F.; Guillemin, F.; Dubertret, B.; Marchal, F. Visualisation of Sentinel Lymph Node with Indium-Based near Infrared Emitting Quantum Dots in a Murine Metastatic Breast Cancer Model. PLoS ONE 2012, 7, e44433. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.R.; Cheng, Z.; De, A.; Koh, A.L.; Sinclair, R.; Gambhir, S.S. Real-Time Intravital Imaging of RGD–Quantum Dot Binding to Luminal Endothelium in Mouse Tumor Neovasculature. Nano Lett. 2008, 8, 2599–2606. [Google Scholar] [CrossRef] [Green Version]
- Pons, T.; Bouccara, S.; Loriette, V.; Lequeux, N.; Pezet, S.; Fragola, A. In Vivo Imaging of Single Tumor Cells in Fast-Flowing Bloodstream Using Near-Infrared Quantum Dots and Time-Gated Imaging. ACS Nano 2019, 13, 3125–3131. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, G.; Zhang, Y.; Chen, G.; Li, F.; Dai, H.; Wang, Q. Ag2S Quantum Dot: A Bright and Biocompatible Fluorescent Nanoprobe in the Second Near-Infrared Window. ACS Nano 2012, 6, 3695–3702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruns, O.T.; Bischof, T.S.; Harris, D.K.; Franke, D.; Shi, Y.; Riedemann, L.; Bartelt, A.; Jaworski, F.B.; Carr, J.A.; Rowlands, C.J.; et al. Next-Generation in Vivo Optical Imaging with Short-Wave Infrared Quantum Dots. Nat. Biomed. Eng. 2017, 1, 0056. [Google Scholar] [CrossRef] [PubMed]
- Mulder, W.J.M.; Koole, R.; Brandwijk, R.J.; Storm, G.; Chin, P.T.K.; Strijkers, G.J.; de Mello Donegá, C.; Nicolay, K.; Griffioen, A.W. Quantum Dots with a Paramagnetic Coating as a Bimodal Molecular Imaging Probe. Nano Lett. 2006, 6, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Jarrett, B.R.; Kauzlarich, S.M.; Louie, A.Y. Core/Shell Quantum Dots with High Relaxivity and Photoluminescence for Multimodality Imaging. J. Am. Chem. Soc. 2007, 129, 3848–3856. [Google Scholar] [CrossRef] [Green Version]
- Ducongé, F.; Pons, T.; Pestourie, C.; Hérin, L.; Thézé, B.; Gombert, K.; Mahler, B.; Hinnen, F.; Kühnast, B.; Dollé, F.; et al. Fluorine-18-Labeled Phospholipid Quantum Dot Micelles for in Vivo Multimodal Imaging from Whole Body to Cellular Scales. Bioconj. Chem. 2008, 19, 1921–1926. [Google Scholar] [CrossRef]
- Sitbon, G.; Bouccara, S.; Tasso, M.; Francois, A.; Bezdetnaya, L.; Marchal, F.; Beaumont, M.; Pons, T. Multimodal Mn-Doped I-III-VI Quantum Dots for near Infrared Fluorescence and Magnetic Resonance Imaging: From Synthesis to in Vivo Application. Nanoscale 2014, 6, 9264–9272. [Google Scholar] [CrossRef] [Green Version]
- Samia, A.C.S.; Chen, X.; Burda, C. Semiconductor Quantum Dots for Photodynamic Therapy. J. Am. Chem. Soc. 2003, 125, 15736–15737. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Slowing, I.I.; Trewyn, B.G.; Lin, V.S.-Y. Mesoporous Silica Nanoparticles for Intracellular Controlled Drug Delivery. Small 2010, 6, 1952–1967. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.-W. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef]
- Ma, X.; Lepoitevin, M.; Serre, C. Metal–Organic Frameworks towards Bio-Medical Applications. Mater. Chem. Front. 2021, 5, 5573–5594. [Google Scholar] [CrossRef]
- Kim, C.K.; Ghosh, P.; Pagliuca, C.; Zhu, Z.-J.; Menichetti, S.; Rotello, V.M. Entrapment of Hydrophobic Drugs in Nanoparticle Monolayers with Efficient Release into Cancer Cells. J. Am. Chem. Soc. 2009, 131, 1360–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, W.; Sharma, C.P. 13—Inorganic Nanoparticles for Targeted Drug Delivery. In Biointegration of Medical Implant Materials, 2nd ed.; Sharma, C.P., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2020; pp. 333–373. ISBN 978-0-08-102680-9. [Google Scholar]
- Kayal, S.; Ramanujan, R.V. Doxorubicin Loaded PVA Coated Iron Oxide Nanoparticles for Targeted Drug Delivery. Mater. Sci. Eng. C 2010, 30, 484–490. [Google Scholar] [CrossRef]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Yuan, J.; Leung, K.C.F.; Lee, S.F.; Sham, K.W.Y.; Cheng, C.H.K.; Au, D.W.T.; Teng, G.J.; Ahuja, A.T.; Wang, Y.X.J. Hollow Superparamagnetic Iron Oxide Nanoshells as a Hydrophobic Anticancer Drug Carrier: Intracelluar PH-Dependent Drug Release and Enhanced Cytotoxicity. Nanoscale 2012, 4, 5744–5754. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Lin, Y.P.; Wang, C.W.; Tzeng, H.C.; Wu, C.H.; Chen, Y.C.; Chen, C.P.; Chen, L.C.; Wu, Y.C. DNA-Gold Nanorod Conjugates for Remote Control of Localized Gene Expression by near Infrared Irradiation. J. Am. Chem. Soc. 2006, 128, 3709–3715. [Google Scholar] [CrossRef]
- Wijaya, A.; Schaffer, S.B.; Pallares, I.G.; Hamad-Schifferli, K. Selective Release of Multiple DNA Oligonucleotides from Gold Nanorods. ACS Nano 2009, 3, 80–86. [Google Scholar] [CrossRef]
- Sanson, C.; Diou, O.; Thevenot, J.; Ibarboure, E.; Soum, A.; Brulet, A.; Miraux, S.; Thiaudiere, E.; Tan, S.; Brisson, A.; et al. Doxorubicin Loaded Magnetic Polymersomes: Theranostic Nanocarriers for MR Imaging and Magneto-Chemotherapy. ACS Nano 2011, 5, 1122–1140. [Google Scholar] [CrossRef] [Green Version]
- Bollhorst, T.; Rezwan, K.; Maas, M. Colloidal Capsules: Nano- and Microcapsules with Colloidal Particle Shells. Chem. Soc. Rev. 2017, 46, 2091–2126. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Gong, L.; Zhu, S.; Yong, Y.; Gu, Z.; Zhao, Y. Emerging Strategies of Nanomaterial-Mediated Tumor Radiosensitization. Adv. Mater. 2019, 31, 1802244. [Google Scholar] [CrossRef]
- Retif, P.; Pinel, S.; Toussaint, M.; Frochot, C.; Chouikrat, R.; Bastogne, T.; Barberi-Heyob, M. Nanoparticles for Radiation Therapy Enhancement: The Key Parameters. Theranostics 2015, 5, 1030–1044. [Google Scholar] [CrossRef] [Green Version]
- Ghaemi, B.; Mashinchian, O.; Mousavi, T.; Karimi, R.; Kharrazi, S.; Amani, A. Harnessing the Cancer Radiation Therapy by Lanthanide-Doped Zinc Oxide Based Theranostic Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 3123–3134. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.K.; Mitov, M.I.; Daley, E.F.; McGarry, R.C.; Anderson, K.W.; Hilt, J.Z. Targeted Iron Oxide Nanoparticles for the Enhancement of Radiation Therapy. Biomaterials 2016, 105, 127–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Zheng, X.; Yong, Y.; Yu, J.; Dong, X.; Zhang, C.; Zhou, R.; Li, B.; Yan, L.; Chen, C.; et al. Design of TPGS-Functionalized Cu3BiS3 Nanocrystals with Strong Absorption in the Second near-Infrared Window for Radiation Therapy Enhancement. Nanoscale 2017, 9, 8229–8239. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Zhang, C.; Gu, Z.; Du, J.; Guo, Z.; Dong, X.; Xie, J.; Zhang, G.; Liu, X.; Zhao, Y. Polyoxometalate-Based Radiosensitization Platform for Treating Hypoxic Tumors by Attenuating Radioresistance and Enhancing Radiation Response. ACS Nano 2017, 11, 7164–7176. [Google Scholar] [CrossRef]
- Yi, X.; Chen, L.; Zhong, X.; Gao, R.; Qian, Y.; Wu, F.; Song, G.; Chai, Z.; Liu, Z.; Yang, K. Core–Shell Au@MnO2 Nanoparticles for Enhanced Radiotherapy via Improving the Tumor Oxygenation. Nano Res. 2016, 9, 3267–3278. [Google Scholar] [CrossRef]
- Roa, W.; Zhang, X.; Guo, L.; Shaw, A.; Hu, X.; Xiong, Y.; Gulavita, S.; Patel, S.; Sun, X.; Chen, J.; et al. Gold Nanoparticle Sensitize Radiotherapy of Prostate Cancer Cells by Regulation of the Cell Cycle. Nanotechnology 2009, 20, 375101. [Google Scholar] [CrossRef] [Green Version]
