Proton Therapy for Squamous Cell Carcinoma of the Head and Neck: Early Clinical Experience and Current Challenges †
Abstract
:Simple Summary
Abstract
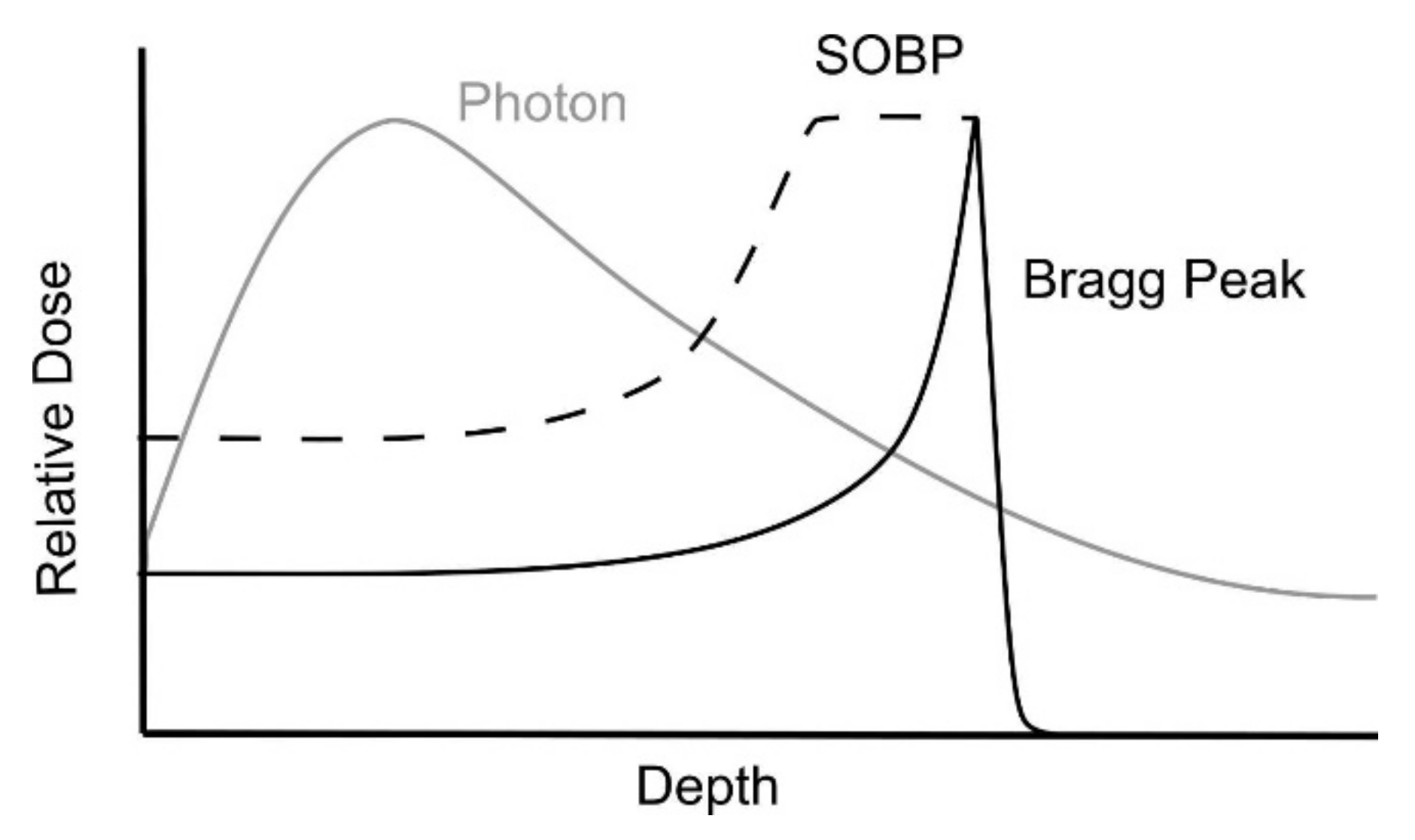
1. Introduction
2. Early Clinical Experience
2.1. Proton Therapy in the Definitive Setting
2.2. Proton Therapy for Dose (De-)Escalation, Adjuvant Treatment and Re-Irradiation
3. The Challenge of Patient Selection
4. Technical Limitations
5. The Issue of Clinical Trials and Reimbursement
6. Costs-Effectiveness of Proton Therapy
7. Future Directions
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Borras, J.M.; Barton, M.; Grau, C.; Corral, J.; Verhoeven, R.; Lemmens, V.; Van Eycken, L.; Henau, K.; Primic-Zakelj, M.; Strojan, P.; et al. The Impact of Cancer Incidence and Stage on Optimal Utilization of Radiotherapy: Methodology of a Population Based Analysis by the ESTRO-HERO Project. Radiother. Oncol. 2015, 116, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 873–898. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.P.; le Maître, A.; Maillard, E.; Bourhis, J. Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC): An Update on 93 Randomised Trials and 17,346 Patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; Bhide, S.A.; Clark, C.; Miles, E.A.; Miah, A.B.; Newbold, K.; Tanay, M.A.; et al. Parotid-Sparing Intensity Modulated versus Conventional Radiotherapy in Head and Neck Cancer (PARSPORT): A Phase 3 Multicentre Randomised Controlled Trial. Lancet Oncol. 2011, 12, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, D.I.; Chambers, M.S.; Fuller, C.D.; Rebueno, N.C.S.; Garcia, J.; Kies, M.S.; Morrison, W.H.; Ang, K.K.; Garden, A.S. Beam Path Toxicities to Non-Target Structures during Intensity-Modulated Radiation Therapy for Head and Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 747–755. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [Green Version]
- De Felice, F.; de Vincentiis, M.; Luzzi, V.; Magliulo, G.; Tombolini, M.; Ruoppolo, G.; Polimeni, A. Late Radiation-Associated Dysphagia in Head and Neck Cancer Patients: Evidence, Research and Management. Oral Oncol. 2018, 77, 125–130. [Google Scholar] [CrossRef]
- Beddok, A.; Vela, A.; Calugaru, V.; Tessonnier, T.; Kubes, J.; Dutheil, P.; Gerard, A.; Vidal, M.; Goudjil, F.; Florescu, C.; et al. Proton Therapy for Head and Neck Squamous Cell Carcinomas: A Review of the Physical and Clinical Challenges. Radiother. Oncol. 2020, 147, 30–39. [Google Scholar] [CrossRef]
- Van de Water, T.A.; Bijl, H.P.; Schilstra, C.; Pijls, J.M.; Langendijk, J.A. The Potential Benefit of Radiotherapy with Protons in Head and Neck Cancer with Respect to Normal Tissue Sparing: A Systematic Review of Literature. Oncologist 2011, 16, 366–377. [Google Scholar] [CrossRef] [Green Version]
- Simone, C.B.; Ly, D.; Dan, T.D.; Ondos, J.; Ning, H.; Belard, A.; O’Connell, J.; Miller, R.W.; Simone, N.L. Comparison of Intensity-Modulated Radiotherapy, Adaptive Radiotherapy, Proton Radiotherapy, and Adaptive Proton Radiotherapy for Treatment of Locally Advanced Head and Neck Cancer. Radiother. Oncol. 2011, 101, 376–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taheri-Kadkhoda, Z.; Björk-Eriksson, T.; Nill, S.; Wilkens, J.J.; Oelfke, U.; Johansson, K.A.; Huber, P.E.; Münter, M.W. Intensity-Modulated Radiotherapy of Nasopharyngeal Carcinoma: A Comparative Treatment Planning Study of Photons and Protons. Radiat. Oncol. 2008, 3, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widesott, L.; Pierelli, A.; Fiorino, C. Intensity-Modulated Proton Therapy versus Helical Tomotherapy in Nasopharynx Cancer: Planning Comparison and NTCP Evaluation. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Van De Water, T.A.; Lomax, A.J.; Bijl, H.P.; De Jong, M.E.; Schilstra, C.; Hug, E.B.; Langendijk, J.A. Potential Benefits of Scanned Intensity-Modulated Proton Therapy versus Advanced Photon Therapy with Regard to Sparing of the Salivary Glands in Oropharyngeal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1216–1224. [Google Scholar] [CrossRef]
- Van De Water, T.A.; Lomax, A.J.; Bijl, H.P.; Schilstra, C.; Hug, E.B.; Langendijk, J.A. Using a Reduced Spot Size for Intensity-Modulated Proton Therapy Potentially Improves Salivary Gland-Sparing in Oropharyngeal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e313–e319. [Google Scholar] [CrossRef]
- Lewis, G.D.; Holliday, E.B.; Kocak-Uzel, E.; Hernandez, M.; Garden, A.S.; Rosenthal, D.I.; Frank, S.J. Intensity-Modulated Proton Therapy for Nasopharyngeal Carcinoma: Decreased Radiation Dose to Normal Structures and Encouraging Clinical Outcomes. Head Neck 2016, 38 (Suppl. 1), E1886–E1895. [Google Scholar] [CrossRef]
