Innovative Diagnostic and Therapeutic Interventions in Cervical Dysplasia: A Systematic Review of Controlled Trials
Abstract
Simple Summary
Abstract
1. Introduction
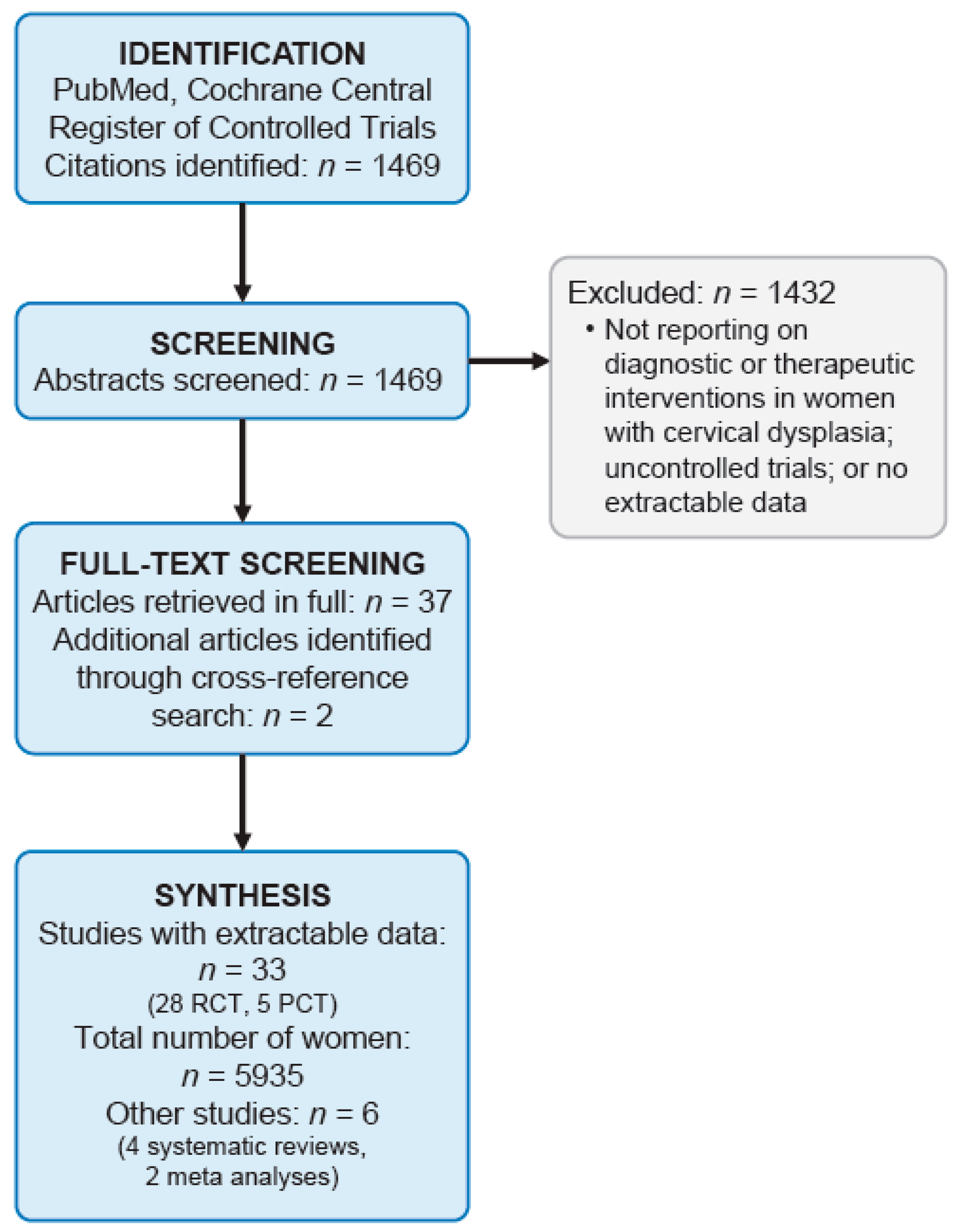
2. Materials and Methods
3. Results
3.1. Diagnostic Studies in Women with Suspected or Proven Cervical Dysplasia
3.2. Therapeutic Studies in Women with Suspected or Proven Cervical Dysplasia
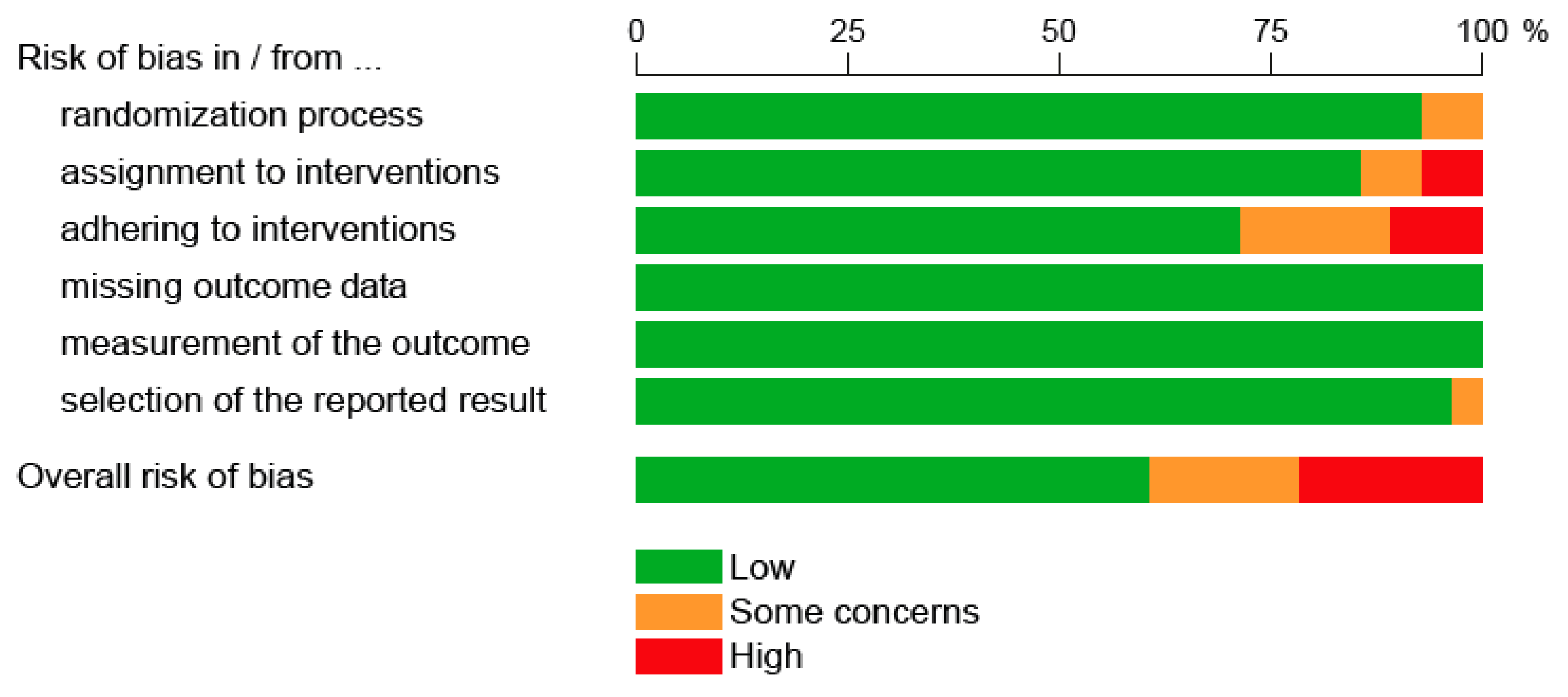
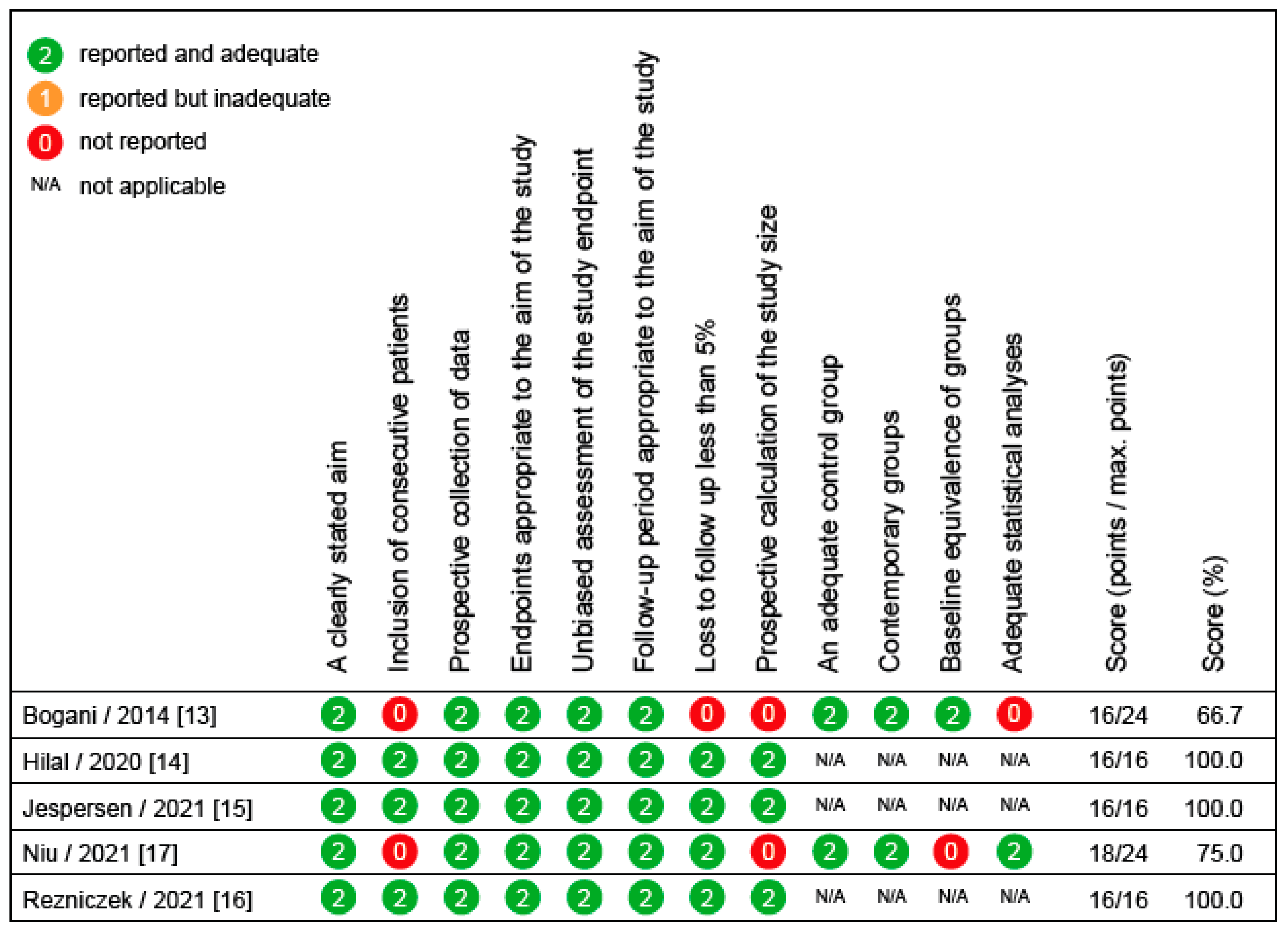
3.3. Methodological Assessment of Diagnostic and Therapeutic Studies in Women with Cervical Dysplasia
3.4. Systematic Reviews of Diagnostic or Therapeutic Interventions in Women with Cervical Dysplasia
3.5. Ongoing Studies
4. Discussion
5. Future Research Needs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; Kubik, M.; et al. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 320, 674–686. [Google Scholar] [CrossRef]
- Loopik, D.L.; Bentley, H.A.; Eijgenraam, M.N.; IntHout, J.; Bekkers, R.L.M.; Bentley, J.R. The Natural History of Cervical Intraepithelial Neoplasia Grades 1, 2, and 3: A Systematic Review and Meta-analysis. J. Low. Genit. Tract Dis. 2021, 25, 221–231. [Google Scholar] [CrossRef]
- Jain, M.A.; Limaiem, F. Cervical Intraepithelial Squamous Cell Lesion; StatPearls: Treasure Island, FL, USA, 2021.
- American Cancer Society. Cancer Facts & Figures 2021. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf (accessed on 4 April 2022).
- Liverani, C.A.; Di Giuseppe, J.; Clemente, N.; Delli Carpini, G.; Monti, E.; Fanetti, F.; Bolis, G.; Ciavattini, A. Length but not transverse diameter of the excision specimen for high-grade cervical intraepithelial neoplasia (CIN 2-3) is a predictor of pregnancy outcome. Eur. J. Cancer Prev. 2016, 25, 416–422. [Google Scholar] [CrossRef]
- Bitonti, G.; Clemente, N.; Del Fabro, A.; Manna, P.; Buttignol, M.; Cadel, M.; Di Carlo, C.; Giorda, G.; Zullo, F.; Sopracordevole, F. CIN 2 in childbearing-age women: May colposcopy help in choosing the proper management? Minerva Obstet. Gynecol. 2021. [Google Scholar] [CrossRef]
- Monti, M.; D’Aniello, D.; Scopelliti, A.; Tibaldi, V.; Santangelo, G.; Colagiovanni, V.; Giannini, A.; Di Donato, V.; Palaia, I.; Perniola, G.; et al. Relationship between cervical excisional treatment for cervical intraepithelial neoplasia and obstetrical outcome. Minerva Obstet. Gynecol. 2021, 73, 233–246. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Athanasiou, A.; Paraskevaidi, M.; Mitra, A.; Kalliala, I.; Martin-Hirsch, P.; Arbyn, M.; Bennett, P.; Paraskevaidis, E. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: Systematic review and meta-analysis. BMJ 2016, 354, i3633. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Thomas, J.; Kneale, D.; McKenzie, J.E.; Brennan, S.E.; Bhaumik, S. Chapter 2: Determining the scope of the review and the questions it will address. In Cochrane Handbook for Systematic Reviews of Interventions: Version 6.3 (Updated February 2022), 2nd ed.; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; John Wiley & Sons: Chichester, UK, 2022. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Bogani, G.; Serati, M.; Cromi, A.; Di Naro, E.; Casarin, J.; Pinelli, C.; Rossi, T.; Ghezzi, F. Local anesthetic versus forced coughing at colposcopic-guided biopsy: A prospective study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 15–19. [Google Scholar] [CrossRef]
- Hilal, Z.; Tempfer, C.B.; Burgard, L.; Rehman, S.; Rezniczek, G.A. How long is too long? Application of acetic acid during colposcopy: A prospective study. Am. J. Obstet. Gynecol. 2020, 223, 101.e1–101.e8. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, M.M.; Booth, B.B.; Petersen, L.K. Can biopsies be omitted after normal colposcopy in women referred with low-grade cervical cytology? A prospective cohort study. BMC Women’s Health 2021, 21, 394. [Google Scholar] [CrossRef] [PubMed]
- Rezniczek, G.A.; Ertan, S.; Rehman, S.; Tempfer, C.B. Sequential Application of Lugol’s Iodine Test after Acetic Acid for Detecting Cervical Dysplasia: A Prospective Cohort Study. Diagnostics 2021, 11, 1598. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Cheng, M.; Hong, Z.; Ling, J.; Di, W.; Gu, L.; Qiu, L. The effect of 5-Aminolaevulinic Acid Photodynamic Therapy versus CO2 laser in the Treatment of Cervical Low-grade Squamous Intraepithelial Lesions with High-Risk HPV Infection: A non-randomized, controlled pilot study. Photodiagnosis Photodyn. Ther. 2021, 36, 102548. [Google Scholar] [CrossRef]
- Öz, M.; Korkmaz, E.; Cetinkaya, N.; Baş, S.; Özdal, B.; Meydanl, M.M.; Güngör, T. Comparison of Topical Lidocaine Spray with Placebo for Pain Relief in Colposcopic Procedures: A Randomized, Placebo-Controlled, Double-Blind Study. J. Low. Genit. Tract Dis. 2015, 19, 212–214. [Google Scholar] [CrossRef]
- Hilal, Z.; Rezniczek, G.A.; Tettenborn, Z.; Hefler, L.A.; Tempfer, C.B. Efficacy of Monsel Solution After Cervical Biopsy: A Randomized Trial. J. Low. Genit. Tract Dis. 2016, 20, 312–316. [Google Scholar] [CrossRef]
- Hilal, Z.; Alici, F.; Tempfer, C.B.; Seebacher, V.; Rezniczek, G.A. Video Colposcopy for Reducing Patient Anxiety During Colposcopy: A Randomized Controlled Trial. Obstet. Gynecol. 2017, 130, 411–419. [Google Scholar] [CrossRef]
- Kiviharju, M.; Kalliala, I.; Nieminen, P.; Dyba, T.; Riska, A.; Jakobsson, M. Pain Sensation During Colposcopy and Cervical Biopsy, with or without Local Anesthesia: A Randomized Trial. J. Low. Genit. Tract Dis. 2017, 21, 102–107. [Google Scholar] [CrossRef]