- Davis, K.; Cole, B.; Ghelardini, M.; Powell, B.A.; Mefford, O.T. Quantitative Measurement of Ligand Exchange with Small-Molecule Ligands on Iron Oxide Nanoparticles via Radioanalytical Techniques. Langmuir 2016, 32, 13716–13727. [Google Scholar] [CrossRef]
- Lattuada, M.; Hatton, T.A. Functionalization of Monodisperse Magnetic Nanoparticles. Langmuir 2007, 23, 2158–2168. [Google Scholar] [CrossRef]
- Palma, S.I.C.J.; Marciello, M.; Carvalho, A.; Veintemillas-Verdaguer, S.; Morales, M.D.P.; Roque, A.C.A. Effects of Phase Transfer Ligands on Monodisperse Iron Oxide Magnetic Nanoparticles. J. Colloid Interface Sci. 2015, 437, 147–155. [Google Scholar] [CrossRef]
- Smolensky, E.D.; Park, H.Y.E.; Berquó, T.S.; Pierre, V.C. Surface Functionalization of Magnetic Iron Oxide Nanoparticles for MRI Applications—Effect of Anchoring Group and Ligand Exchange Protocol. Contrast Media Mol. Imaging 2011, 6, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Amstad, E.; Gillich, T.; Bilecka, I.; Textor, M.; Reimhult, E. Ultrastable Iron Oxide Nanoparticle Colloidal Suspensions Using Dispersants with Catechol-Derived Anchor Groups. Nano Lett. 2009, 9, 4042–4048. [Google Scholar] [CrossRef] [PubMed]
- Amstad, E.; Gehring, A.U.; Fischer, H.; Nagaiyanallur, V.V.; Hähner, G.; Textor, M.; Reimhult, E. Influence of Electronegative Substituents on the Binding Affinity of Catechol-Derived Anchors to Fe3O4 Nanoparticles. J. Phys. Chem. C 2011, 115, 683–691. [Google Scholar] [CrossRef]
- Yuen, A.K.L.; Hutton, G.A.; Masters, A.F.; Maschmeyer, T. The Interplay of Catechol Ligands with Nanoparticulate Iron Oxides. Dalton Trans. 2012, 41, 2545–2559. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, M.; Camilo, R.L.; Sampaio, L.C.; Macêdo, M.A.; Nakamura, M.; Toma, H.E. Preparation and Characterization of (3-Aminopropyl)Triethoxysilane-Coated Magnetite Nanoparticles. J. Magn. Magn. Mater. 2004, 279, 210–217. [Google Scholar] [CrossRef]
- De Palma, R.; Peeters, S.; Van Bael, M.J.; Van Den Rul, H.; Bonroy, K.; Laureyn, W.; Mullens, J.; Borghs, G.; Maes, G. Silane Ligand Exchange to Make Hydrophobic Superparamagnetic Nanoparticles Water-Dispersible. Chem. Mater. 2007, 19, 1821–1831. [Google Scholar] [CrossRef]
- Huang, P.; Li, Z.; Lin, J.; Yang, D.; Gao, G.; Xu, C.; Bao, L.; Zhang, C.; Wang, K.; Song, H.; et al. Photosensitizer-Conjugated Magnetic Nanoparticles for in Vivo Simultaneous Magnetofluorescent Imaging and Targeting Therapy. Biomaterials 2011, 32, 3447–3458. [Google Scholar] [CrossRef]
- Neouze, M.A.; Schubert, U. Surface Modification and Functionalization of Metal and Metal Oxide Nanoparticles by Organic Ligands. Mon. Fur Chem. 2008, 139, 183–195. [Google Scholar] [CrossRef]
- Lalatonne, Y.; Paris, C.; Serfaty, J.M.; Weinmann, P.; Lecouvey, M.; Motte, L. Bis-Phosphonates–Ultra Small Superparamagnetic Iron Oxide Nanoparticles: A Platform towards Diagnosis and Therapy. Chem. Commun. 2008, 2553–2555. [Google Scholar] [CrossRef]
- Walter, A.; Garofalo, A.; Bonazza, P.; Meyer, F.; Martinez, H.; Fleutot, S.; Billotey, C.; Taleb, J.; Felder-Flesch, D.; Begin-Colin, S. Effect of the Functionalization Process on the Colloidal, Magnetic Resonance Imaging, and Bioelimination Properties of Mono- or Bisphosphonate-Anchored Dendronized Iron Oxide Nanoparticles. Chempluschem 2017, 82, 647–659. [Google Scholar] [CrossRef]
- Basly, B.; Felder-Flesch, D.; Perriat, P.; Pourroy, G.; Bégin-Colin, S. Properties and Suspension Stability of Dendronized Iron Oxide Nanoparticles for MRI Applications. Contrast Media Mol. Imaging 2011, 6, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Sandiford, L.; Phinikaridou, A.; Protti, A.; Meszaros, L.K.; Cui, X.; Yan, Y.; Frodsham, G.; Williamson, P.A.; Gaddum, N.; Botnar, R.M.; et al. Bisphosphonate-Anchored Pegylation and Radiolabeling of Superparamagnetic Iron Oxide: Long-Circulating Nanoparticles for in Vivo Multimodal (T1 MRI-SPECT) Imaging. ACS Nano 2013, 7, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Won, N.; Bang, J.; Jin, H.; Park, J.; Jung, S.; Jung, S.; Park, Y.; Kim, S. Surface Engineering of Inorganic Nanoparticles for Imaging and Therapy. Adv. Drug Deliv. Rev. 2013, 65, 622–648. [Google Scholar] [CrossRef] [PubMed]
- Cotin, G.; Piant, S.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Iron Oxide Nanoparticles for Biomedical Applications: Synthesis, Functionalization, and Application; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081019252. [Google Scholar]
- Bhatt, N.; Huang, P.J.J.; Dave, N.; Liu, J. Dissociation and degradation of thiol-modified DNA on gold nanoparticles in aqueous and organic solvents. Langmuir 2011, 27, 6132–6137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An Overview of N-Heterocyclic Carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Borsari, R.K.; Schuster-Little, N.; Strausser, S.L.; Whelan, R.J.; Jenkins, D.M.; Camden, J.P. N-Heterocyclic Carbene Ligand Stability on Gold Nanoparticles in Biological Media. ACS Omega 2022, 7, 1444–1451. [Google Scholar] [CrossRef]
- Nosratabad, N.A.; Jin, Z.; Du, L.; Thakur, M.; Mattoussi, H. N-Heterocyclic Carbene-Stabilized Gold Nanoparticles: Mono- Versus Multidentate Ligands. Chem. Mater. 2021, 33, 921–933. [Google Scholar] [CrossRef]
- MacLeod, M.J.; Goodman, A.J.; Ye, H.Z.; Nguyen, H.V.T.; Van Voorhis, T.; Johnson, J.A. Robust gold nanorods stabilized by bidentate N-heterocyclic-carbene–thiolate ligands. Nat. Chem. 2019, 11, 57–63. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Salmi, Z.; Dahoumane, S.A.; Mekki, A.; Carbonnier, B.; Chehimi, M.M. Functionalization of Nanomaterials with Aryldiazonium Salts. Adv. Colloid Interface Sci. 2015, 225, 16–36. [Google Scholar] [CrossRef]
- Ahmad, R.; Boubekeur-Lecaque, L.; Nguyen, M.; Lau-Truong, S.; Lamouri, A.; Decorse, P.; Galtayries, A.; Pinson, J.; Felidj, N.; Mangeney, C. Tailoring the Surface Chemistry of Gold Nanorods through Au-C/Ag-C Covalent Bonds Using Aryl Diazonium Salts. J. Phys. Chem. C 2014, 118, 19098–19105. [Google Scholar] [CrossRef]
- Ahmad, A.A.L.; Marutheri Parambath, J.B.; Postnikov, P.S.; Guselnikova, O.; Chehimi, M.M.; Bruce, M.R.M.; Bruce, A.E.; Mohamed, A.A. Conceptual Developments of Aryldiazonium Salts as Modifiers for Gold Colloids and Surfaces. Langmuir 2021, 37, 8897–8907. [Google Scholar] [CrossRef]
- Calzada, R.; Thompson, C.M.; Westmoreland, D.E.; Edme, K.; Weiss, E.A. Organic-to-Aqueous Phase Transfer of Cadmium Chalcogenide Quantum Dots Using a Sulfur-Free Ligand for Enhanced Photoluminescence and Oxidative Stability. Chem. Mater. 2016, 28, 6716–6723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uyeda, H.T.; Medintz, I.L.; Jaiswal, J.K.; Simon, S.M.; Mattoussi, H. Synthesis of Compact Multidentate Ligands to Prepare Stable Hydrophilic Quantum Dot Fluorophores. J. Am. Chem. Soc. 2005, 127, 3870–3878. [Google Scholar] [CrossRef] [PubMed]
- Susumu, K.; Uyeda, H.; Medintz, I.; Pons, T.; Delehanty, J.B.; Mattoussi, H. Enhancing the Stability and Biological Functionalities of Quantum Dots via Compact Multifunctional Ligands. J. Am. Chem. Soc 2007, 129, 13987–13996. [Google Scholar] [CrossRef] [PubMed]