- Mohamed, N.; Lee, A.; Lee, N.Y. Proton Beam Radiation Therapy Treatment for Head and Neck Cancer. Precis. Radiat. Oncol. 2022, 6, 59–68. [Google Scholar] [CrossRef]
- Tambas, M.; van der Laan, H.P.; Steenbakkers, R.J.H.M.; Doyen, J.; Timmermann, B.; Orlandi, E.; Hoyer, M.; Haustermans, K.; Georg, P.; Burnet, N.G.; et al. Current Practice in Proton Therapy Delivery in Adult Cancer Patients across Europe. Radiother. Oncol. 2022, 167, 7–13. [Google Scholar] [CrossRef]
- Hug, E.B.; Loredo, L.N.; Slater, J.D.; DeVries, A.; Grove, R.I.; Schaefer, R.A.; Rosenberg, A.E.; Slater, J.M. Proton Radiation Therapy for Chordomas and Chondrosarcomas of the Skull Base. J. Neurosurg. 1999, 91, 432–439. [Google Scholar] [CrossRef]
- Romesser, P.B.; Cahlon, O.; Scher, E.D.; Hug, E.B.; Sine, K.; Deselm, C.; Fox, J.L.; Mah, D.; Garg, M.K.; Han-Chih Chang, J.; et al. Proton Beam Re-Irradiation for Recurrent Head and Neck Cancer: Multi-Institutional Report on Feasibility and Early Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 386. [Google Scholar] [CrossRef] [Green Version]
- Dagan, R.; Bryant, C.; Li, Z.; Yeung, D.; Justice, J.; Dzieglewiski, P.; Werning, J.; Fernandes, R.; Pirgousis, P.; Lanza, D.C.; et al. Outcomes of Sinonasal Cancer Treated With Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Kitpanit, S.; Marqueen, K.E.; Sine, K.; Kang, J.J.; Fan, D.; Fan, M.; Wang, H.; Li, X.; Gelblum, D.; et al. Proton Therapy for Non-Skull Base Head and Neck Adenoid Cystic Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 1196. [Google Scholar] [CrossRef]
- Alterio, D.; D’Ippolito, E.; Vischioni, B.; Fossati, P.; Gandini, S.; Bonora, M.; Ronchi, S.; Vitolo, V.; Mastella, E.; Magro, G.; et al. Mixed-Beam Approach in Locally Advanced Nasopharyngeal Carcinoma: IMRT Followed by Proton Therapy Boost versus IMRT-Only. Evaluation of Toxicity and Efficacy. Acta Oncol. 2020, 59, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Fuller, C.D.; Gunn, G.B.; Beadle, B.M.; Phan, J.; Frank, S.J.; Garden, A.S.; Morrison, W.H.; Ang, K.K.; Rosenthal, D.I.; et al. A Phase II Trial of Proton Radiation Therapy With Chemotherapy for Nasopharyngeal Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, S151–S152. [Google Scholar] [CrossRef]
- Holliday, E.B.; Garden, A.S.; Rosenthal, D.I.; Fuller, C.D.; Morrison, W.H.; Gunn, G.B.; Phan, J.; Beadle, B.M.; Zhu, X.R.; Zhang, X.; et al. Proton Therapy Reduces Treatment-Related Toxicities for Patients with Nasopharyngeal Cancer: A Case-Match Control Study of Intensity-Modulated Proton Therapy and Intensity-Modulated Photon Therapy. Int. J. Part. Ther. 2015, 2, 19–28. [Google Scholar] [CrossRef]
- Sio, T.T.; Lin, H.K.; Shi, Q.; Gunn, G.B.; Cleeland, C.S.; Lee, J.J.; Hernandez, M.; Blanchard, P.; Thaker, N.G.; Phan, J.; et al. Intensity Modulated Proton Therapy Versus Intensity Modulated Photon Radiation Therapy for Oropharyngeal Cancer: First Comparative Results of Patient-Reported Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1107–1114. [Google Scholar] [CrossRef] [Green Version]
- Gunn, G.B.; Blanchard, P.; Garden, A.S.; Zhu, X.R.; Fuller, C.D.; Mohamed, A.S.; Morrison, W.H.; Phan, J.; Beadle, B.M.; Skinner, H.D.; et al. Clinical Outcomes and Patterns of Disease Recurrence after Intensity Modulated Proton Therapy for Oropharyngeal Squamous Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, P.; Garden, A.S.; Gunn, G.B.; Rosenthal, D.I.; Morrison, W.H.; Hernandez, M.; Crutison, J.; Lee, J.J.; Ye, R.; Fuller, C.D.; et al. Intensity Modulated Proton Beam Therapy (IMPT) versus Intensity Photon Therapy (IMRT) for Oropharynx Cancer Patients—A Case Analysis. Radiother. Oncol. 2016, 120, 48. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, X.; Yang, P.; Blanchard, P.; Garden, A.S.; Gunn, B.; Fuller, C.D.; Chambers, M.; Hutcheson, K.A.; Ye, R.; et al. Intensity-Modulated Proton Therapy and Osteoradionecrosis in Oropharyngeal Cancer. Radiother. Oncol. 2017, 123, 401–405. [Google Scholar] [CrossRef]
- Aljabab, S.; Liu, A.; Wong, T.; Liao, J.J.; Laramore, G.E.; Parvathaneni, U. Proton Therapy for Locally Advanced Oropharyngeal Cancer: Initial Clinical Experience at the University of Washington. Int. J. Part. Ther. 2020, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Manzar, G.S.; Lester, S.C.; Routman, D.M.; Harmsen, W.S.; Petersen, M.M.; Sloan, J.A.; Mundy, D.W.; Hunzeker, A.E.; Amundson, A.C.; Anderson, J.L.; et al. Comparative Analysis of Acute Toxicities and Patient Reported Outcomes between Intensity-Modulated Proton Therapy (IMPT) and Volumetric Modulated Arc Therapy (VMAT) for the Treatment of Oropharyngeal Cancer. Radiother. Oncol. 2020, 147, 64–74. [Google Scholar] [CrossRef]
