A Patient Selection Approach Based on NTCP Models and DVH Parameters for Definitive Proton Therapy in Locally Advanced Sinonasal Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Characteristics
2.2. Volumes Definition, Dose Prescription, and Planning Objectives
2.3. Plan Optimization
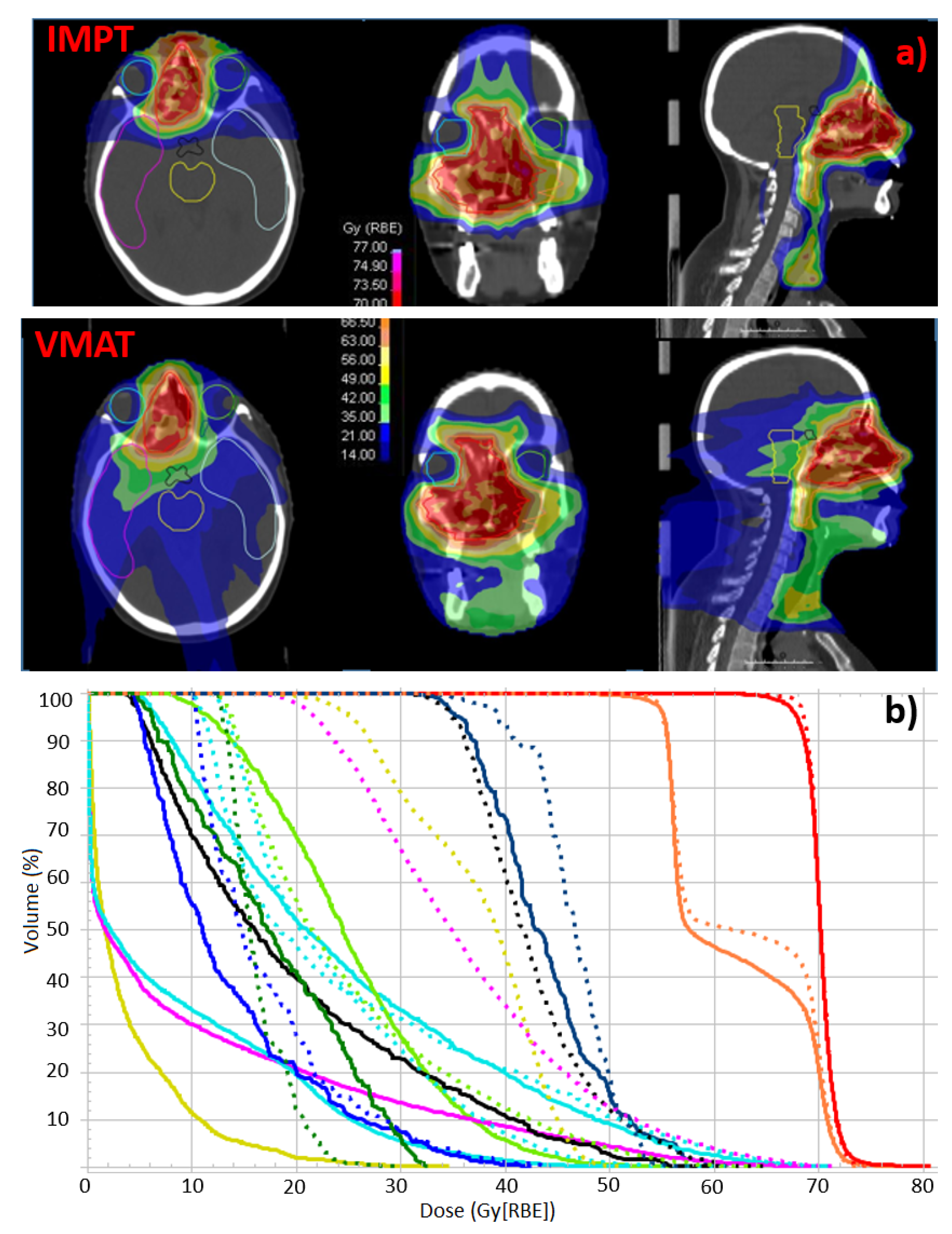
2.4. Plan Analysis and Comparison
- 1
- (a) ΔNTCP exceeded a threshold of 20% (similar to [10]) for at least three of all the investigated intermediate toxicities side effects.(b) NTCP exceeded a threshold of for a single severe toxicity.