- Giovanelli, E.; Muro, E.; Sitbon, G.; Hanafi, M.; Pons, T.; Dubertret, B.; Lequeux, N. Highly Enhanced Affinity of Multidentate versus Bidentate Zwitterionic Ligands for Long-Term Quantum Dot Bioimaging. Langmuir 2012, 28, 15177–15184. [Google Scholar] [CrossRef] [PubMed]
- Gravel, E.; Tanguy, C.; Cassette, E.; Pons, T.; Knittel, F.; Bernards, N.; Garofalakis, A.; Ducongé, F.; Dubertret, B.; Doris, E. Compact Tridentate Ligands for Enhanced Aqueous Stability of Quantum Dots and in Vivo Imaging. Chem. Sci. 2013, 4, 411–417. [Google Scholar] [CrossRef]
- Stewart, M.H.; Susumu, K.; Mei, B.C.; Medintz, I.L.; Delehanty, J.B.; Blanco-Canosa, J.B.; Dawson, P.E.; Mattoussi, H. Multidentate Poly(Ethylene Glycol) Ligands Provide Colloidal Stability to Semiconductor and Metallic Nanocrystals in Extreme Conditions. J. Am. Chem. Soc. 2010, 132, 9804–9813. [Google Scholar] [CrossRef]
- Pinaud, F.; King, D.; Moore, H.-P.; Weiss, S. Bioactivation and Cell Targeting of Semiconductor CdSe/ZnS Nanocrystals with Phytochelatin-Related Peptides. J. Am. Chem. Soc. 2004, 126, 6115–6123. [Google Scholar] [CrossRef] [Green Version]
- Aldana, J.; Wang, Y.A.; Peng, X. Photochemical Instability of CdSe Nanocrystals Coated by Hydrophilic Thiols. J. Am. Chem. Soc. 2001, 123, 8844–8850. [Google Scholar] [CrossRef]
- Kim, S.; Bawendi, M.G. Oligomeric Ligands for Luminescent and Stable Nanocrystal Quantum Dots. J. Am. Chem. Soc. 2003, 125, 14652–14653. [Google Scholar] [CrossRef]
- Liu, W.; Greytak, A.B.; Lee, J.; Wong, C.R.; Park, J.; Marshall, L.F.; Jiang, W.; Curtin, P.N.; Ting, A.Y.; Nocera, D.G.; et al. Compact Biocompatible Quantum Dots via RAFT-Mediated Synthesis of Imidazole-Based Random Copolymer Ligand. J. Am. Chem. Soc. 2010, 132, 472–483. [Google Scholar] [CrossRef] [Green Version]
- Tasso, M.; Giovanelli, E.; Zala, D.; Bouccara, S.; Fragola, A.; Hanafi, M.; Lenkei, Z.; Pons, T.; Lequeux, N. Sulfobetaine-Vinylimidazole Block Copolymers: A Robust Quantum Dot Surface Chemistry Expanding Bioimaging’s Horizons. ACS Nano 2015, 9, 11479–11489. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Clapp, A.R.; Brunel, F.M.; Tiefenbrunn, T.; Tetsuo Uyeda, H.; Chang, E.L.; Deschamps, J.R.; Dawson, P.E.; Mattoussi, H. Proteolytic Activity Monitored by Fluorescence Resonance Energy Transfer through Quantum-Dot–Peptide Conjugates. Nat. Mater. 2006, 5, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.; Pons, T.; Medintz, I. Kinetics of Metal-Affinity Driven Self-Assembly between Proteins or Peptides and CdSe-ZnS Quantum Dots. J. Phys. Chem. C 2007, 111, 11528–11538. [Google Scholar] [CrossRef]
- Medintz, I.L.; Clapp, A.R.; Mattoussi, H.; Goldman, E.R.; Fisher, B.; Mauro, J.M. Self-Assembled Nanoscale Biosensors Based on Quantum Dot FRET Donors. Nat. Mater. 2003, 2, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Berti, L.; Pons, T.; Grimes, A.F.; English, D.S.; Alessandrini, A.; Facci, P.; Mattoussi, H. A Reactive Peptidic Linker for Self-Assembling Hybrid Quantum Dot-DNA Bioconjugates. Nano Lett. 2007, 7, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Baldim, V.; Bia, N.; Graillot, A.; Loubat, C.; Berret, J.F. Monophosphonic versus Multiphosphonic Acid Based PEGylated Polymers for Functionalization and Stabilization of Metal (Ce, Fe, Ti, Al) Oxide Nanoparticles in Biological Media. Adv. Mater. Interfaces 2019, 6, 1801814. [Google Scholar] [CrossRef]
- Dunlap, J.H.; Loszko, A.F.; Flake, R.A.; Huang, Y.; Benicewicz, B.C.; Greytak, A.B. Multiply-Binding Polymeric Imidazole Ligands: Influence of Molecular Weight and Monomer Sequence on Colloidal Quantum Dot Stability. J. Phys. Chem. C 2018, 122, 26756–26763. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M.; Giersig, M.; Mulvaney, P. Synthesis of Nanosized Gold−Silica Core−Shell Particles. Langmuir 1996, 12, 4329–4335. [Google Scholar] [CrossRef]
- Gerion, D.; Pinaud, F.; Williams, S.C.; Parak, W.J.; Zanchet, D.; Weiss, S.; Alivisatos, A.P. Synthesis and Properties of Biocompatible Water-Soluble Silica-Coated CdSe/ZnS Semiconductor Quantum Dots. J. Phys. Chem. B 2001, 105, 8861–8871. [Google Scholar] [CrossRef] [Green Version]
- Philipse, A.P.; van Bruggen, M.P.B.; Pathmamanoharan, C. Magnetic Silica Dispersions: Preparation and Stability of Surface-Modified Silica Particles with a Magnetic Core. Langmuir 1994, 10, 92–99. [Google Scholar] [CrossRef]
- Selvan, S.T.; Tan, T.T.; Ying, J.Y. Robust, Non-Cytotoxic, Silica-Coated CdSe Quantum Dots with Efficient Photoluminescence. Adv. Mater. 2005, 17, 1620–1625. [Google Scholar] [CrossRef]
- Graf, C.; Vossen, D.L.J.; Imhof, A.; van Blaaderen, A. A General Method To Coat Colloidal Particles with Silica. Langmuir 2003, 19, 6693–6700. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.S.; Lee, N.; Kim, T.; Kim, H.; Yu, T.; Song, I.C.; Moon, W.K.; Hyeon, T. Multifunctional Uniform Nanoparticles Composed of a Magnetite Nanocrystal Core and a Mesoporous Silica Shell for Magnetic Resonance and Fluorescence Imaging and for Drug Delivery. Angew. Chem. Int. Ed. 2008, 47, 8438–8441. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.-J.; Kim, J.S.; Kim, B.G.; Yu, K.N.; Cho, M.-H.; Lee, J.-K. Multifunctional Nanoparticles Possessing A “Magnetic Motor Effect” for Drug or Gene Delivery. Angew. Chem. Int. Ed. 2005, 44, 1068–1071. [Google Scholar] [CrossRef]
- Estephan, Z.G.; Jaber, J.A.; Schlenoff, J.B. Zwitterion-Stabilized Silica Nanoparticles: Toward Nonstick Nano. Langmuir 2010, 26, 16884–16889. [Google Scholar] [CrossRef]
- Dembele, F.; Tasso, M.; Trapiella-Alfonso, L.; Xu, X.; Hanafi, M.; Lequeux, N.; Pons, T. Zwitterionic Silane Copolymer for Ultra-Stable and Bright Biomolecular Probes Based on Fluorescent Quantum Dot Nanoclusters. ACS Appl. Mater. Interfaces 2017, 9, 18161–18169. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Frey, W.; Kim, S.; Homan, K.; Kruizinga, P.; Sokolov, K.; Emelianov, S. Enhanced Thermal Stability of Silica-Coated Gold Nanorods for Photoacoustic Imaging and Image-Guided Therapy. Opt. Express 2010, 18, 8867–8878. [Google Scholar] [CrossRef]
- Israelachvili, J. Intermolecular and Surface Forces, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011; ISBN 978-0-12-391927-4. [Google Scholar]
- Nikam, D.S.; Jadhav, S.V.; Khot, V.M.; Ningthoujam, R.S.; Hong, C.K.; Mali, S.S.; Pawar, S.H. Colloidal Stability of Polyethylene Glycol Functionalized Co 0.5Zn0.5Fe2O4 Nanoparticles: Effect of PH, Sample and Salt Concentration for Hyperthermia Application. RSC Adv. 2014, 4, 12662–12671. [Google Scholar] [CrossRef]
- Ensing, B.; Tiwari, A.; Tros, M.; Hunger, J.; Domingos, S.R.; Pérez, C.; Smits, G.; Bonn, M.; Bonn, D.; Woutersen, S. On the Origin of the Extremely Different Solubilities of Polyethers in Water. Nat. Commun. 2019, 10, 2893. [Google Scholar] [CrossRef] [Green Version]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Özdemir, C.; Güner, A. Solution Thermodynamics of Poly(Ethylene Glycol)/Water Systems. J. Appl. Polym. Sci. 2006, 101, 203–216. [Google Scholar] [CrossRef]
- Howard, M.D.; Jay, M.; Dziubla, T.D.; Lu, X. PEGylation of Nanocarrier Drug Delivery Systems: State of the Art. J. Biomed. Nanotechnol. 2008, 4, 133–148. [Google Scholar] [CrossRef]