- Hilal, Z.; Alici, F.; Tempfer, C.B.; Rath, K.; Nar, K.; Rezniczek, G.A. Mozart for Reducing Patient Anxiety During Colposcopy: A Randomized Controlled Trial. Obstet. Gynecol. 2018, 132, 1047–1055. [Google Scholar] [CrossRef]
- Karaman, E.; Kolusarı, A.; Alkış, İ.; Çetin, O. Comparison of topical lidocaine spray with forced coughing in pain relief during colposcopic biopsy procedure: A randomised trial. J. Obstet. Gynaecol. 2019, 39, 534–538. [Google Scholar] [CrossRef]
- Comba, C.; Demirayak, G.; Erdogan, S.V.; Karaca, I.; Demir, O.; Guler, O.; Ozdemir, I.A. Comparison of pain and proper sample status according to usage of tenaculum and analgesia: A randomized clinical trial. Obstet. Gynecol. Sci. 2020, 63, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Topdaği, Y.E.; Topdagi Yilmaz, E.P.; Aydin, M.E.; Ates, I.; Oral Ahiskalioglu, E. Does intravenous lidocaine added to nonsteroidal anti-inflammatory drugs reduce pain during colposcopy? A prospective randomized double-blind study. Ginekol. Pol. 2021, 92, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Boonlikit, S.; Thitisagulwong, S. C-LETZ versus large loop excision of the transformation zone for the treatment of cervical intraepithelial neoplasia: A randomized controlled trial. Arch. Gynecol. Obstet. 2012, 286, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.J.; Russomano, F.B.; Tristão, M.A.; Huf, G.; Prendiville, W. Large loop versus straight-wire excision of the transformation zone for treatment of cervical intraepithelial neoplasia: A randomised controlled trial of electrosurgical techniques. BJOG 2015, 122, 552–557. [Google Scholar] [CrossRef]
- Russomano, F.; Tristao, M.A.P.; Côrtes, R.; de Camargo, M.J. A comparison between type 3 excision of the transformation zone by straight wire excision of the transformation zone (SWETZ) and large loop excision of the transformation zone (LLETZ): A randomized study. BMC Womens. Health 2015, 15, 12. [Google Scholar] [CrossRef][Green Version]
- Hilal, Z.; Mavrommati, G.; Foerster, C.; Rezniczek, G.A.; Hefler, L.A.; Tempfer, C.B. Spray Versus Forced Coagulation in Large Loop Excision of the Transformation Zone: A Randomized Trial. J. Low. Genit. Tract Dis. 2016, 20, 169–173. [Google Scholar] [CrossRef][Green Version]
- Firnhaber, C.; Swarts, A.; Goeieman, B.; Rakhombe, N.; Mulongo, M.; Williamson, A.-L.; Michelow, P.; Ramotshela, S.; Faesen, M.; Levin, S.; et al. Cryotherapy Reduces Progression of Cervical Intraepithelial Neoplasia Grade 1 in South African HIV-Infected Women: A Randomized, Controlled Trial. J. Acquir. Immune Defic. Syndr. 2017, 76, 532–538. [Google Scholar] [CrossRef]
- Hilal, Z.; Rezniczek, G.A.; El-Fizazi, N.; Tempfer, C.B. Large Loop Excision of the Transformation Zone Versus True Cone Biopsy Electrode Excision: A Randomized Trial. J. Low. Genit. Tract Dis. 2017, 21, 272–278. [Google Scholar] [CrossRef]
- Smith, J.S.; Sanusi, B.; Swarts, A.; Faesen, M.; Levin, S.; Goeieman, B.; Ramotshela, S.; Rakhombe, N.; Williamson, A.L.; Michelow, P.; et al. A randomized clinical trial comparing cervical dysplasia treatment with cryotherapy vs. loop electrosurgical excision procedure in HIV-seropositive women from Johannesburg, South Africa. Am. J. Obstet. Gynecol. 2017, 217, 183.e1–183.e11. [Google Scholar] [CrossRef]
- Hilal, Z.; Rezniczek, G.A.; Alici, F.; Kumpernatz, A.; Dogan, A.; Alieva, L.; Tempfer, C.B. Loop electrosurgical excision procedure with or without intraoperative colposcopy: A randomized trial. Am. J. Obstet. Gynecol. 2018, 219, 377.e1–377.e7. [Google Scholar] [CrossRef]
- Greene, S.A.; de Vuyst, H.; John-Stewart, G.C.; Richardson, B.A.; McGrath, C.J.; Marson, K.G.; Trinh, T.T.; Yatich, N.; Kiptinness, C.; Cagle, A.; et al. Effect of Cryotherapy vs. Loop Electrosurgical Excision Procedure on Cervical Disease Recurrence Among Women with HIV and High-Grade Cervical Lesions in Kenya: A Randomized Clinical Trial. JAMA 2019, 322, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Kolben, T.M.; Etzel, L.T.; Bergauer, F.; Hagemann, I.; Hillemanns, P.; Repper, M.; Kaufmann, A.M.; Sotlar, K.; Kolben, T.; Helms, H.J.; et al. A randomized trial comparing limited-excision conisation to Large Loop Excision of the Transformation Zone (LLETZ) in cervical dysplasia patients. J. Gynecol. Oncol. 2019, 30, e42. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Mandal, R.; Mandal, A.; Ghosh, I.; Mittal, S.; Muwonge, R.; Lucas, E.; Basu, P. A Prospective Randomized Trial to Compare Safety, Acceptability and Efficacy of Thermal Ablation and Cryotherapy in a Screen and Treat Setting. Asian Pac. J. Cancer Prev. 2020, 21, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Chong, G.O.; Lee, Y.H.; Jeon, S.Y.; Yang, H.-Y.; An, S.-H. Efficacy of a chitosan tampon in the loop electrosurgical excision procedure: A prospective randomized controlled study. Sci. Rep. 2020, 10, 6017. [Google Scholar] [CrossRef]
- Rezniczek, G.A.; Hecken, J.M.; Rehman, S.; Dogan, A.; Tempfer, C.B.; Hilal, Z. Syringe or mask? Loop electrosurgical excision procedure under local or general anesthesia: A randomized trial. Am. J. Obstet. Gynecol. 2020, 223, 888.e1–888.e9. [Google Scholar] [CrossRef]
- Duan, L.; Du, H.; Belinson, J.L.; Liu, Z.; Xiao, A.; Liu, S.; Zhao, L.; Wang, C.; Qu, X.; Wu, R. Thermocoagulation versus cryotherapy for the treatment of cervical precancers. J. Obstet. Gynaecol. Res. 2021, 47, 279–286. [Google Scholar] [CrossRef]