- 2
- TS was higher than a certain arbitrary threshold of 250.
3. Results
3.1. Dosimetric Analysis
3.2. NTCP Analysis
3.3. TS Calculation and Overall Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrari, M.; Migliorati, S.; Tomasoni, M.; Crisafulli, V.; Nocivelli, G.; Paderno, A.; Rampinelli, V.; Taboni, S.; Schreiber, A.; Mattavelli, D.; et al. Sinonasal cancer encroaching the orbit: Ablation or preservation? Oral Oncol. 2021, 114, 105185. [Google Scholar] [CrossRef] [PubMed]
- Newhauser, W.D.; Zhang, R. The physics of proton therapy. Phys. Med. Biol. 2015, 60, R155–R209. [Google Scholar] [CrossRef] [PubMed]
- Fiani, B.; Quadri, S.A.; Cathel, A.; Farooqui, M.; Ramachandran, A.; Siddiqi, I.; Ghanchi, H.; Zafar, A.; Berman, B.W.; Siddiqi, J. Esthesioneuroblastoma: A comprehensive review of diagnosis, management, and current treatment options. World Neurosurg. 2019, 126, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.Y.; Gamez, M.E.; Hartsell, W.F.; Tsai, H.K.; Laramore, G.E.; Larson, G.L.; Simone, C.B.; Rossi, C.; Katz, S.R.; Buras, M.R.; et al. A multi-institutional experience of proton beam therapy for sinonasal tumors. Adv. Radiat. Oncol. 2019, 4, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Kang, J.J.; Lee, A.; Fan, D.; Wang, H.; Kitpanit, S.; Fox, P.; Sine, K.; Mah, D.; McBride, S.M.; et al. Outcomes and toxicities of definitive radiotherapy and reirradiation using 3-dimensional conformal or intensity-modulated (pencil beam) proton therapy for patients with nasal cavity and paranasal sinus malignancies. Cancer 2020, 126, 1905–1916. [Google Scholar] [CrossRef]
- Pasalic, D.; Ludmir, E.B.; Allen, P.K.; Thaker, N.G.; Chapman, B.V.; Hanna, E.Y.; Su, S.Y.; Ferrarotto, R.; Glisson, B.S.; Reddy, J.P.; et al. Patient-reported outcomes, physician-reported toxicities, and treatment outcomes in a modern cohort of patients with sinonasal cancer treated using proton beam therapy. Radiother. Oncol. 2020, 148, 258–266. [Google Scholar] [CrossRef]
- Dagan, R.; Uezono, H.; Bryant, C.; Holtzman, A.L.; Morris, C.G.; Mendenhall, W.M. Long-term outcomes from proton therapy for sinonasal cancers. Int. J. Part. Ther. 2021, 8, 200–212. [Google Scholar] [CrossRef]
- Langendijk, J.A.; Boersma, L.J.; Rasch, C.R.N.; van Vulpen, M.; Reitsma, J.B.; van der Schaaf, A.; Schuit, E. Clinical trial strategies to compare protons with photons. Semin. Radiat. Oncol. 2018, 28, 79–87. [Google Scholar] [CrossRef]
- Vai, A.; Molinelli, S.; Rossi, E.; Iacovelli, N.A.; Magro, G.; Cavallo, A.; Pignoli, E.; Rancati, T.; Mirandola, A.; Russo, S.; et al. Proton radiation therapy for nasopharyngeal cancer patients: Dosimetric and NTCP evaluation supporting clinical decision. Cancers 2022, 14, 1109. [Google Scholar] [CrossRef]
- Langendijk, J.A.; Lambin, P.; Ruysscher, D.D.; Widder, J.; Bos, M.; Verheij, M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: The model-based approach. Radiother. Oncol. 2013, 107, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Batth, S.S.; Sreeraman, R.; Dienes, E.; Beckett, L.A.; Daly, M.E.; Cui, J.; Mathai, M.; Purdy, J.A.; Chen, A.M. Clinical-dosimetric relationship between lacrimal gland dose and ocular toxicity after intensity-modulated radiotherapy for sinonasal tumours. Br. J. Radiol. 2013, 86, 20130459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burman, C.; Kutcher, G.J.; Emami, B.; Goitein, M. Fitting of normal tissue tolerance data to an analytic function. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 123–135. [Google Scholar] [CrossRef]
