PET-CT in Clinical Adult Oncology—VI. Primary Cutaneous Cancer, Sarcomas and Neuroendocrine Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Primary Cutaneous Malignancies
2.1. Background and General Considerations
2.2. Challenges Related to Immunotherapy
2.3. Melanoma
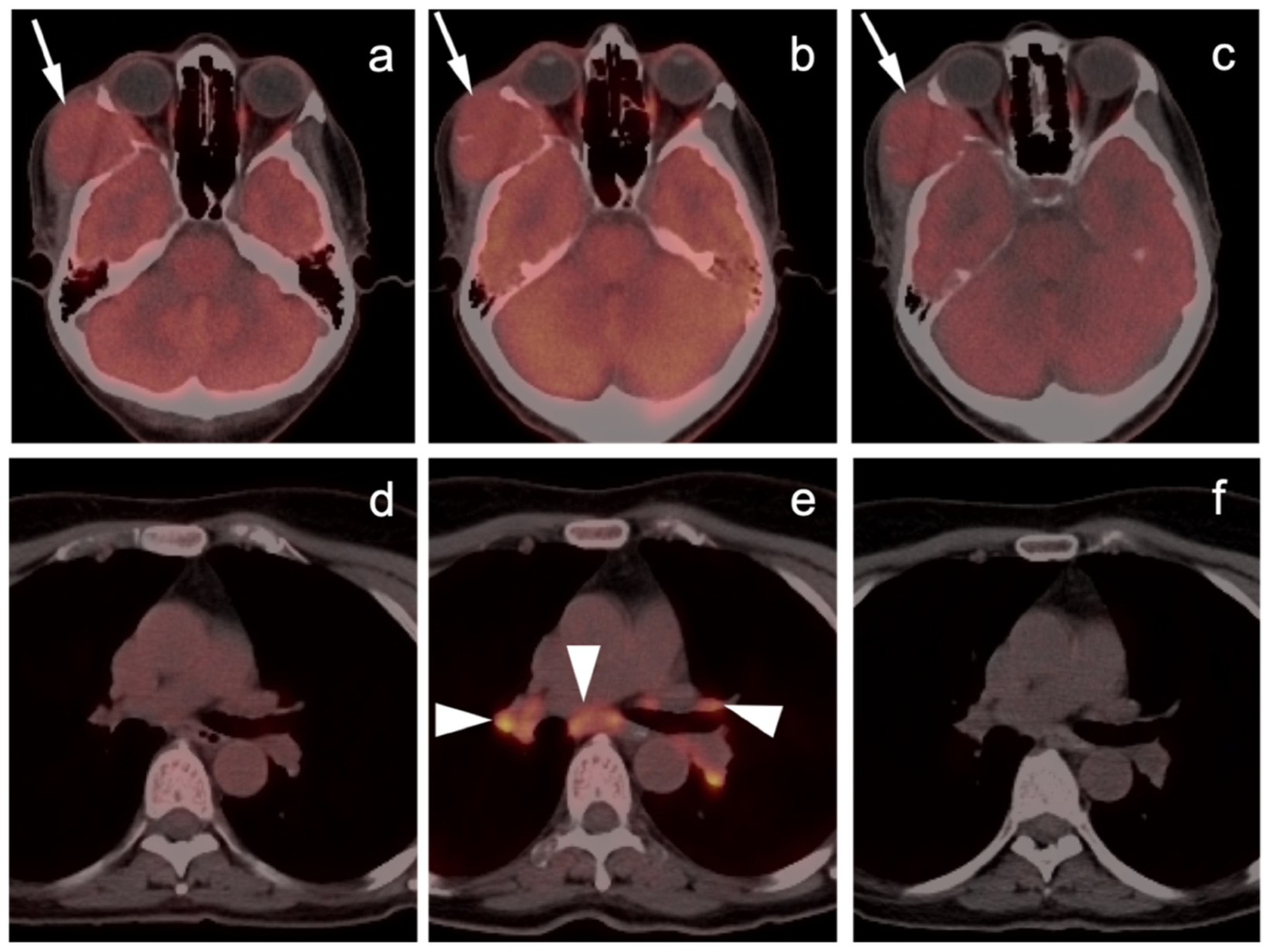
2.3.1. Cutaneous Melanoma
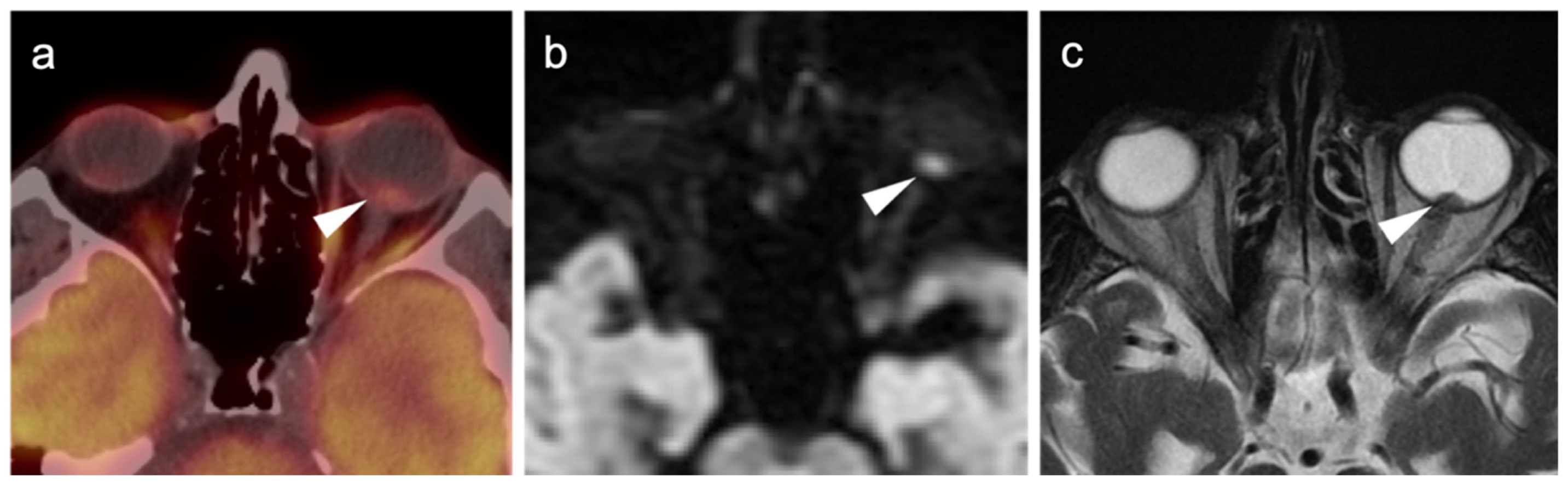
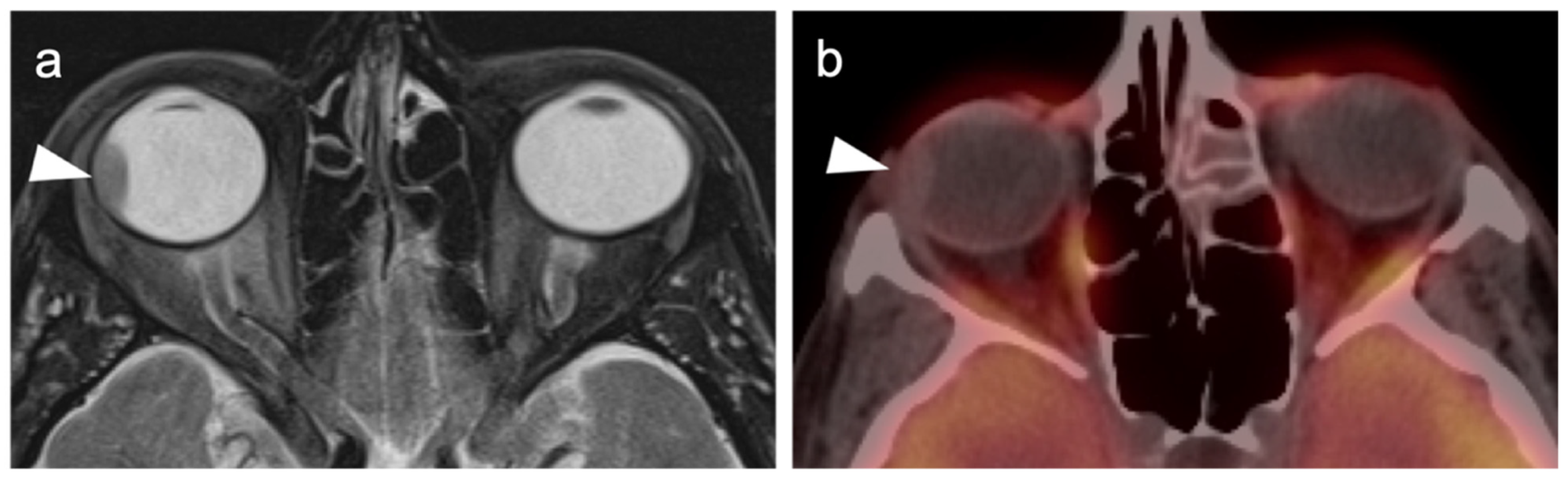
2.3.2. Ocular Melanoma
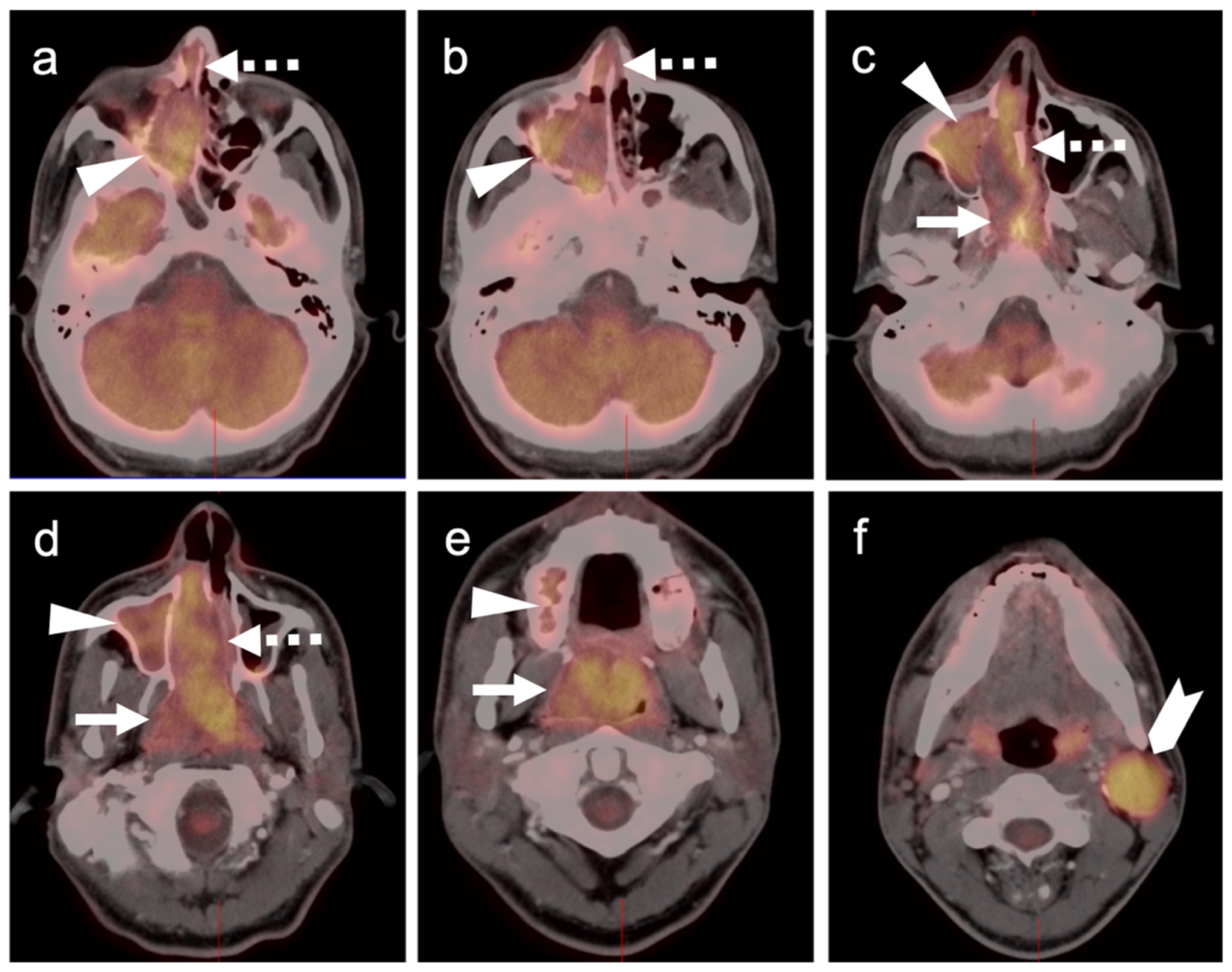
2.3.3. Mucosal Melanoma
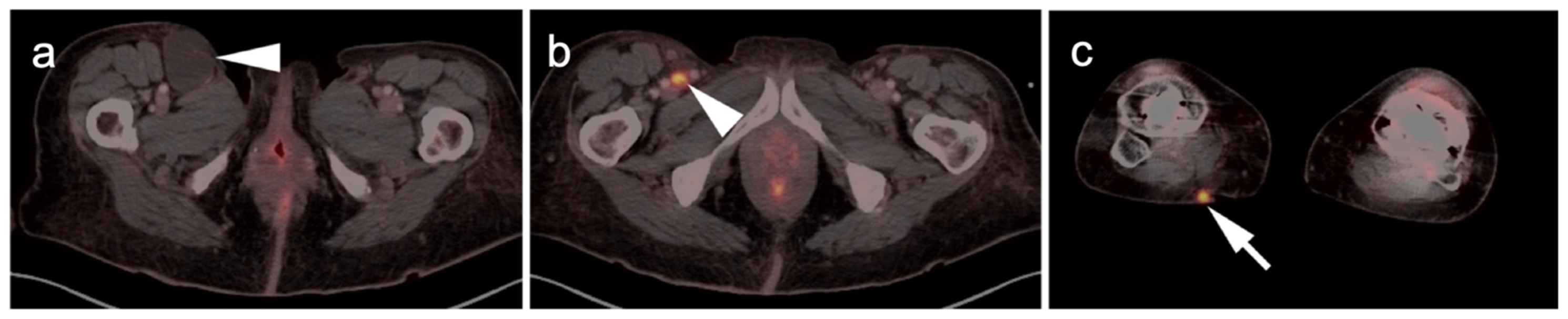
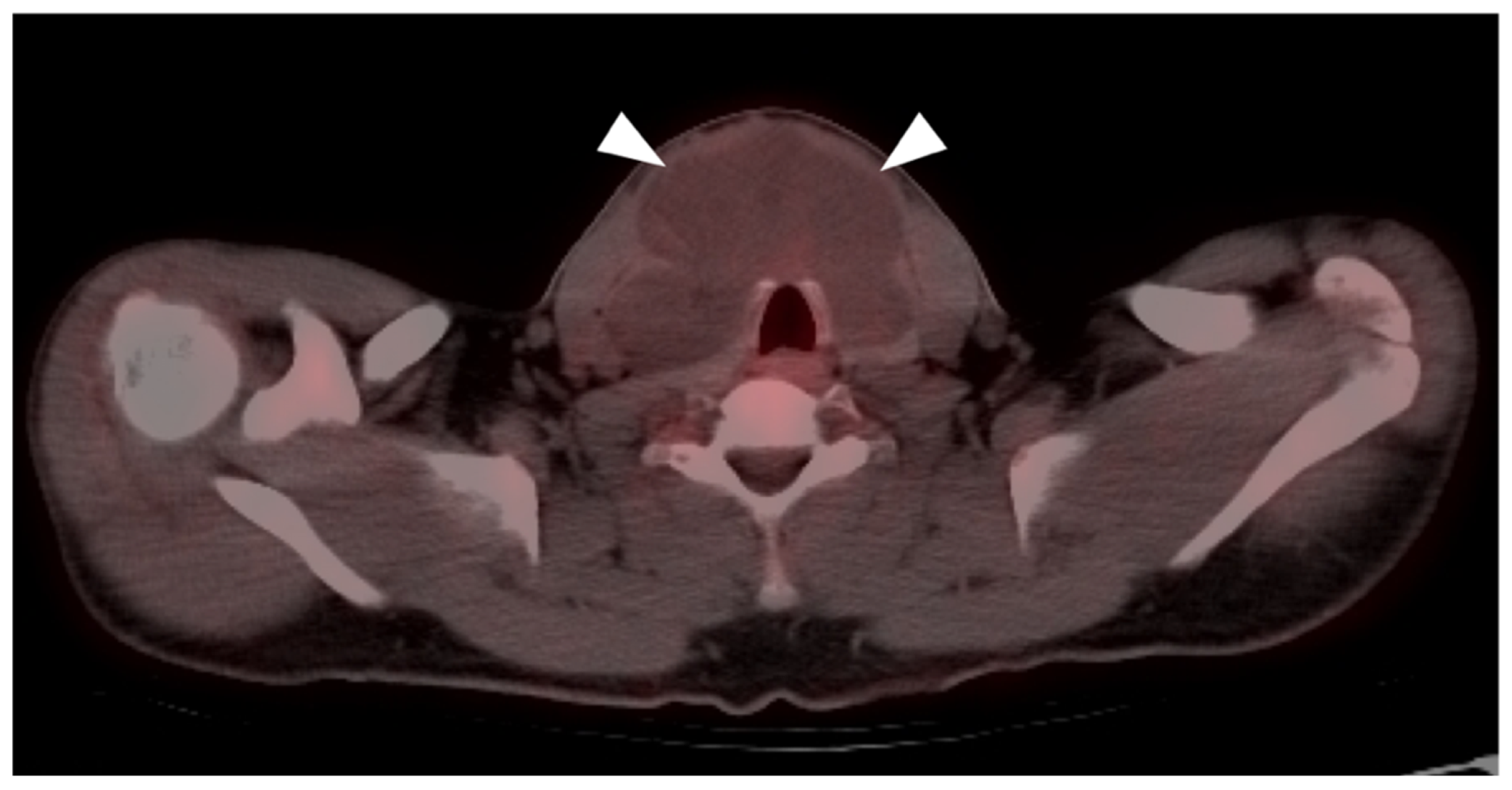
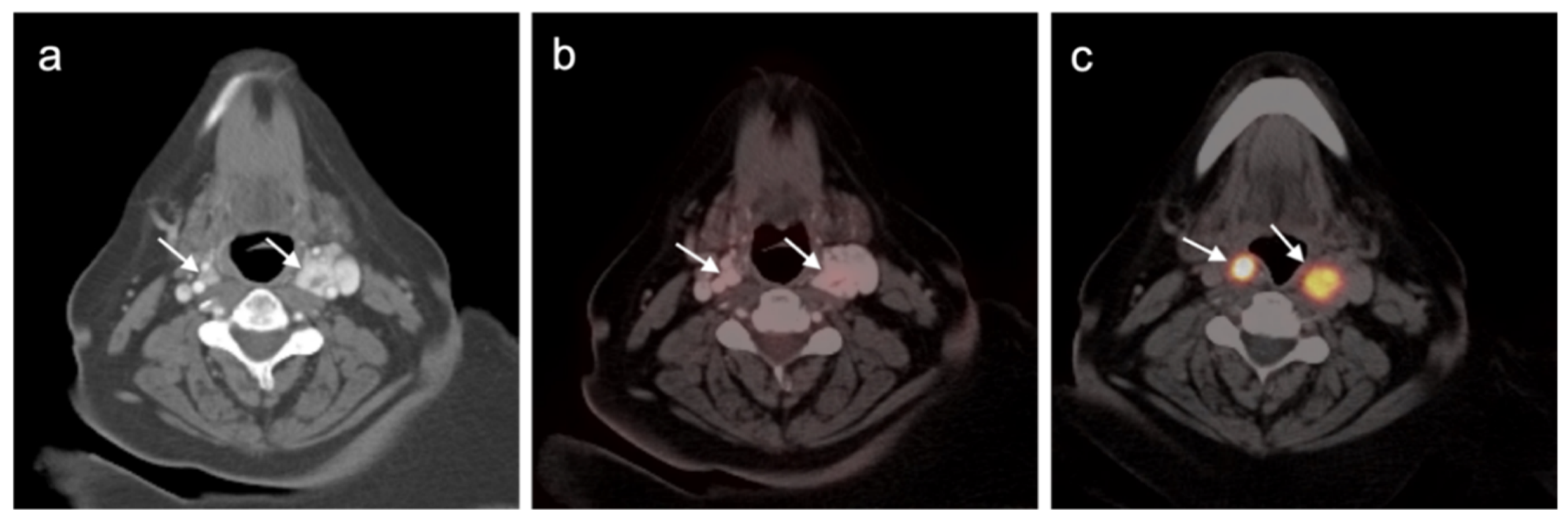
2.4. Merkel Cell Carcinoma
2.5. Cutaneous Squamous Cell Carcinoma
3. Sarcomas
3.1. Soft Tissue Sarcomas
3.1.1. Undifferentiated Pleomorphic Sarcoma
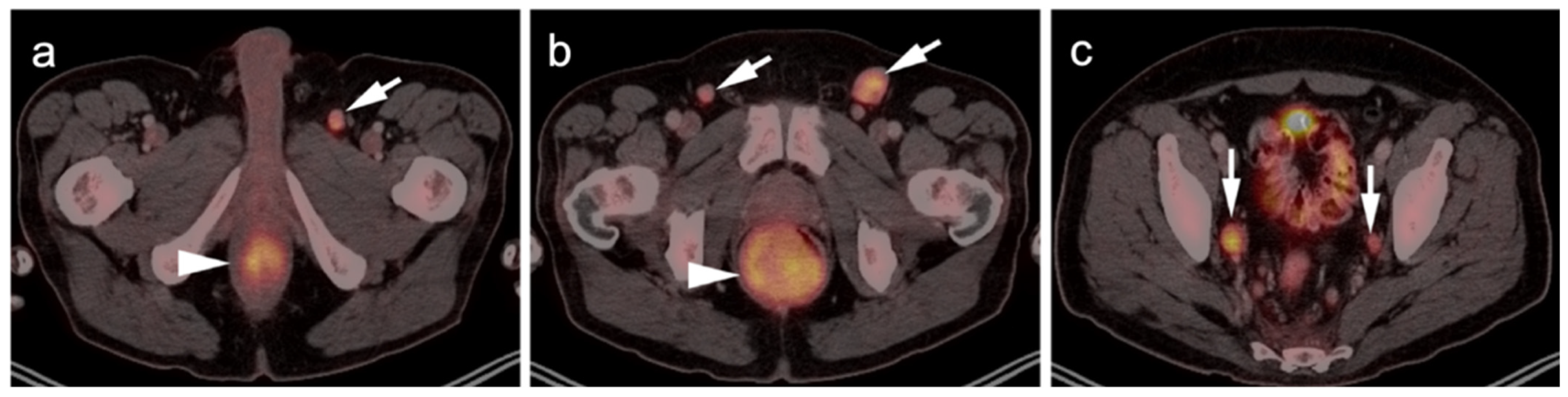
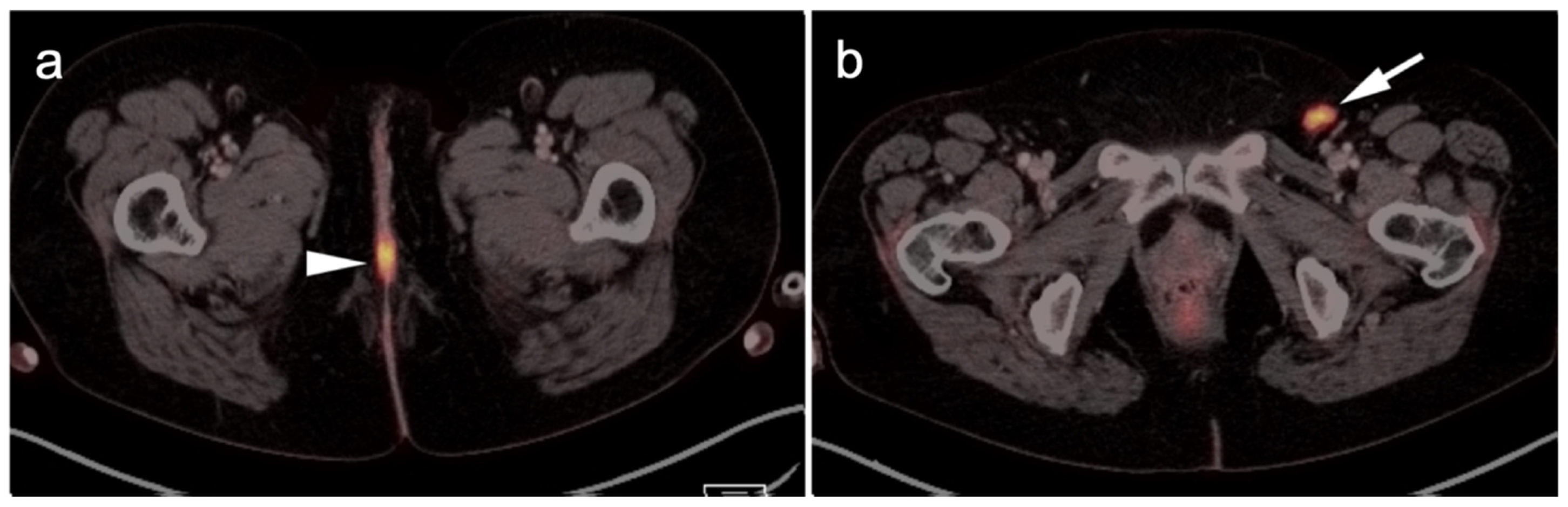
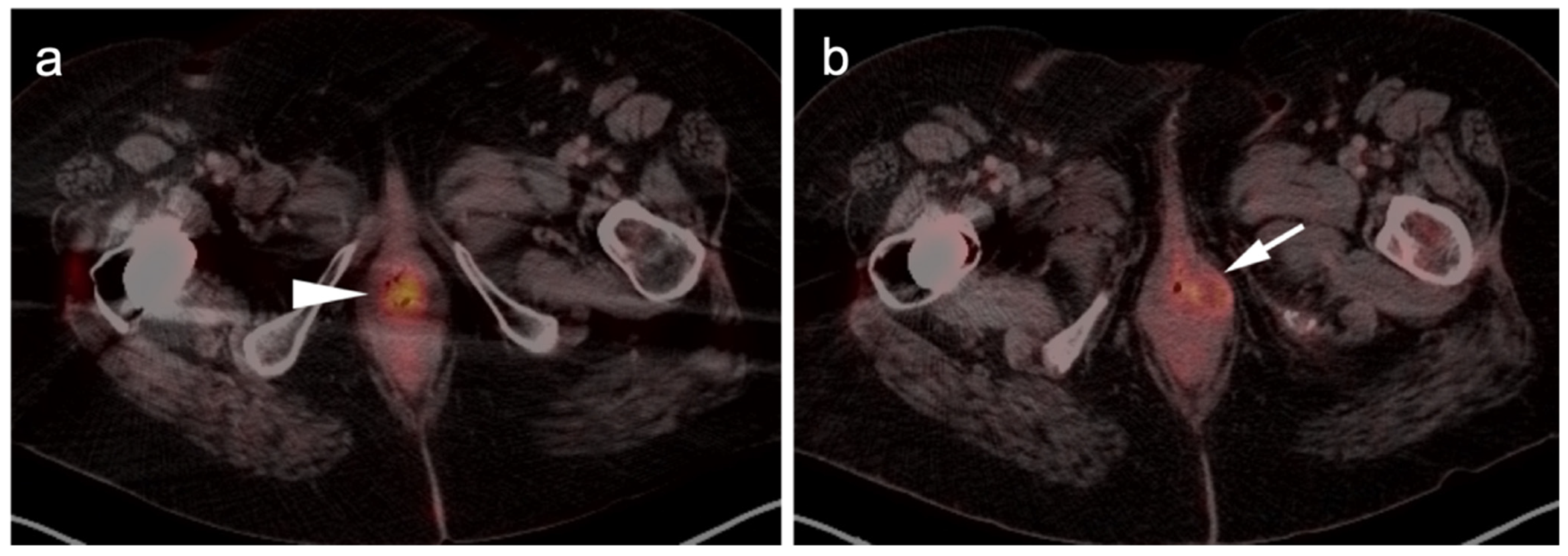
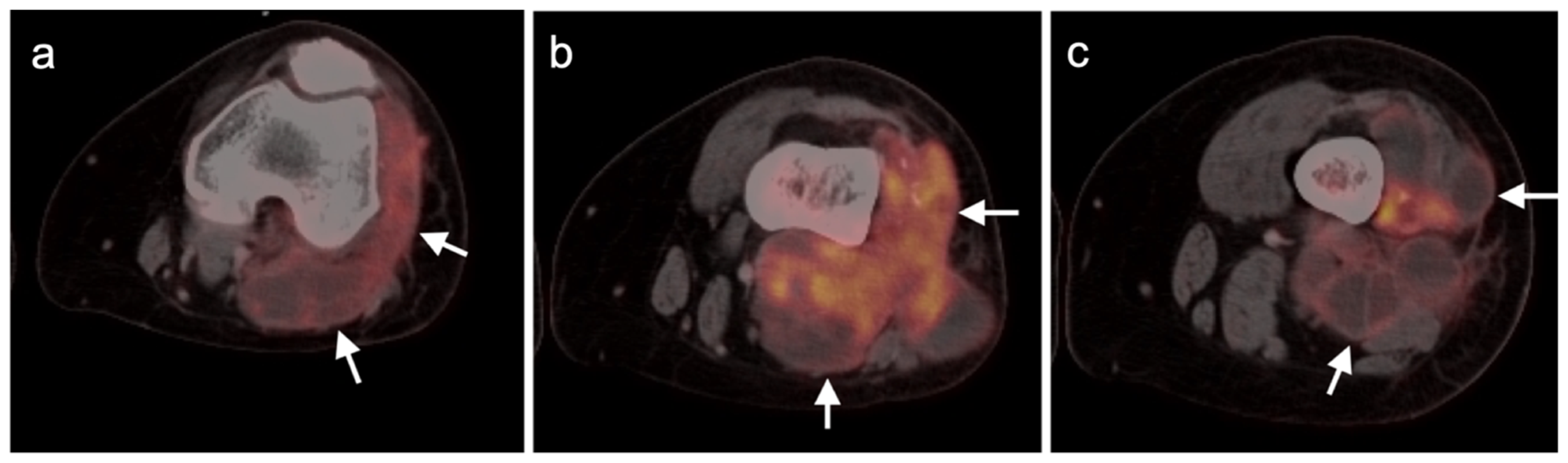
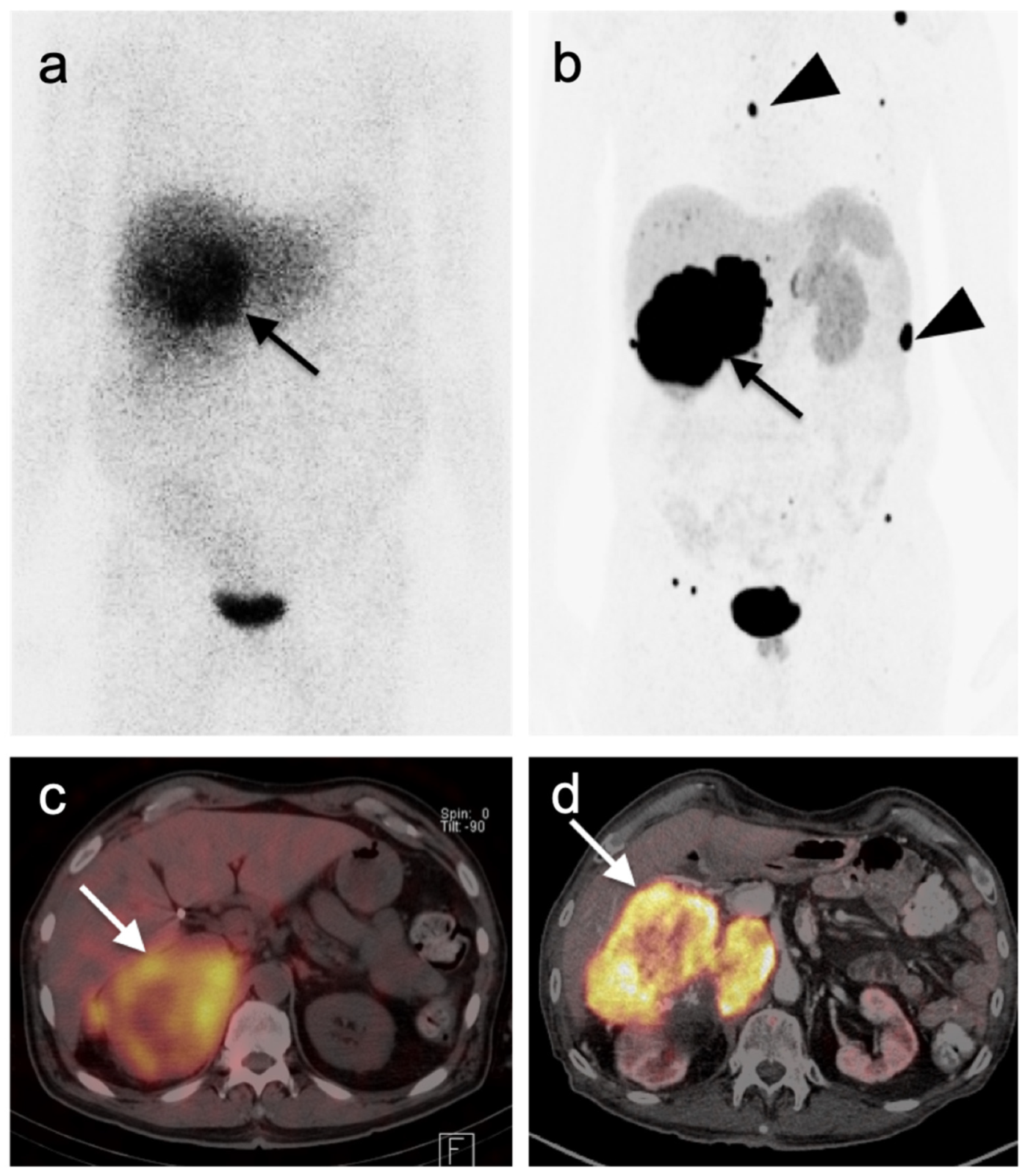
3.1.2. Leiomyosarcoma
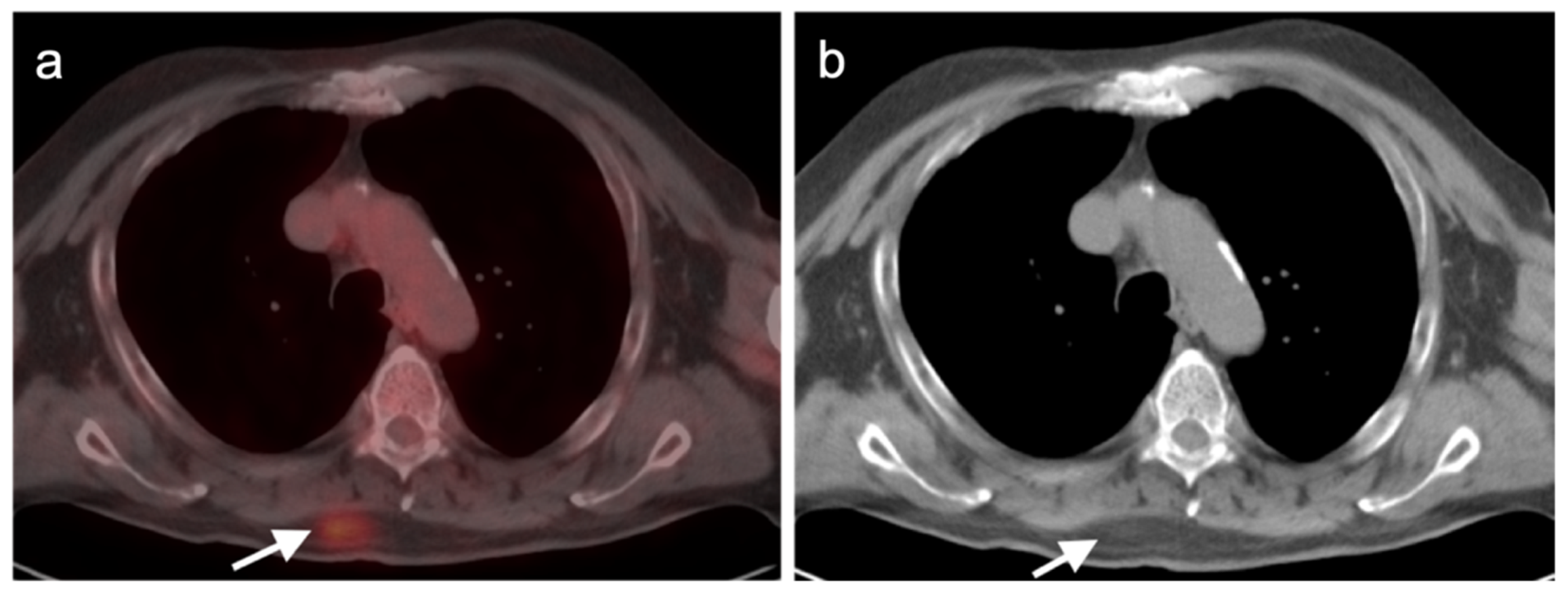
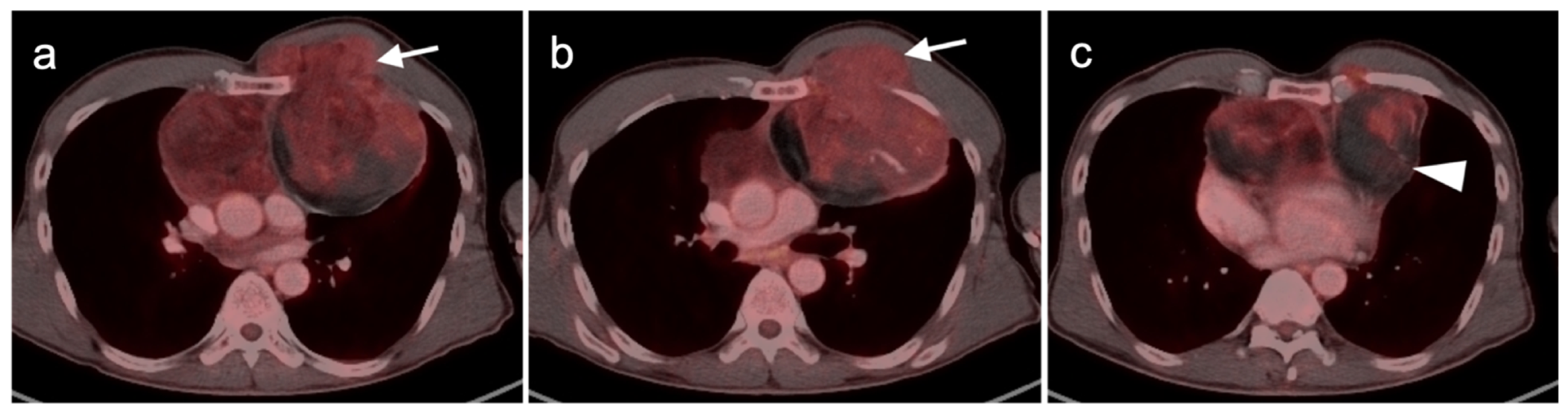
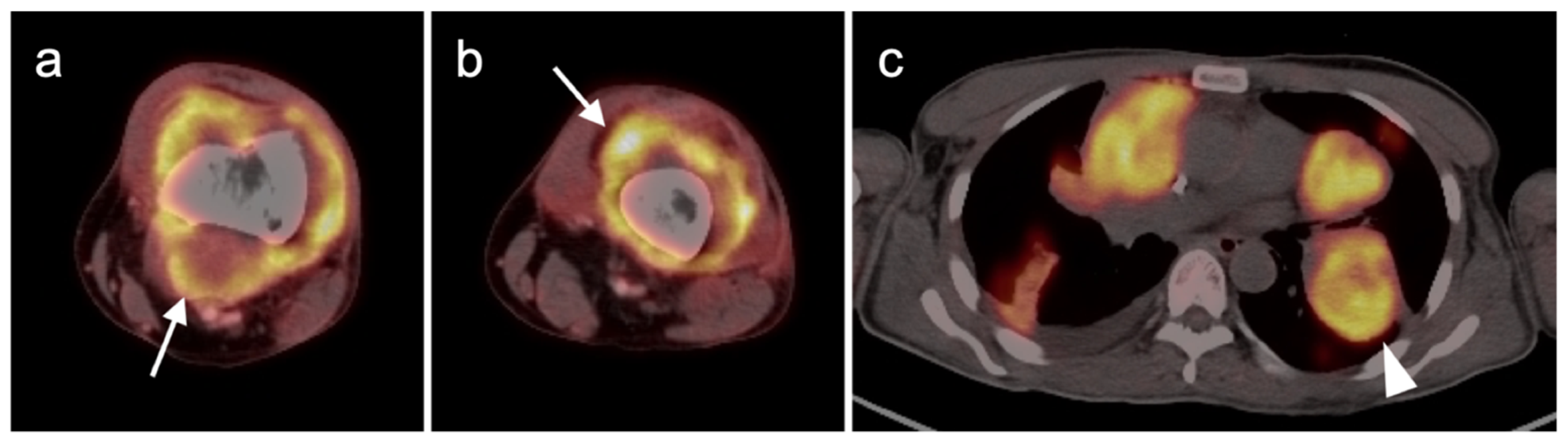
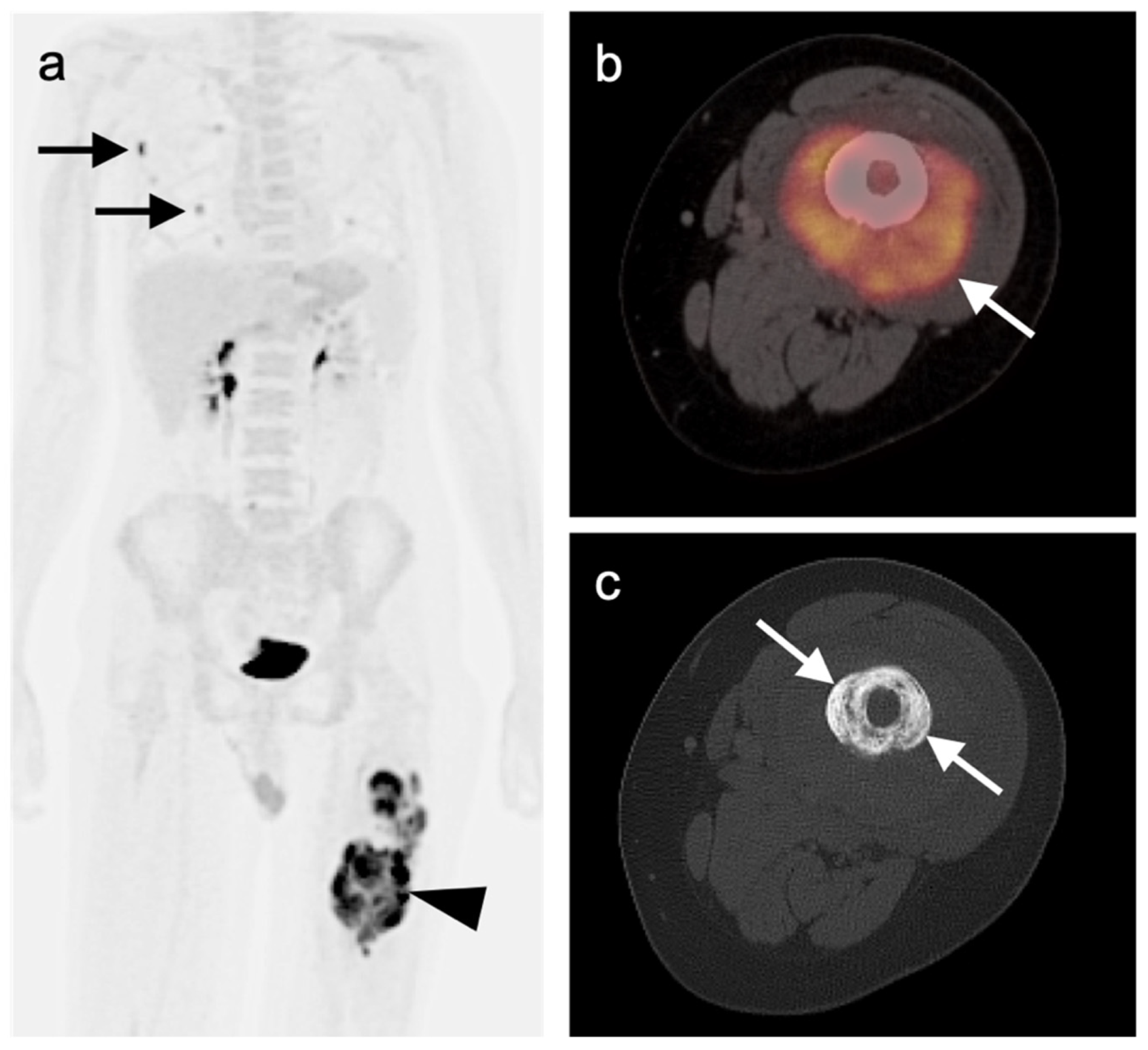
3.1.3. Angiosarcoma
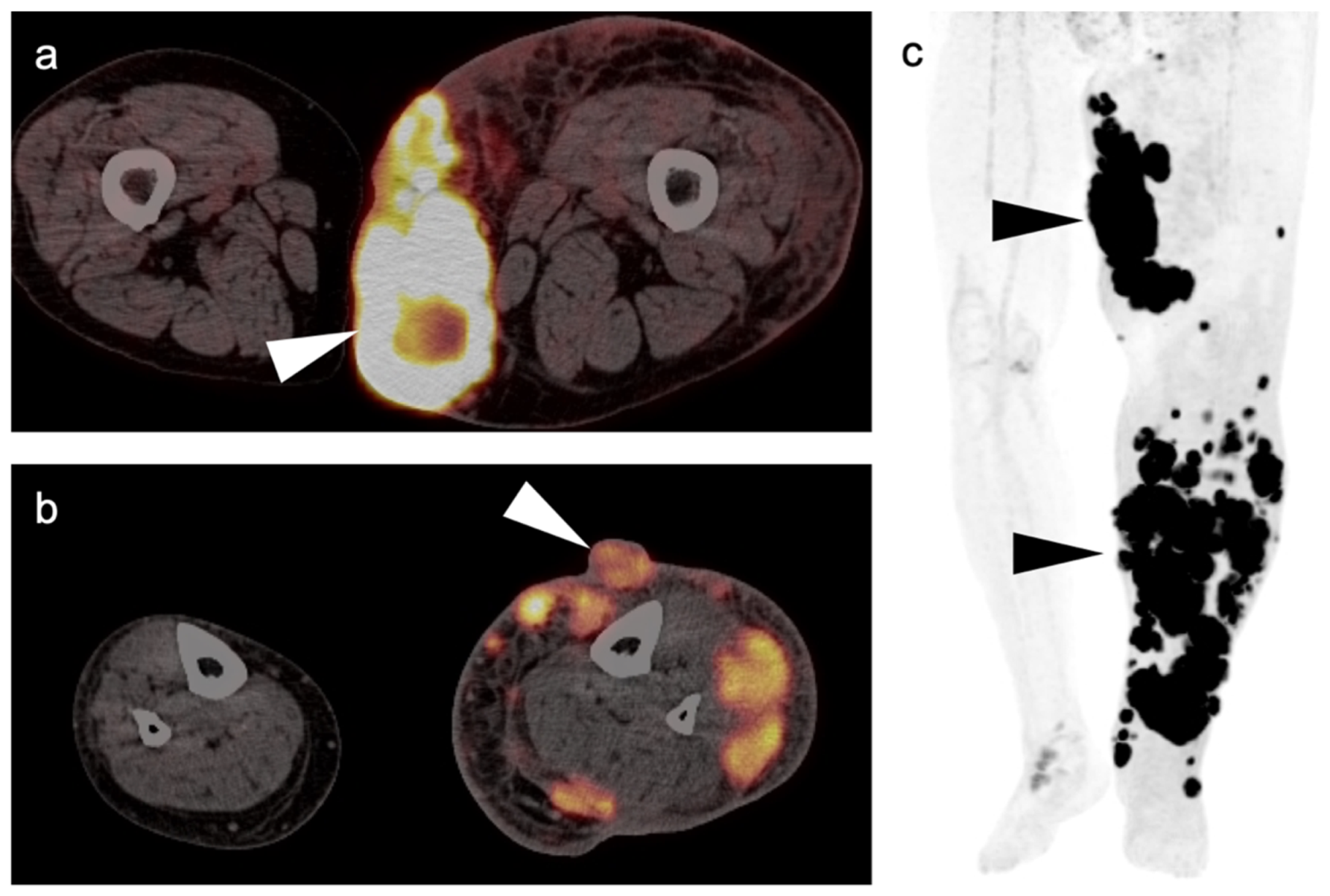
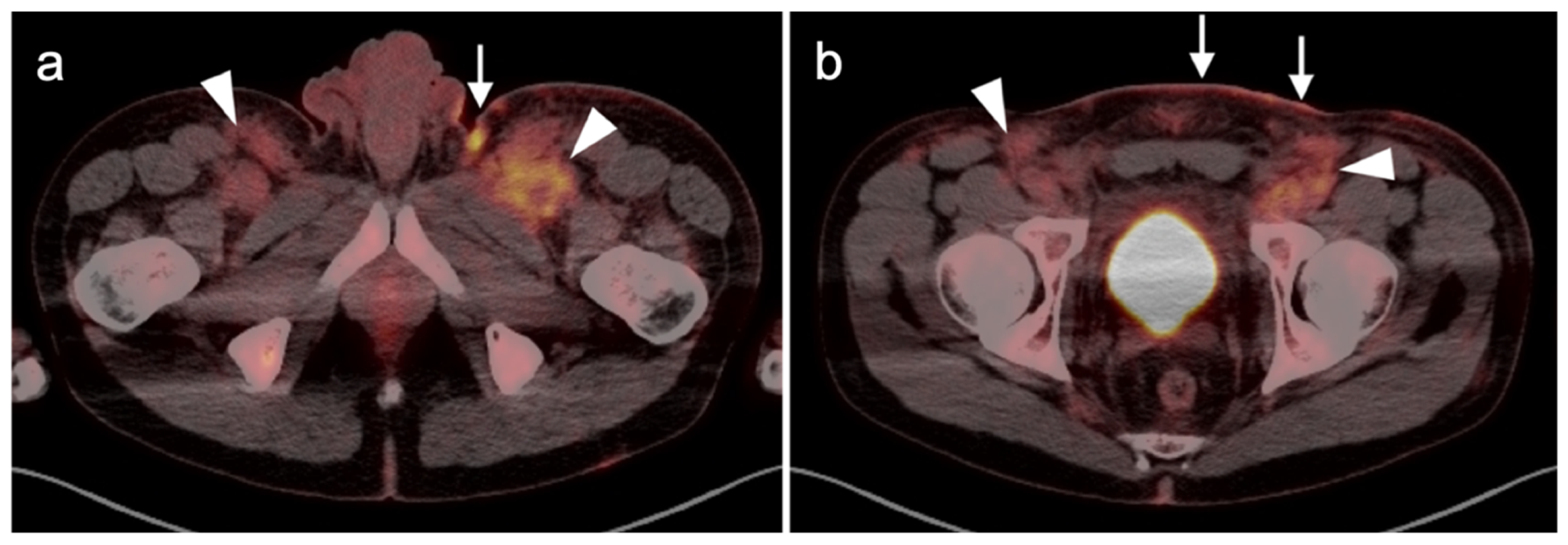
3.1.4. Kaposi Sarcoma
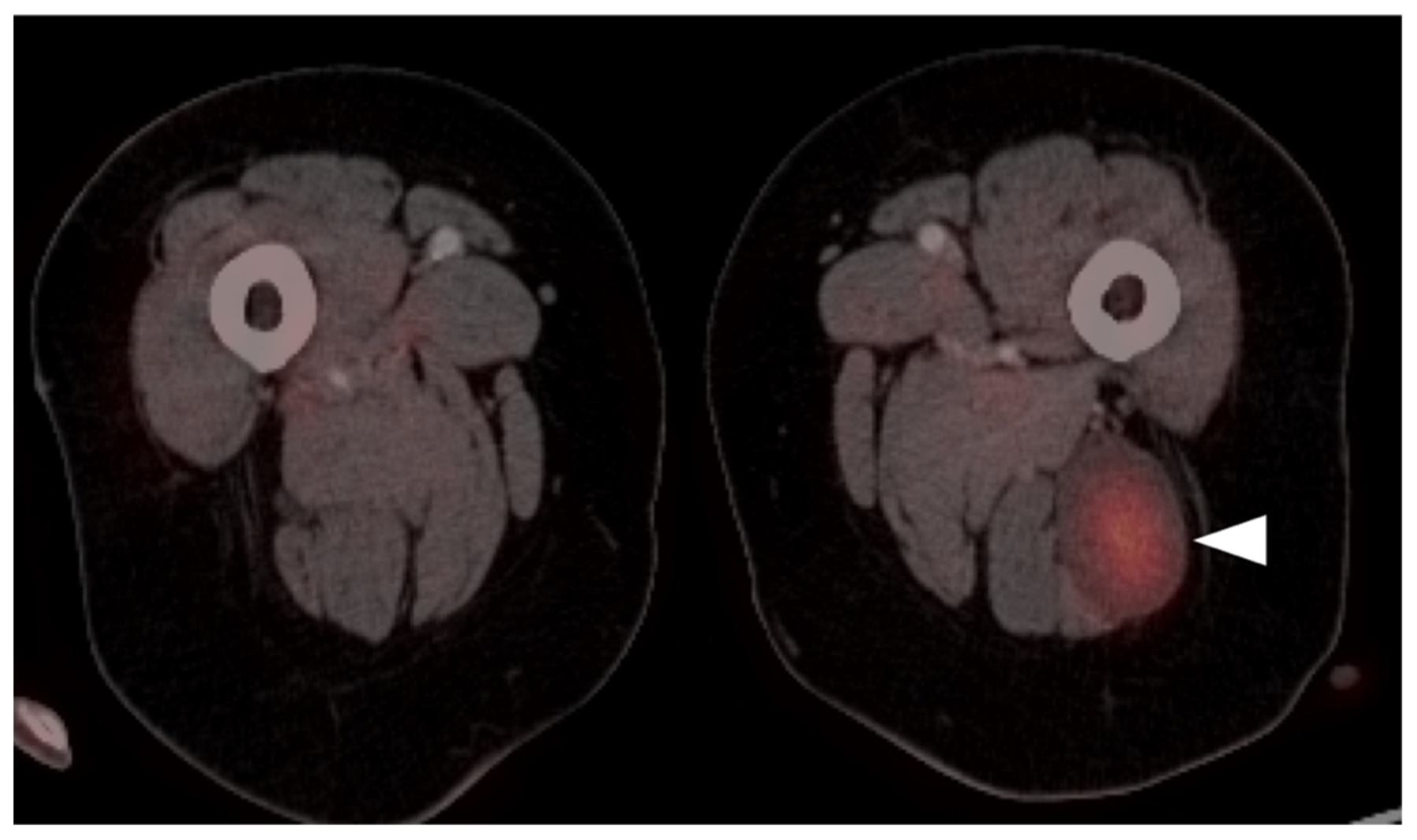
3.1.5. Rhabdomyosarcoma
3.1.6. Liposarcoma
3.1.7. Synovial Sarcoma
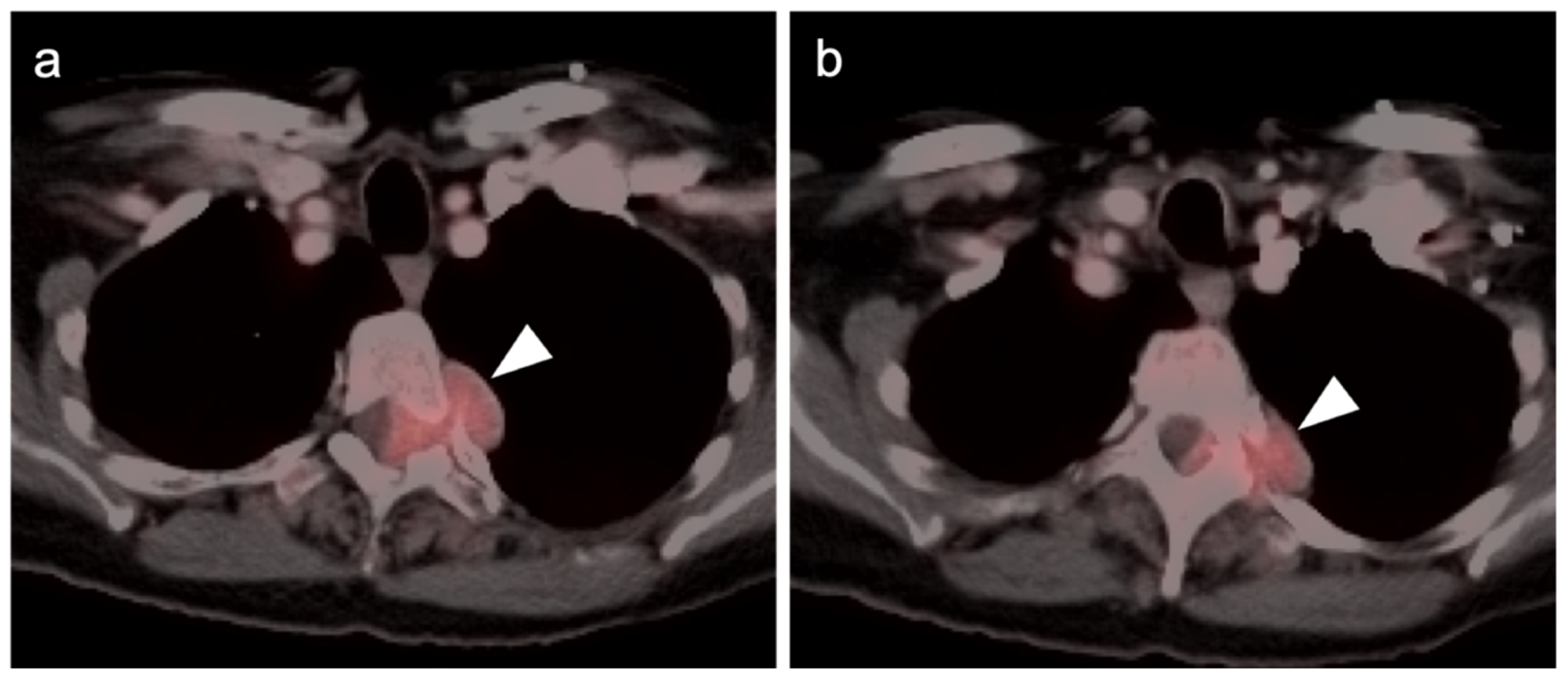
3.1.8. Nerve Sheath Tumors
3.2. Bone Sarcomas
3.2.1. Chondrosarcoma
3.2.2. Osteosarcoma
3.2.3. Ewing Sarcoma
4. Neuroendocrine Tumors
4.1. Epidemiology and Agents
4.2. Normal Physiologic and Benign Patterns
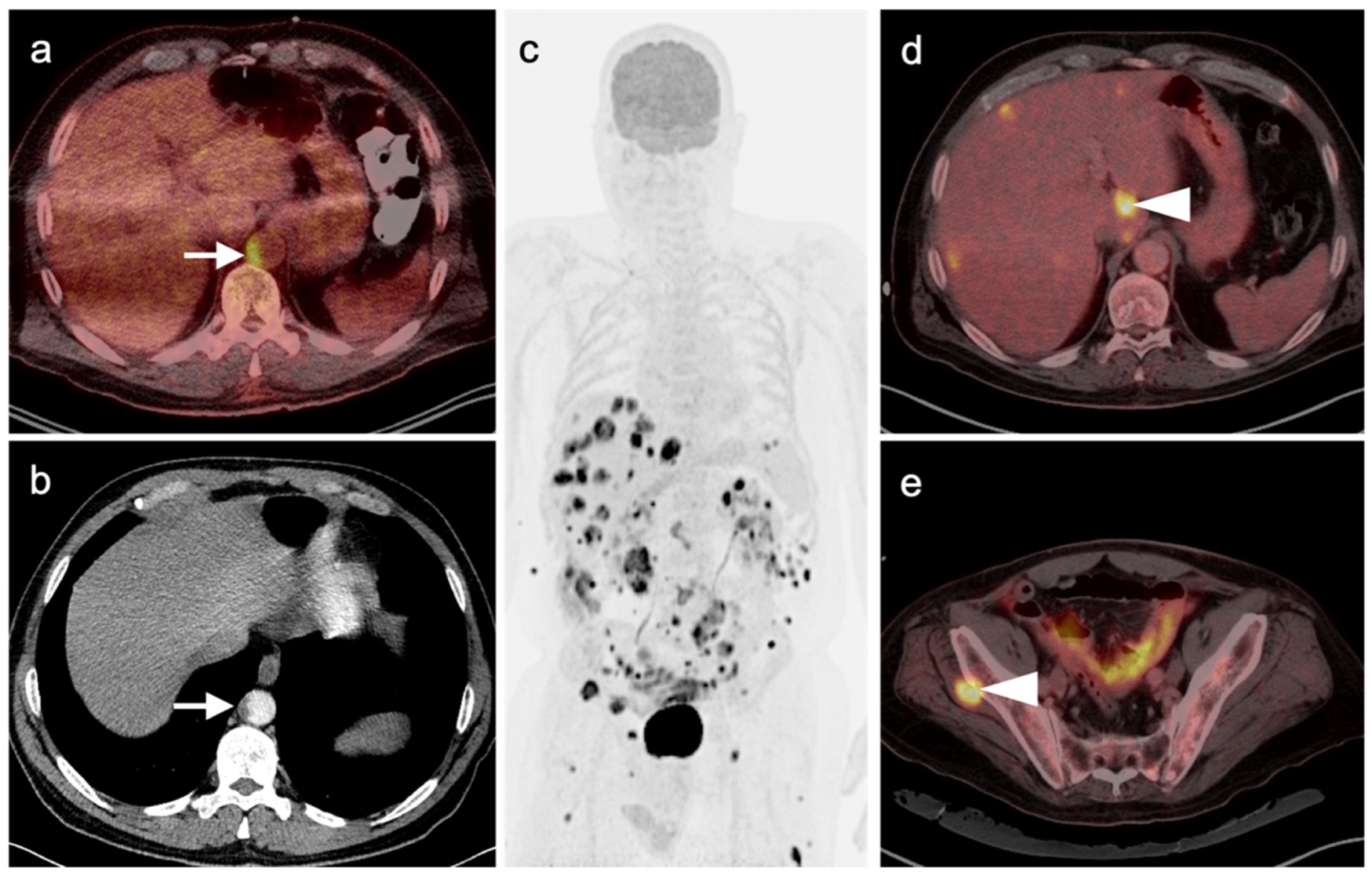
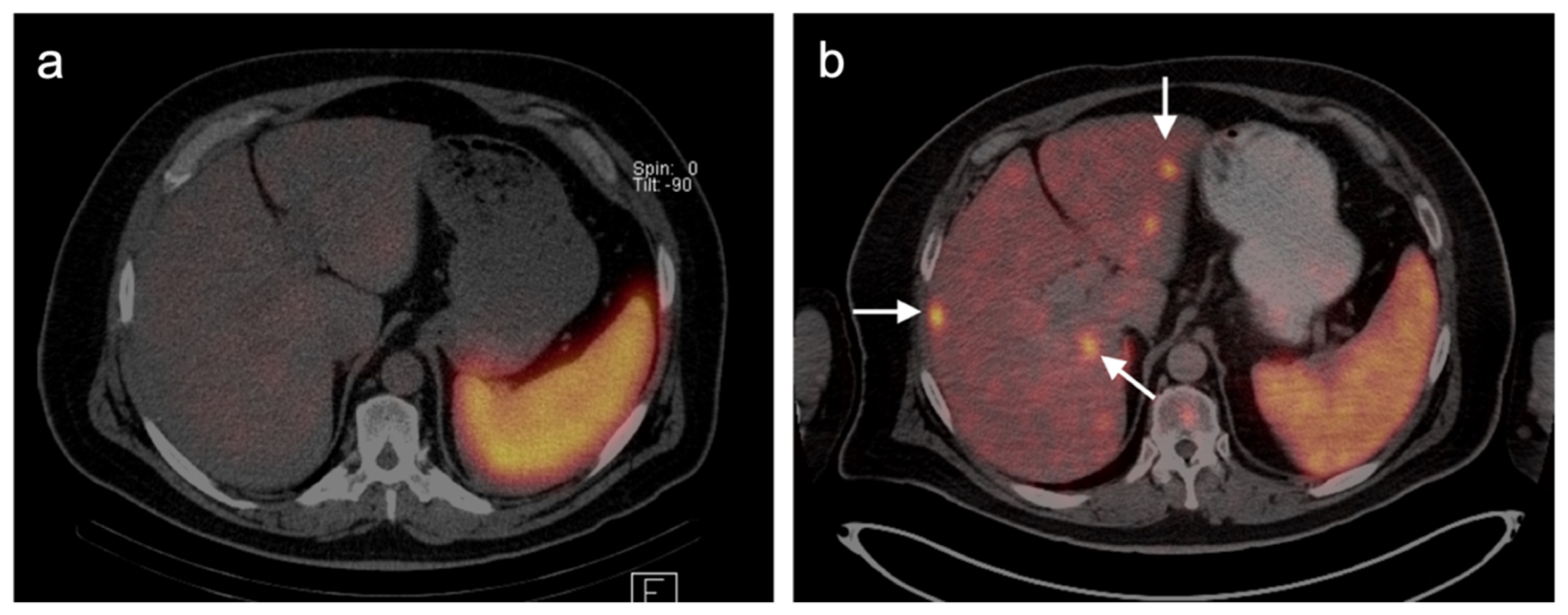