- Kitpanit, S.; Lee, A.; Fan, D.; Fan, M.; Wang, H.; Mohamed, N.; Spielsinger, D.; Gelblum, D.; Sherman, E.; Dunn, L.; et al. Clinical Outcomes and Toxicities in Oropharyngeal Cancer (OPC) Patients Treated with Proton Therapy: A Single Institutional Experience. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 1152–1153. [Google Scholar] [CrossRef]
- Jiří, K.; Vladimír, V.; Michal, A.; Matěj, N.; Silvia, S.; Pavel, V.; Kateřina, D.; Jana, P.; Barbora, O.; Eliška, R.; et al. Proton Pencil-Beam Scanning Radiotherapy in the Treatment of Nasopharyngeal Cancer: Dosimetric Parameters and 2-Year Results. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 763–769. [Google Scholar] [CrossRef]
- Li, X.; Kitpanit, S.; Lee, A.; Mah, D.; Sine, K.; Sherman, E.J.; Dunn, L.A.; Michel, L.S.; Fetten, J.; Zakeri, K.; et al. Toxicity Profiles and Survival Outcomes Among Patients With Nonmetastatic Nasopharyngeal Carcinoma Treated with Intensity-Modulated Proton Therapy vs. Intensity-Modulated Radiation Therapy. JAMA Netw. Open 2021, 4, e2113205. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.M.; Parvathaneni, U.; Laramore, G.E.; Aljabab, S.; Wong, T.P.; Liao, J.J. Intensity-Modulated Proton Therapy for Nasopharynx Cancer: 2-Year Outcomes from a Single Institution. Int. J. Part. Ther. 2021, 8, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, X.; Jiang, B.; Chen, J.; Wang, X.; Wang, L.; Sahoo, N.; Zhu, X.R.; Ye, R.; Blanchard, P.; et al. Intensity-Modulated Proton Therapy for Oropharyngeal Cancer Reduces Rates of Late Xerostomia. Radiother. Oncol. 2021, 160, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.C.; Fan, K.H.; Lin, C.Y.; Hung, T.M.; Huang, B.S.; Chang, K.P.; Kang, C.J.; Huang, S.F.; Chang, P.H.; Hsu, C.L.; et al. Intensity Modulated Proton Beam Therapy versus Volumetric Modulated Arc Therapy for Patients with Nasopharyngeal Cancer: A Propensity Score-Matched Study. Cancers 2021, 13, 3555. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L.; Corry, J.; Ringash, J.; Rischin, D. Quality of Life, Toxicity and Unmet Needs in Nasopharyngeal Cancer Survivors. Front. Oncol. 2020, 10, 930. [Google Scholar] [CrossRef]
- Lee, N.; Harris, J.; Garden, A.S.; Straube, W.; Glisson, B.; Xia, P.; Bosch, W.; Morrison, W.H.; Quivey, J.; Thorstad, W.; et al. Intensity-Modulated Radiation Therapy with or without Chemotherapy for Nasopharyngeal Carcinoma: Radiation Therapy Oncology Group Phase II Trial 0225. J. Clin. Oncol. 2009, 27, 3684–3690. [Google Scholar] [CrossRef] [Green Version]
- Sapir, E.; Tao, Y.; Feng, F.; Samuels, S.; El Naqa, I.; Murdoch-Kinch, C.A.; Feng, M.; Schipper, M.; Eisbruch, A. Predictors of Dysgeusia in Patients With Oropharyngeal Cancer Treated With Chemotherapy and Intensity Modulated Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 354–361. [Google Scholar] [CrossRef]
- DeCesaris, C.M.; Rice, S.R.; Bentzen, S.M.; Jatczak, J.; Mishra, M.V.; Nichols, E.M. Quantification of Acute Skin Toxicities in Patients With Breast Cancer Undergoing Adjuvant Proton versus Photon Radiation Therapy: A Single Institutional Experience. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Romesser, P.B.; Cahlon, O.; Scher, E.; Zhou, Y.; Berry, S.L.; Rybkin, A.; Sine, K.M.; Tang, S.; Sherman, E.J.; Wong, R.; et al. Proton Beam Radiation Therapy Results in Significantly Reduced Toxicity Compared with Intensity-Modulated Radiation Therapy for Head and Neck Tumors That Require Ipsilateral Radiation. Radiother. Oncol. 2016, 118, 286–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ausat, N.; Rosenthal, D.; Gunn, G.; Ye, R.; Garden, A.; Morrison, W.; Fuller, C.; Phan, J.; Reddy, J.; Moreno, A.; et al. Outcomes Following Proton Therapy for Squamous Cell Carcinoma of the Larynx. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 1213. [Google Scholar] [CrossRef]
- Patel, S.H.; Wang, Z.; Wong, W.W.; Murad, M.H.; Buckey, C.R.; Mohammed, K.; Alahdab, F.; Altayar, O.; Nabhan, M.; Schild, S.E.; et al. Charged Particle Therapy versus Photon Therapy for Paranasal Sinus and Nasal Cavity Malignant Diseases: A Systematic Review and Meta-Analysis. Lancet. Oncol. 2014, 15, 1027–1038. [Google Scholar] [CrossRef]