- Martel, M.K.; Sandler, H.M.; Cornblath, W.T.; Marsh, L.H.; Hazuka, M.B.; Roa, W.H.; Fraass, B.A.; Lichter, A.S. Dose-volume complication analysis for visual pathway structures of patients with advanced paranasal sinus tumors. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 273–284. [Google Scholar] [CrossRef]
- Mayo, C.; Martel, M.K.; Marks, L.B.; Flickinger, J.; Nam, J.; Kirkpatrick, J. Radiation dose–volume effects of optic nerves and chiasm. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S28–S35. [Google Scholar] [CrossRef] [PubMed]
- Bender, E.T. Brain necrosis after fractionated radiation therapy: Is the halftime for repair longer than we thought? Med. Phys. 2012, 39, 7055–7061. [Google Scholar] [CrossRef] [PubMed]
- Niyazi, M.; Niemierko, A.; Paganetti, H.; Söhn, M.; Schapira, E.; Goldberg, S.; Adams, J.; Kim, V.; Oh, K.S.; Hwang, W.L.; et al. Volumetric and actuarial analysis of brain necrosis in proton therapy using a novel mixture cure model. Radiother. Oncol. 2020, 142, 154–161. [Google Scholar] [CrossRef]
- Gondi, V.; Hermann, B.P.; Mehta, M.P.; Tomé, W.A. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e487–e493. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, Y.R.; Li, X.A.; El Naqa, I.; Hahn, C.A.; Marks, L.B.; Merchant, T.E.; Dicker, A.P. Radiation dose–volume effects in the brain. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S20–S27. [Google Scholar] [CrossRef] [Green Version]
- Orlandi, E.; Giandini, T.; Iannacone, E.; Ponti, E.D.; Carrara, M.; Mongioj, V.; Stucchi, C.; Tana, S.; Bossi, P.; Licitra, L.; et al. Radiotherapy for unresectable sinonasal cancers: Dosimetric comparison of intensity modulated radiation therapy with coplanar and non-coplanar volumetric modulated arc therapy. Radiother. Oncol. 2014, 113, 260–266. [Google Scholar] [CrossRef]
- Bossi, P.; Farina, D.; Gatta, G.; Lombardi, D.; Nicolai, P.; Orlandi, E. Paranasal sinus cancer. Crit. Rev. Oncol. Hematol. 2016, 98, 45–61. [Google Scholar] [CrossRef]
- Claus, F.; Gersem, W.D.; Wagter, C.D.; Severen, R.V.; Vanhoutte, I.; Duthoy, W.; Remouchamps, V.; Duyse, B.V.; Vakaet, L.; Lemmerling, M.; et al. An implementation strategy for IMRT of ethmoid sinus cancer with bilateral sparing of the optic pathways. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 318–331. [Google Scholar] [CrossRef]
- Grégoire, V.; Ang, K.; Budach, W.; Grau, C.; Hamoir, M.; Langendijk, J.A.; Lee, A.; Le, Q.T.; Maingon, P.; Nutting, C.; et al. Delineation of the neck node levels for head and neck tumors: A 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother. Oncol. 2014, 110, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Mirandola, A.; Molinelli, S.; Freixas, G.V.; Mairani, A.; Gallio, E.; Panizza, D.; Russo, S.; Ciocca, M.; Donetti, M.; Magro, G.; et al. Dosimetric commissioning and quality assurance of scanned ion beams at the italian national center for oncological hadrontherapy. Med. Phys. 2015, 42, 5287–5300. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, A.; Forsgren, A.; Hårdemark, B. Minimax optimization for handling range and setup uncertainties in proton therapy. Med. Phys. 2011, 38, 1672–1684. [Google Scholar] [CrossRef]