4.3. Gastroenteropancreatic (GEP) Neuroendocrine Tumors
4.4. Bronchial Carcinoid
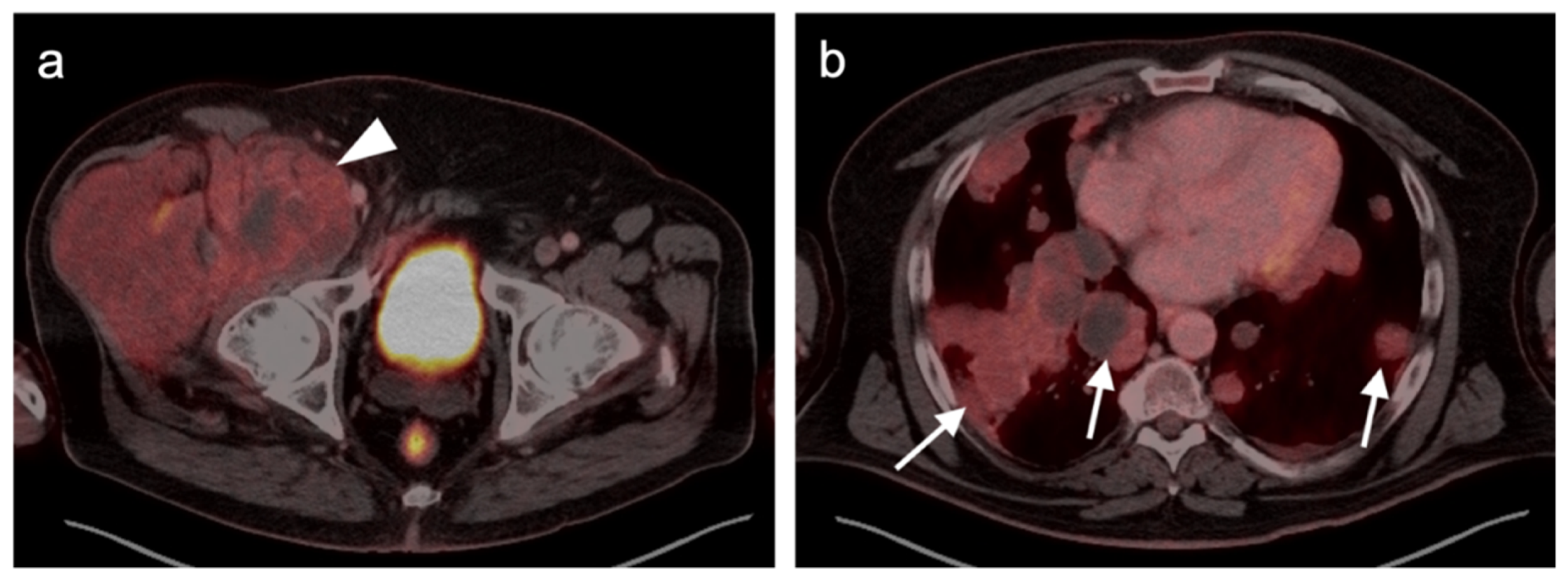
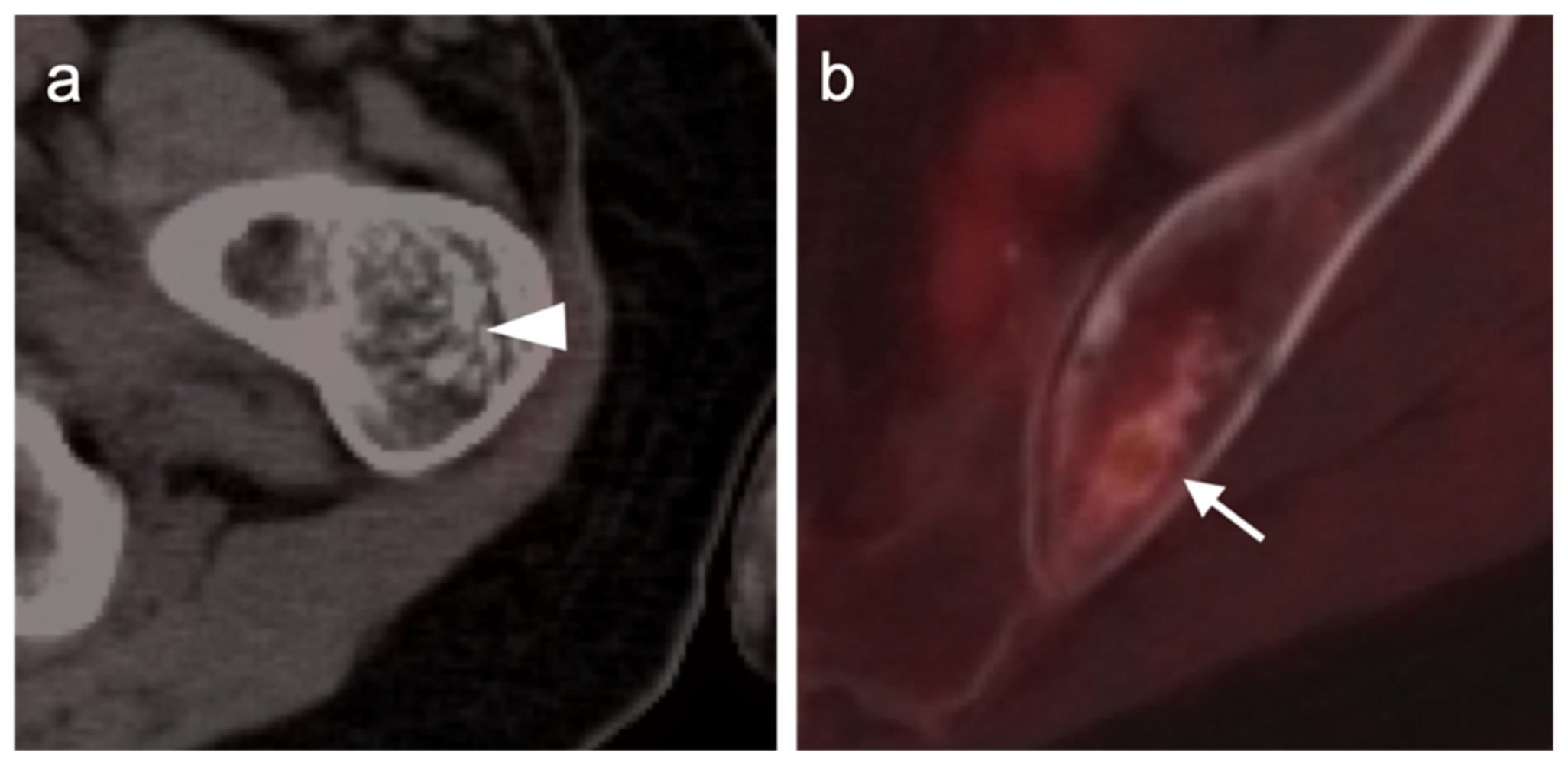
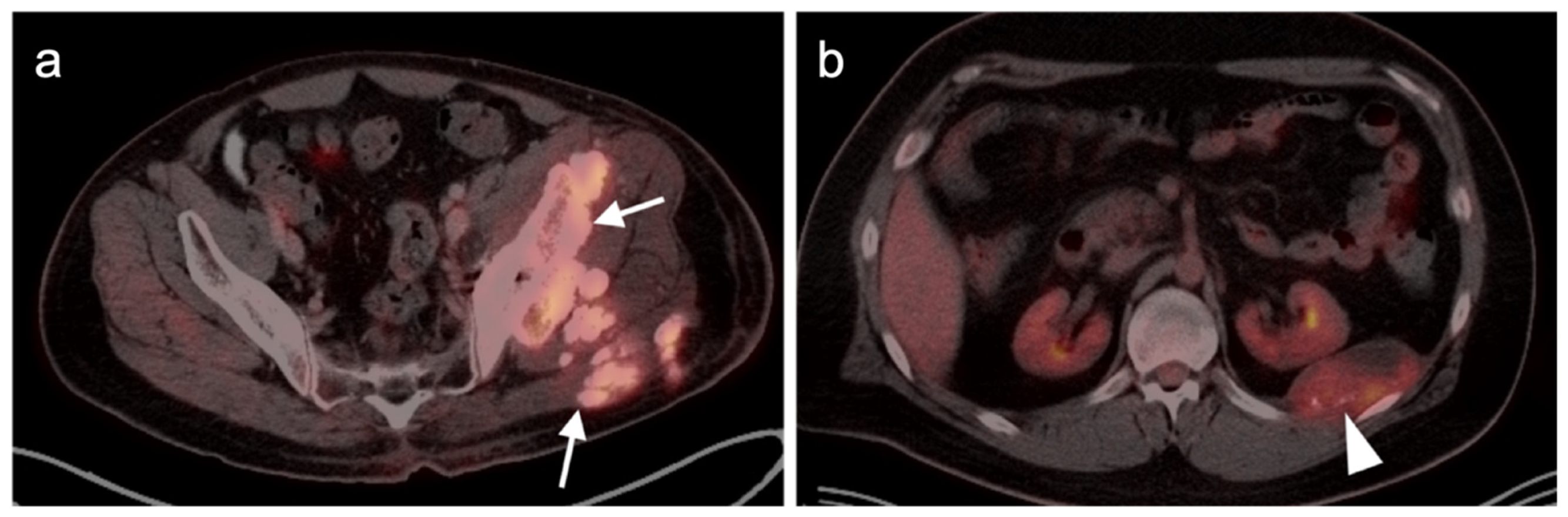
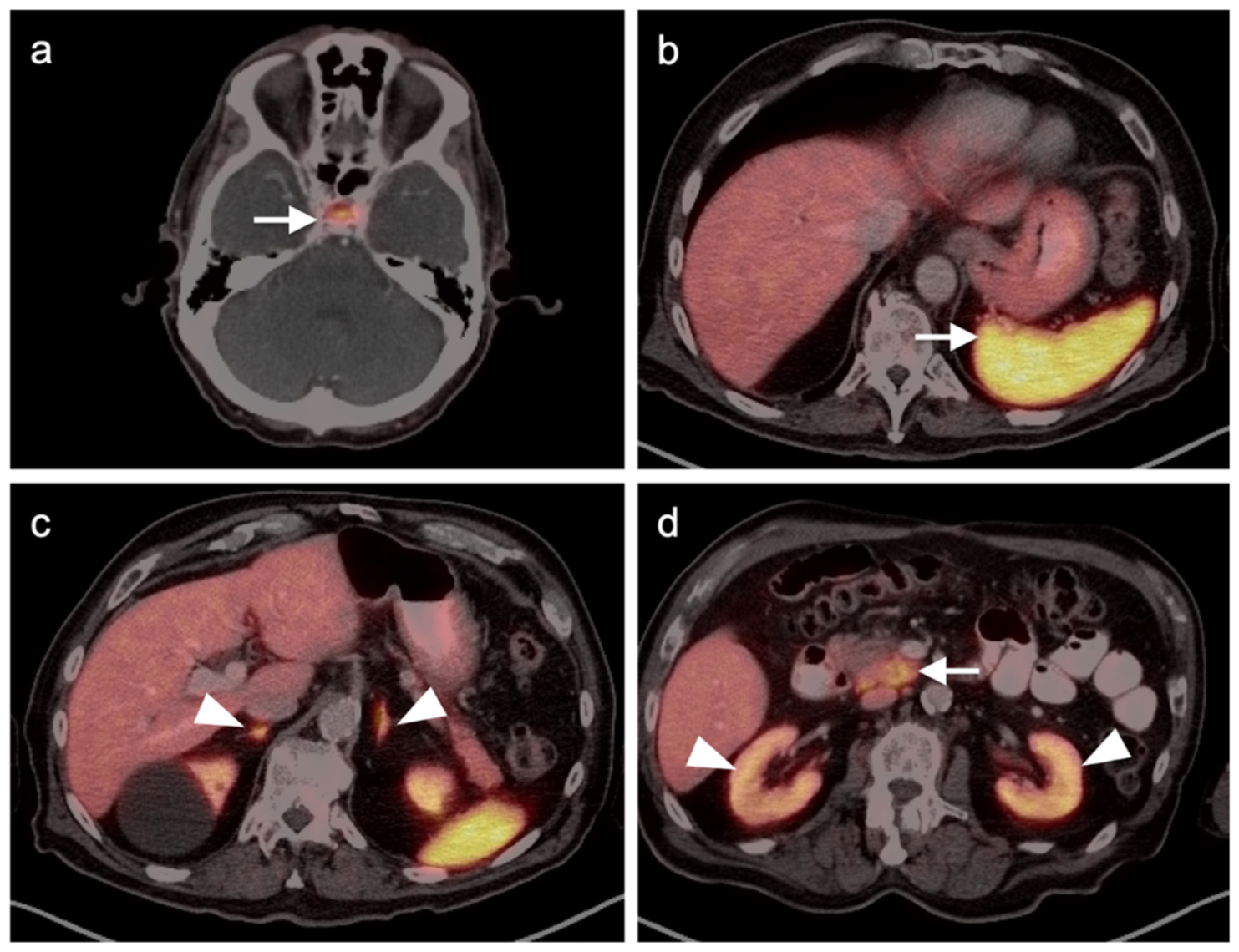
4.5. Pheochromocytoma and Paraganglioma
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Davey, M.G.; Miller, N.; McInerney, N.M. A Review of Epidemiology and Cancer Biology of Malignant Melanoma. Cureus 2021, 13, e15087. [Google Scholar] [CrossRef] [PubMed]
- Coggshall, K.; Tello, T.L.; North, J.P.; Yu, S.S. Merkel cell carcinoma: An update and review: Pathogenesis, diagnosis, and staging. J. Am. Acad. Dermatol. 2018, 78, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fania, L.; Didona, D.; Di Pietro, F.; Verkhovskaia, S.; Morese, R.; Paolino, G.; Donati, M.; Ricci, F.; Coco, V.; Ricci, F.; et al. Cutaneous Squamous Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Webb, H.R.; Latifi, H.R.; Griffeth, L.K. Utility of whole-body (head-to-toe) PET/CT in the evaluation of melanoma and sarcoma patients. Nucl. Med. Commun. 2018, 39, 68–73. [Google Scholar] [CrossRef]
- Alberti, A.; Bossi, P. Immunotherapy for Cutaneous Squamous Cell Carcinoma: Results and Perspectives. Front. Oncol. 2022, 11, 727027. [Google Scholar] [CrossRef]
- Ralli, M.; Botticelli, A.; Visconti, I.C.; Angeletti, D.; Fiore, M.; Marchetti, P.; Lambiase, A.; De Vincentiis, M.; Greco, A. Immunotherapy in the Treatment of Metastatic Melanoma: Current Knowledge and Future Directions. J. Immunol. Res. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Shalhout, S.Z.; Kaufman, H.L.; Emerick, K.S.; Miller, D.M. Immunotherapy for Nonmelanoma skin cancer: Facts and Hopes. Clin. Cancer Res. 2022, 28, 2211–2220. [Google Scholar] [CrossRef]