- McDonald, M.W.; Liu, Y.; Moore, M.G.; Johnstone, P.A.S. Acute Toxicity in Comprehensive Head and Neck Radiation for Nasopharynx and Paranasal Sinus Cancers: Cohort Comparison of 3D Conformal Proton Therapy and Intensity Modulated Radiation Therapy. Radiat. Oncol. 2016, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Nuyts, S.; Bollen, H.; Eisbruch, A.; Corry, J.; Strojan, P.; Mäkitie, A.A.; Langendijk, J.A.; Mendenhall, W.M.; Smee, R.; DeBree, R.; et al. Unilateral versus Bilateral Nodal Irradiation: Current Evidence in the Treatment of Squamous Cell Carcinoma of the Head and Neck. Head Neck 2021, 43, 2807–2821. [Google Scholar] [CrossRef]
- Dagan, R.; Bryant, C.M.; Bradley, J.A.; Indelicato, D.J.; Rutenberg, M.; Rotondo, R.; Morris, C.G.; Mendenhall, W.M. A Prospective Evaluation of Acute Toxicity from Proton Therapy for Targets of the Parotid Region. Int. J. Part. Ther. 2016, 3, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Holliday, E.; Bhattasali, O.; Kies, M.S.; Hanna, E.; Garden, A.S.; Rosenthal, D.I.; Morrison, W.H.; Gunn, G.B.; Phan, J.; Zhu, X.R.; et al. Postoperative Intensity-Modulated Proton Therapy for Head and Neck Adenoid Cystic Carcinoma. Int. J. Part. Ther. 2016, 2, 533–543. [Google Scholar] [CrossRef] [Green Version]
- Chuong, M.; Bryant, J.; Hartsell, W.; Larson, G.; Badiyan, S.; Laramore, G.E.; Katz, S.; Tsai, H.; Vargas, C. Minimal Acute Toxicity from Proton Beam Therapy for Major Salivary Gland Cancer. Acta Oncol. 2020, 59, 196–200. [Google Scholar] [CrossRef]
- Jeans, E.B.; Shiraishi, S.; Manzar, G.; Morris, L.K.; Amundson, A.; McGee, L.A.; Rwigema, J.C.; Neben-Wittich, M.; Routman, D.M.; Ma, D.J.; et al. An Comparison of Acute Toxicities and Patient-Reported Outcomes between Intensity-Modulated Proton Therapy and Volumetric-Modulated Arc Therapy after Ipsilateral Radiation for Head and Neck Cancers. Head Neck 2022, 44, 359–371. [Google Scholar] [CrossRef]
- Slater, J.D.; Yonemoto, L.T.; Mantik, D.W.; Bush, D.A.; Preston, W.; Grove, R.I.; Miller, D.W.; Slater, J.M. Proton Radiation for Treatment of Cancer of the Oropharynx: Early Experience at Loma Linda University Medical Center Using a Concomitant Boost Technique. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Beddok, A.; Feuvret, L.; Noel, G.; Bolle, S.; Deberne, M.; Mammar, H.; Chaze, A.; Le Tourneau, C.; Goudjil, F.; Zefkili, S.; et al. Efficacy and Toxicity of Proton with Photon Radiation for Locally Advanced Nasopharyngeal Carcinoma. Acta Oncol. 2019, 58, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Zhou, O.; Thompson, R.; Gabriel, P.; Chalian, A.; Rassekh, C.; Weinstein, G.S.; O’Malley, B.W.; Aggarwal, C.; Bauml, J.; et al. Quality of Life of Postoperative Photon versus Proton Radiation Therapy for Oropharynx Cancer. Int. J. Part. Ther. 2018, 5, 11. [Google Scholar] [CrossRef]
- Lee, A.; Kitpanit, S.; Chilov, M.; Langendijk, J.A.; Lu, J.; Lee, N.Y. A Systematic Review of Proton Therapy for the Management of Nasopharyngeal Cancer. Int. J. Part. Ther. 2021, 8, 119–130. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.W.; Zolali-Meybodi, O.; Lehnert, S.J.; Estabrook, N.C.; Liu, Y.; Cohen-Gadol, A.A.; Moore, M.G. Reirradiation of Recurrent and Second Primary Head and Neck Cancer With Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Phan, J.; Sio, T.T.; Nguyen, T.P.; Takiar, V.; Gunn, G.B.; Garden, A.S.; Rosenthal, D.I.; Fuller, C.D.; Morrison, W.H.; Beadle, B.; et al. Reirradiation of Head and Neck Cancers With Proton Therapy: Outcomes and Analyses. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 30–41. [Google Scholar] [CrossRef]
- Inskip, P.D.; Sigurdson, A.J.; Veiga, L.; Bhatti, P.; Ronckers, C.; Rajaraman, P.; Boukheris, H.; Stovall, M.; Smith, S.; Hammond, S.; et al. Radiation-Related New Primary Solid Cancers in the Childhood Cancer Survivor Study: Comparative Radiation Dose Response and Modification of Treatment Effects. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 800–807. [Google Scholar] [CrossRef] [Green Version]
- Berrington De Gonzalez, A.; Gilbert, E.; Curtis, R.; Inskip, P.; Kleinerman, R.; Morton, L.; Rajaraman, P.; Little, M.P. Second Solid Cancers after Radiation Therapy: A Systematic Review of the Epidemiologic Studies of the Radiation Dose-Response Relationship. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Luitel, K.; Bozeman, R.; Kaisani, A.; Kim, S.B.; Barron, S.; Richardson, J.A.; Shay, J.W. Proton Radiation-Induced Cancer Progression. Life Sci. Space Res. 2018, 19, 31–42. [Google Scholar] [CrossRef]
- American Society of Radiation Oncology (ASTRO). Model Policies for Proton Beam Therapy. Available online: www.astro.org/uploadedFiles/_MAIN_SITE/Daily_Practice/Reimbursement/Model_Policies/Content_Pieces/ASTROPBTModelPolicy (accessed on 25 April 2022).