- Stuschke, M.; Kaiser, A.; Pöttgen, C.; Lübcke, W.; Farr, J. Potentials of robust intensity modulated scanning proton plans for locally advanced lung cancer in comparison to intensity modulated photon plans. Radiother. Oncol. 2012, 104, 45–51. [Google Scholar] [CrossRef]
- Tambas, M.; Steenbakkers, R.J.H.M.; van der Laan, H.P.; Wolters, A.M.; Kierkels, R.G.J.; Scandurra, D.; Korevaar, E.W.; Oldehinkel, E.; van Zon-Meijer, T.W.H.; Both, S.; et al. First experience with model-based selection of head and neck cancer patients for proton therapy. Radiother. Oncol. 2020, 151, 206–213. [Google Scholar] [CrossRef]
- Lambrecht, M.; Eekers, D.B.P.; Alapetite, C.; Burnet, N.G.; Calugaru, V.; Coremans, I.E.M.; Fossati, P.; Høyer, M.; Langendijk, J.A.; Romero, A.M.; et al. Radiation dose constraints for organs at risk in neuro-oncology; the european particle therapy network consensus. Radiother. Oncol. 2018, 128, 26–36. [Google Scholar] [CrossRef]
- Parsons, J.T.; Bova, F.J.; Mendenhall, W.M.; Million, R.R.; Fitzgerald, C.R. Response of the normal eye to high dose radiotherapy. Oncology 1996, 10, 837–847. [Google Scholar]
- Marks, L.B.; Yorke, E.D.; Jackson, A.; Haken, R.K.T.; Constine, L.S.; Eisbruch, A.; Bentzen, S.M.; Nam, J.; Deasy, J.O. Use of normal tissue complication probability models in the clinic. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S10–S19. [Google Scholar] [CrossRef] [Green Version]
- Mayo, C.; Yorke, E.; Merchant, T.E. Radiation associated brainstem injury. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S36–S41. [Google Scholar] [CrossRef] [Green Version]
- Lemp, M.A.; Foulks, G.N. The Definition and Classification of Dry Eye Disease: Report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul. Surf. 2007, 5, 75–92. [Google Scholar] [CrossRef]
- Stephens, L.C.; Schultheiss, T.E.; Peters, L.J.; Ang, K.K.; Gray, K.N. Acute radiation injury of ocular adnexa. Arch. Ophthalmol. 1988, 106, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Sreeraman, R.; Doshi, S.; Cui, J.; Mathai, M.; Yang, C.C.; Purdy, J.A.; Chen, A.M. Clinical-dosimetric analysis of lacrimal gland dysfunction among patients treated by intensity-modulated radiotherapy for sinonasal tumors. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, S417–S418. [Google Scholar] [CrossRef]
- Dutz, A.; Lühr, A.; Agolli, L.; Bütof, R.; Valentini, C.; Troost, E.G.C.; Baumann, M.; Vermeren, X.; Geismar, D.; Lamba, N.; et al. Modelling of late side-effects following cranial proton beam therapy. Radiother. Oncol. 2021, 157, 15–23. [Google Scholar] [CrossRef]
- Kong, C.; Zhu, X.Z.; Lee, T.F.; Feng, P.B.; Xu, J.H.; Qian, P.D.; Zhang, L.F.; He, X.; Huang, S.F.; Zhang, Y.Q. LASSO-based NTCP model for radiation-induced temporal lobe injury developing after intensity-modulated radiotherapy of nasopharyngeal carcinoma. Sci. Rep. 2016, 6, 26378. [Google Scholar] [CrossRef] [PubMed]
- Bhandare, N.; Moiseenko, V.; Song, W.Y.; Morris, C.G.; Bhatti, M.T.; Mendenhall, W.M. Severe dry eye syndrome after radiotherapy for head-and-neck tumors. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1501–1508. [Google Scholar] [CrossRef]