- Stonesifer, C.J.; Djavid, A.R.; Grimes, J.M.; Khaleel, A.E.; Soliman, Y.S.; Maisel-Campbell, A.; Garcia-Saleem, T.J.; Geskin, L.J.; Carvajal, R.D. Immune Checkpoint Inhibition in Non-Melanoma Skin Cancer: A Review of Current Evidence. Front. Oncol. 2021, 11, 734354. [Google Scholar] [CrossRef]
- Gandy, N.; Arshad, M.A.; Wallitt, K.L.; Dubash, S.; Khan, S.; Barwick, T.D. Immunotherapy-related adverse effects on 18F-FDG PET/CT imaging. Br. J. Radiol. 2020, 93, 20190832. [Google Scholar] [CrossRef]
- Johkoh, T.; Lee, K.S.; Nishino, M.; Travis, W.D.; Ryu, J.H.; Lee, H.Y.; Ryerson, C.J.; Franquet, T.; Bankier, A.A.; Brown, K.K.; et al. Chest CT Diagnosis and Clinical Management of Drug-Related Pneumonitis in Patients Receiving Molecular Targeting Agents and Immune Checkpoint Inhibitors. Chest 2021, 159, 1107–1125. [Google Scholar] [CrossRef]
- Thomas, R.; Sebastian, B.; George, T.; Majeed, N.F.; Akinola, T.; Laferriere, S.L.; Braschi-Amirfarzan, M. A review of the imaging manifestations of immune check point inhibitor toxicities. Clin. Imaging 2020, 64, 70–79. [Google Scholar] [CrossRef]
- Widmann, G.; Nguyen, V.A.; Plaickner, J.; Jaschke, W. Imaging Features of Toxicities by Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Radiol. Rep. 2017, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Malaty, S.; Bastian, C.M.; Ramirez-Cibes, I.; Shahlapour, M.; Dhillon, W. Pembrolizumab-Induced Sarcoid-Like Reaction: FDG-PET Scan Interpretation in the Era of Immunotherapy. Cureus 2020, 12, e9449. [Google Scholar] [CrossRef]