- Fonseca, B.O.; Possati-Resende, J.C.; Salcedo, M.P.; Schmeler, K.M.; Accorsi, G.S.; Fregnani, J.H.T.G.; Antoniazzi, M.; Pantano, N.P.; Santana, I.V.V.; Matsushita, G.M.; et al. Topical Imiquimod for the Treatment of High-Grade Squamous Intraepithelial Lesions of the Cervix: A Randomized Controlled Trial. Obstet. Gynecol. 2021, 137, 1043–1053. [Google Scholar] [CrossRef]
- Firnhaber, C.; Swarts, A.; Jezile, V.; Mulongo, M.; Goeieman, B.; Williams, S.; Faesen, M.; Michelow, P.; Wilkin, T. Human Papillomavirus Vaccination Prior to Loop Electroexcision Procedure Does Not Prevent Recurrent Cervical High-grade Squamous Intraepithelial Lesions in Women Living with Human Immunodeficiency Virus: A Randomized, Double-blind, Placebo-controlled Trial. Clin. Infect. Dis. 2021, 73, e2211–e2216. [Google Scholar] [CrossRef]
- Gungorduk, K.; Ozdemir, A.; Sahin, O. Optimal timing of the loop electrosurgical excision procedure according to different phases of the menstrual cycle. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101888. [Google Scholar] [CrossRef]
- Vieira, M.D.A.; de Araújo, R.L.C.; Da Cunha Andrade, C.E.M.; Schmidt, R.L.; Filho, A.L.; Reis, R.D. A randomized clinical trial of a new anti-cervical stenosis device after conization by loop electrosurgical excision. PLoS ONE 2021, 16, e0242067. [Google Scholar] [CrossRef]
- Rezniczek, G.A.; Neghabian, N.; Rehman, S.; Tempfer, C.B. Video colposcopy versus headlight for large loop excision of the transformation zone (LLETZ): A randomised trial. Arch. Gynecol. Obstet. 2022, 305, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Polterauer, S.; Reich, O.; Widschwendter, A.; Hadjari, L.; Bogner, G.; Reinthaller, A.; Joura, E.; Trutnovsky, G.; Ciresa-Koenig, A.; Ganhoer-Schimboeck, J.; et al. Topical imiquimod compared with conization to treat cervical high-grade squamous intraepithelial lesions: Multicenter, randomized controlled trial. Gynecol. Oncol. 2022, 165, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Martin-Hirsch, P.P.L.; Bryant, A. Interventions for preventing blood loss during the treatment of cervical intraepithelial neoplasia. Cochrane Database Syst. Rev. 2013, CD001421. [Google Scholar] [CrossRef]
- Gajjar, K.; Martin-Hirsch, P.P.L.; Bryant, A.; Owens, G.L. Pain relief for women with cervical intraepithelial neoplasia undergoing colposcopy treatment. Cochrane Database Syst. Rev. 2016, 7, CD006120. [Google Scholar] [CrossRef]
- Santesso, N.; Mustafa, R.A.; Wiercioch, W.; Kehar, R.; Gandhi, S.; Chen, Y.; Cheung, A.; Hopkins, J.; Khatib, R.; Ma, B.; et al. Systematic reviews and meta-analyses of benefits and harms of cryotherapy, LEEP, and cold knife conization to treat cervical intraepithelial neoplasia. Int. J. Gynaecol. Obstet. 2016, 132, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, A.; Sun, W.; Yue, Y.; Li, H. Efficacy and safety of photodynamic therapy for cervical intraepithelial neoplasia and human papilloma virus infection: A systematic review and meta-analysis of randomized clinical trials. Medicine 2018, 97, e10864. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, P.; Arduino, B.; Borgo, M.; Saccone, G.; Venturella, R.; Di Cello, A.; Zullo, F. Loop Electrosurgical Excision Procedure versus Cryotherapy in the Treatment of Cervical Intraepithelial Neoplasia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Gynecol. Minim. Invasive Ther. 2018, 7, 145–151. [Google Scholar] [CrossRef]
- Unanyan, A.; Pivazyan, L.; Davydova, J.; Murvatova, K.; Khrapkova, A.; Movsisyan, R.; Ishchenko, A.; Ishchenko, A. Efficacy of photodynamic therapy in women with HSIL, LSIL and early stage squamous cervical cancer: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2021, 36, 102530. [Google Scholar] [CrossRef]

| Author/Year | Clinical Trial Registration | Study Type | Sample Size | Objective | Primary Endpoint | Numerical Results | Main Conclusions |
|---|---|---|---|---|---|---|---|
| Bogani et al., 2014 [13] | None | PCT | 100 | To compare the effect of 2 mL of intracervical lidocaine 1% vs. forced coughing for pain control during colposcopically guided biopsy | Procedure-related pain; using a 100-mm visual analogue scale | No between-group differences were observed in terms of pain related to speculum insertion, biopsies and pain recorded after the procedure (p > 0.05) | Forced coughing should be preferred over local anesthesia |
| Öz et al., 2015 [18] | None | RCT | 214 | To compare the effectiveness of topical lidocaine spray vs. placebo for relieving pain during colposcopically guided biopsy and ECC | Pain level immediately after the cervical biopsy and ECC, measured using the Wong-Baker FACES Pain Rating Scale | Pain scores were similar; mean ± SD pain scores were 2.18 ± 1.7 in the lidocaine group and 2.31 ± 1.6 in the control group | Routine use of a lidocaine spray before cervical punch biopsy or ECC is not recommended |
| Hilal et al., 2016 [19] | NCT02486471 | RCT | 145 | To estimate the efficacy and side effects of Monsel’s solution for hemostasis after colposcopically guided biopsy | Vaginal bleeding after 15 min measured by scoring a sanitary pad with a 5-level pictogram | Mean bleeding score after 15 min with Monsel’s solution was 1.2 ± 0.6 vs. 1.8 ± 1.0 without Monsel’s solution (p < 0.001) | Monsel’s solution significantly reduces bleeding |
| Hilal et al., 2017 [20] | NCT02697175 | RCT | 225 | To test whether video colposcopy reduces anxiety among patients undergoing colposcopically guided biopsy | Reduction of situation-specific anxiety scores (∆S = S2−S1) measured before (S1) and after (S2) colposcopy, using the State-Trait Anxiety Inventory | The mean ∆S was −10.3 ± 11.3 SD in the video colposcopy group and −10.3 ± 11.0 SD in controls (p = 0.50) | Video colposcopy does not reduce anxiety |