- Patel, S.; Parliament, M.; Kostaras, X.; Hagen, N.; Olivotto, I.A.; Nordal, R.; Aronyk, K. Recommendations for the Referral of Patients for Proton-Beam Therapy, an Alberta Health Services Report: A Model for Canada? Curr. Oncol. 2014, 21, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Grau, C.; Baumann, M.; Weber, D.C. Optimizing Clinical Research and Generating Prospective High-Quality Data in Particle Therapy in Europe: Introducing the European Particle Therapy Network (EPTN). Radiother. Oncol. 2018, 128, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bekelman, J.E.; Asch, D.A.; Tochner, Z.; Friedberg, J.; Vaughn, D.J.; Rash, E.; Raksowski, K.; Hahn, S.M. Principles and Reality of Proton Therapy Treatment Allocation. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 499–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Q.; Roelofs, E.; Ramaekers, B.L.T.; Eekers, D.; Van Soest, J.; Lustberg, T.; Hendriks, T.; Hoebers, F.; Van Der Laan, H.P.; Korevaar, E.W.; et al. Development and Evaluation of an Online Three-Level Proton vs Photon Decision Support Prototype for Head and Neck Cancer—Comparison of Dose, Toxicity and Cost-Effectiveness. Radiother. Oncol. 2016, 118, 281–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, A.M.; Douglass, M.J.J.; Nguyen, G.T.; Penfold, S.N. A Radiobiological Markov Simulation Tool for Aiding Decision Making in Proton Therapy Referral. Phys. Med. 2017, 44, 72–82. [Google Scholar] [CrossRef]
- Jain, V.; Irmen, P.; O’Reilly, S.; Vogel, J.H.; Lin, L.; Lin, A. Predicted Secondary Malignancies Following Proton versus Photon Radiation for Oropharyngeal Cancers. Int. J. Part. Ther. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Langendijk, J.A.; Hoebers, F.J.P.; De Jong, M.A.; Doornaert, P.; Terhaard, C.H.J.; Steenbakkers, R.J.H.M.; Hamming-Vrieze, O.; Van De Kamer, J.B.; Verbakel, W.F.A.R.; Keskin-Cambay, F.; et al. National Protocol for Model-Based Selection for Proton Therapy in Head and Neck Cancer. Int. J. Part. Ther. 2021, 8, 354–365. [Google Scholar] [CrossRef]
- Ramaekers, B.L.T.; Grutters, J.P.C.; Pijls-Johannesma, M.; Lambin, P.; Joore, M.A.; Langendijk, J.A. Protons in Head-and-Neck Cancer: Bridging the Gap of Evidence. Int. J. Radiat. Oncol. 2013, 85, 1282–1288. [Google Scholar] [CrossRef] [Green Version]
- Van den Bosch, L.; van der Schaaf, A.; van der Laan, H.P.; Hoebers, F.J.P.; Wijers, O.B.; van den Hoek, J.G.M.; Moons, K.G.M.; Reitsma, J.B.; Steenbakkers, R.J.H.M.; Schuit, E.; et al. Comprehensive Toxicity Risk Profiling in Radiation Therapy for Head and Neck Cancer: A New Concept for Individually Optimised Treatment. Radiother. Oncol. 2021, 157, 147–154. [Google Scholar] [CrossRef]
- Tambas, M.; Steenbakkers, R.J.H.M.; van der Laan, H.P.; Wolters, A.M.; Kierkels, R.G.J.; Scandurra, D.; Korevaar, E.W.; Oldehinkel, E.; van Zon-Meijer, T.W.H.; Both, S.; et al. First Experience with Model-Based Selection of Head and Neck Cancer Patients for Proton Therapy. Radiother. Oncol. 2020, 151, 206–213. [Google Scholar] [CrossRef]
- Jakobi, A.; Bandurska-Luque, A.; Stützer, K.; Haase, R.; Löck, S.; Wack, L.J.; Mönnich, D.; Thorwarth, D.; Perez, D.; Lühr, A.; et al. Identification of Patient Benefit From Proton Therapy for Advanced Head and Neck Cancer Patients Based on Individual and Subgroup Normal Tissue Complication Probability Analysis. Int. J. Radiat. Oncol. 2015, 92, 1165–1174. [Google Scholar] [CrossRef]
- Beetz, I.; Schilstra, C.; Van Der Schaaf, A.; Van Den Heuvel, E.R.; Doornaert, P.; Van Luijk, P.; Vissink, A.; Van Der Laan, B.F.A.M.; Leemans, C.R.; Bijl, H.P.; et al. NTCP Models for Patient-Rated Xerostomia and Sticky Saliva after Treatment with Intensity Modulated Radiotherapy for Head and Neck Cancer: The Role of Dosimetric and Clinical Factors. Radiother. Oncol. 2012, 105, 101–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christianen, M.E.M.C.; Schilstra, C.; Beetz, I.; Muijs, C.T.; Chouvalova, O.; Burlage, F.R.; Doornaert, P.; Koken, P.W.; Leemans, C.R.; Rinkel, R.N.P.M.; et al. Predictive Modelling for Swallowing Dysfunction after Primary (Chemo)Radiation: Results of a Prospective Observational Study. Radiother. Oncol. 2012, 105, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wopken, K.; Bijl, H.P.; Van Der Schaaf, A.; Van Der Laan, H.P.; Chouvalova, O.; Steenbakkers, R.J.H.M.; Doornaert, P.; Slotman, B.J.; Oosting, S.F.; Christianen, M.E.M.C.; et al. Development of a Multivariable Normal Tissue Complication Probability (NTCP) Model for Tube Feeding Dependence after Curative Radiotherapy/Chemo-Radiotherapy in Head and Neck Cancer. Radiother. Oncol. 2014, 113, 95–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijman, R.G.; Breedveld, S.; Arts, T.; Astreinidou, E.; de Jong, M.A.; Granton, P.V.; Petit, S.F.; Hoogeman, M.S. Impact of Model and Dose Uncertainty on Model-Based Selection of Oropharyngeal Cancer Patients for Proton Therapy. Acta Oncol. 2017, 56, 1444–1450. [Google Scholar] [CrossRef] [Green Version]