- Ge, X.; Fu, Q.; Su, L.; Li, Z.; Zhang, W.; Chen, T.; Yang, H.; Song, J. Light-Activated Gold Nanorod Vesicles with NIR-II Fluorescence and Photoacoustic Imaging Performances for Cancer Theranostics. Theranostics 2020, 10, 4809. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Zhao, Y.; Yoon, S.J.; Gambhir, S.S.; Emelianov, S. Miniature Gold Nanorods for Photoacoustic Molecular Imaging in the Second Near-Infrared Optical Window. Nat. Nanotechnol. 2019, 14, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for Imaging and Therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [Green Version]
- Owens, D.E.; Peppas, N.A. Opsonization, Biodistribution, and Pharmacokinetics of Polymeric Nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Shenoy, D.; Fu, W.; Li, J.; Crasto, C.; Jones, G.; DiMarzio, C.; Sridhar, S.; Amiji, M. Surface Functionalization of Gold Nanoparticles Using Hetero-Bifunctional Poly(Ethylene Glycol) Spacer for Intracellular Tracking and Delivery. Int. J. Nanomed. 2006, 1, 51–57. [Google Scholar] [CrossRef]
- Otsuka, H.; Akiyama, Y.; Nagasaki, Y.; Kataoka, K. Quantitative and Reversible Lectin-Induced Association of Gold Nanoparticles Modified with α-Lactosyl-ω-Mercapto-Poly(Ethylene Glycol). J. Am. Chem. Soc. 2001, 123, 8226–8230. [Google Scholar] [CrossRef]
- Xue, W.; Liu, Y.; Zhang, N.; Yao, Y.; Ma, P.; Wen, H.; Huang, S.; Luo, Y.; Fan, H. Effects of Core Size and PEG Coating Layer of Iron Oxide Nanoparticles on the Distribution and Metabolism in Mice. Int. J. Nanomed. 2018, 13, 5719–5731. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Yuan, Z.; Hung, H.; Ma, J.; Jain, P.; Tsao, C.; Xie, J.; Zhang, P.; Lin, X.; Wu, K.; et al. Revealing the Immunogenic Risk of Polymers. Angew. Chem. Int. Ed. 2018, 57, 13873–13876. [Google Scholar] [CrossRef]
- Jiang, S.; Cao, Z. Ultralow-Fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, J.; Jordan, R.; Luxenhofer, R. On the Biodegradability of Polyethylene Glycol, Polypeptoids and Poly(2-Oxazoline)s. Biomaterials 2014, 35, 4848–4861. [Google Scholar] [CrossRef] [PubMed]
- Tassa, C.; Shaw, S.Y.; Weissleder, R. Dextran-Coated Iron Oxide Nanoparticles: A Versatile Platform for Targeted Molecular Imaging, Molecular Diagnostics, and Therapy. Acc. Chem. Res. 2011, 44, 842–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, H.; Konopka, C.J.; Prabhu, S.; Sarkar, S.; Medina, N.G.; Fayyaz, M.; Arogundade, O.H.; Vidana Gamage, H.E.; Shahoei, S.H.; Nall, D.; et al. Dextran-Mimetic Quantum Dots for Multimodal Macrophage Imaging In Vivo, Ex Vivo, and In Situ. ACS Nano 2022, 16, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Mehvar, R. Dextrans for Targeted and Sustained Delivery of Therapeutic and Imaging Agents. J. Control. Release 2000, 69, 1–25. [Google Scholar] [CrossRef]
- Pustylnikov, S.; Sagar, D.; Jain, P.; Khan, Z.K. Targeting the C-Type Lectins-Mediated Host-Pathogen Interactions with Dextran. J. Pharm. Pharm. Sci. 2014, 17, 371–392. [Google Scholar] [CrossRef] [Green Version]
- Vasey, P.A.; Kaye, S.B.; Morrison, R.; Twelves, C.; Wilson, P.; Duncan, R.; Thomson, A.H.; Murray, L.S.; Hilditch, T.E.; Murray, T.; et al. Phase I Clinical and Pharmacokinetic Study of PK1 [N-(2-Hydroxypropyl)Methacrylamide Copolymer Doxorubicin]: First Member of a New Class of Chemotherapeutic Agents—Drug-Polymer Conjugates. Clin. Cancer Res. 1999, 5, 83–94. [Google Scholar]
- Lammers, T.; Subr, V.; Ulbrich, K.; Hennink, W.E.; Storm, G.; Kiessling, F. Polymeric Nanomedicines for Image-Guided Drug Delivery and Tumor-Targeted Combination Therapy. Nano Today 2010, 5, 197–212. [Google Scholar] [CrossRef]
- Yuan, W.; Yuan, J.; Zhou, L.; Wu, S.; Hong, X. Fe3O4@poly(2-Hydroxyethyl Methacrylate)-Graft-Poly(ɛ-Caprolactone) Magnetic Nanoparticles with Branched Brush Polymeric Shell. Polymer 2010, 51, 2540–2547. [Google Scholar] [CrossRef]
- Sundhoro, M.; Park, J.; Jayawardana, K.W.; Chen, X.; Jayawardena, H.S.N.; Yan, M. Poly(HEMA-Co-HEMA-PFPA): Synthesis and Preparation of Stable Micelles Encapsulating Imaging Nanoparticles. J. Colloid Interface Sci. 2017, 500, 1–8. [Google Scholar] [CrossRef]
- Sasikala, A.R.K.; GhavamiNejad, A.; Unnithan, A.R.; Thomas, R.G.; Moon, M.; Jeong, Y.Y.; Park, C.H.; Kim, C.S. A Smart Magnetic Nanoplatform for Synergistic Anticancer Therapy: Manoeuvring Mussel-Inspired Functional Magnetic Nanoparticles for PH Responsive Anticancer Drug Delivery and Hyperthermia. Nanoscale 2015, 7, 18119–18128. [Google Scholar] [CrossRef] [PubMed]
- Horák, D.; Shagotova, T.; Mitina, N.; Trchová, M.; Boiko, N.; Babič, M.; Stoika, R.; Kovářová, J.; Hevus, O.; Beneš, M.J.; et al. Surface-Initiated Polymerization of 2-Hydroxyethyl Methacrylate from Heterotelechelic Oligoperoxide-Coated γ-Fe2O3 Nanoparticles and Their Engulfment by Mammalian Cells. Chem. Mater. 2011, 23, 2637–2649. [Google Scholar] [CrossRef]
- Viegas, T.X.; Bentley, M.D.; Harris, J.M.; Fang, Z.; Yoon, K.; Dizman, B.; Weimer, R.; Mero, A.; Pasut, G.; Veronese, F.M. Polyoxazoline: Chemistry, Properties, and Applications in Drug Delivery. Bioconj. Chem. 2011, 22, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Koshkina, O.; Westmeier, D.; Lang, T.; Bantz, C.; Hahlbrock, A.; Würth, C.; Resch-Genger, U.; Braun, U.; Thiermann, R.; Weise, C.; et al. Tuning the Surface of Nanoparticles: Impact of Poly(2-Ethyl-2-Oxazoline) on Protein Adsorption in Serum and Cellular Uptake. Macromol. Biosci. 2016, 16, 1287–1300. [Google Scholar] [CrossRef]
- Morgese, G.; Shirmardi Shaghasemi, B.; Causin, V.; Zenobi-Wong, M.; Ramakrishna, S.N.; Reimhult, E.; Benetti, E.M. Next-Generation Polymer Shells for Inorganic Nanoparticles Are Highly Compact, Ultra-Dense, and Long-Lasting Cyclic Brushes. Angew. Chem. Int. Ed. 2017, 56, 4507–4511. [Google Scholar] [CrossRef]
- Klein, T.; Parkin, J.; de Jongh, P.A.J.M.; Esser, L.; Sepehrizadeh, T.; Zheng, G.; De Veer, M.; Alt, K.; Hagemeyer, C.E.; Haddleton, D.M.; et al. Functional Brush Poly(2-Ethyl-2-Oxazine)s: Synthesis by CROP and RAFT, Thermoresponsiveness and Grafting onto Iron Oxide Nanoparticles. Macromol. Rapid Commun. 2019, 40, 1800911. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Tyeb, S.; Manzar, N.; Behera, L.; Ateeq, B.; Verma, V. Entropically Driven Controlled Release of Paclitaxel from Poly(2-Ethyl-2-Oxazoline) Coated Maghemite Nanostructures for Magnetically Guided Cancer Therapy. Soft Matter 2018, 14, 6537–6553. [Google Scholar] [CrossRef]
- Moreira, A.F.; Rodrigues, C.F.; Reis, C.A.; Costa, E.C.; Ferreira, P.; Correia, I.J. Development of Poly-2-Ethyl-2-Oxazoline Coated Gold-Core Silica Shell Nanorods for Cancer Chemo-Photothermal Therapy. Nanomedicine 2018, 13, 2611–2627. [Google Scholar] [CrossRef]
- Kurzhals, S.; Pretzner, B.; Reimhult, E.; Zirbs, R. Thermoresponsive Polypeptoid-Coated Superparamagnetic Iron Oxide Nanoparticles by Surface-Initiated Polymerization. Macromol. Chem. Phys. 2017, 218, 1700116. [Google Scholar] [CrossRef]