| Kiviharju et al., 2017 [21] | SRCTN20548888 | RCT | 204 | To compare the effect of an intracervical anesthetic vs. no intervention for pain control during colposcopically guided biopsy | Pain experienced during colposcopic examination, using a 10-cm visual analog scale | Mean VAS score for the local anesthetic was 2.7 vs. 3.5 in controls (p = 0.017; 95% CI = 0.1–1.5) | Injection of a local anesthetic reduces pain compared to no intervention during colposcopically guided biopsy |
| Hilal et al., 2018 [22] | NCT03005795 | RCT | 212 | To test whether music by Mozart reduces anxiety among patients undergoing during colposcopically guided biopsy | Reduction of the situation-specific anxiety of women hearing Mozart’s Symphony No. 40 during colposcopy, measured before and after colposcopy using the State-Trait Anxiety Inventory | The mean anxiety reduction was −9.4 ± 10.8 SD in the music group and −9.0 ± 10.6 in controls (p = 0.40) | Mozart’s Symphony No. 40 does not reduce anxiety in women undergoing during colposcopically guided biopsy |
| Karaman et al., 2019 [23] | NCT03100565 | RCT | 86 | To compare the effectiveness of a local lidocaine spray compared to forced coughing for pain control during colposcopically guided cervical biopsy | Differences in pain perceived at four different consecutive steps during colposcopically guided cervical biopsies, assessed by using a 10 cm visual analogue scale | The mean ± SD pain scores after biopsy were 3.25 ± 1.4 in the lidocaine spray group and 4.4 ± 1.3 in the forced coughing group (p < 0.05) | Lidocaine spray can be recommended for pain relief during colposcopically directed cervical biopsy |
| Comba et al., 2020 [24] | NCT03279666 | RCT | 228 | To compare pain perception during colposcopy with/ without tenaculum and with/without intracervical lidocaine/adrenaline | Pain perception during colposcopy assessed using a linear visual analogue scale and biopsy specimen size measured in millimeters in 4 arms (with/without tenaculum and with/without intracervical lidocaine plus adrenaline) | Tenaculum use increased pain perception in the without analgesic group; no differences were noted when the local analgesic was used; size and number of biopsy specimens did not affect pain | Administration of an intracervical analgesic reduces pain when a tenaculum is used |
| Hilal et al., 2020 [14] | None | PCT | 300 | To define the optimal timing for the colposcopic assessment of acetowhite lesions | Most severe colposcopic lesion 1, 3, and 5 min after application of acetic acid, using a standardized colposcopy protocol | After 1 min, 290 of 300 patients (96.7%) were diagnosed with the most severe colposcopic lesion; this proportion did not improve after 3 min (290/300 [96.7%]) or after 5 min (233/264 [88.3%]) | The best time to identify lesions is 1 min after the application of acetic acid; fading of acetowhite lesions is common and supports a recommendation of not prolonging colposcopy beyond 3 min |
| Jespersen et al., 2021 [15] | NCT04249856 | PCT | 173 | To determine the yield of CIN2+ from one to four cervical biopsies in women with cytology of LSIL or ASCUS and a normal colposcopic impression | CIN2+ in women with cytology of LSIL or ASCUS and a normal colposcopic impression | Four biopsies significantly increases CIN2+ cases vs. one biopsy (11.0% and 22.0%, p = 0.006) | Four random cervical biopsies at the squamocolumnar junction should be performed in women with cytology of LSIL or ASCUS who had a normal colposcopic impression |
| Rezniczek et al., 2021 [16] | None | PCT | 320 | To assess the performance of Lugol’s iodine test to identify HSIL/LSIL | Sensitivity/specificity of most severe iodine-negative lesions for the detection of LSIL/HSIL | The sensitivity and specificity of most severe iodine-negative lesions for the detection of LSIL/HSIL was 81.4 (95%-CI 77.3–85.0)% and 29.5 (24.2–35.5)%, respectively | Lugol’s iodine showed moderate sensitivity and poor specificity, but it changed clinical management in 5% of cases when used in addition to acetic acid |
| Topdaği et al., 2021 [25] | None | RCT | 76 | To investigate the effectiveness of intravenous lidocaine use in pain management during colposcopic cervical biopsy and ECC | Pain levels after i.v. lidocaine vs. no intervention measured using visual analogue scale scores | Pain scores were significantly lower in the lidocaine group than in the control group (p < 0.001) | Intravenous lidocaine administration can be used as an alternative approach to reduce pain and increase operator and patient satisfaction during colposcopy-directed biopsy and ECC |
| Author/Year | Clinical Trial Registration | Study Type | Sample Size | Objective | Primary Endpoint | Numerical Results | Main Conclusions |
|---|---|---|---|---|---|---|---|
| Boonlikit et al., 2012 [26] | None | RCT | 98 | To compare LLETZ with C-LETZ in the surgical management of CIN | Fragmentation of the operative specimen | C-LETZ was more likely to result in a single pathologic specimen (76 vs. 29.16%, p < 0.001); the incidence of incomplete excision and complications were similar in both groups | C-LETZ results in a higher rate of a single pathologic specimen but removes more cervical tissue than LLETZ |
| Camargo et al., 2015 [27] | NCT00995020 | RCT | 103 | To compare SWETZ and LLETZ, for the surgical management of CIN | Rate of free endocervical margins | 42 women in the LLETZ-cone group had free endocervical margin vs. 43 women in the SWETZ group (relative risk 1.04, 95% CI 0.87–1.25; p = 0.64) | SWETZ and LLETZ were equal with no difference regarding endocervical margin involvement |