- Aide, N.; Hicks, R.J.; Le Tourneau, C.; Lheureux, S.; Fanti, S.; Lopci, E. FDG PET/CT for assessing tumour response to immunotherapy: Report on the EANM symposium on immune modulation and recent review of the literature. Eur. J. Pediatr. 2018, 46, 238–250. [Google Scholar] [CrossRef] [Green Version]
- Sachpekidis, C.; Kopp-Schneider, A.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. Assessment of early metabolic progression in melanoma patients under immunotherapy: An 18F-FDG PET/CT study. EJNMMI Res. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Lopci, E.; Hicks, R.J.; Dimitrakopoulou-Strauss, A.; Dercle, L.; Iravani, A.; Seban, R.D.; Sachpekidis, C.; Humbert, O.; Gheysens, O.; Glaudemans, A.W.J.M.; et al. Joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards on recommended use of [18F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors version 1.0. Eur. J. Pediatr. 2022, 49, 2323–2341. [Google Scholar] [CrossRef]
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.M.; Marabelle, A.; Soria, J.-C.; et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L. Clin. Cancer Res. 2017, 23, 1920–1928. [Google Scholar] [CrossRef] [Green Version]
- McLean, L.S.; Cavanagh, K.; Hicks, R.J.; Callahan, J.; Xie, J.; Cardin, A.; Lim, A.M.; Rischin, D. FDG-PET/CT imaging for evaluating durable responses to immune check point inhibitors in patients with advanced cutaneous squamous cell carcinoma. Cancer Imaging 2021, 21, 1–6. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J. Nucl. Med. 2009, 50 (Suppl. S1), 122S–150S. [Google Scholar] [CrossRef] [Green Version]
- Elder, D.E.; Bastian, B.C.; Cree, I.A.; Massi, D.; Scolyer, R.A. The 2018 World Health Organization Classification of Cutaneous, Mucosal, and Uveal Melanoma: Detailed Analysis of 9 Distinct Subtypes Defined by Their Evolutionary Pathway. Arch. Pathol. Lab. Med. 2020, 144, 500–522. [Google Scholar] [CrossRef] [Green Version]