- Fokina, A.; Klinker, K.; Braun, L.; Jeong, B.G.; Bae, W.K.; Barz, M.; Zentel, R. Multidentate Polysarcosine-Based Ligands for Water-Soluble Quantum Dots. Macromolecules 2016, 49, 3663–3671. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Y.; Yan, F.-J.; Chen, J.; Tao, X.-F.; Ling, J.; Yang, B.; He, Q.-J.; Mao, Z.-W. Polysarcosine Brush Stabilized Gold Nanorods for in Vivo Near-Infrared Photothermal Tumor Therapy. Acta Biomater. 2017, 50, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Z.; Zhu, D.; Tao, X.; Gao, Y.; Zhu, H.; Mao, Z.; Ling, J. Gold Nanoparticles Coated with Polysarcosine Brushes to Enhance Their Colloidal Stability and Circulation Time in Vivo. J. Colloid Interface Sci. 2016, 483, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Mary, P.; Bendejacq, D.D.; Labeau, M.-P.; Dupuis, P. Reconciling Low- and High-Salt Solution Behavior of Sulfobetaine Polyzwitterions. J. Phys. Chem. B 2007, 111, 7767–7777. [Google Scholar] [CrossRef] [PubMed]
- Vaisocherová, H.; Zhang, Z.; Yang, W.; Cao, Z.; Cheng, G.; Taylor, A.D.; Piliarik, M.; Homola, J.; Jiang, S. Functionalizable Surface Platform with Reduced Nonspecific Protein Adsorption from Full Blood Plasma—Material Selection and Protein Immobilization Optimization. Biosens. Bioelectron. 2009, 24, 1924–1930. [Google Scholar] [CrossRef]
- Park, J.; Kurosawa, S.; Watanabe, J.; Ishihara, K. Evaluation of 2-Methacryloyloxyethyl Phosphorylcholine Polymeric Nanoparticle for Immunoassay of C-Reactive Protein Detection. Anal. Chem. 2004, 76, 2649–2655. [Google Scholar] [CrossRef]
- Goda, T.; Ishihara, K.; Miyahara, Y. Critical Update on 2-Methacryloyloxyethyl Phosphorylcholine (MPC) Polymer Science. J. Appl. Polym. Sci. 2015, 132, 41766. [Google Scholar] [CrossRef]
- Ashraf, S.; Park, J.; Bichelberger, M.A.; Kantner, K.; Hartmann, R.; Maffre, P.; Said, A.H.; Feliu, N.; Lee, J.; Lee, D.; et al. Zwitterionic Surface Coating of Quantum Dots Reduces Protein Adsorption and Cellular Uptake. Nanoscale 2016, 8, 17794–17800. [Google Scholar] [CrossRef]
- Debayle, M.; Balloul, E.; Dembele, F.; Xu, X.; Hanafi, M.; Ribot, F.; Monzel, C.; Coppey, M.; Fragola, A.; Dahan, M.; et al. Zwitterionic Polymer Ligands: An Ideal Surface Coating to Totally Suppress Protein-Nanoparticle Corona Formation? Biomaterials 2019, 219, 119357. [Google Scholar] [CrossRef] [Green Version]
- Erfani, A.; Seaberg, J.; Aichele, C.P.; Ramsey, J.D. Interactions between Biomolecules and Zwitterionic Moieties: A Review. Biomacromolecules 2020, 21, 2557–2573. [Google Scholar] [CrossRef]
- García, K.P.; Zarschler, K.; Barbaro, L.; Barreto, J.A.; O’Malley, W.; Spiccia, L.; Stephan, H.; Graham, B. Zwitterionic-Coated “Stealth” Nanoparticles for Biomedical Applications: Recent Advances in Countering Biomolecular Corona Formation and Uptake by the Mononuclear Phagocyte System. Small 2014, 10, 2516–2529. [Google Scholar] [CrossRef]
- Tatumi, R.; Fujihara, H. Remarkably Stable Gold Nanoparticles Functionalized with a Zwitterionic Liquid Based on Imidazolium Sulfonate in a High Concentration of Aqueous Electrolyte and Ionic Liquid. Chem. Commun. 2005, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Niidome, Y.; Niidome, T.; Kaneko, K.; Kawasaki, H.; Yamada, S. Modification of Gold Nanorods Using Phosphatidylcholine to Reduce Cytotoxicity. Langmuir 2006, 22, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Rouhana, L.L.; Jaber, J.A.; Schlenoff, J.B. Aggregation-Resistant Water-Soluble Gold Nanoparticles. Langmuir 2007, 23, 12799–12801. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, L.; Wang, S.; White, A.D.; Jiang, S. Functionalizable and Ultra Stable Nanoparticles Coated with Zwitterionic Poly(Carboxybetaine) in Undiluted Blood Serum. Biomaterials 2009, 30, 5617–5621. [Google Scholar] [CrossRef]
- Wei, H.; Bruns, O.T.; Kaul, M.G.; Hansen, E.C.; Barch, M.; Wiśniowska, A.; Chen, O.; Chen, Y.; Li, N.; Okada, S.; et al. Exceedingly Small Iron Oxide Nanoparticles as Positive MRI Contrast Agents. Proc. Natl. Acad. Sci. USA 2017, 114, 2325–2330. [Google Scholar] [CrossRef] [Green Version]
- Muro, E.; Pons, T.; Lequeux, N.; Fragola, A.; Sanson, N.; Lenkei, Z.; Dubertret, B. Small and Stable Sulfobetaine Zwitterionic Quantum Dots for Functional Live-Cell Imaging. J. Am. Chem. Soc. 2010, 132, 4556–4557. [Google Scholar] [CrossRef]
- Zhan, N.; Palui, G.; Safi, M.; Ji, X.; Mattoussi, H. Multidentate Zwitterionic Ligands Provide Compact and Highly Biocompatible Quantum Dots. J. Am. Chem. Soc. 2013, 135, 13786–13795. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, B. Analytical Strategies for Characterizing the Surface Chemistry of Nanoparticles. Anal. Bioanal. Chem. 2010, 396, 973–982. [Google Scholar] [CrossRef] [Green Version]
- Hens, Z.; Martins, J.C. A Solution NMR Toolbox for Characterizing the Surface Chemistry of Colloidal Nanocrystals. Chem. Mater. 2013, 25, 1211–1221. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Baldelli Bombelli, F.; Dawson, K.A. Physical−Chemical Aspects of Protein Corona: Relevance to in Vitro and in Vivo Biological Impacts of Nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular Coronas Provide the Biological Identity of Nanosized Materials. Nat. Nanotech. 2012, 7, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, S.L.; McKenzie, D.R.; Nosworthy, N.J.; Denman, J.A.; Sezerman, O.U.; Bilek, M.M.M. The Vroman Effect: Competitive Protein Exchange with Dynamic Multilayer Protein Aggregates. Colloids Surf. B Biointerfaces 2013, 103, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Jedlovszky-Hajdú, A.; Bombelli, F.B.; Monopoli, M.P.; Tombácz, E.; Dawson, K.A. Surface Coatings Shape the Protein Corona of SPIONs with Relevance to Their Application in Vivo. Langmuir 2012, 28, 14983–14991. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shang, L.; Maffre, P.; Hohmann, S.; Kirschhöfer, F.; Brenner-Weiß, G.; Nienhaus, G.U. The Nature of a Hard Protein Corona Forming on Quantum Dots Exposed to Human Blood Serum. Small 2016, 12, 5836–5844. [Google Scholar] [CrossRef]
- Miotto, G.; Magro, M.; Terzo, M.; Zaccarin, M.; Da Dalt, L.; Bonaiuto, E.; Baratella, D.; Gabai, G.; Vianello, F. Protein Corona as a Proteome Fingerprint: The Example of Hidden Biomarkers for Cow Mastitis. Colloids Surf. B Biointerfaces 2016, 140, 40–49. [Google Scholar] [CrossRef]
- Costa-Fernández, J.M.; Menéndez-Miranda, M.; Bouzas-Ramos, D.; Encinar, J.R.; Sanz-Medel, A. Mass Spectrometry for the Characterization and Quantification of Engineered Inorganic Nanoparticles. TrAC Trends Anal. Chem. 2016, 84, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Bonvin, D.; Chiappe, D.; Moniatte, M.; Hofmann, H.; Mionić Ebersold, M. Methods of Protein Corona Isolation for Magnetic Nanoparticles. Analyst 2017, 142, 3805–3815. [Google Scholar] [CrossRef]
- Singh, N.; Marets, C.; Boudon, J.; Millot, N.; Saviot, L.; Maurizi, L. In Vivo Protein Corona on Nanoparticles: Does the Control of All Material Parameters Orient the Biological Behavior? Nanoscale Adv. 2021, 3, 1209–1229. [Google Scholar] [CrossRef]
- Guerrini, L.; Arenal, R.; Mannini, B.; Chiti, F.; Pini, R.; Matteini, P.; Alvarez-Puebla, R.A. SERS Detection of Amyloid Oligomers on Metallorganic-Decorated Plasmonic Beads. ACS Appl. Mater. Interfaces 2015, 7, 9420–9428. [Google Scholar] [CrossRef]
- Fischer, K.; Schmidt, M. Pitfalls and Novel Applications of Particle Sizing by Dynamic Light Scattering. Biomaterials 2016, 98, 79–91. [Google Scholar] [CrossRef]