| Russomano et al., 2015 [28] | NCT01929993 | RCT | 164 | To compare SWETZ and LLETZ in women with a type 3 transformation zone regarding incomplete excision and other surgical outcomes | Resection margin status | LLETZ resulted in a higher risk of compromised or damaged endocervical margins compared to SWETZ (RR 1.72, 95% CI: 1.14 to 2.6); absolute risk reduction 26.4% | This study showed a lower proportion of compromised or damaged endocervical surgical margin in specimens resulting from SWETZ in relation to LLETZ |
| Hilal et al., 2016 [29] | NCT02330471 | RCT | 151 | To evaluate spray and forced coagulation to achieve local hemostasis in women undergoing LLETZ | Time to complete local hemostasis | Mean (SD) time to complete local hemostasis with forced and spray coagulation was 43.3 (38.5) and 28.9 (22.9) s (p < 0.001) | Spray coagulation is superior to forced coagulation in women undergoing LLETZ; Spray coagulation should be used as the standard approach |
| Firnhaber et al., 2017 [30] | NCT02250716 | RCT | 220 | To compare cryotherapy vs. no treatment in HIV-infected women with LSIL | Progression to HSIL after 12 m | Cryotherapy reduced progression to HSIL: 2/99 (2%) in the cryotherapy arm and 15/103 (15%) in the no treatment arm (86% reduction; 95% CI: 41% to 97%; p = 0.002) | Treatment of cervical LSIL with cryotherapy decreased progression to HSIL among HIV-infected women especially if high-risk HPV positive |
| Hilal et al., 2017 [31] | NCT02515162 | RCT | 172 | To compare two conization techniques, LLETZ and TCBEE | Resection margin status | No difference in involved margin status between LLETZ and TCBEE was observed (12/91 [13%] vs. 7/81 [9%]; p = 0.4). Specimen fragmentation and surgeon preference favored LLETZ | LLETZ and TCBEE are equally safe and efficacious, but specimen fragmentation and surgeon preference favor LLETZ |
| Smith et al., 2017 [32] | NCT01723956 | RCT | 166 | To compare the efficacy of LEEP vs. cryotherapy for the treatment of HSIL in HIV-seropositive women | 6- and 12-m cumulative incidence of CIN2+ | Cumulative CIN2+ incidence was higher for cryotherapy (24.3%; 95% CI, 16.1–35.8) than LEEP at 6 m (10.8%; 95% CI, 5.7–19.8) (p = 0.02), although by 12 m, the difference was not significant (27.2%; 95% CI, 18.5–38.9 vs. 18.5%; 95% CI, 11.6–28.8, p = 0.2) | Although rates of cumulative CIN2+ were lower after LEEP than cryotherapy treatment at 6 m, both treatments were equally effective in reducing CIN2+ by >70% by 12 m |
| Hilal et al., 2018 [33] | NCT02910388 | RCT | 182 | To assess the benefits of performing LEEP under colposcopic guidance vs. no colposcopy | Resected cone mass | Women undergoing LEEP under colposcopic vision had significantly smaller cone specimens vs. controls (weight: median 1.86 (interquartile range 1.20–2.72) vs. 2.37 (interquartile range 1.63–3.31) g, p = 0.006) | LEEP with intra-operative colposcopy leads to significantly smaller cone specimens without compromising margin status |
| Greene et al., 2019 [34] | NCT01298596 | RCT | 400 | To evaluate whether cryotherapy or LEEP is a more effective treatment for HSIL in women with HIV | Disease recurrence defined as CIN2 or higher on cervical biopsy during a 24-m follow-up | After 2 y, 60 women (30%) randomized to cryotherapy had recurrent CIN2 or higher vs. 37 (19%) in the LEEP group (relative risk, 1.71 (95% CI, 1.12–2.65); risk difference, 7.9% (95% CI, 1.9%–14.0%); p = 0.01) | Treatment with LEEP compared with cryotherapy resulted in a significantly lower rate of CIN recurrence over 24 m in women with HIV |
| Kolben et al., 2019 [35] | DRKS00006169 | RCT | 100 | To show noninferiority of a limited-excision (resection of the dysplastic lesion only) vs. classical LLETZ | Rate of negative HPV tests after 6 m; trial was prematurely terminated | Patients in the limited-excision group did not show a lower number of negative HPV-tests (78% (LLETZ)-80% (limited-excision) = −2%; 90% confidence interval = −15%–12%) | Limited-excision may be an option to reduce surgical extent of cervical surgery; the trial was not sufficiently powered after premature termination due to lack of recruitment |
| Banerjee et al., 2020 [36] | CTRI/2017/06/008731 | RCT | 286 | To compare the safety, acceptability, and efficacy of thermal ablation vs. cryotherapy in a screen and treat setting for CIN1+ | Intensity of pain experienced during the procedure | Significantly more women treated by cryotherapy (75.3%) had pain compared to thermal ablation (61.0%), although intensity was mild in most cases | Thermal ablation reduces pain vs. cryotherapy in women with CIN1+; cure rates were comparable |
| Chong et al., 2020 [37] | KCT0003696 | RCT | 62 | To evaluate the efficacy and feasibility of using a chitosan tampon (Hemoblock®) in preventing hemorrhage and enhancing wound healing after LEEP | Vaginal bleeding 2 w after surgery; measured daily with a pictorial blood assessment chart | The bleeding count was significantly lower in the chitosan group vs. controls (21.37 ± 16.86 vs. 40.52 ± 16.55, p = 0.0014) | The use of chitosan tampons can reduce hemorrhage, vaginal discharge, abdominal pain, and impairment of daily living after LEEP |