- Bortfeld, T.; Paganetti, H. The Biologic Relevance of Daily Dose Variations in Adaptive Treatment Planning. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 899–906. [Google Scholar] [CrossRef]
- Kouwenberg, J.; Penninkhof, J.; Habraken, S.; Zindler, J.; Hoogeman, M.; Heijmen, B. Model Based Patient Pre-Selection for Intensity-Modulated Proton Therapy (IMPT) Using Automated Treatment Planning and Machine Learning. Radiother. Oncol. 2021, 158, 224–229. [Google Scholar] [CrossRef]
- Tambas, M.; van der Laan, H.P.; van der Schaaf, A.; Steenbakkers, R.J.H.M.; Langendijk, J.A. A Decision Support Tool to Optimize Selection of Head and Neck Cancer Patients for Proton Therapy. Cancers 2022, 14, 681. [Google Scholar] [CrossRef]
- Lomax, A.J. Intensity Modulated Proton Therapy and Its Sensitivity to Treatment Uncertainties 2: The Potential Effects of Inter-Fraction and Inter-Field Motions. Phys. Med. Biol. 2008, 53, 1043–1056. [Google Scholar] [CrossRef]
- Paganetti, H. Range Uncertainties in Proton Therapy and the Role of Monte Carlo Simulations. Phys. Med. Biol. 2012, 57, R99. [Google Scholar] [CrossRef]
- Kraan, A.C.; Van De Water, S.; Teguh, D.N.; Al-Mamgani, A.; Madden, T.; Kooy, H.M.; Heijmen, B.J.M.; Hoogeman, M.S. Dose Uncertainties in IMPT for Oropharyngeal Cancer in the Presence of Anatomical, Range, and Setup Errors. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 888–896. [Google Scholar] [CrossRef] [Green Version]
- Müller, B.S.; Duma, M.N.; Kampfer, S.; Nill, S.; Oelfke, U.; Geinitz, H.; Wilkens, J.J. Impact of Interfractional Changes in Head and Neck Cancer Patients on the Delivered Dose in Intensity Modulated Radiotherapy with Protons and Photons. Phys. Med. 2015, 31, 266–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stützer, K.; Jakobi, A.; Bandurska-Luque, A.; Barczyk, S.; Arnsmeyer, C.; Löck, S.; Richter, C. Potential Proton and Photon Dose Degradation in Advanced Head and Neck Cancer Patients by Intratherapy Changes. J. Appl. Clin. Med. Phys. 2017, 18, 104. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, L.V.; Steenbakkers, R.J.H.M.; Ten Haken, B.; Van Der Laan, H.P.; Van’t Veld, A.A.; Langendijk, J.A.; Korevaar, E.W. Robust Intensity Modulated Proton Therapy (IMPT) Increases Estimated Clinical Benefit in Head and Neck Cancer Patients. PLoS ONE 2016, 11, e0152477. [Google Scholar] [CrossRef]
- Liu, W.; Frank, S.J.; Li, X.; Li, Y.; Park, P.C.; Dong, L.; Ronald Zhu, X.; Mohan, R. Effectiveness of Robust Optimization in Intensity-Modulated Proton Therapy Planning for Head and Neck Cancers. Med. Phys. 2013, 40, 051711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albertini, F.; Matter, M.; Nenoff, L.; Zhang, Y.; Lomax, A. Online Daily Adaptive Proton Therapy. Br. J. Radiol. 2020, 93, 20190594. [Google Scholar] [CrossRef] [PubMed]
- Placidi, L.; Bolsi, A.; Lomax, A.J.; Schneider, R.A.; Malyapa, R.; Weber, D.C.; Albertini, F. Effect of Anatomic Changes on Pencil Beam Scanned Proton Dose Distributions for Cranial and Extracranial Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Sato, Y.; Minami, H.; Yasukawa, Y.; Yamamoto, K.; Tamamura, H.; Shibata, S.; Bou, S.; Sasaki, M.; Tameshige, Y.; et al. Positioning Accuracy and Daily Dose Assessment for Prostate Cancer Treatment Using In-Room CT Image Guidance at a Proton Therapy Facility. Med. Phys. 2018, 45, 1832–1843. [Google Scholar] [CrossRef]
- Sun, B.; Yang, D.; Lam, D.; Zhang, T.; Dvergsten, T.; Bradley, J.; Mutic, S.; Zhao, T. Toward Adaptive Proton Therapy Guided with a Mobile Helical CT Scanner. Radiother. Oncol. 2018, 129, 479–485. [Google Scholar] [CrossRef]
- Nenoff, L.; Matter, M.; Hedlund Lindmar, J.; Weber, D.C.; Lomax, A.J.; Albertini, F. Daily Adaptive Proton Therapy—The Key to Innovative Planning Approaches for Paranasal Cancer Treatments. Acta Oncol. 2019, 58, 1423–1428. [Google Scholar] [CrossRef] [Green Version]
- Van De Water, S.; Albertini, F.; Weber, D.C.; Heijmen, B.J.M.; Hoogeman, M.S.; Lomax, A.J. Anatomical Robust Optimization to Account for Nasal Cavity Filling Variation during Intensity-Modulated Proton Therapy: A Comparison with Conventional and Adaptive Planning Strategies. Phys. Med. Biol. 2018, 63, 025020. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, X.; Wang, X.; Zhu, X.R.; Gunn, B.; Frank, S.J.; Chang, Y.; Li, Q.; Yang, K.; Wu, G.; et al. Multiple-CT Optimization: An Adaptive Optimization Method to Account for Anatomical Changes in Intensity-Modulated Proton Therapy for Head and Neck Cancers. Radiother. Oncol. 2020, 142, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, A.; Bobić, M.; Winey, B.; Verburg, J.; Sharp, G.C.; Paganetti, H. Anatomic Changes in Head and Neck Intensity-Modulated Proton Therapy: Comparison between Robust Optimization and Online Adaptation. Radiother. Oncol. 2021, 159, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Scandurra, D.; Meijer, T.W.H.; Free, J.; van den Hoek, J.G.M.; Kelder, L.; Oldehinkel, E.; Steenbakkers, R.J.H.M.; Both, S.; Langendijk, J.A. Evaluation of Robustly Optimised Intensity Modulated Proton Therapy for Nasopharyngeal Carcinoma. Radiother. Oncol. 2022, 168, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Hague, C.; Aznar, M.; Dong, L.; Fotouhi-Ghiam, A.; Lee, L.W.; Li, T.; Lin, A.; Lowe, M.; Lukens, J.N.; McPartlin, A.; et al. Inter-Fraction Robustness of Intensity-Modulated Proton Therapy in the Post-Operative Treatment of Oropharyngeal and Oral Cavity Squamous Cell Carcinomas. Br. J. Radiol. 2020, 93, 20190638. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Mesías, M.; Troost, E.G.C.; Lohaus, F.; Agolli, L.; Rehm, M.; Richter, C.; Stützer, K. Including Anatomical Variations in Robust Optimization for Head and Neck Proton Therapy Can Reduce the Need of Adaptation. Radiother. Oncol. 2019, 131, 127–134. [Google Scholar] [CrossRef]