- Altieri, L.; Eguchi, M.; Peng, D.H.; Cockburn, M. Predictors of mucosal melanoma survival in a population-based setting. J. Am. Acad. Dermatol. 2018, 81, 136–142.e2. [Google Scholar] [CrossRef]
- Ossio, R.; Roldán-Marín, R.; Martínez-Said, H.; Adams, D.J.; Espinoza, C.D.R. Melanoma: A global perspective. Nat. Cancer 2017, 17, 393–394. [Google Scholar] [CrossRef]
- Reinhardt, M.J. Cutaneous Melanoma. In PET in Oncology; Recent Results in Cancer Research; Dresel, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 170. [Google Scholar] [CrossRef]
- Sinnamon, A.J.; Neuwirth, M.G.; Bartlett, E.K.; Zaheer, S.; Etherington, M.S.; Xu, X.; Elder, D.E.; Czerniecki, B.J.; Fraker, D.L.; Karakousis, G.C. Predictors of false negative sentinel lymph node biopsy in trunk and extremity melanoma. J. Surg. Oncol. 2017, 116, 848–855. [Google Scholar] [CrossRef]
- Hinz, T.; Voth, H.; Ahmadzadehfar, H.; Hoeller, T.; Wenzel, J.; Bieber, T.; Schmid-Wendtner, M.-H. Role of High-Resolution Ultrasound and PET/CT Imaging for Preoperative Characterization of Sentinel Lymph Nodes in Cutaneous Melanoma. Ultrasound Med. Biol. 2013, 39, 30–36. [Google Scholar] [CrossRef]
- Topuz, V.; Görtan, F.A.; Döner, Z.R.K.; Önsel, Ç.; Sayman, H.B. Usefulness of 18F-FDG PET/CT in Cutaneous Melanoma Patients with Negative Sentinel Lymph Nodes and High Clark Levels. Mol. Imaging Radionucl. Ther. 2018, 27, 66–72. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Cutaneous Melanoma. NCCN. Version 2.2022. 26 January 2022. Available online: https://www.nccn.org/professionals/physician_gls/PDF/melanoma.pdf. (accessed on 14 February 2022).