- Balog, S.; Rodriguez-Lorenzo, L.; Monnier, C.A.; Obiols-Rabasa, M.; Rothen-Rutishauser, B.; Schurtenberger, P.; Petri-Fink, A. Characterizing Nanoparticles in Complex Biological Media and Physiological Fluids with Depolarized Dynamic Light Scattering. Nanoscale 2015, 7, 5991–5997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carril, M.; Padro, D.; Del Pino, P.; Carrillo-Carrion, C.; Gallego, M.; Parak, W.J. In Situ Detection of the Protein Corona in Complex Environments. Nat. Commun. 2017, 8, 1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, P.M.; Åberg, C.; Polo, E.; O’Connell, A.; Cookman, J.; Fallon, J.; Krpetić, Ž.; Dawson, K.A. Mapping Protein Binding Sites on the Biomolecular Corona of Nanoparticles. Nat. Nanotechnol. 2015, 10, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Faserl, K.; Chetwynd, A.J.; Lynch, I.; Thorn, J.A.; Lindner, H.H. Corona Isolation Method Matters: Capillary Electrophoresis Mass Spectrometry Based Comparison of Protein Corona Compositions Following on-Particle versus in-Solution or in-Gel Digestion. Nanomaterials 2019, 9, 898. [Google Scholar] [CrossRef] [Green Version]
- Matczuk, M.; Anecka, K.; Scaletti, F.; Messori, L.; Keppler, B.K.; Timerbaev, A.R.; Jarosz, M. Speciation of Metal-Based Nanomaterials in Human Serum Characterized by Capillary Electrophoresis Coupled to ICP-MS: A Case Study of Gold Nanoparticles. Metallomics 2015, 7, 1364–1370. [Google Scholar] [CrossRef]
- Gianneli, M.; Yan, Y.; Polo, E.; Peiris, D.; Aastrup, T.; Dawson, K.A. Novel QCM-Based Method to Predict in Vivo Behaviour of Nanoparticles. Procedia Technol. 2017, 27, 197–200. [Google Scholar] [CrossRef]
- Santos-Martinez, M.J.; Inkielewicz-Stepniak, I.; Medina, C.; Rahme, K.; Arcy, D.; Fox, D.; Holmes, J.D.; Zhang, H.; Radomski, M.W. The Use of Quartz Crystal Microbalance with Dissipation (QCM-D) for Studying Nanoparticle-Induced Platelet Aggregation. Int. J. Nanomed. 2012, 7, 243–255. [Google Scholar] [CrossRef] [Green Version]
- Carrillo-Carrion, C.; Carril, M.; Parak, W.J. Techniques for the Experimental Investigation of the Protein Corona. Curr. Opin. Biotechnol. 2017, 46, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Ovais, M.; Nethi, S.K.; Ullah, S.; Ahmad, I.; Mukherjee, S.; Chen, C. Recent Advances in the Analysis of Nanoparticle-Protein Coronas. Nanomedicine 2020, 15, 1037–1061. [Google Scholar] [CrossRef]
- Sakulkhu, U.; Maurizi, L.; Mahmoudi, M.; Motazacker, M.; Vries, M.; Gramoun, A.; Ollivier Beuzelin, M.-G.; Vallée, J.-P.; Rezaee, F.; Hofmann, H. Ex Situ Evaluation of the Composition of Protein Corona of Intravenously Injected Superparamagnetic Nanoparticles in Rats. Nanoscale 2014, 6, 11439–11450. [Google Scholar] [CrossRef] [Green Version]
- Lundqvist, M.; Cedervall, T. Three Decades of Research about the Corona Around Nanoparticles: Lessons Learned and Where to Go Now. Small 2020, 16, e2000892. [Google Scholar] [CrossRef] [PubMed]
- Corbo, C.; Molinaro, R.; Tabatabaei, M.; Farokhzad, O.C.; Mahmoudi, M. Personalized Protein Corona on Nanoparticles and Its Clinical Implications. Biomater. Sci. 2017, 5, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Lynch, I.; Ejtehadi, M.R.; Monopoli, M.P.; Bombelli, F.B.; Laurent, S. Protein−Nanoparticle Interactions: Opportunities and Challenges. Chem. Rev. 2011, 111, 5610–5637. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, S.; Simon, J.; Paßlick, D.; Frey, M.-L.; Wagner, M.; Mailänder, V.; Crespy, D.; Landfester, K. Brush Conformation of Polyethylene Glycol Determines the Stealth Effect of Nanocarriers in the Low Protein Adsorption Regime. Nano Lett. 2021, 21, 1591–1598. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In Vivo Delivery, Pharmacokinetics, Biodistribution and Toxicity of Iron Oxide Nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef]
- Soo Choi, H.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal Clearance of Quantum Dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Zheng, J. Clearance Pathways and Tumor Targeting of Imaging Nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef] [Green Version]
- Mohammadpour, R.; Yazdimamaghani, M.; Cheney, D.L.; Jedrzkiewicz, J.; Ghandehari, H. Subchronic Toxicity of Silica Nanoparticles as a Function of Size and Porosity. J. Control Release 2019, 304, 216–232. [Google Scholar] [CrossRef]
- Frank, M.M.; Fries, L.F. The Role of Complement in Inflammation and Phagocytosis. Immunol. Today 1991, 12, 322–326. [Google Scholar] [CrossRef]
- Fam, S.Y.; Chee, C.F.; Yong, C.Y.; Ho, K.L.; Mariatulqabtiah, A.R.; Tan, W.S. Stealth Coating of Nanoparticles in Drug-Delivery Systems. Nanomaterials 2020, 10, 787. [Google Scholar] [CrossRef] [Green Version]
- Juliano, R.L. Factors Affecting the Clearance Kinetics and Tissue Distribution of Liposomes, Microspheres and Emulsions. Adv. Drug Deliv. Rev. 1988, 2, 31–54. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, P.R.; Martinez-Pomares, L.; Stacey, M.; Lin, H.-H.; Brown, G.D.; Gordon, S. MACROPHAGE RECEPTORS AND IMMUNE RECOGNITION. Annu. Rev. Immunol. 2005, 23, 901–944. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Kamakshi Sundaram, S.; Gary Teeguarden, J.; Joseph Riley, B.; Sheldon Fifield, L.; Morrell Jacobs, J.; Raymond Addleman, S.; Alan Kaysen, G.; Mohan Moudgil, B.; Joseph Weberkj, T. Adsorbed Proteins Influence the Biological Activity and Molecular Targeting of Nanomaterials. Toxicol. Sci. 2007, 100, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Saha, K.; Rahimi, M.; Yazdani, M.; Kim, S.T.; Moyano, D.F.; Hou, S.; Das, R.; Mout, R.; Rezaee, F.; Mahmoudi, M.; et al. Regulation of Macrophage Recognition through the Interplay of Nanoparticle Surface Functionality and Protein Corona. ACS Nano 2016, 10, 4421–4430. [Google Scholar] [CrossRef] [Green Version]
- Schöttler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailänder, V.; Wurm, F.R. Protein Adsorption Is Required for Stealth Effect of Poly(Ethylene Glycol)- and Poly(Phosphoester)-Coated Nanocarriers. Nat. Nanotechnol. 2016, 11, 372–377. [Google Scholar] [CrossRef]
- Ritz, S.; Schöttler, S.; Kotman, N.; Baier, G.; Musyanovych, A.; Kuharev, J.; Landfester, K.; Schild, H.; Jahn, O.; Tenzer, S.; et al. Protein Corona of Nanoparticles: Distinct Proteins Regulate the Cellular Uptake. Biomacromolecules 2015, 16, 1311–1321. [Google Scholar] [CrossRef]
- Vert, M.; Domurado, D. Poly(Ethylene Glycol): Protein-Repulsive or Albumin-Compatible? J. Biomater. Sci. Polym. Ed. 2000, 11, 1307–1317. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, S.; Yang, Q.; Zhang, T.; Wei, X.-Q.; Jiang, L.; Zhang, C.-L.; Chen, Q.-M.; Zhang, Z.-R.; Lin, Y.-F. Preformed Albumin Corona, a Protective Coating for Nanoparticles Based Drug Delivery System. Biomaterials 2013, 34, 8521–8530. [Google Scholar] [CrossRef]
- Ouyang, B.; Poon, W.; Zhang, Y.N.; Lin, Z.P.; Kingston, B.R.; Tavares, A.J.; Zhang, Y.; Chen, J.; Valic, M.S.; Syed, A.M.; et al. The Dose Threshold for Nanoparticle Tumour Delivery. Nat. Mater. 2020, 19, 1362–1371. [Google Scholar] [CrossRef]
- Nikitin, M.P.; Zelepukin, I.V.; Shipunova, V.O.; Sokolov, I.L.; Deyev, S.M.; Nikitin, P.I. Enhancement of the Blood-Circulation Time and Performance of Nanomedicines via the Forced Clearance of Erythrocytes. Nat. Biomed. Eng. 2020, 4, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Zelepukin, I.V.; Yaremenko, A.V.; Shipunova, V.O.; Babenyshev, A.V.; Balalaeva, I.V.; Nikitin, P.I.; Deyev, S.M.; Nikitin, M.P. Nanoparticle-Based Drug Delivery: Via RBC-Hitchhiking for the Inhibition of Lung Metastases Growth. Nanoscale 2019, 11, 1636–1646. [Google Scholar] [CrossRef]