- Lühr, A.; Löck, S.; Roth, K.; Helmbrecht, S.; Jakobi, A.; Petersen, J.B.; Just, U.; Krause, M.; Enghardt, W.; Baumann, M. Concept for Individualized Patient Allocation: ReCompare-Remote Comparison of Particle and Photon Treatment Plans. Radiat. Oncol. 2014, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and Neck Cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef]
- Goitein, M.; Cox, J.D. Should Randomized Clinical Trials Be Required for Proton Radiotherapy? J. Clin. Oncol. 2008, 26, 175–176. [Google Scholar] [CrossRef]
- Bollen, H.; van der Veen, J.; Laenen, A.; Nuyts, S. Recurrence Patterns After IMRT/VMAT in Head and Neck Cancer. Front. Oncol. 2021, 11, 3688. [Google Scholar] [CrossRef]
- Taylor, P.A.; Kry, S.F.; Alvarez, P.; Keith, T.; Lujano, C.; Hernandez, N.; Followill, D.S. Results From the Imaging and Radiation Oncology Core Houston’s Anthropomorphic Phantoms Used for Proton Therapy Clinical Trial Credentialing. Int. J. Radiat. Oncol. 2016, 95, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Langendijk, J.A.; Boersma, L.J.; Rasch, C.R.N.; van Vulpen, M.; Reitsma, J.B.; van der Schaaf, A.; Schuit, E. Clinical Trial Strategies to Compare Protons With Photons. Semin. Radiat. Oncol. 2018, 28, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Relton, C.; Torgerson, D.; O’Cathain, A.; Nicholl, J. Rethinking Pragmatic Randomised Controlled Trials: Introducing the “Cohort Multiple Randomised Controlled Trial” Design. BMJ 2010, 340, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.S.; Gomez, D.R.; Shah, A.K.; Kim, C.R.; Palmer, M.B.; Thaker, N.G.; Grosshans, D.R.; Liao, Z.; Chapman, B.V.; Brooks, E.D.; et al. The Insurance Approval Process for Proton Radiation Therapy: A Significant Barrier to Patient Care. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Ricci, K.I.; Efstathiou, J.A. Beyond a Moonshot: Insurance Coverage for Proton Therapy. Lancet Oncol. 2016, 17, 559–561. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Khan, A.J.; Goyal, S.; Millevoi, R.; Elsebai, N.; Jabbour, S.K.; Yue, N.J.; Haffty, B.G.; Parikh, R.R. Insurance Approval for Proton Beam Therapy and Its Impact on Delays in Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 714–723. [Google Scholar] [CrossRef]
- Lievens, Y.; Pijls-Johannesma, M. Health Economic Controversy and Cost-Effectiveness of Proton Therapy. Semin. Radiat. Oncol. 2013, 23, 134–141. [Google Scholar] [CrossRef]
- Lievens, Y.; Van Den Bogaert, W. Proton Beam Therapy: Too Expensive to Become True? Radiother. Oncol. 2005, 75, 131–133. [Google Scholar] [CrossRef]
- Goitein, M.; Jermann, M. The Relative Costs of Proton and X-ray Radiation Therapy. Clin. Oncol. 2003, 15, S37–S50. [Google Scholar] [CrossRef]
- Hawkins, P.G.; Kadam, A.S.; Jackson, W.C.; Eisbruch, A. Organ-Sparing in Radiotherapy for Head-and-Neck Cancer: Improving Quality of Life. Semin. Radiat. Oncol. 2018, 28, 46–52. [Google Scholar] [CrossRef]
- Brodin, N.P.; Kabarriti, R.; Pankuch, M.; Schechter, C.B.; Gondi, V.; Kalnicki, S.; Guha, C.; Garg, M.K.; Tomé, W.A. A Quantitative Clinical Decision-Support Strategy Identifying Which Patients With Oropharyngeal Head and Neck Cancer May Benefit the Most From Proton Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 540–552. [Google Scholar] [CrossRef]
- Sher, D.J.; Tishler, R.B.; Pham, N.L.; Punglia, R.S. Cost-Effectiveness Analysis of Intensity Modulated Radiation Therapy Versus Proton Therapy for Oropharyngeal Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Frank, S.J.; Verma, V.; Thaker, N.G.; Brooks, E.D.; Palmer, M.B.; Harrison, R.F.; Deshmukh, A.A.; Ning, M.S. Cost-Effectiveness Models of Proton Therapy for Head and Neck: Evaluating Quality and Methods to Date. Int. J. Part. Ther. 2021, 8, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Thaker, N.G.; Boyce-Fappiano, D.; Ning, M.S.; Pasalic, D.; Guzman, A.; Smith, G.; Holliday, E.B.; Incalcaterra, J.; Garden, A.S.; Shaitelman, S.F.; et al. Activity-Based Costing of Intensity-Modulated Proton versus Photon Therapy for Oropharyngeal Cancer. Int. J. Part. Ther. 2021, 8, 374. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 5 April 2022).
- PTCOG—Facilities in Operation. Available online: https://www.ptcog.ch/index.php/facilities-in-operation (accessed on 25 April 2022).