- Schröer-Günther, M.A.; Wolff, R.F.; Westwood, M.E.; Scheibler, F.J.; Schürmann, C.; Baumert, B.G.; Sauerland, S.; Kleijnen, J. F-18-fluoro-2-deoxyglucose positron emission tomography (PET) and PET/computed tomography imaging in primary staging of patients with malignant melanoma: A systematic review. Syst. Rev. 2012, 1, 62. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.Y.; Huff, D.T.; Jeraj, R.; Albertini, M.R. FDG PET/CT for Assessment of Immune Therapy: Opportunities and Understanding Pitfalls. Semin Nucl Med. 2020, 50, 18–531. [Google Scholar] [CrossRef]
- Sharma, R.; Shah, P.; Narendran, V. Poor uptake of fluorodeoxyglucose in positron emission tomography-computed tomography scan for intraocular choroidal melanoma in Asian Indian Eyes. World J. Nucl. Med. 2016, 15, 53–55. [Google Scholar] [CrossRef]
- Reddy, S.; Kurli, M.; Tena, L.B.; Finger, P.T. PET/CT imaging: Detection of choroidal melanoma. Br. J. Ophthalmol. 2005, 89, 1265–1269. [Google Scholar] [CrossRef] [Green Version]
- Zurcher, K.S.; Houghton, O.M.; Shen, J.F.; Seetharam, M.; Roarke, M.C.; Yang, M. Nuclear Medicine and Molecular Imaging in Nodal Staging and Surveillance of Ocular Melanoma: Case Reports and Review of the Literature. J. Nucl. Med. Technol. 2021, 49, 275–280. [Google Scholar] [CrossRef]
- Orcurto, V.; Denys, A.; Voelter, V.; Schalenbourg, A.; Schnyder, P.; Zografos, L.; Leyvraz, S.; Delaloye, A.B.; Prior, J. 18F-fluorodeoxyglucose positron emission tomography/computed tomography and magnetic resonance imaging in patients with liver metastases from uveal melanoma. Melanoma Res. 2012, 22, 63–69. [Google Scholar] [CrossRef] [PubMed]
- The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma III: Local complications and observations following enucleation COMS report no. 11. Am. J. Ophthalmol. 1998, 126, 362–372. [CrossRef]
- The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma II: Initial mortality findings COMS report no. 10. Am. J. Ophthalmol. 1998, 125, 779–796. [CrossRef]
- Damato, B.E.; Dukes, J.; Goodall, H.; Carvajal, R.D. Tebentafusp: T Cell Redirection for the Treatment of Metastatic Uveal Melanoma. Cancers 2019, 11, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovanovic, P.; Mihajlovic, M.; Djordjevic-Jocic, J.; Vlajkovic, S.; Cekic, S.; Stefanovic, V. Ocular melanoma: An overview of the current status. Int. J. Clin. Exp. Pathol. 2013, 6, 1230–1244. [Google Scholar]
- Boggs, W. Possible That Early Treatment of Choroidal Melanoma Might Prevent Metastatic Death. Medscape Medical News. Available online: http://www.medscape.com/viewarticle/822126 (accessed on 22 January 2022).
- John, A.R.; Jain, A.; Kishore, B.; Vishnoi, M.; Pandit, A.G.; Sharma, A.; Jaimini, A.; Jain, M.; Singh, A. F-18 fluorodeoxyglucose positron emission tomography/computed tomography in conjunctival melanoma with recurrence. J. Cancer Res. Ther. 2020, 16, 240–242. [Google Scholar] [CrossRef]
- Cassou-Mounat, T.; Luporsi, M.; Huchet, V.; Jehanno, N. Gallbladder Metastasis from Conjunctival Melanoma. Clin. Nucl. Med. 2019, 44, e107–e109. [Google Scholar] [CrossRef]
- Kurli, M.; Chin, K.; Finger, P.T. Whole-body 18 FDG PET/CT imaging for lymph node and metastatic staging of conjunctival melanoma. Br. J. Ophthalmol. 2008, 92, 479–482. [Google Scholar] [CrossRef]
- Bishop, K.D.; Olszewski, A.J. Epidemiology and survival outcomes of ocular and mucosal melanomas: A population-based analysis. Int. J. Cancer 2013, 134, 2961–2971. [Google Scholar] [CrossRef]
- Keraliya, A.R.; Krajewski, K.M.; Braschi-Amirfarzan, M.; Tirumani, S.H.; Shinagare, A.B.; Jagannathan, J.P.; Ramaiya, N.H. Extracutaneous melanomas: A primer for the radiologist. Insights Imaging 2015, 6, 707–717. [Google Scholar] [CrossRef] [Green Version]
- Wong, V.K.; Lubner, M.G.; Menias, C.O.; Mellnick, V.M.; Kennedy, T.A.; Bhalla, S.; Pickhardt, P.J. Clinical and Imaging Features of Noncutaneous Melanoma. Am. J. Roentgenol. 2017, 208, 942–959. [Google Scholar] [CrossRef]
- Goerres, G.W.; Stoeckli, S.J.; Von Schulthess, G.K.; Steinert, H.C. FDG PET for Mucosal Malignant Melanoma of the Head and Neck. Laryngoscope 2002, 112, 381–385. [Google Scholar] [CrossRef]
- Wang, S.; Tachimori, Y.; Hokamura, N.; Igaki, H.; Kishino, T.; Kushima, R. Diagnosis and Surgical Outcomes for Primary Malignant Melanoma of the Esophagus: A Single-Center Experience. Ann. Thorac. Surg. 2013, 96, 1002–1006. [Google Scholar] [CrossRef]
- Falch, C.; Stojadinovic, A.; Hann-Von-Weyhern, C.; Protic, M.; Nissan, A.; Faries, M.B.; Daumer, M.; Bilchik, A.J.; Itzhak, A.; Brücher, B.L. Anorectal Malignant Melanoma: Extensive 45-Year Review and Proposal for a Novel Staging Classification. J. Am. Coll. Surg. 2013, 217, 324–335. [Google Scholar] [CrossRef]
- Murphy, G.; Hussey, D.; Metser, U. Non-cutaneous melanoma: Is there a role for18F-FDG PET-CT? Br. J. Radiol. 2014, 87, 20140324. [Google Scholar] [CrossRef] [Green Version]
- Falch, C.; Mueller, S.; Kirschniak, A.; Braun, M.; Koenigsrainer, A.; Klumpp, B. Anorectal malignant melanoma: Curative abdominoperineal resection: Patient selection with 18F-FDG-PET/CT. World J. Surg. Oncol. 2016, 14, 185. [Google Scholar] [CrossRef] [Green Version]
- Lebbe, C.; Becker, J.C.; Grob, J.-J.; Malvehy, J.; del Marmol, V.; Pehamberger, H.; Peris, K.; Saiag, P.; Middleton, M.R.; Bastholt, L.; et al. Diagnosis and treatment of Merkel Cell Carcinoma. European consensus-based interdisciplinary guideline. Eur. J. Cancer 2015, 51, 2396–2403. [Google Scholar] [CrossRef]
- Mahajan, S.; Barker, C.A.; Mauguen, A.; D’Angelo, S.P.; Yeh, R.; Pandit-Taskar, N. 18F-FDG PET/CT for post-treatment surveillance imaging of patients with stage III Merkel cell carcinoma. J. Nucl. Med. 2021. [Google Scholar] [CrossRef]
- Prior, V.L.; Cussac, B.L. Papel de la 18F-FDG PET/TC en el manejo del carcinoma de células de Merkel: Experiencia de 51 estudios en nuestra institución. Rev. Esp. Med. Nucl. Imagen. Mol. 2021, 40, 139–148. [Google Scholar] [CrossRef]
- Weppler, A.M.; Pattison, A.; Bhave, P.; De Ieso, P.; Raleigh, J.; Hatzimihalis, A.; Gill, A.J.; Balachander, S.; Callahan, J.; Chua, M.; et al. Clinical, FDG-PET and molecular markers of immune checkpoint inhibitor response in patients with metastatic Merkel cell carcinoma. J. Immunother. Cancer 2020, 8, e000700. [Google Scholar] [CrossRef]
- Taralli, S.; Sollini, M.; Milella, M.; Perotti, G.; Filice, A.; Menga, M.; Versari, A.; Rufini, V. 18F-FDG and 68Ga-somatostatin analogs PET/CT in patients with Merkel cell carcinoma: A comparison study. EJNMMI Res. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Eigentler, T.K.; Leiter, U.; Häfner, H.-M.; Garbe, C.; Röcken, M.; Breuninger, H. Survival of Patients with Cutaneous Squamous Cell Carcinoma: Results of a Prospective Cohort Study. J. Investig. Dermatol. 2017, 137, 2309–2315. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, S.; Barker, C.A.; Singh, B.; Pandit-Taskar, N. Clinical value of 18F-FDG-PET/CT in staging cutaneous squamous cell carcinoma. Nucl. Med. Commun. 2019, 40, 744–751. [Google Scholar] [CrossRef]
- Fujiwara, M.; Suzuki, T.; Takiguchi, T.; Fukamizu, H.; Tokura, Y. Evaluation of positron emission tomography imaging to detect lymph node metastases in patients with high-risk cutaneous squamous cell carcinoma. J. Dermatol. 2016, 43, 1314–1320. [Google Scholar] [CrossRef]
- Supriya, M.; Suat-Chin, N.; Sizeland, A. Use of positron emission tomography scanning in metastatic head and neck cutaneous squamous cell cancer: Does it add to patient management? Am. J. Otolaryngol. 2014, 35, 347–352. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Squamous Cell Skin Cancer. NCCN. Version 1.2022. 17 November 2021. Available online: https://www.nccn.org/professionals/physician_gls/PDF/squamous.pdf (accessed on 16 February 2022).
- Mahajan, S.; Barker, C.A.; Mauguen, A.; Singh, B.; Pandit-Taskar, N. Restaging [18F] fludeoxyglucose positron emission tomography/computed tomography scan in recurrent cutaneous squamous cell carcinoma: Diagnostic performance and prognostic significance. J. Am. Acad. Dermatol. 2019, 82, 878–886. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours, 5th ed.; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2020; Volume 3. [Google Scholar]
- MacPherson, R.E.; Pratap, S.; Tyrrell, H.; Khonsari, M.; Wilson, S.; Gibbons, M.; Whitwell, D.; Giele, H.; Critchley, P.; Cogswell, L.; et al. Retrospective audit of 957 consecutive 18F-FDG PET-CT scans compared to CT and MRI in 493 patients with different histological subtypes of bone and soft tissue sarcoma. Clin. Sarcoma Res. 2018, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Vaz, S.C.; Oliveira, C.; Castanheira, J.C.; Silva, F.; Costa, D.C. Gastric GIST Incidentally Detected on 68Ga-PSMA-PET/CT: Correlation Between Functional Imaging and Histology. Clin. Nucl. Med. 2018, 43, e488–e491. [Google Scholar] [CrossRef]
- Mathew, B.; Agrawal, A.; Purandare, N.C.; Ramadwar, M.; Menon, S.; Bakshi, G.; Joshi, A.; Puranik, A.; Shah, S.; Rangarajan, V. Incidental Detection of Pleomorphic Sarcoma on 68Ga-PSMA PET/CT in a Patient with Prostate Cancer. Clin. Nucl. Med. 2019, 45, e120–e121. [Google Scholar] [CrossRef]
- Jackson, T.; Mosci, C.; von Eyben, R.; Mittra, E.; Ganjoo, K.; Biswal, S.; Gambhir, S.S.; Iagaru, A. Combined 18F-NaF and 18F-FDG PET/CT in the Evaluation of Sarcoma Patients. Clin. Nucl. Med. 2015, 40, 720–724. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Soft Tissue Sarcoma. NCCN. Version 3.2021. 26 January 2022. Available online: https://www.nccn.org/professionals/physician_gls/PDF/sarcoma.pdf (accessed on 16 February 2022).
- Schuetze, S.M.; Rubin, B.P.; Vernon, C.; Hawkins, D.S.; Bruckner, J.D.; Conrad, E.U.; Eary, J.F. Use of positron emission tomography in localized extremity soft tissue sarcoma treated with neoadjuvant chemotherapy. Cancer 2005, 103, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Benz, M.R.; Czernin, J.; Allen-Auerbach, M.S.; Tap, W.D.; Dry, S.M.; Elashoff, D.; Chow, K.; Evilevitch, V.; Eckardt, J.J.; Phelps, M.E.; et al. FDG-PET/CT Imaging Predicts Histopathologic Treatment Responses after the Initial Cycle of Neoadjuvant Chemotherapy in High-Grade Soft-Tissue Sarcomas. Clin. Cancer Res. 2009, 15, 2856–2863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uslu, L.; Asa, S.; Sager, S.; Halaç, M. Multiple cardiac masses and distant metastatic foci in a patient with high grade pleomorphic sarcoma of the heart revealed by follow-up FDG PET/CT. Nukl.-Nucl. 2014, 53, N8–N9. [Google Scholar] [CrossRef]
- Roberge, D.; Vakilian, S.; Alabed, Y.Z.; Turcotte, R.E.; Freeman, C.R.; Hickeson, M. FDG PET/CT in Initial Staging of Adult Soft-Tissue Sarcoma. Sarcoma 2012, 2012, 960194. [Google Scholar] [CrossRef] [Green Version]
- Punt, S.E.; Eary, J.F.; O’Sullivan, J.; Conrad, E.U. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: Imaging characteristics. Nucl. Med. Commun. 2009, 30, 546–549. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.-J.; Yang, Z.; Yu, J.-Y.; Li, N.; Wang, X.-J.; Zhou, N.-N. Potential application value of PET/computed tomography in retroperitoneal leiomyosarcoma and a literature review. Nucl. Med. Commun. 2021, 42, 800–810. [Google Scholar] [CrossRef]
- Subramaniam, S.; Callahan, J.; Bressel, M.; Hofman, M.S.; Mitchell, C.; Hendry, S.; Vissers, F.L.; Van der Hiel, B.; Patel, D.; Van Houdt, W.J.; et al. The role of 18 F-FDG PET/CT in retroperitoneal sarcomas—A multicenter retrospective study. J. Surg. Oncol. 2021, 123, 1081–1087. [Google Scholar] [CrossRef]
- Qiu, S.; Zou, S.; Cheng, S.; Song, S.; Zhu, X. Positive FAPI PET/CT in a Bilateral Mammary Angiosarcoma Patient with Less Impressive FDG PET/CT Images. Clin. Nucl. Med. 2022. [Google Scholar] [CrossRef]
- Camoni, L.; Albano, D. Contrast-enhanced 18F-FDG PET/CT to differentiate primary cardiac lymphoma from primary cardiac angiosarcoma. J. Nucl. Cardiol. 2021, 1–3. [Google Scholar] [CrossRef]
- Umemura, H.; Yamasaki, O.; Kaji, T.; Hamada, T.; Otsuka, M.; Asagoe, K.; Iwatsuki, K. Prognostic value of 18 F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with cutaneous angiosarcoma: A retrospective study of 18 cases. J. Dermatol. 2017, 44, 1046–1049. [Google Scholar] [CrossRef] [Green Version]
- Tokmak, E.; Özkan, E.; Yağcı, S.; Kır, K.M. F18-FDG PET/CT Scanning in Angiosarcoma: Report of Two Cases. Mol. Imaging Radionucl. Ther. 2011, 20, 63–66. [Google Scholar] [CrossRef]
- Mankia, S.K.; Miller, R.F.; Edwards, S.G.; Ramsay, A.; Lee, S.M. The Response of HIV-Associated Lymphadenopathic Kaposi Sarcoma to Highly Active Antiretroviral Therapy Evaluated by 18F-FDG PET/CT. Clin. Nucl. Med. 2012, 37, 692–693. [Google Scholar] [CrossRef]
- Morooka, M.; Ito, K.; Kubota, K.; Minamimoto, R.; Shida, Y.; Hasuo, K.; Ito, T.; Tasato, D.; Honda, H.; Teruya, K.; et al. Whole-body 18F-fluorodeoxyglucose positron emission tomography/computed tomography images before and after chemotherapy for Kaposi sarcoma and highly active antiretrovirus therapy. Jpn. J. Radiol. 2010, 28, 759–762. [Google Scholar] [CrossRef]
- Polizzotto, M.N.; Millo, C.; Uldrick, T.S.; Aleman, K.; Whatley, M.; Wyvill, K.M.; O’Mahony, D.; Marshall, V.; Whitby, D.; Maass-Moreno, R.; et al. 18F-fluorodeoxyglucose Positron Emission Tomography in Kaposi Sarcoma Herpesvirus–Associated Multicentric Castleman Disease: Correlation with Activity, Severity, Inflammatory and Virologic Parameters. J. Infect. Dis. 2015, 212, 1250–1260. [Google Scholar] [CrossRef] [Green Version]
- Thompson, L.D.R.; Jo, V.Y.; Agaimy, A.; Llombart-Bosch, A.; Morales, G.N.; Machado, I.; Flucke, U.; Wakely, P.E., Jr.; Miettinen, M.; Bishop, J.A. Sinonasal Tract Alveolar Rhabdomyosarcoma in Adults: A Clinicopathologic and Immunophenotypic Study of Fifty-Two Cases with Emphasis on Epithelial Immunoreactivity. Head Neck Pathol. 2018, 12, 181–192. [Google Scholar] [CrossRef]
- Mercolini, F.; Zucchetta, P.; Jehanno, N.; Corradini, N.; Van Rijn, R.R.; Rogers, T.; Cameron, A.; Scarzello, G.; Coppadoro, B.; Minard-Colin, V.; et al. Role of 18F-FDG-PET/CT in the staging of metastatic rhabdomyosarcoma: A report from the European paediatric Soft tissue sarcoma Study Group. Eur. J. Cancer 2021, 155, 155–162. [Google Scholar] [CrossRef]
- Tateishi, U.; Hosono, A.; Makimoto, A.; Nakamoto, Y.; Kaneta, T.; Fukuda, H.; Murakami, K.; Terauchi, T.; Suga, T.; Inoue, T.; et al. Comparative study of FDG PET/CT and conventional imaging in the staging of rhabdomyosarcoma. Ann. Nucl. Med. 2009, 23, 155–161. [Google Scholar] [CrossRef]
- Donner, D.; Feraco, P.; Meneghello, L.; Rombi, B.; Picori, L.; Chierichetti, F. Usefulness of 18f-FDG PET-CT in Staging, Restaging, and Response Assessment in Pediatric Rhabdomyosarcoma. Diagnostics 2020, 10, 1112. [Google Scholar] [CrossRef]
- Harrison, D.J.; Chi, Y.; Tian, J.; Hingorani, P.; Mascarenhas, L.; McCowage, G.B.; Weigel, B.J.; Venkatramani, R.; Wolden, S.L.; Yock, T.I.; et al. Metabolic response as assessed by 18 F-fluorodeoxyglucose positron emission tomography-computed tomography does not predict outcome in patients with intermediate- or high-risk rhabdomyosarcoma: A report from the Children’s Oncology Group Soft Tissue Sarcoma Committee. Cancer Med. 2020, 10, 857–866. [Google Scholar] [CrossRef]
- Parkes, A.; Urquiola, E.; Bhosale, P.; Lin, H.; Watson, K.; Wang, W.-L.; Feig, B.; Torres, K.; Roland, C.L.; Conley, A.P.; et al. PET/CT Imaging as a Diagnostic Tool in Distinguishing Well-Differentiated versus Dedifferentiated Liposarcoma. Sarcoma 2020, 2020, 8363986. [Google Scholar] [CrossRef]
- Dalal, K.M.; Antonescu, C.R.; Singer, S. Diagnosis and management of lipomatous tumors. J. Surg. Oncol. 2008, 97, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Lisle, J.W.; Eary, J.F.; O’Sullivan, J.; Conrad, E.U. Risk Assessment Based on FDG-PET Imaging in Patients with Synovial Sarcoma. Clin. Orthop. Relat. Res. 2008, 467, 1605–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, K.J.; Lim, I.; Park, J.Y.; Jo, A.R.; Kong, C.B.; Song, W.S.; Jo, W.H.; Lee, S.Y.; Koh, J.S.; Kim, B.I.; et al. The Role of 18F-FDG PET/CT as a Prognostic Factor in Patients with Synovial Sarcoma. Nucl. Med. Mol. Imaging 2014, 49, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, K.M.; Woodruff, J.M.; Ellis, F.T.; Posner, J.B. Radiation-induced malignant and atypical peripheral nerve sheath tumors. Ann. Neurol. 1980, 7, 311–318. [Google Scholar] [CrossRef]
- Farid, M.; Demicco, E.G.; Garcia, R.; Ahn, L.; Merola, P.R.; Cioffi, A.; Maki, R.G. Malignant Peripheral Nerve Sheath Tumors. Oncologist 2014, 19, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Broski, S.M.; Johnson, G.; Howe, B.M.; Nathan, M.A.; Wenger, D.E.; Spinner, R.J.; Amrami, K.K. Evaluation of 18F-FDG PET and MRI in differentiating benign and malignant peripheral nerve sheath tumors. Skelet. Radiol. 2016, 45, 1097–1105. [Google Scholar] [CrossRef]
- Li, C.-S.; Huang, G.-S.; Wu, H.-D.; Chen, W.-T.; Shih, L.-S.; Lii, J.-M.; Duh, S.-J.; Chen, R.-C.; Tu, H.-Y.; Chan, W.P. Differentiation of soft tissue benign and malignant peripheral nerve sheath tumors with magnetic resonance imaging. Clin. Imaging 2008, 32, 121–127. [Google Scholar] [CrossRef]
- Enneking, W.F.; Spanier, S.S.; Goodman, M.A. A system for the surgical staging of musculoskeletal sarcoma. Clin. Orthop. Relat. Res. 1980, 62, 1027–1030. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Bone Cancer. Version 2.NCCN. 8 October 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/bone.pdf (accessed on 9 February 2022).