| Rezniczek et al., 2020 [38] | NCT03494686 | RCT | 208 | To compare LEEP under local anesthesia vs. general anesthesia | Patient satisfaction assessed on the day of surgery and 14 d thereafter, using a Likert scale (score 0–100) and a questionnaire | Patient satisfaction did not differ between the study groups directly after surgery (Likert scale 100 (90–100) vs. 100 (90–100); p = 0.077) and 14 d thereafter (Likert scale 100 (80–100) vs. 100 (90–100); p = 0.079) | LEEP under local anesthesia is equally well tolerated and offers patient-reported and procedure-related benefits over general anesthesia |
| Duan et al., 2021 [39] | None | RCT | 149 | To compare thermocoagulation and cryotherapy for treatment of HSIL | Cytology-negative rate and HPV negative rate at follow-up at 4 and 8 m | No difference between thermocoagulation and cryotherapy regarding HPV-negative rates (4/8 m: 72.5%/86.2% vs. 68.6%/80.6%) (all p > 0.05); the cytology-negative rate was similar at 4 m (79.7% vs. 78.9%, p > 0.05), but higher for thermocoagulation at 8 m (100% vs. 88.7%, p < 0.05) | Thermocoagulation was as effective and safe as cryotherapy and might be easily applied to treat HSIL |
| Fonseca et al., 2021 [40] | NCT03233412 | RCT | 90 | To evaluate the histologic response rate of HSIL after topical application of a 5% imiquimod cream | Rate of histologic regression (to CIN1 or less) in LEEP specimens | Histologic regression was observed in 23 of 38 participants (61%) in the experimental group compared with 9 of 40 (23%) in the controls (p = 0.001) | Weekly topical treatment with imiquimod is effective in promoting regression of HSIL |
| Firnhaber et al., 2021 [41] | NCT01928225 | RCT | 180 | To evaluate if HPV vaccination improves response to treatment of cervical HSIL in women with HIV | Cervical HSIL by histology or cytology 26 and 52 w after HPV vaccine or placebo | HSIL was similar in the vaccine and placebo groups (53% vs. 45%; relative risk, 1.18 (95% CI, 0.87–1.6); p = 0.29) | This study did not support HPV vaccination to prevent recurrent HSIL after LEEP in women with HIV |
| Gungorduk et al., 2021 [42] | NCT03952975 | RCT | 73 | To determine whether treatment of LSIL/HSIL in the follicular phase or luteal phase of the menstrual cycle affects peri- and post-operative blood loss during LEEP | Median early post-operative blood loss | Blood loss was lower during the follicular phase than during the luteal phase (209.2 (67.7–468.6) vs. 289.0 (120.3–552.8) mL; p = 0.01) | Performing LEEP during the follicular phase of the menstrual cycle significantly reduces intra-operative blood loss, early post-operative blood loss, and late post-operative blood loss |
| Niu et al., 2021 [17] | None | PCT | 297 | To compare the efficacy of 5-aminolaevulinic acid photodynamic therapy (5-ALA PDT) and CO2 laser in the treatment of LSIL with high-risk HPV | Complete remission rates at 4–6 and 12 m | After 4–6 m, there was no significant difference between the two groups, but after 12 m, complete remission rates were higher in the 5-ALA PDT group | The effect of 5-ALA PDT is similar to CO2 laser at 4–6 m; the long-term efficacy of 5-ALA PDT appears better |
| Vieira et al., 2021 [43] | NCT02500966 | RCT | 240 | To compare the role of a new endocervical device to prevent cervical stenosis after LEEP in patients with HSIL | Rate of cervical stenosis at 30 d and 3, 6, and 12 m after intervention | The rate of cervical stenosis inDUDA group was (4–7.3%), and in No DUDA group was (4.3–5.8%) (p = 0.5) | The rate of cervical stenosis after LEEP was not reduced by an endocervical device |
| Rezniczek et al., 2022 [44] | NCT04326049 | RCT | 218 | To compare LLETZ using video colposcopy vs. a headlight | Resected cone mass | LLETZ-video colposcopy and LLETZ-headlight (109 women each) had comparable cone masses (1.57 [0.98–2.37] vs. 1.67 [1.15–2.46] grams; p = 0.454) | Intra-operative video colposcopy for LLETZ results in equal cone masses |
| Polterauer et al., 2022 [45] | NCT01283763 | RCT | 93 | To establish non-inferiority of a 16-w, self-applied topical imiquimod therapy vs. LLETZ in patients with HSIL | Negative HPV high-risk test 6 m after the start of treatment | In the imiquimod group, negative HPV test after 6 m was observed in 22/51 (43.1%) vs. 27/42 (64.3%) patients in the LLETZ group (rate difference 21.2%-points, 95% two-sided CI: 0.8 to 39.1) | In women with HSIL, imiquimod treatment results in lower HPV clearance rates when compared to LLETZ; LLETZ remains the standard of care |
| Location | Title | NCT | Study Type | Sample Size | Study Population | Interventions | Primary Endpoint(s) |
|---|---|---|---|---|---|---|---|
| Germany, Ruhr University Bochum | Comparison of Two Surgical Approaches in the Treatment of Cervical Dysplasia: Complete Removal of the Transformation Zone (LLETZ) vs. Isolated Resection of the Colposcopically Visible Lesion (LEEP) | 04772937 | RCT | 206 | Women with a LSIL/HSIL undergoing cervical surgery | LLETZ vs. limited cervical resection of LSIL/HSIL only | Rate of involved resection margins |
| Germany, Ruhr University Bochum | Large Loop Excision of the Transformation Zone (LLETZ) with vs. without Intra-operative Application of Lugol’s Iodine in Women with Cervical Dysplasia: a Prospective Randomized Trial | 05132114 | RCT | 216 | Women with a LSIL/HSIL undergoing LLETZ | Intra-operative Application of Lugol’s Iodine solution to define resection borders vs. standard LLETZ without application of Lugol’s Iodine solution | Rate of involved resection margins |