- Chen, Z.A.; Wu, S.H.; Chen, P.; Chen, Y.P.; Mou, C.Y. Critical Features for Mesoporous Silica Nanoparticles Encapsulated into Erythrocytes. ACS Appl. Mater. Interfaces 2019, 11, 4790–4798. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dong, H.; Li, M.; Cao, Y.; Yang, F.; Zhang, K.; Dai, W.; Wang, C.; Zhang, X. Erythrocyte-Cancer Hybrid Membrane Camouflaged Hollow Copper Sulfide Nanoparticles for Prolonged Circulation Life and Homotypic-Targeting Photothermal/Chemotherapy of Melanoma. ACS Nano 2018, 12, 5241–5252. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.L.; Harada, T.; Christian, D.A.; Pantano, D.A.; Tsai, R.K.; Discher, D.E. Minimal “Self” Peptides That Inhibit Phagocytic Clearance and Enhance Delivery of Nanoparticles. Science 2013, 339, 971–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle–Liver Interactions: Cellular Uptake and Hepatobiliary Elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Khlebtsov, N.; Dykman, L. Biodistribution and Toxicity of Engineered Gold Nanoparticles: A Review of in Vitro and in Vivo Studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, Distribution, Clearance, and Toxicity of Iron Oxide Nanoparticles with Different Sizes and Coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a Definition of Inorganic Nanoparticles from an Environmental, Health and Safety Perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef]
- Oh, E.; Liu, R.; Nel, A.; Gemill, K.B.; Bilal, M.; Cohen, Y.; Medintz, I.L. Meta-Analysis of Cellular Toxicity for Cadmium-Containing Quantum Dots. Nat. Nanotechnol. 2016, 11, 479–486. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the Cytotoxicity Of Semiconductor Quantum Dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardman, R. A Toxicologic Review of Quantum Dots: Toxicity Depends on Physicochemical and Environmental Factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.A.J.; Andreko, S.K.; Ernst, L.A.; Waggoner, A.S.; Ballou, B.; Bruchez, M.P. Long-Term Persistence and Spectral Blue Shifting of Quantum Dots in Vivo. Nano Lett. 2009, 9, 2736–2741. [Google Scholar] [CrossRef] [Green Version]
- Kays, J.C.; Saeboe, A.M.; Toufanian, R.; Kurant, D.E.; Dennis, A.M. Shell-Free Copper Indium Sulfide Quantum Dots Induce Toxicity in Vitro and in Vivo. Nano Lett. 2020, 20, 1980–1991. [Google Scholar] [CrossRef]
- Du, Y.; Zhong, Y.; Dong, J.; Qian, C.; Sun, S.; Gao, L.; Yang, D. The Effect of PEG Functionalization on the: In Vivo Behavior and Toxicity of CdTe Quantum Dots. RSC Adv. 2019, 9, 12218–12225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storey, P.; Lim, R.P.; Chandarana, H.; Rosenkrantz, A.B.; Kim, D.; Stoffel, D.R.; Lee, V.S. MRI Assessment of Hepatic Iron Clearance Rates after USPIO Administration in Healthy Adults. Investig. Radiol. 2012, 47, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Zelepukin, I.V.; Yaremenko, A.V.; Ivanov, I.N.; Yuryev, M.V.; Cherkasov, V.R.; Deyev, S.M.; Nikitin, P.I.; Nikitin, M.P. Long-Term Fate of Magnetic Particles in Mice: A Comprehensive Study. ACS Nano 2021, 15, 11341–11357. [Google Scholar] [CrossRef] [PubMed]
- Kolosnjaj-Tabi, J.; Lartigue, L.; Javed, Y.; Luciani, N.; Pellegrino, T.; Wilhelm, C.; Alloyeau, D.; Gazeau, F. Biotransformations of Magnetic Nanoparticles in the Body. Nano Today 2016, 11, 280–284. [Google Scholar] [CrossRef]
- Stepien, G.; Moros, M.; Pérez-Hernández, M.; Monge, M.; Gutiérrez, L.; Fratila, R.M.; De Las Heras, M.; Menao Guillén, S.; Puente Lanzarote, J.J.; Solans, C.; et al. Effect of Surface Chemistry and Associated Protein Corona on the Long-Term Biodegradation of Iron Oxide Nanoparticles in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 4548–4560. [Google Scholar] [CrossRef] [Green Version]
- Lartigue, L.; Alloyeau, D.; Kolosnjaj-Tabi, J.; Javed, Y.; Guardia, P.; Riedinger, A.; Péchoux, C.; Pellegrino, T.; Wilhelm, C.; Gazeau, F. Biodegradation of Iron Oxide Nanocubes: High-Resolution in Situ Monitoring. ACS Nano 2013, 7, 3939–3952. [Google Scholar] [CrossRef]
- Lin, Z.; Monteiro-Riviere, N.A.; Riviere, J.E. Pharmacokinetics of Metallic Nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Semmler-Behnke, M.; Kreyling, W.G.; Lipka, J.; Fertsch, S.; Wenk, A.; Takenaka, S.; Schmid, G.; Brandau, W. Biodistribution of 1.4- and 18-Nm Gold Particles in Rats. Small 2008, 4, 2108–2111. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of Colloidal Gold Nanoparticles after Intravenous Administration: Effect of Particle Size. Colloids Surf. B Biointerfaces 2008, 66, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Balfourier, A.; Luciani, N.; Wang, G.; Lelong, G.; Ersen, O.; Khelfa, A.; Alloyeau, D.; Gazeau, F.; Carn, F. Unexpected Intracellular Biodegradation and Recrystallization of Gold Nanoparticles. Proc. Natl. Acad. Sci. USA 2020, 117, 103–113. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs1. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and Fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef]
- Danhier, F. To Exploit the Tumor Microenvironment: Since the EPR Effect Fails in the Clinic, What Is the Future of Nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Nichols, J.W.; Bae, Y.H. Odyssey of a Cancer Nanoparticle: From Injection Site to Site of Action. Nano Today 2012, 7, 606–618. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Wang, T.; Li, L.; Li, X.; Zhai, Y.; Zhang, J.; Li, W. The Application of Inorganic Nanoparticles in Molecular Targeted Cancer Therapy: EGFR Targeting. Front. Pharmacol. 2021, 12, 702445. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Shen, S.; Liao, Z.; Shi, W.; Wang, Y.; Zhao, J.; Hu, Y.; Yang, J.; Chen, J.; Mei, H.; et al. Targeting Fibronectins of Glioma Extracellular Matrix by CLT1 Peptide-Conjugated Nanoparticles. Biomaterials 2014, 35, 4088–4098. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, X.; Liu, L.; Zhang, Y.; Lu, Y.; Zhang, Y.; Chen, Q.; Ruan, C.; Guo, Q.; Li, C.; et al. Sequentially Triggered Nanoparticles with Tumor Penetration and Intelligent Drug Release for Pancreatic Cancer Therapy. Adv. Sci. 2018, 5, 1701070. [Google Scholar] [CrossRef] [PubMed]
- Goossens, J.; Sein, H.; Lu, S.; Radwanska, M.; Muyldermans, S.; Sterckx, Y.G.J.; Magez, S. Functionalization of Gold Nanoparticles with Nanobodies through Physical Adsorption. Anal. Methods 2017, 9, 3430–3440. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Functionalizing Nanoparticles with Cancer-Targeting Antibodies: A Comparison of Strategies. J. Control. Release 2020, 320, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Bartczak, D.; Kanaras, A.G. Preparation of Peptide-Functionalized Gold Nanoparticles Using One Pot EDC/Sulfo-NHS Coupling. Langmuir 2011, 27, 10119–10123. [Google Scholar] [CrossRef]
- Sukhanova, A.; Even-Desrumeaux, K.; Kisserli, A.; Tabary, T.; Reveil, B.; Millot, J.-M.; Chames, P.; Baty, D.; Artemyev, M.; Oleinikov, V.; et al. Oriented Conjugates of Single-Domain Antibodies and Quantum Dots: Toward a New Generation of Ultrasmall Diagnostic Nanoprobes. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.M.; Peça, I.N.; Roque, A.C.A. Antibody-Conjugated Nanoparticles for Therapeutic Applications. Curr. Med. Chem. 2012, 19, 3103–3127. [Google Scholar] [CrossRef]