- Subhawong, T.K.; Winn, A.; Shemesh, S.; Pretell-Mazzini, J. F-18 FDG PET differentiation of benign from malignant chondroid neoplasms: A systematic review of the literature. Skelet. Radiol. 2017, 46, 1233–1239. [Google Scholar] [CrossRef]
- Brenner, W.; Conrad, E.U.; Eary, J.F. FDG PET imaging for grading and prediction of outcome in chondrosarcoma patients. Eur. J. Pediatr. 2004, 31, 189–195. [Google Scholar] [CrossRef]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef] [Green Version]
- Aryal, A.; Kumar, V.S.; Shamim, S.A.; Gamanagatti, S.; Alam Khan, S. What Is the Comparative Ability of 18F-FDG PET/CT, 99mTc-MDP Skeletal Scintigraphy, and Whole-body MRI as a Staging Investigation to Detect Skeletal Metastases in Patients with Osteosarcoma and Ewing Sarcoma? Clin. Orthop. Relat. Res. 2021, 479, 1768–1779. [Google Scholar] [CrossRef]
- Strauss, S.J.; Frezza, A.M.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; Bonvalot, S.; et al. Bone sarcomas: Esmo-Euracan-Genturis-Ern PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1520–1536. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, W.; Wang, Z. The Role of Chemotherapy for Metastatic, Relapsed and Refractory Osteosarcoma. Pediatr. Drugs 2014, 16, 503–512. [Google Scholar] [CrossRef]
- Hongtao, L.; Hui, Z.; Bingshun, W.; Xiaojin, W.; Zhiyu, W.; Shuier, Z.; Aina, H.; Yuanjue, S.; Daliu, M.; Zan, S.; et al. 18F-FDG positron emission tomography for the assessment of histological response to neoadjuvant chemotherapy in osteosarcomas: A meta-analysis. Surg. Oncol. 2012, 21, e165–e170. [Google Scholar] [CrossRef]
- Treglia, G.; Salsano, M.; Stefanelli, A.; Mattoli, M.V.; Giordano, A.; Bonomo, L. Diagnostic accuracy of 18 F-FDG-PET and PET/CT in patients with Ewing sarcoma family tumours: A systematic review and a meta-analysis. Skelet. Radiol. 2011, 41, 249–256. [Google Scholar] [CrossRef]
- Campbell, K.M.; Shulman, D.S.; Grier, H.E.; DuBois, S.G. Role of bone marrow biopsy for staging new patients with Ewing sarcoma: A systematic review. Pediatr. Blood Cancer 2020, 68, e28807. [Google Scholar] [CrossRef]
- Hawkins, D.S.; Schuetze, S.M.; Butrynski, J.E.; Rajendran, J.G.; Vernon, C.B.; Conrad, E.U.; Eary, J.F. [18F]Fluorodeoxyglucose Positron Emission Tomography Predicts Outcome for Ewing Sarcoma Family of Tumors. J. Clin. Oncol. 2005, 23, 8828–8834. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.M.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Krenning, E.; Breeman, W.; Kooij, P.; Lameris, J.; Bakker, W.; Koper, J.; Ausema, L.; Reubi, J.; Lamberts, S. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet 1989, 333, 242–244. [Google Scholar] [CrossRef]
- Hicks, R. Use of molecular targeted agents for the diagnosis, staging and therapy of neuroendocrine malignancy. Cancer Imaging 2010, 10, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, A.; El-Khouli, R.; Waits, T.; Agrawal, R.; Siddiqui, F.; Tarter, Z.; Horn, M.; Weiss, H.; Oates, E.; Evers, B.M.; et al. Post FDA approval analysis of 200 gallium-68 DOTATATE imaging: A retrospective analysis in neuroendocrine tumor patients. Oncotarget 2020, 11, 3061–3068. [Google Scholar] [CrossRef]
- Walker, R.C.; Smith, G.T.; Liu, E.; Moore, B.; Clanton, J.; Stabin, M. Measured Human Dosimetry of 68Ga-DOTATATE. J. Nucl. Med. 2013, 54, 855–860. [Google Scholar] [CrossRef] [Green Version]
- Sandström, M.; Velikyan, I.; Garske-Román, U.; Sörensen, J.; Eriksson, B.; Granberg, D.; Lundqvist, H.; Sundin, A.; Lubberink, M. Comparative Biodistribution and Radiation Dosimetry of 68Ga-DOTATOC and 68Ga-DOTATATE in Patients with Neuroendocrine Tumors. J. Nucl. Med. 2013, 54, 1755–1759. [Google Scholar] [CrossRef] [Green Version]
- Hofman, M.S.; Lau, W.F.E.; Hicks, R.J. Somatostatin Receptor Imaging with68Ga DOTATATE PET/CT: Clinical Utility, Normal Patterns, Pearls, and Pitfalls in Interpretation. RadioGraphics 2015, 35, 500–516. [Google Scholar] [CrossRef] [Green Version]
- Jais, P.; Terris, B.; Ruszniewski, P.; LeROMANCER, M.; Reyl-Desmars, F.; Vissuzaine, C.; Cadiot, G.; Mignon, M.; Lewin, M.J.M. Somatostatin receptor subtype gene expression in human endocrine gastroentero-pancreatic tumours. Eur. J. Clin. Investig. 1997, 27, 639–644. [Google Scholar] [CrossRef]
- Al Musaimi, O.; Al Shaer, D.; Albericio, F.; de la Torre, B. 2020 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals 2021, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Johnbeck, C.B.; Knigge, U.; Loft, A.; Berthelsen, A.K.; Mortensen, J.; Oturai, P.; Langer, S.W.; Elema, D.R.; Kjaer, A. Head-to-Head Comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: A Prospective Study of 59 Patients with Neuroendocrine Tumors. J. Nucl. Med. 2017, 58, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Kuyumcu, S.; Özkan, Z.G.; Sanli, Y.; Yilmaz, E.; Mudun, A.; Adalet, I.; Unal, S. Physiological and tumoral uptake of 68Ga-DOTATATE: Standardized uptake values and challenges in interpretation. Ann. Nucl. Med. 2013, 27, 538–545. [Google Scholar] [CrossRef]
- Sansone, A.; Lauretta, R.; Vottari, S.; Chiefari, A.; Barnabei, A.; Romanelli, F.; Appetecchia, M. Specific and Non-Specific Biomarkers in Neuroendocrine Gastroenteropancreatic Tumors. Cancers 2019, 11, 1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimstra, D.S.; Klöppel, G.; La Rosa, S.; Rindi, G. Classification of neuroendocrine neoplasms of the digestive system. In WHO Classification of Tumours, 5th ed.; WHO Classification of Tumours Editorial Board, Ed.; Digestive System Tumours; IARC: Lyon, France, 2019; pp. 16–19. [Google Scholar]
- Geijer, H.; Breimer, L.H. Somatostatin receptor PET/CT in neuroendocrine tumours: Update on systematic review and meta-analysis. Eur. J. Pediatr. 2013, 40, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.H.; Langer, S.W.; Federspiel, B.H.; Oxbøl, J.; Loft, A.; Berthelsen, A.K.; Mortensen, J.; Oturai, P.; Knigge, U.; Kjær, A. (68)Ga-DOTATOC PET and gene expression profile in patients with neuroendocrine carcinomas: Strong correlation between PET tracer uptake and gene expression of somatostatin receptor subtype 2. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 59–72. [Google Scholar] [PubMed]
- Boy, C.; Heusner, T.A.; Poeppel, T.D.; Redmann-Bischofs, A.; Unger, N.; Jentzen, W.; Brandau, W.; Mann, K.; Antoch, G.; Bockisch, A.; et al. 68Ga-DOTATOC PET/CT and somatostatin receptor (sst1–sst5) expression in normal human tissue: Correlation of sst2 mRNA and SUVmax. Eur. J. Pediatr. 2011, 38, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Kaemmerer, D.; Peter, L.; Lupp, A.; Schulz, S.; Sänger, J.; Prasad, V.; Kulkarni, H.; Haugvik, S.-P.; Hommann, M.; Baum, R.P. Molecular imaging with 68Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur. J. Pediatr. 2011, 38, 1659–1668. [Google Scholar] [CrossRef]