| Germany, Ruhr University Bochum | Impact of a VR Headset on Pain Perception and Satisfaction During Colposcopic Workup of Cervical Precancerous Lesions: a Multicenter Randomized-controlled Trial | 04751799 | RCT | 286 | Women undergoing colposcopy for suspected LSIL/HSIL | Virtual reality device before or before and during colposcopy vs. standard colposcopy | Patient anxiety and satisfaction |
| Denmark, University of Aarhus | See and Treat in an Outpatient Setting in Women above 45 Y with Cervical Dysplasia | 04298957 | PCT | 150 | Women ≥45 y with a positive cervical screening test and a T2/T3 type transformation zone | See-and-treat cone biopsy | Prevalence of CIN2+ lesions |
| Italy, Azienda USL Toscana Nord Ovest | A Randomised, Double-Blind, Placebo-Controlled, Phase III Study to Investigate the Efficacy of Presurgical 9-valent HPV Vaccination in Women Treated with LEEP for CIN2+ and Initially Invasive Cervical Cancer | 03848039 | RCT | 1220 | Women with histologically proven CIN2+ to early invasive cervical cancer ≤1a1 | HPV vaccination (Gardasil9®) prior to cervical surgery and 2 m thereafter vs. placebo | CIN recurrence 5 y after surgical treatment |
| Austria, University of Vienna | TRICIN: Prospective Study on the Efficacy of Single Topical Trichloroacetic Acid (TCA) 85% in the Treatment of CIN1/2 | 04400578 | PCT | 101 | Women with histologically proven CIN1/2 | A single topical intervention of Trichloroacetic Acid (TCA) 85% on the cervix | CIN remission rate 6 m after intervention; safety and efficacy |
| USA, Guided Therapeutics, Inc. | The Use of the LuViva Advanced Cervical Scan to Identify Women at High Risk for Cervical Neoplasia | 04915495 | PCT | 400 | Scheduled for colposcopy for suspected LSIL/HSIL | Standardized colposcopy protocol + additional cervical biopsies based on LuViva + random biopsies | Sensitivity and specificity of the experimental device for CIN2+ |
| Germany, University of Tübingen | Treatment of Cervical Intraepithelial Neoplasia (CIN) Grade III with Non-invasive Physical Plasma | 04753073 | RCT | 40 | Women with histologically proven CIN3 | Topical cervical treatment with low temperature physical plasma followed by LEEP within 8 w vs. LEEP | Rate of complete CIN3 remission at the time of LEEP |
| Denmark, University of Copenhagen | Improving Diagnostic in Cervical Dysplasia: A Randomized Study with Local Estrogen Prior to Colposcopy | 05283421 | RCT | 150 | Women scheduled for colposcopy | Vaginal application of estrogen 30 µg once a day for 14 d prior to colposcopy vs. placebo | Visibility of the squamo-columnar junction at colposcopy |
| USA, Yale University | Treatment of High-Grade Pre-Neoplastic Cervical Lesions (CIN2/3) Using a Novel “Prime and Pull” Strategy | 02864147 | RCT | 138 | Women with HPV-positive CIN2/3 | 9-valent HPV vaccination twice (baseline and after 8 w) vs. weekly topical imiquimod 6.25 mg vaginal suppository for 16 w vs. observation | Regression to CIN1 or less after 20–24 m |
| USA, University of California at Los Angeles | A Phase II Open-Label, Single Arm Pilot Study to Evaluate the Safety and Efficacy of Pembrolizumab for High-Grade Cervical Intraepithelial Neoplasia | 04712851 | PCT | 25 | Women with histologically proven CIN2/3 | Pembrolizumab every 6 w for 24 w | Pathological response rate at 6 m |
| China, Shanxi Academy of Medical Sciences | A Randomized Controlled Trial Comparing Cure Rates of Cervical Intraepithelial Neoplasia Grade 2 and Higher (CIN2+) Treated with CO2-based Cryotherapy, CropPen, and Thermoablation (UH3) | 03084081 | RCT | 1152 | Women with histologically proven CIN2/3 | One 5 min freezing therapy (Cryopen) vs. 60 s thermoablation at 100 °C (thermoablation) vs. standard (ablative CO2 laser) | Residual CIN2+ at 12 m |
| USA, Frantz Viral Therapeutics, Inc. | A Phase II Double Blind, Placebo-controlled, Randomized Trial of Artesunate Vaginal Inserts for the Treatment of Patients with Cervical Intraepithelial Neoplasia (CIN2/3) | 04098744 | RCT | 78 | Women with histologically proven CIN2/3 | Artesunate vaginal inserts, 200 mg/d for three 5-d cycles | Histological regression after 15 w |
| China, Peking University | Comparison of Cervical Intraepithelial Neoplasia 2/3 Treatment Outcomes with a Portable LMIC-adapted Thermal Ablation Device vs. Gas-based Cryotherapy | 03429582 | RCT | 1282 | Women with histologically proven CIN2/3 | Thermoablation (cone tip) vs. thermoablation (detachable probe) vs. standard (cryotherapy) | Residual CIN2+ at 12 m |
| Zambia, International Agency for Research on Cancer and University of North Carolina Global Project Zambia and Liger Medical Llc | Development, Field Testing and Evaluation of the Efficacy of a Hand-held, Portable and Affordable Thermo-coagulator to Prevent Cervical Cancer in Low- and Middle-income Countries | 02956239 | RCT | 450 | Women with suspected cervical dysplasia | Thermoablation vs. cryotherapy vs. standard (LEEP) | |
| China, Peking Union Medical College Hospital | A Double Blind, Prospective, Randomized, Placebo Controlled, Multi-center Phase 3 Study to Evaluate Efficacy and Safety of Cevira® in Patients with Cervical Histologic High-grade Squamous Intraepithelial Lesions (HSIL) | 03870113 | RCT | 384 | Women with histologically proven HSIL | Cevira® (topical ointment + a single-use, disposable, LED-based red light source with continuous photoactivation of 125 J/cm2 over 4.6 h) | Histological response rates after 6 m |
| Spain, Hospital de la Santa Creu i Sant Pau | Conservative Management of Patients Diagnosed with High-grade Squamous Intraepithelial Lesions (H-SIL) Who Have Pregnancy Intentions: a Prospective Observational Study | 04783805 | PCT | 200 | Women with histologically proven CIN2/3 | Conservative management with regular follow-up every 4 m with colposcopy and cytology at each visit | CIN2/3 regression after 2 y |