- Jeong, S.; Park, J.Y.; Cha, M.G.; Chang, H.; Kim, Y.I.; Kim, H.M.; Jun, B.H.; Lee, D.S.; Lee, Y.S.; Jeong, J.M.; et al. Highly Robust and Optimized Conjugation of Antibodies to Nanoparticles Using Quantitatively Validated Protocols. Nanoscale 2017, 9, 2548–2555. [Google Scholar] [CrossRef]
- Pola, R.; Braunová, A.; Laga, R.; Pechar, M.; Ulbrich, K. Click Chemistry as a Powerful and Chemoselective Tool for the Attachment of Targeting Ligands to Polymer Drug Carriers. Polym. Chem. 2014, 5, 1340–1350. [Google Scholar] [CrossRef]
- Tasso, M.; Singh, M.K.; Giovanelli, E.; Fragola, A.; Loriette, V.; Regairaz, M.; Dautry, F.; Treussart, F.; Lenkei, Z.; Lequeux, N.; et al. Oriented Bioconjugation of Unmodified Antibodies to Quantum Dots Capped with Copolymeric Ligands as Versatile Cellular Imaging Tools. ACS Appl. Mater. Interfaces 2015, 7, 26904–26913. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Upreti, M.; Jyoti, A.; Sethi, P. Tumor Microenvironment and Nanotherapeutics. Transl. Cancer Res. 2013, 2, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.F.; Dias, D.R.; Correia, I.J. Stimuli-Responsive Mesoporous Silica Nanoparticles for Cancer Therapy: A Review. Microporous Mesoporous Mater. 2016, 236, 141–157. [Google Scholar] [CrossRef]
- Park, S.; Lee, W.J.; Park, S.; Choi, D.; Kim, S.; Park, N. Reversibly PH-Responsive Gold Nanoparticles and Their Applications for Photothermal Cancer Therapy. Sci. Rep. 2019, 9, 20180. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sun, J.; Li, F.; Wang, J.; Liu, J.; Kim, D.; Fan, C.; Hyeon, T.; Ling, D. Highly Sensitive Diagnosis of Small Hepatocellular Carcinoma Using PH-Responsive Iron Oxide Nanocluster Assemblies. J. Am. Chem. Soc. 2018, 140, 10071–10074. [Google Scholar] [CrossRef]
- Piao, J.-G.; Gao, F.; Li, Y.; Yu, L.; Liu, D.; Tan, Z.-B.; Xiong, Y.; Yang, L.; You, Y.-Z. PH-Sensitive Zwitterionic Coating of Gold Nanocages Improves Tumor Targeting and Photothermal Treatment Efficacy. Nano Res. 2018, 11, 3193–3204. [Google Scholar] [CrossRef]
- Yao, X.; Chen, X.; He, C.; Chen, L.; Chen, X. Dual PH-Responsive Mesoporous Silica Nanoparticles for Efficient Combination of Chemotherapy and Photodynamic Therapy. J. Mater. Chem. B 2015, 3, 4707–4714. [Google Scholar] [CrossRef]
- Coussens, L.M.; Fingleton, B.; Matrisian, L.M. Matrix Metalloproteinase Inhibitors and Cancer: Trials and Tribulations. Science 2002, 295, 2387–2392. [Google Scholar] [CrossRef]
- Ansari, C.; Tikhomirov, G.A.; Hong, S.H.; Falconer, R.A.; Loadman, P.M.; Gill, J.H.; Castaneda, R.; Hazard, F.K.; Tong, L.; Lenkov, O.D.; et al. Development of Novel Tumor-Targeted Theranostic Nanoparticles Activated by Membrane-Type Matrix Metalloproteinases for Combined Cancer Magnetic Resonance Imaging and Therapy. Small 2014, 10, 566–575. [Google Scholar] [CrossRef]
- Gallo, J.; Kamaly, N.; Lavdas, I.; Stevens, E.; De Nguyen, Q.; Wylezinska-Arridge, M.; Aboagye, E.O.; Long, N.J. CXCR4-Targeted and MMP-Responsive Iron Oxide Nanoparticles for Enhanced Magnetic Resonance Imaging. Angew. Chem. Int. Ed. 2014, 53, 9550–9554. [Google Scholar] [CrossRef] [Green Version]
- Gotov, O.; Battogtokh, G.; Ko, Y.T. Docetaxel-Loaded Hyaluronic Acid-Cathepsin B-Cleavable-Peptide-Gold Nanoparticles for the Treatment of Cancer. Mol. Pharm. 2018, 15, 4668–4676. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yang, H.; Mao, Z.; Wang, B. Cathepsin B-Responsive Multifunctional Peptide Conjugated Gold Nanorods for Mitochondrial Targeting and Precise Photothermal Cancer Therapy. J. Colloid Interface Sci. 2021, 601, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.M.; Ortega, A.; Obrador, E. Glutathione in Cancer Biology and Therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Hu, J.J.; Xu, Q.; Chen, S.; Jia, H.Z.; Sun, Y.X.; Zhuo, R.X.; Zhang, X.Z. A Redox-Responsive Drug Delivery System Based on RGD Containing Peptide-Capped Mesoporous Silica Nanoparticles. J. Mater. Chem. B 2015, 3, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A Review of Stimuli-Responsive Nanocarriers for Drug and Gene Delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; He, D.; He, X.; Wang, K.; Zou, Z.; Li, X.; Shi, H.; Luo, J.; Yang, X. Glutathione-Mediated Degradation of Surface-Capped MnO2 for Drug Release from Mesoporous Silica Nanoparticles to Cancer Cells. Part. Part. Syst. Charact. 2015, 32, 205–212. [Google Scholar] [CrossRef]
- Kawano, T.; Niidome, Y.; Mori, T.; Katayama, Y.; Niidome, T. PNIPAM Gel-Coated Gold Nanorods, for Targeted Delivery Responding to a Near-Infrared Laser. Bioconjug. Chem. 2009, 20, 209–212. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]

| Name | Structure | Applicability |
|---|---|---|
| Carboxylate | 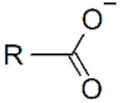 | IONPs |
| Phosphonate | 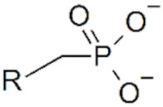 | IONPs |
| Thiol/thiolate | 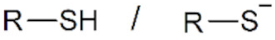 | AuNPs, QDs |
| Imidazole | 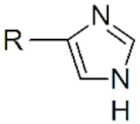 | QDs |
| Catechol | 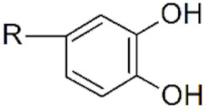 | IONPs |
| Aryldiazonium | 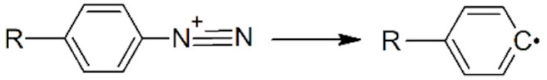 | AuNPs |
| N-heterocyclic carbene |  | AuNPs |
| Silane | 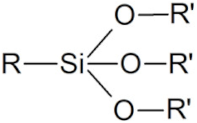 | All NPs |
| NPs | Applied Strategy | Model | Dose | Results | Ref. |
|---|---|---|---|---|---|
| Gold, 50 nm | Kupffer cell saturation by NPs | Mice | 50 trillion NPs | Prolonged circulation time from 2 min to 8 h—Improved tumor delivery efficiency from 0.03% to 12% | [233] |
| Iron oxide, 100–200 nm | Kupffer cell saturation by forced clearance of erythrocytes | Mice | 1.25 mg/kg of allogeneic anti-erythrocyte antibodies; Then, 25 µg NPs | Prolonged NP half-life time from ~1 min to ~15 min | [234] |
| Iron oxide, 100 nm | RBC-hitchhiking | Mice | 200 µg NPs | Improved tumor delivery efficiency from 0.6% to 41% | [235] |
| Silica NP, 25–200 nm | RBC-hitchhiking | Mice | 108 cells | NP half-life time 3 h | [236] |
| Gold NPs, 2 nm | Macrophage recognition regulation by protein corona | In vitro | 100 μL of AuNPs (1 μM) mixed with 400 μL of human serum | Correlated macrophage uptake induced by specific complement surface proteins | [228] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delille, F.; Pu, Y.; Lequeux, N.; Pons, T. Designing the Surface Chemistry of Inorganic Nanocrystals for Cancer Imaging and Therapy. Cancers 2022, 14, 2456. https://doi.org/10.3390/cancers14102456
Delille F, Pu Y, Lequeux N, Pons T. Designing the Surface Chemistry of Inorganic Nanocrystals for Cancer Imaging and Therapy. Cancers. 2022; 14(10):2456. https://doi.org/10.3390/cancers14102456
Chicago/Turabian StyleDelille, Fanny, Yuzhou Pu, Nicolas Lequeux, and Thomas Pons. 2022. "Designing the Surface Chemistry of Inorganic Nanocrystals for Cancer Imaging and Therapy" Cancers 14, no. 10: 2456. https://doi.org/10.3390/cancers14102456
APA StyleDelille, F., Pu, Y., Lequeux, N., & Pons, T. (2022). Designing the Surface Chemistry of Inorganic Nanocrystals for Cancer Imaging and Therapy. Cancers, 14(10), 2456. https://doi.org/10.3390/cancers14102456