- Binderup, T.; Knigge, U.; Mogensen, A.M.; Hansen, C.P.; Kjaer, A. Quantitative Gene Expression of Somatostatin Receptors and Noradrenaline Transporter Underlying Scintigraphic Results in Patients with Neuroendocrine Tumors. Neuroendocrinology 2008, 87, 223–232. [Google Scholar] [CrossRef]
- Lee, H.; Nakamoto, R.; Moore, S.E.; Pantel, A.R.; Eads, J.R.; Aparici, C.M.; Pryma, D.A. Combined Quantification of 18F-FDG and 68Ga-DOTATATE PET/CT for Prognosis in High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms. Acad. Radiol. 2021. [Google Scholar] [CrossRef]
- Panagiotidis, E.; Alshammari, A.; Michopoulou, S.; Skoura, E.; Naik, K.; Maragkoudakis, E.; Mohmaduvesh, M.; Al-Harbi, M.; Belda, M.; Caplin, M.E.; et al. Comparison of the Impact of 68Ga-DOTATATE and 18F-FDG PET/CT on Clinical Management in Patients with Neuroendocrine Tumors. J. Nucl. Med. 2016, 58, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Mapelli, P.; Partelli, S.; Salgarello, M.; Doraku, J.; Muffatti, F.; Lena, M.S.; Pasetto, S.; Bezzi, C.; Bettinardi, V.; Andreasi, V.; et al. Dual Tracer 68Ga-DOTATOC and 18F-FDG PET Improve Preoperative Evaluation of Aggressiveness in Resectable Pancreatic Neuroendocrine Neoplasms. Diagnostics 2021, 11, 192. [Google Scholar] [CrossRef]
- Ambrosini, V.; Zanoni, L.; Filice, A.; Lamberti, G.; Argalia, G.; Fortunati, E.; Campana, D.; Versari, A.; Fanti, S. Radiolabeled Somatostatin Analogues for Diagnosis and Treatment of Neuroendocrine Tumors. Cancers 2022, 14, 1055. [Google Scholar] [CrossRef]
- Cingarlini, S.; Ortolani, S.; Salgarello, M.; Butturini, G.; Malpaga, A.; Malfatti, V.; D’Onofrio, M.; Davì, M.V.; Vallerio, P.; Ruzzenente, A.; et al. Role of Combined 68Ga-DOTATOC and 18F-FDG Positron Emission Tomography/Computed Tomography in the Diagnostic Workup of Pancreas Neuroendocrine Tumors. Pancreas 2017, 46, 42–47. [Google Scholar] [CrossRef]
- Mapelli, P.; Partelli, S.; Salgarello, M.; Doraku, J.; Pasetto, S.; Rancoita, P.M.; Muffatti, F.; Bettinardi, V.; Presotto, L.; Andreasi, V.; et al. Dual tracer 68Ga-DOTATOC and 18F-FDG PET/computed tomography radiomics in pancreatic neuroendocrine neoplasms: An endearing tool for preoperative risk assessment. Nucl. Med. Commun. 2020, 41, 896–905. [Google Scholar] [CrossRef]
- Kayani, I.; Conry, B.G.; Groves, A.M.; Win, T.; Dickson, J.; Caplin, M.; Bomanji, J.B. A Comparison of 68Ga-DOTATATE and 18F-FDG PET/CT in Pulmonary Neuroendocrine Tumors. J. Nucl. Med. 2009, 50, 1927–1932. [Google Scholar] [CrossRef] [Green Version]
- Zidan, L.; Iravani, A.; Kong, G.; Akhurst, T.; Michael, M.; Hicks, R.J. Theranostic implications of molecular imaging phenotype of well-differentiated pulmonary carcinoid based on 68Ga-DOTATATE PET/CT and 18F-FDG PET/CT. Eur. J. Pediatr. 2020, 48, 204–216. [Google Scholar] [CrossRef]
- Tatci, E.; Ozmen, O.; Gokcek, A.; Biner, I.U.; Ozaydin, E.; Kaya, S.; Arslan, N. 18F-FDG PET/CT rarely provides additional information other than primary tumor detection in patients with pulmonary carcinoid tumors. Ann. Thorac. Med. 2014, 9, 227–231. [Google Scholar] [CrossRef]
- Venkitaraman, B.; Karunanithi, S.; Kumar, A.; Khilnani, G.C.; Kumar, R. Role of 68Ga-DOTATOC PET/CT in initial evaluation of patients with suspected bronchopulmonary carcinoid. Eur. J. Pediatr. 2014, 41, 856–864. [Google Scholar] [CrossRef]
- Komek, H.; Can, C.; Urakçi, Z.; Kepenek, F. Comparison of (18F)FDG PET/CT and (68Ga)DOTATATE PET/CT imaging methods in terms of detection of histological subtype and related SUVmax values in patients with pulmonary carcinoid tumors. Nucl. Med. Commun. 2019, 40, 517–524. [Google Scholar] [CrossRef]
- Lamberts, S.; Barker, W.; Reubi, J.-C.; Krenning, E. Somatostatin-Receptor Imaging in the Localization of Endocrine Tumors. N. Engl. J. Med. 1990, 323, 1246–1249. [Google Scholar] [CrossRef]
- Sharma, P.; Dhull, V.S.; Arora, S.; Gupta, P.; Kumar, R.; Durgapal, P.; Malhotra, A.; Chumber, S.; Ammini, A.C.; Kumar, R.; et al. Diagnostic accuracy of 68Ga-DOTANOC PET/CT imaging in pheochromocytoma. Eur. J. Pediatr. 2013, 41, 494–504. [Google Scholar] [CrossRef]
- Han, S.; Suh, C.H.; Woo, S.; Kim, Y.J.; Lee, J.J. Performance of 68Ga-DOTA–Conjugated Somatostatin Receptor–Targeting Peptide PET in Detection of Pheochromocytoma and Paraganglioma: A Systematic Review and Metaanalysis. J. Nucl. Med. 2018, 60, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Rana, M.U.; Østhus, A.A.; Heimdal, K.; Jebsen, P.; Revheim, M.-E.R.; Osnes, T.A. Head and neck paragangliomas in Norway, importance of genetics, updated diagnostic workup and treatment. Acta Oto-Laryngol. 2020, 141, 303–308. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Frezza, A.M.; Blay, J.; Baldini, E.H.; Bonvalot, S.; Bovée, J.V.M.G.; Callegaro, D.; Casali, P.G.; Chiang, R.C.; Demetri, G.D.; et al. Ultra-rare sarcomas: A consensus paper from the Connective Tissue Oncology Society community of experts on the incidence threshold and the list of entities. Cancer 2021, 127, 2934–2942. [Google Scholar] [CrossRef]

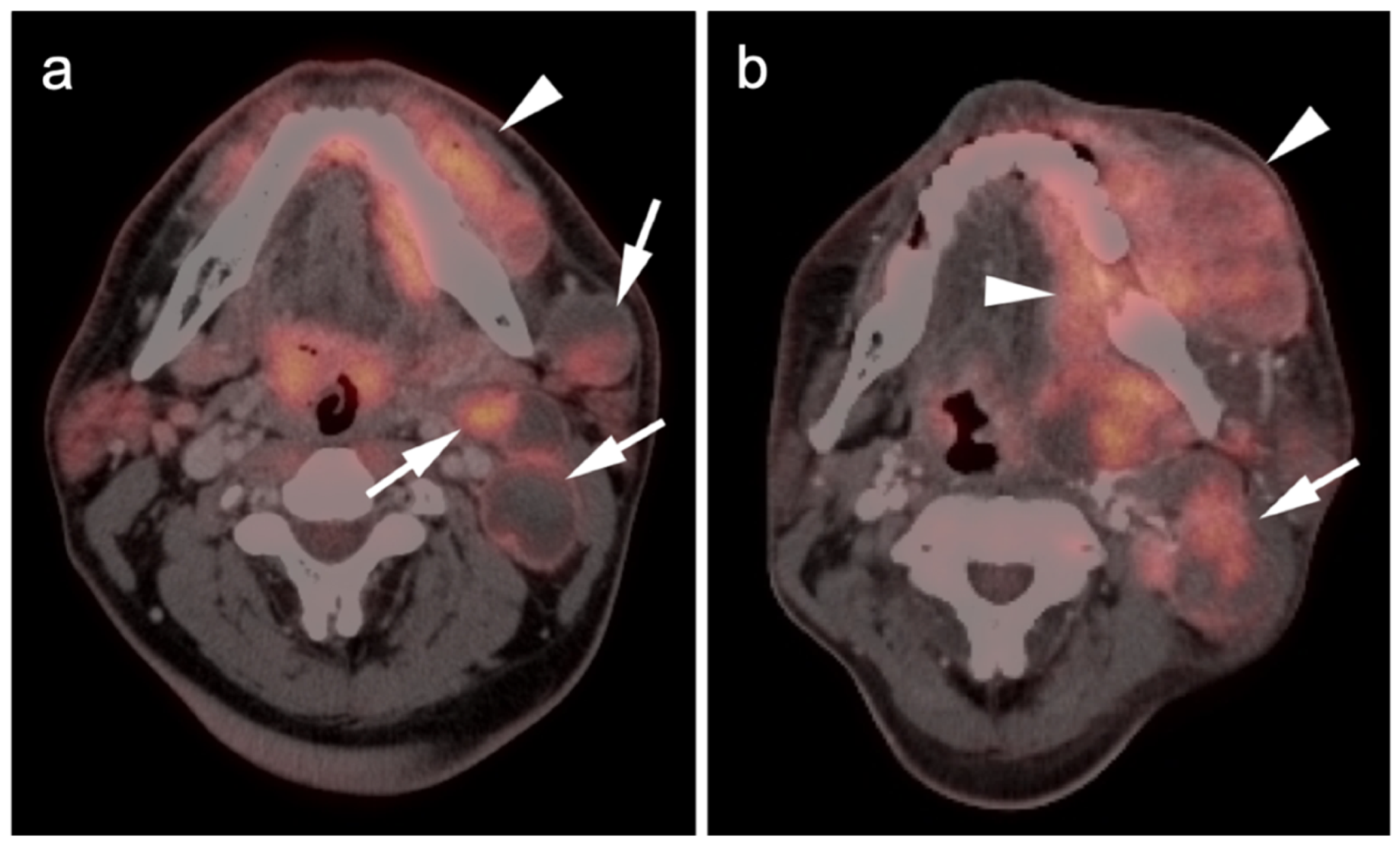




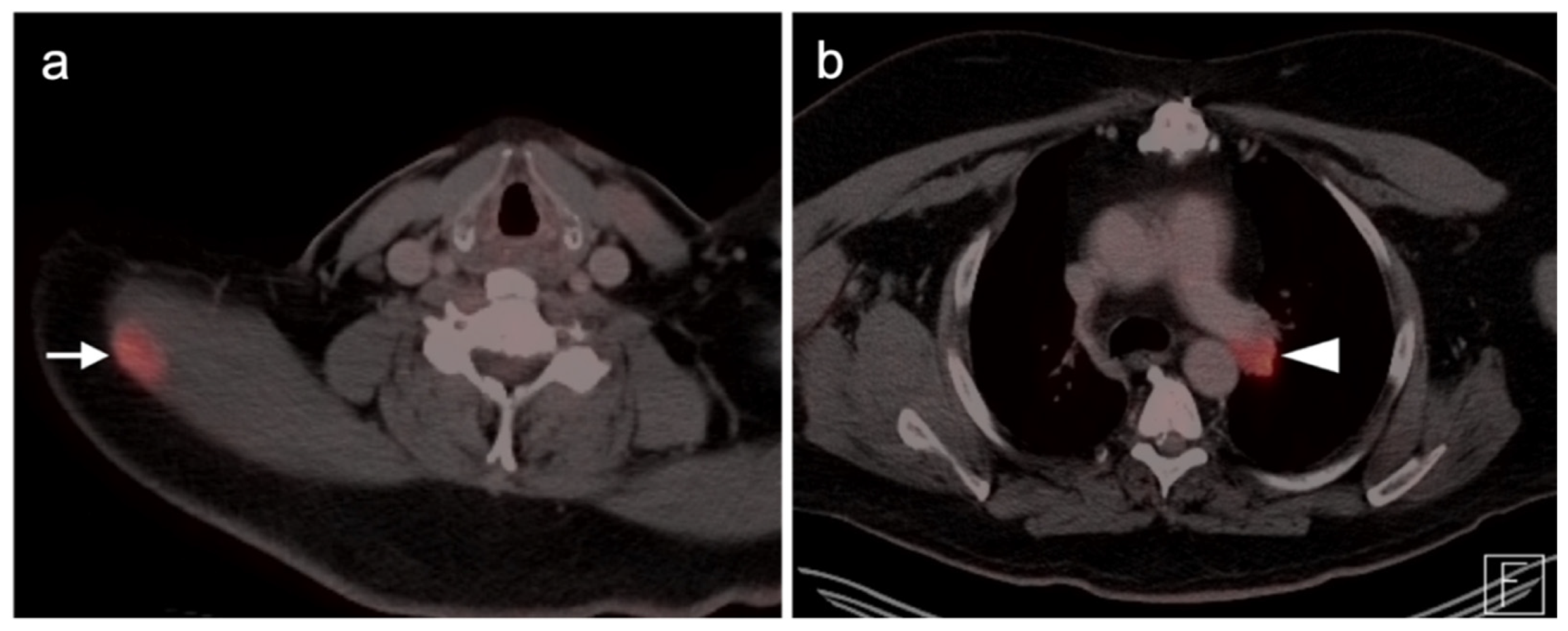
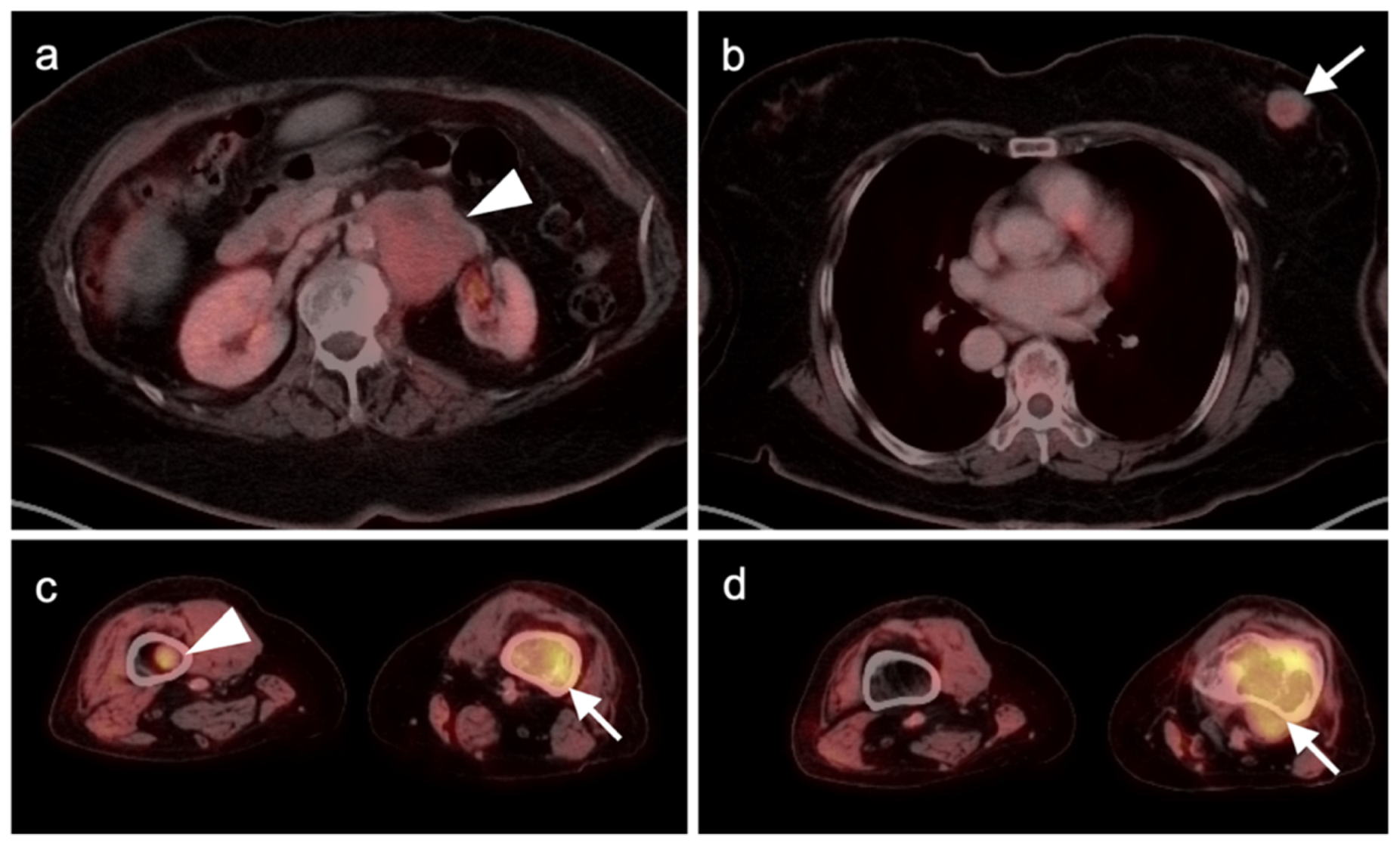
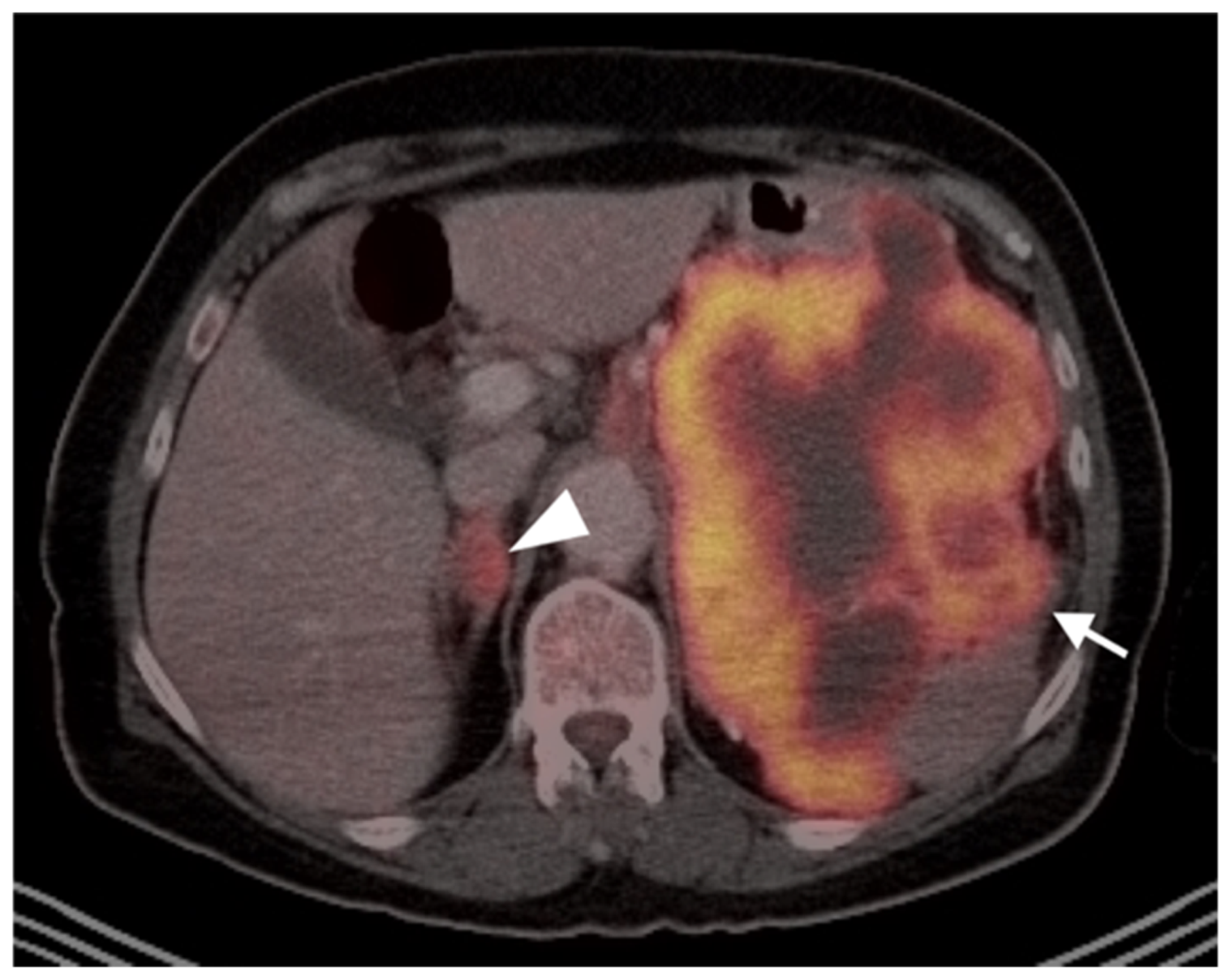





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fine, G.C.; Covington, M.F.; Koppula, B.R.; Salem, A.E.; Wiggins, R.H.; Hoffman, J.M.; Morton, K.A. PET-CT in Clinical Adult Oncology—VI. Primary Cutaneous Cancer, Sarcomas and Neuroendocrine Tumors. Cancers 2022, 14, 2835. https://doi.org/10.3390/cancers14122835
Fine GC, Covington MF, Koppula BR, Salem AE, Wiggins RH, Hoffman JM, Morton KA. PET-CT in Clinical Adult Oncology—VI. Primary Cutaneous Cancer, Sarcomas and Neuroendocrine Tumors. Cancers. 2022; 14(12):2835. https://doi.org/10.3390/cancers14122835
Chicago/Turabian StyleFine, Gabriel C., Matthew F. Covington, Bhasker R. Koppula, Ahmed Ebada Salem, Richard H. Wiggins, John M. Hoffman, and Kathryn A. Morton. 2022. "PET-CT in Clinical Adult Oncology—VI. Primary Cutaneous Cancer, Sarcomas and Neuroendocrine Tumors" Cancers 14, no. 12: 2835. https://doi.org/10.3390/cancers14122835
APA StyleFine, G. C., Covington, M. F., Koppula, B. R., Salem, A. E., Wiggins, R. H., Hoffman, J. M., & Morton, K. A. (2022). PET-CT in Clinical Adult Oncology—VI. Primary Cutaneous Cancer, Sarcomas and Neuroendocrine Tumors. Cancers, 14(12), 2835. https://doi.org/10.3390/cancers14122835