| USA, Johns Hopkins University | A Phase I Efficacy and Safety Study of HPV16-specific Therapeutic DNA-vaccinia Vaccination in Combination with Topical Imiquimod, in Patients with HPV16+ High Grade Cervical Dysplasia (CIN3) | 00788164 | PCT | 75 | Women with HPV 16-positive CIN3 | Dose escalation study of a TA-HPV vaccine; pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine intramuscularly in weeks 0 and 4 and TA-HPV vaccine IM in week 8 vs. topical imiquimod once in weeks 0, 4, and 8 vs. pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine and TA-HPV vaccine + imiquimod | Safety, tolerability, and feasibility |
| USA, Johns Hopkins University | A Phase I Open Label, Dose Escalation Clinical Trial Assessing the Safety, Tolerability, and Feasibility of pNGVL4aCRTE6E7L2 HPV DNA Vaccine Administration Via Intramuscular TriGrid™ Electroporation Delivery System to Patients with HPV16-Positive High-Grade Cervical Intraepithelial Neoplasia | 04131413 | PCT | 48 | Women with HPV 16-positive CIN2 or HPV 16-positive CIN3 | Dose escalation of an experimental vaccine, pNGVL4aCRTE6E7L2 with three escalating doses; Level 1 dose will be 0.3 mg | Dose-limiting toxicity |
| Sweden, University of Gothenburg | Expectancy as Alternative to Treatment for Cervical Intraepithelial Neoplasia Grade 2 Among Women 25–30 Y of Age. A Multicenter Clinical Study | 03177863 | PCT | 160 | Women with histologically proven CIN2 | Expectant management with clinical visits every 6 m | Rate of regression after 24 m |
| France, University of Bordeaux | Therapeutic Abstention and Surveillance of Intra-epithelial Histological Lesions of High Grade Cervical CIN2 (Cervical Intraepithelial Neoplasia Grade 2). SUIVICIN | 04057924 | PCT | 100 | Women with histologically proven CIN2 | Expectant management for 24 m | Rate of regression after 24 m |
| Israel, Tel Aviv Sourasky Medical Center | Virtual Reality During Conization of Cervix Uterus Under Local Anesthesia | 04742543 | RCT | 100 | Women undergoing cervical conization for dysplasia | Performance of conization with the use of virtual reality glasses vs. standard | Pain assessed by a defined score |
| USA, Emory University | An Investigation in the Use of Curcumin Topical Herbal Agent for the Treatment of Cervical Intraepithelial Neoplasia | 04266275 | RCT | 200 | Women with LSIL or recently treated HSIL | 2000 mg of intravaginal curcumin once a week for 20 w vs. placebo | HPV clearance after 6 m |
| USA, University of Southern California | A Two-Cohort Randomized Phase 2 Trial of the IRX-2 Regimen in Women with Squamous Cervical Intraepithelial Neoplasia 3 (CIN3) or Vulvar Intraepithelial Neoplasia 3 (VIN 3) | 03267680 | RCT | 60 | Women with histologically confirmed CIN3 or usual type VIN 3 | Cyclophosphamide IV on day 1 and IRX-2 via submucosal injections in the cervix or SC for vulvar lesions on days 4–7 plus indomethacin, multivitamins and omeprazole every 6 w for up to 2 courses | Pathological complete or partial remission after 25 w |
| Cuba, Our Lady of Rule No. 52 hospital | Evaluation of the Effect of the Combination of the Natural Products Glizigen® and Ocoxin®-Viusid® in the Treatment of High-grade Cervical Intraepithelial Lesions (Phase II) | 03549273 | PCT | 62 | Women with colposcopically diagnosed major change and HPV hr-positivity | Glizigen® spray, topical use, 2 times a day for 6 m with an interruption for 2 m at the end of the third month and oral Ocoxin®-Viusid® 60 mL daily for 8 m | Lesion progression on colposcopy after 9 m |
| China, Huazhong University of Science and Technology | Safety Study of Transcription Activator-like Effector Nucleases T512 in HPV16-infected Subjects | 03226470 | PCT | 40 | Women with HPV 16-infection | Biological T512 suppository contain 500 µg of T512 and suppocire (TALEN-T512) | Safety during 6 m |
| France, Centre Hospitalier Régional d’Orléans | Papilocare®: Effects on Regression of Histologically Confirmed Cervical Intraepithelial Lesions 1 and Tolerance | 04624568 | RCT | 90 | Women with histologically confirmed LSIL or ASC-US or LSIL cervical-cytology | Papilocare® (hyaluronic acid and pre-biotics—Coriolus Versicolor—for 6 m with a single dose per day for 21 d over 28 during the first month, then 1 d over 2 during the following 5 m | Cervical cytology normalization after 12 m |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hecken, J.M.; Rezniczek, G.A.; Tempfer, C.B. Innovative Diagnostic and Therapeutic Interventions in Cervical Dysplasia: A Systematic Review of Controlled Trials. Cancers 2022, 14, 2670. https://doi.org/10.3390/cancers14112670
Hecken JM, Rezniczek GA, Tempfer CB. Innovative Diagnostic and Therapeutic Interventions in Cervical Dysplasia: A Systematic Review of Controlled Trials. Cancers. 2022; 14(11):2670. https://doi.org/10.3390/cancers14112670
Chicago/Turabian StyleHecken, Julia M., Günther A. Rezniczek, and Clemens B. Tempfer. 2022. "Innovative Diagnostic and Therapeutic Interventions in Cervical Dysplasia: A Systematic Review of Controlled Trials" Cancers 14, no. 11: 2670. https://doi.org/10.3390/cancers14112670
APA StyleHecken, J. M., Rezniczek, G. A., & Tempfer, C. B. (2022). Innovative Diagnostic and Therapeutic Interventions in Cervical Dysplasia: A Systematic Review of Controlled Trials. Cancers, 14(11), 2670. https://doi.org/10.3390/cancers14112670