- Lee, T.F.; Yeh, S.A.; Chao, P.J.; Chang, L.; Chiu, C.L.; Ting, H.M.; Wang, H.Y.; Huang, Y.J. Normal tissue complication probability modeling for cochlea constraints to avoid causing tinnitus after head-and-neck intensity-modulated radiation therapy. Radiat Oncol 2015, 10, 194. [Google Scholar] [CrossRef] [Green Version]
- Jeganathan, V.S.E.; Wirth, A.; MacManus, M.P. Ocular risks from orbital and periorbital radiation therapy: A critical review. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 650–659. [Google Scholar] [CrossRef]
- Mock, U.; Georg, D.; Bogner, J.; Auberger, T.; Pötter, R. Treatment planning comparison of conventional, 3d conformal, and intensity-modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 147–154. [Google Scholar] [CrossRef]
- Lewis, L.; Kreinbrink, P.; Richardson, M.; Westerfield, M.; Doberstein, M.; Zhang, Y.; Redmond, K.; Takiar, V. Intensity modulated proton therapy better spares non-adjacent organs and reduces the risk of secondary malignant neoplasms in the treatment of sinonasal cancers. Med. Dosim. 2021, 47, 117–122. [Google Scholar] [CrossRef]
- Zenda, S.; Kohno, R.; Kawashima, M.; Arahira, S.; Nishio, T.; Tahara, M.; Hayashi, R.; Kishimoto, S.; Ogino, T. Proton beam therapy for unresectable malignancies of the nasal cavity and paranasal sinuses. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Toyomasu, Y.; Demizu, Y.; Matsuo, Y.; Sulaiman, N.S.; Mima, M.; Nagano, F.; Terashima, K.; Tokumaru, S.; Hayakawa, T.; Daimon, T.; et al. Outcomes of patients with sinonasal squamous cell carcinoma treated with particle therapy using protons or carbon ions. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1096–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holliday, E.B.; Esmaeli, B.; Pinckard, J.; Garden, A.S.; Rosenthal, D.I.; Morrison, W.H.; Kies, M.S.; Gunn, G.B.; Fuller, C.D.; Phan, J.; et al. A multidisciplinary orbit-sparing treatment approach that includes proton therapy for epithelial tumors of the orbit and ocular adnexa. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 344–352. [Google Scholar] [CrossRef]
- Köthe, A.; van Luijk, P.; Safai, S.; Kountouri, M.; Lomax, A.J.; Weber, D.C.; Fattori, G. Combining clinical and dosimetric features in a PBS proton therapy cohort to develop a NTCP model for radiation-induced optic neuropathy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Abdelmeguid, A.S.; Watcherporn, T.; Takahashi, H.; Tam, S.; Bell, D.; Ferrarotto, R.; Glisson, B.; Kupferman, M.E.; Roberts, D.B.; et al. Induction chemotherapy response as a guide for treatment optimization in sinonasal undifferentiated carcinoma. J. Clin. Oncol. 2019, 37, 504–512. [Google Scholar] [CrossRef] [PubMed]
| Structure | Planning Objectives/Constraints | DVH Indices |
|---|---|---|
| Optic Chiasm | D < 60 Gy(RBE) | V |
| Contralateral Optical Nerve | D < 60 Gy(RBE), D ALAP | V |
| Brainstem | D < 54 Gy(RBE) | |
| Spinal Cord | D < 45 Gy(RBE) | |
| HR-CTV | D > 95% | |
| D < 107% | ||
| V > 95% | ||
| LR-CTV | D > 95% | |
| V > 95% | ||
| Ipsilateral Optical Nerve | D < 60 Gy(RBE) | V |
| Ipsilateral Retina | D < 30 Gy(RBE); D < 60 Gy(RBE) | V, V |
| Ipsilateral Eye | D < 30 Gy(RBE); D < 65 Gy(RBE) | |