| TUMOR SITE | STUDY TYPE | NUMBER OF PATIENTS | MEDIAN FOLLOW-UP (IMRT-PT) | TOXICITY IMRT VS. PT | ONCOLOGIC OUTCOME IMRT VS. PT | |
|---|---|---|---|---|---|---|
| Holliday et al., (2015) [25] | NPC | RETROSPECTIVE CASE-MATCHED COHORT | 10 IMPT 20 IMRT | 21.6 MO 25.8 MO | ACUTE G3 TOXICITY: 90% vs. 50% * LATE G2 DYSPHAGIA: 15% vs. 0% ACUTE G3 DERMATITIS: 25% vs. 40% G-TUBE: 65% vs. 20% * | AT LATEST FUP: LRC: 95 vs. 100% OS: 95% vs. 90% |
| Sio et al., (2016) [26] | OPC | RETROSPECTIVE | 35 IMPT 46 IMRT | 7.7 MO 2.7 MO | TOP 11 MD ANDERSON SYMPTOMS: NO DIFFERENCE IN ACUTE AND CHRONIC SYMPTOMS SIGNIFICANT DIFFERENCE IN FAVOR OF PT FOR SUBACUTE APPETITE AND MUCOSITIS * G-TUBE: 48% vs. 20% * | - |
| Gunn et al., (2016) [27] | OPC | PROSPECTIVE | 50 IMPT | 29 MO | ACUTE G3 MUCOSITIS: 46% ACUTE AND LATE G3 DYSPHAGIA: 24% and 6.12% G-TUBE: 22% | 2Y LRC: 92% 2Y PFS: 88.6% 2Y OS: 94.5% |
| Blanchard et al., (2016) [28] | OPC | RETROSPECTIVE CASE-MATCHED COHORT | 50 IMPT 100 IMRT | 33 MO 29 MO | ACUTE XEROSTOMIA GRADE 2–3: 60% vs. 42%* G-TUBE OF WEIGHT LOSS > 20% 3MONTHS AFTER RT: 34% vs. 18%* | 3Y LRC: 89.7% vs. 91% 3Y PFS: 86.4% vs. 85.8% 3Y OS: 89.3% vs. 94.3% |
| Lewis et al., (2016) [16] | NPC | PROSPECTIVE | 10 IMPT | 24.5 MO | ACUTE G3 DERMATITIS: 44% LATE G2 XEROSTOMIA: 22% LATE ≥ G3 TOXICITIES: 0% | 2Y LRC: 100% 2Y OS: 88.9% |
| Zhang et al., (2017) [29] | OPC | RETROSPECTIVE | 50 IMPT 534 IMRT | 33.8 MO | MANDIBULAR ORN: 7.7% vs. 2% MEAN MANDIBULAR DOSE: 41.2% vs. 25.6%* | - |
| Aljabab et al., (2020) [30] | OPC | RETROSPECTIVE | 46 IMPT (28 DEFINITIVE RT, 18 PORT) | 19.2 MO | G3 DERMATITIS: 76% LATE G2 DYSPHAGIA: 2% LATE G2 XEROSTOMIA: 30% | AT LATEST FUP: LRC: 100% PFS: 93.5% OS: 95.7% |
| Manzar et al., (2020) [31] | OPC | RETROSPECTIVE | 46 IMPT 259 IMRT (138 DEFINITIVE RT, 167 PORT) | 30 MO 12 MO | G-TUBE: 56% vs. 25% * ACUTE G3 DYSPHAGIA: 44.2% vs. 23.3% ACUTE ≥ G2 DERMATITIS: 68.3% vs. 82.9% * | 1Y OS: 91.3% vs. 92.6% |
| Kitpanit et al., (2020) [32] | OPC | RETROSPECTIVE | 27 IMPT (18 DEFINITIVE RT, 9 PORT) | 19 MO | ACUTE G1-2 DERMATITIS: 92.6% ACUTE G1-2 DYSPHAGIA: 81.5% ACUTE G3 TOXICITIES: 3.7% LATE G1 XEROSTOMIA: 77.8% | 1Y LRC: 100% 1Y OS: 100% |
| Jiří et al., (2021) [33] | NPC | RETROSPECTIVE | 40 IMPT | 24 MO | ACUTE G3 DERMATITIS: 14% G-TUBE: 9.3% G2 XEROSTOMIA: 7% G2 DYSPHAGIA: 5% | 2Y LRC: 84% 2Y PFS: 75% 2Y OS: 80% |
| Li et al., (2021) [34] | NPC | RETROSPECTIVE CASE-MATCHED COHORT | 28 IMPT 49 IMRT | 37 MO 23 MO | ACUTE ≥ G2 TOXICITIES: 93.9% vs. 69.9% * LATE G3 TOXICITIES: 16.3% vs. 3.8% | 2Y LRC: 86.2% vs. 100% 2Y PFS: 76.7% vs. 95.7% 3Y OS: 94% vs. 100% |
| Williams et al., (2021) [35] | NPC | RETROSPECTIVE | 26 IMPT | 25 MO | ACUTE G3 DERMATITIS: 42% LATE G2 XEROSTOMIA: 8% LATE G2 DYSPHAGIA: 4% NO G3 TOXICITIES | 2Y LRC: 92% 2Y OS: 85% |
| Cao et al., (2021) [36] | OPC | RETROSPECTIVE | 103 IMPT 429 IMRT | 36.2 MO | G2-3 XEROSTOMIA: − < 18 MO: 16% vs. 9% −18–24 MO: 20% vs. 6% * −24–36 MO: 20% vs. 6% * | - |
| Chou et al., (2021) [37] | NPC | RETROSPECTIVE CASE-MATCHED COHORT | 80 IMPT 80 IMRT | 24.1 MO 42.2 MO | G-TUBE: 15% vs. 5%* WEIGHT LOSS > 7%: 6.21 vs. 4.87 * G3 DERMATITIS: 7.5% vs. 35%* NO DIFFERENCES FOR OTHER ACUTE ≥ G2 TOXICITIES | 2Y LRC: 95% vs. 97.5% 2Y PFS: 83.7% vs. 94.4% 2Y OS: 89.5% vs. 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuyts, S.; Bollen, H.; Ng, S.P.; Corry, J.; Eisbruch, A.; Mendenhall, W.M.; Smee, R.; Strojan, P.; Ng, W.T.; Ferlito, A. Proton Therapy for Squamous Cell Carcinoma of the Head and Neck: Early Clinical Experience and Current Challenges. Cancers 2022, 14, 2587. https://doi.org/10.3390/cancers14112587
Nuyts S, Bollen H, Ng SP, Corry J, Eisbruch A, Mendenhall WM, Smee R, Strojan P, Ng WT, Ferlito A. Proton Therapy for Squamous Cell Carcinoma of the Head and Neck: Early Clinical Experience and Current Challenges. Cancers. 2022; 14(11):2587. https://doi.org/10.3390/cancers14112587
Chicago/Turabian StyleNuyts, Sandra, Heleen Bollen, Sweet Ping Ng, June Corry, Avraham Eisbruch, William M Mendenhall, Robert Smee, Primoz Strojan, Wai Tong Ng, and Alfio Ferlito. 2022. "Proton Therapy for Squamous Cell Carcinoma of the Head and Neck: Early Clinical Experience and Current Challenges" Cancers 14, no. 11: 2587. https://doi.org/10.3390/cancers14112587
APA StyleNuyts, S., Bollen, H., Ng, S. P., Corry, J., Eisbruch, A., Mendenhall, W. M., Smee, R., Strojan, P., Ng, W. T., & Ferlito, A. (2022). Proton Therapy for Squamous Cell Carcinoma of the Head and Neck: Early Clinical Experience and Current Challenges. Cancers, 14(11), 2587. https://doi.org/10.3390/cancers14112587