| Contralateral Retina | D < 30 Gy(RBE); D ALAP | |
| Contralateral Eye | D, D ALAP | |
| Ipsilateral Ant. Chamber | D < 30 Gy(RBE) | |
| Contralateral Ant. Chamber | D ALAP | |
| Ipsilateral Lacrimal Gland | D < 30 Gy(RBE) | V |
| Contralateral Lacrimal Gland | D ALAP | |
| Left Temp. Lobe | D < 68 Gy(RBE) | V, V |
| Right Temp. Lobe | D < 68 Gy(RBE) | V, V |
| Frontal Lobe | D < 68 Gy(RBE) | V, V |
| Brain minus LR-CTV | D < 68 Gy(RBE) | V, V |
| Ipsilateral Cochlea | D < 40 Gy(RBE) | |
| Contralateral Cochlea | D ALAP |
| Toxicity Endpoint (Scoring) | Author | NTCP Model | OAR |
|---|---|---|---|
| Blindness (Late/Severe) | Burman et al. [12] | Optic Chiasm, Left/Right Optical Nerve | |
| Brain Necrosis (Late/Severe) | Bender et al. [15] | Brainstem, Brain outside CTV | |
| Overall Ocular Toxicities (Acute/Intermediate) | Batth et al. [11] | Left/Right Lacrimal Gland | |
| Temporal Lobe Necrosis (Late/Severe) | Kong et al. [35] | Left/Right/Frontal Lobe | |
| Tinnitus (Late/Intermediate) | Lee et al. [37] | Left/Right Cochlea | |
| Cataract Requiring Intervention (Late/Intermediate) | Burman et al. [12] | Left/Right Lens | |
| Dry Eye Syndrome (Late/Severe) | Jeganathan et al. [38] | Left/Right Lacrimnal Gland | |
| Brain Necrosis (Late/Intermediate) | Niyazi et al. [16] | Brain oustide CTV |
| Target Volume | Dose Parameter | VMAT | IMPT |
|---|---|---|---|
| D | 68.4 ± 0.7 | 67.4 ± 0.7 | |
| D | 67.2 ± 1.4 | 65.9 ± 0.8 | |
| V | 98.5 ± 1.4 | 97.1 ± 1.4 | |
| HR-CTV | D | 72.9 ± 0.6 | 73.4 ± 0.7 |
| D | 70.4 ± 0.3 | 70.3 ± 0.4 | |
| HI | 0.08 ± 0.03 | 0.11 ± 0.02 | |
| CI | 1.64 ± 0.19 | 1.28 ± 0.28 | |
| D | 55.0 ± 0.4 | 55.0 ± 0.4 | |
| D | 54.5 ± 0.4 | 53.5 ± 1.0 | |
| LR-CTV | V | 99.6 ± 0.4 | 98.3 ± 0.4 |
| CI | 1.45 ± 0.4 | 1.14 ± 0.3 | |
| D | 57.6 ± 2.3 | 57.0 ± 1.2 |
| DVH for V | 1 | 0 | −1 | DVH for V | 1 | 0 | −1 |
|---|---|---|---|---|---|---|---|
| Ipsilat. Lacrimal gland | 6 | 1 | 1 | Ipsilat. Retina | 13 | 8 | 0 |
| Contralat. Lacrimal gland | 3 | 0 | 0 | Contralat. Retina | 11 | 7 | 1 |
| DVH for V | 1 | 0 | −1 | DVH for V | 1 | 0 | −1 |
| Left Temporal Lobe | 22 | 0 | 0 | Left Temporal Lobe | 22 | 0 | 0 |
| Right Temporal Lobe | 22 | 0 | 0 | Right Temporal Lobe | 22 | 0 | 0 |
| Frontal Lobe | 22 | 0 | 0 | Frontal Lobe | 22 | 0 | 0 |
| DVH for V | 1 | 0 | −1 | DVH for D | 1 | 0 | −1 |
| Optic Chiasm | 11 | 10 | 1 | Ipsilat. Lacrimal gland | 6 | 16 | 0 |
| Ipsilat. Optical Nerve | 7 | 15 | 0 | Contralat. Lacrimal gland | 7 | 14 | 1 |
| Contralat. Optical Nerve | 12 | 8 | 0 | Ipsilat. Anterior chamber | 6 | 15 | 1 |
| Ipsilat. Retina | 11 | 7 | 1 | Contralat. Anterior chamber | 7 | 14 | 1 |
| Contralat. Retina | 10 | 6 | 1 |
| OAR | 0% ≤ NTCP < 1% | 1% ≤ NTCP < 3% | NTCP≥ 3% |
|---|---|---|---|
| Contralateral Lacrimal Gland | 22 (100%) | - | - |
| Ipsilateral Lacrimal Gland | 18 (81.8%) | 3 (13.6%) | 1 (4.5%) |
| Optic chiasm | 20 (90.9%) | 2 (9.1%) | - |
| Contralateral optic nerve | 22 (100%) | - | - |
| Ipsilateral optic nerve | 22 (100%) | - | - |
| Left Temporal Lobe | 10 (45.5%) | 10 (45.5%) | 2 (9.1%) |
| Right Temporal Lobe | 11 (50.0%) | 10 (45.5%) | 1 (4.5%) |
| Frontal Lobe | 17 (77.3%) | 4 (18.2%) | 1 (4.5%) |
| Brainstem | 22 (100%) | - | - |
| Brain | 22 (100%) | - | - |
| OAR | NTCP < | NTCP < | NTCP < | NTCP < | NTCP | NTCP |
|---|---|---|---|---|---|---|
| Brain | - | - | 2 (9.1%) | 12 (54.5%) | 8 (36.4%) | 17.3 |
| Ipsilateral Lens | 2 (9.1%) | 2 (9.1%) | 9 (40.9%) | 5 (22.7%) | 4 (18.2%) | 3.4 |
| Contralateral Lens | 2 (9.1%) | 3 (13.6%) | 5 (22.7%) | 5 (22.7%) | 7 (31.9%) | 10.0 |
| Ipsilateral Lacrimal Gland | 3 (13.6%) | 4 (18.2%) | 5 (22.7%) | 7 (31.9%) | 3 (13.6%) | 1.4 |
| Contralateral Lacrimal Gland | - | 7 (31.9%) | 6 (27.2%) | 4 (18.2%) | 5 (22.7%) | 5.9 |
| Ispilateral Cochlea | - | - | 4 (18.2%) | 13 (59.1%) | 5 (22.7%) | 12.5 |
| Contralateral Cochlea | - | - | 5 (22.7%) | 10 (45.4%) | 7 (31.9%) | 16.1 |
| Patient | NTCP > 20% | NTCP > 3% | w | w | w | TS > 250 |
|---|---|---|---|---|---|---|
| P1 | 200 | 65 | 110 | 375 | ||
| P2 | DES | 280 | 72 | 110 | 462 | |
| P3 | 60 | 68 | 120 | 248 | ||
| P4 | 100 | 130 | 140 | 370 | ||
| P5 | 100 | 91 | 120 | 311 | ||
| P6 | 100 | 121 | 80 | 301 | ||
| P7 | Brain Necrosis | 140 | 66 | 130 | 336 | |
| P8 | 60 | 78 | 90 | 228 | ||
| P9 | G2 Brain Necrosis + Tinnitus + Catharact | 160 | 51 | 120 | 331 | |
| P10 | 100 | 38 | 120 | 258 | ||
| P11 | Brain Necrosis | 180 | 19 | 120 | 319 | |
| P12 | 120 | 82 | 40 | 242 | ||
| P13 | G2 Brain Necrosis + Tinnitus + Catharact | 160 | 82 | 100 | 342 | |
| P14 | 80 | 75 | 100 | 255 | ||
| P15 | 140 | -5 | 90 | 225 | ||
| P16 | G2 Brain Necrosis + Tinnitus + Ocular tox | 140 | 93 | 120 | 353 | |
| P17 | 80 | 38 | 140 | 258 | ||
| P18 | 120 | -8 | 60 | 172 | ||
| P19 | Brain Necrosis | 120 | -6 | 70 | 184 | |
| P20 | 120 | 75 | 140 | 335 | ||
| P21 | 100 | 98 | 130 | 328 | ||
| P22 | G2 Brain Necrosis + Tinnitus + Ocular tox | 140 | 145 | 110 | 395 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirandola, A.; Russo, S.; Bonora, M.; Vischioni, B.; Camarda, A.M.; Ingargiola, R.; Molinelli, S.; Ronchi, S.; Rossi, E.; Vai, A.; et al. A Patient Selection Approach Based on NTCP Models and DVH Parameters for Definitive Proton Therapy in Locally Advanced Sinonasal Cancer Patients. Cancers 2022, 14, 2678. https://doi.org/10.3390/cancers14112678
Mirandola A, Russo S, Bonora M, Vischioni B, Camarda AM, Ingargiola R, Molinelli S, Ronchi S, Rossi E, Vai A, et al. A Patient Selection Approach Based on NTCP Models and DVH Parameters for Definitive Proton Therapy in Locally Advanced Sinonasal Cancer Patients. Cancers. 2022; 14(11):2678. https://doi.org/10.3390/cancers14112678
Chicago/Turabian StyleMirandola, Alfredo, Stefania Russo, Maria Bonora, Barbara Vischioni, Anna Maria Camarda, Rossana Ingargiola, Silvia Molinelli, Sara Ronchi, Eleonora Rossi, Alessandro Vai, and et al. 2022. "A Patient Selection Approach Based on NTCP Models and DVH Parameters for Definitive Proton Therapy in Locally Advanced Sinonasal Cancer Patients" Cancers 14, no. 11: 2678. https://doi.org/10.3390/cancers14112678
APA StyleMirandola, A., Russo, S., Bonora, M., Vischioni, B., Camarda, A. M., Ingargiola, R., Molinelli, S., Ronchi, S., Rossi, E., Vai, A., Iacovelli, N. A., Thariat, J., Ciocca, M., & Orlandi, E. (2022). A Patient Selection Approach Based on NTCP Models and DVH Parameters for Definitive Proton Therapy in Locally Advanced Sinonasal Cancer Patients. Cancers, 14(11), 2678. https://doi.org/10.3390/cancers14112678







